Abstract
Tinnitus is perception of sound in the absence of an apparent external acoustic stimulus. The condition is prevalent in adults, especially the elderly (≥65 years), and may be associated with cognitive function decline and significantly impacts on the quality of life, heralding difficulties in managing this challenging disorder. Interventions for tinnitus have been varied. However, drugs have not yet been approved for the treatment of tinnitus and there is no pharmacotherapy recommended by existing guidelines. Still, herbal medicines are used for the treatment of tinnitus in many countries, especially Gingko (G.) biloba. In the current updated literature review, we evaluated the efficacy of herbal medicines in the treatment of tinnitus by reviewing the evidence of relevant randomized controlled trials. The authors also highlight some of the issues in clinical trials of herbal medicines given that currently available evidence on herbal medicines for tinnitus is overall of insufficient quality and the conclusions from existing trials are conflicting. Nevertheless, there is a clear and urgent need for safe and effective pharmacotherapy of tinnitus.
Keywords: tinnitus, clinical trials, herbal medicine, medicinal plants, dementia, depression, mood disorders
Introduction
Tinnitus is prevalent in adults, especially the elderly (≥65 years), and can be categorized to subjective tinnitus and objective tinnitus (Baguley et al., 2013; Han et al., 2021). Subjective tinnitus is defined as conscious awareness of sound in the absence of an apparent external acoustic stimulus which can only be perceived by the affected person while objective tinnitus can also be perceived by the examiner. Tinnitus can be primary without an apparent cause or secondary where a specific cause can be identified. Tinnitus affects approximately one in 10 adults in the United States (Bhatt et al., 2016). In United Kingdom, about 15% of the adult population suffer from the disorder (Biswas and Hall, 2021). According to a cross-sectional analysis of data in the United Kingdom Biobank (Dawes et al., 2020), approximately six percent of 168,348 participants aged between 40 and 69 years with hearing difficulties and tinnitus reported annoying tinnitus. The prevalence of tinnitus varies widely, from 4.3% to 51.3%, in China (Zhang et al., 2021). The presence of chronic tinnitus may be associated with cognitive function decline, especially decline of attention and emotional health, anxiety and depression (Trevis et al., 2018).
In addition, tinnitus can significantly impact on the quality of life of individuals (National Guideline, 2020) and incur an increasing economic cost (Stockdale et al., 2017). The direct and indirect costs of tinnitus treatment are considerable, and there is a direct relationship between tinnitus severity and associated costs (Trochidis et al., 2021). Aging, unhealthy lifestyles, systemic diseases, sleep disorder, exposure to noise, depression and various anxiety disorders can induce or exacerbate tinnitus (Veile et al., 2018; Chamouton and Nakamura, 2021; Dai et al., 2021; Schubert et al., 2021), heralding difficulties in managing this challenging disorder.
There are many interventions for tinnitus, including educational counselling, relaxation therapy, tinnitus retraining therapy, cognitive behavioral therapy, sound therapy, transcranial direct current stimulation, repetitive transcranial magnetic stimulation, transcutaneous electrical nerve stimulation, acupuncture, and pharmacotherapies (Langguth et al., 2019). Hitherto, no drug has been approved for the treatment of tinnitus by regulatory agencies around the world. Due to the lack of effective treatment for tinnitus, it is a common practice in many countries that dietary supplements are used for treatment of tinnitus, especially Gingko (G.) biloba and lipoflavones (Bhatt et al., 2016; Coelho et al., 2016) although these alternative treatments are not endorsed by regulatory bodies such as the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNSF) and the European guidelines. According to alternative medicine theories, dietary therapy is worthwhile considering its potential benefits, good tolerabilities and cultural acceptability (Luetzenberg et al., 2020). Enrico et al. (2007) argue that the use of complementary and alternative medicine products in the treatment of tinnitus often lacks substantial scientific support and that these substances may not be clinically effective either. No definitive conclusions can be drawn regarding the pharmacological approach to complementary and alternative medicine in the treatment of tinnitus. Hofmeister and Coelho also pointed out that further high-quality analytical studies should be conducted before recommendation by clinicians (Coelho et al., 2016; Hofmeister, 2019).
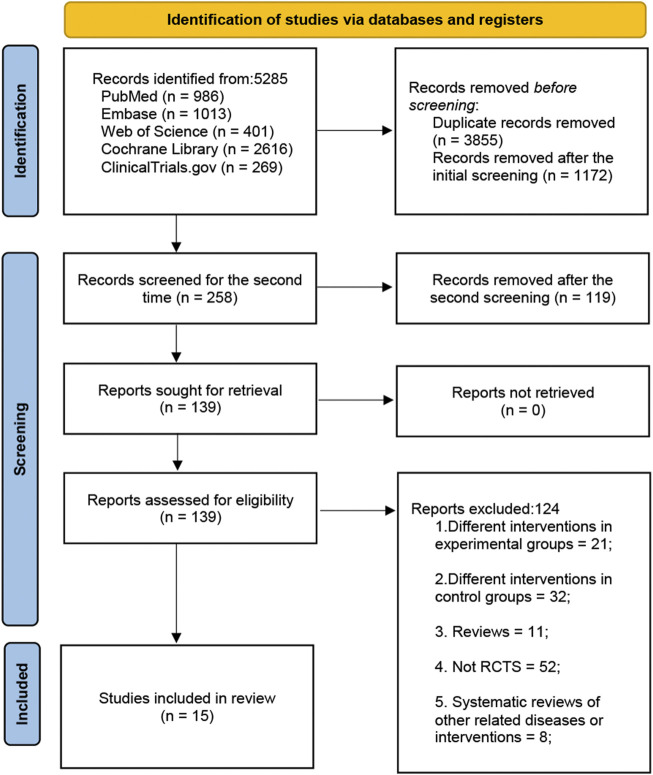
We are interested in whether herbal medicines are safe and effective in the treatment of tinnitus. In the current updated literature review, the authors evaluated the efficacy and safety of herbal medicines in the treatment of tinnitus by reviewing the evidence of relevant randomized controlled trials (RCTs). The authors searched PubMed, Embase, Web of Science, Cochrane Library and ClinicalTrials.gov for original studies on tinnitus from the date of inception to 1 February 2022. Literature with the keyword “tinnitus” in the title/abstract was eligible, with filters for “randomized controlled trial” and “controlled clinical trial.” Randomized placebo-controlled studies or studies with an active comparator were eligible. There was no language restriction. In addition, manual search was performed for additional eligible articles from the reference lists of review articles, clinical guidelines, and pairwise meta-analyses. Measures of tinnitus included Tinnitus Handicap Inventory (THI) score (Newman et al., 1996), tinnitus loudness, Visual Analogue Scale (VAS) score, Tinnitus Functional Index (TFI), Tinnitus Questionnaire (TQ) score (Richard, 1996) and effectiveness. Totally 5,285 records were identified, and 15 studies were eligible. The search process is illustrated in Figure 1. Eligible RCTs on herbal medicines in the treatment of tinnitus are summarized in Supplementary Table S1.
FIGURE 1.
PRISMA flowchart for study.
G. biloba in the treatment of tinnitus
Ginkgo [Ginkgoaceae; Gingko; G. biloba L., synonym, Salisburia biloba, Salisburia adiantifolia] has been used as a medicinal herb for over two thousand years. The leaves of G. biloba have been used for the treatment of central nervous system illnesses (Belwal et al., 2019), including Alzheimer’s disease (Nowak et al., 2021), metabolic syndrome (Eisvand et al., 2020), cardiovascular diseases (Tian et al., 2017) and a variety of other conditions (Roland and Nergård, 2012). G. biloba has been shown to protect against oxidative stress, inhibit platelet-activating factor (PAF), suppress inflammation, affect vascular smooth muscle, inhibit amyloid aggregation, and modulate gene expression (DeKosky et al., 2008). G. biloba has an abundance of bioactive compounds, and the main constituents of G. biloba leaf extract include bioflavonoids and flavonoids (such as quercetin, kaempferol, and isorhamnetine), terpene trilactones (such as ginkgolides and bilobalide), polyprenols, and organic acids (Guo et al., 2020). Standard G. biloba extract, including G. biloba extract EGb 761, (Rökan, Tanakan, Tebonin), contains approximately 24% flavone glycosides and 6% terpene lactones (2.8%–3.4% ginkgolides A, B, and C and 2.6%–3.2% bilobalide) (EGb, 2003; Unger, 2013). Dietary flavonoids are bioactive compounds that have been extensively studied for their relationship to vascular health outcomes. Because tinnitus development is associated with vascular access, dietary flavonoids such as those present in G. biloba extract have antioxidant and vasodilatory effects and may play a role in alleviating tinnitus symptoms. Preclinical and clinical studies have shown that apart from its antioxidant and vasodilatory effects, G. biloba extract may exert its actions by improving cochlear microcirculation, protects against ototoxicities, and alleviates aging associated degeneration (Barth et al., 2021).
G. biloba studies in the treatment of tinnitus are listed in Table 1. Though G. biloba extract has been investigated for the treatment of tinnitus in a number of clinical studies, including RCTs, its efficacy remains inconclusive or questionable according to recent meta-analyses (Mahmoudian-Sani et al., 2017; Kramer and Ortigoza, 2018; Spiegel et al., 2018; Sereda et al., 2022). An early randomized study of 259 patients with tinnitus of less than 1 year duration showed that G. biloba extract (Meyer, 1986) reduced the severity of tinnitus in 70% of the patients. A double-blind placebo-controlled trial of 1,121 healthy subjects with tinnitus showed that 50 mg G biloba extract LI 1370 (containing 25% flavonoids, 3% ginkgolides, and 5% bilobalides) three times daily for 12 weeks resulted in no notable improvement in tinnitus versus placebo (Drew and Davies, 2001; Rejali et al., 2004; Polanski et al., 2016). The authors also failed to find improvement in other symptoms of cerebral insufficiency with G. biloba extract. Overall, G. biloba extract was safe and had no serious side effects. In a randomized placebo-controlled double-blind trial, tinnitus patients received G. biloba extract 120 mg/day or placebo for 12 weeks. No significant difference in changes in THI scores was observed between subjects receiving G. biloba extract and those receiving placebo (p = .51) (Rejali et al., 2004). In a crossover RCT, patients with tinnitus received clonazepam (.5 mg per tablet) or G. biloba (40 mg per tablet) for the first 3 weeks and switched to the other drug after a 2-week washout and for the final 3 weeks, subjects were instructed to increase the dose by 40 mg every 3 days to a maximum of 160 mg daily until they perceived a satisfactory decrease in tinnitus loudness or intolerable side effects. The study found that G. biloba had no significant effect on tinnitus loudness, duration, annoyance, and THI score (Han et al., 2012). In an open phase study followed by a double-blind placebo-controlled study, 80 patients with persistent severe tinnitus received G. biloba (15 mg bd) in the open phase and 20 of 21 patients who reported a positive effect in the open phase went on to receive G. biloba or placebo. No significant effect on tinnitus was observed (Holgers et al., 1994).
TABLE 1.
G. biloba in the treatment of tinnitus.
| Study design (trial registration) | Patients | Interventions | Outcomes | |
|---|---|---|---|---|
| G. biloba extract EGb 761 Morgenstern and Biermann, (2002) | RCT | Chronic tinnitus aurium (n = 60) | Patients receivedG. biloba extract EGb 761 2 × 80 mg/d subsequent to 10-day EGb 761 infusion treatment (35 patients) or placebo (38 patients) | Significant change in tinnitus volume with G. biloba extract EGb 761 |
| G. biloba extract Rejali et al. (2004) | A randomized placebo-controlled double-blind trial | Tinnitus (n = 66) | Patients receivedG. biloba extract 120 mg once daily sustained release formulation or placebo for 12 weeks | A-4.7 ± 12.1 reduction in THI score vs. placebo -2.2 ± 16.7, p = .51 |
| G. biloba extract Han et al. (2012) | An open-label, randomised, crossover study | Tinnitus (n = 38) | Patients received clonazepam or G. biloba for the first 3 weeks. For the next 2 weeks of washout no medication was taken. For the final 3 weeks, subjects were given the other drug. The initial dose of clonazepam and G. biloba was one tablet daily (clonazepam .5 mg; G biloba 40 mg). Subjects were instructed to increase the dose by one tablet every 3 days to a maximum of four tablets daily until they perceived a satisfactory decrease in tinnitus loudness or intolerable side effects | G. biloba had no significant effect on tinnitus loudness, duration, annoyance, and tinnitus handicap inventory score |
| G. biloba Holgers et al. (1994) | An open phase study followed by a double-blind placebo-controlled study | Persistent severe tinnitus (n = 80) | Patients received G. biloba (15 mg bd) in the open phase (80 patients) and 20 of 21 who reported a positive effect went on to receive G. biloba (7 patients), placebo (7 patients), or either (6 patients) | No significant effect on tinnitus |
| G. bilobaextract EGb 761 Procházková et al. (2018) | Double blind RCT | Sub-chronic or chronic tinnitus (n = 200) | Patients received 120 mg G bilobaextract EGb 761 or 600 mg pentoxifylline, each twice a day for 12 weeks | G. biloba led to a significant least square mean (LSM) reduction from baseline in the abridged TQ score (−1.57, 95%CI − 2.25 to − .89; p < .001), the 11-Point Box Scale for tinnitus loudness (−.41, 95% CI − .68 to − .15, p = .002) and annoyance (−.56, 95% CI− .84 to − .27; p < .001) |
| G. biloba dry extract Polanski et al. (2016) | Double-blind RCT | Tinnitus with sensorineural hearing loss (n = 58) | Patients receivedG. biloba dry extract (120 mg/day), α-lipoic acid (60 mg/day) + vitamin C (600 mg/day), papaverine hydrochloride (100 mg/day) + vitamin E (400 mg/day), and placebo | No difference in THI before and after treatment with G. biloba dry extract |
G. biloba extract EGb 761 is the most widely tested drug in both non-clinical tinnitus models as well as in clinical trials. The extract is adjusted to 22.0%–27.0% G. flavonoids calculated as ginkgo flavonole glycosides and 5.0%–7.0% terpene lactones which consist of 2.8%–3.4% ginkgolides A, B, C and 2.6%–3.2% bilobalides and contain less than 5 ppm ginkgolic acids (Barth et al., 2021). A recent double-blind RCT of subjects with sub-chronic or chronic tinnitus showed that G. biloba extract EGb 761,120 mg, twice daily for 12 weeks caused a significant reduction from baseline in the least square mean (LSM) of the abridged TQ score (LSM − 1.57, 95%CI − 2.25 to − .89; p < .001), the 11-Point Box Scale for tinnitus loudness (LSM − .41, 95% CI − .68 to − .15, p = .002) and annoyance (LSM − .56, 95% CI− .84 to − .27; p < .001). The proportion of subjects with an abnormal Hospital Anxiety and Depression Scale (HADS) score decreased from 36% at baseline to 23% post treatment with G. biloba extract EGb 761 (p = .005). Furthermore, G. biloba extract EGb 761 had a lower rate of adverse events than its comparator pentoxifylline. This finding was supported by another parallel group RCT showing that 240 mg G biloba extract EGb 761 significantly improved self-perception of tinnitus loudness and severity after 90 days of treatment in patients with hearing loss (Procházková et al., 2018; Nishad et al., 2019; Radunz et al., 2020). In an earlier RCT of 60 patients with chronic tinnitus, Morgenstern and Biermann demonstrated that 160 mg/day G biloba extract EGb 761 subsequent to 10-day G. biloba extract EGb 761 infusion resulted in significant change in tinnitus volume compared to placebo (Morgenstern and Biermann, 2002). In a systemic review of eight clinical studies involving 1,199 patients, Boetticher found that G. biloba extract EGb 761 in sufficient dosing and treatment duration was effective for tinnitus compared to placebo (von Boetticher, 2011). The author attributed the failure of G. biloba extracts in reducing severity of tinnitus in some trials to the types of Ginkgo biloba extracts used or flaws in the trials. The inconclusive results of G. biloba extracts for tinnitus have led some investigators to question whether G. biloba extracts should be used for treatment of tinnitus (Smith et al., 2005). The inconsistencies in trial results of G. biloba extract highlight the need for further delineation of G. biloba extract components, standardization of G. biloba extract contents, and optimization of G. biloba extract doses and treatment duration in future clinical trials.
G. biloba extract as dietary supplements does not require pre-marketing approval from regulatory agencies around the world. Furthermore, G. biloba extracts prepared by different manufacturers vary in the contents of bioactive compounds. Currently, there are no treatment recommendations of G. biloba extract for tinnitus. Existing evidence from RCTs remains inconclusive for the effectiveness of G. biloba extract.
G. biloba in the treatment of tinnitus in mild to moderate dementia patients
Dementia is prevalent among the elderly and neurosensory symptoms, such as tinnitus and dizziness, are frequently reported in patients with dementia. However, dementia is often of mixed pathologies and defies treatment. G. biloba could offer a treatment option for dementia by reducing tinnitus, which is considered a sign of neurodegenerative disease and has been found to be independently associated with cognitive impairment (Jafari et al., 2019). Tinnitus is found to be a risk for development of Alzheimer’s disease (Chu et al., 2020). G. biloba is used for the treatment of early-stage Alzheimer’s disease and vascular dementia (Sierpina et al., 2003), and measures of tinnitus are now assessed in clinical trials of G. biloba extract in patients with dementia as earlier studies show that G. biloba extract could alleviate dementia-associated pathologies such as mitochondrial dysfunction and impaired hippocampal neurogenesis. In addition, tinnitus in patients with vascular dementia can represent an entity with a treatable cause given that G. biloba extract improves circulation (DeKosky et al., 2008).
G. biloba studies in the treatment of mild to moderate dementia are listed in Table 2. A double-blind RCT of 410 patients with mild to moderate dementia of vascular origin showed that treatment with G. biloba (Herrschaft et al., 2012) EGb 761,240 mg/day led to no improvement in the 11-Point Box Scale tinnitus score versus placebo. In a randomized, placebo-controlled, double-blind, parallel-group, multicenter trial, Schneider et al. demonstrated that G. biloba extract 120 mg or 240 mg for 26 weeks caused a -2.05 ± 2.09 reduction in the 11-Point Box Scale tinnitus score versus -.21 ± 1.69 with the placebo (Schneider et al., 2005). A recent meta-analysis (Spiegel et al., 2018) of five randomized placebo-controlled trials in 1972 patients with mild to moderate dementia showed a mean reduction in tinnitus severity in patients treated with G. biloba extract EGb 761, with a weighted mean difference of -1.06 (95% CI: 1.77, -.36) for tinnitus (p = .003), which corresponded to an improvement over placebo by 27%–40% of baseline severity in the individual studies.
TABLE 2.
G. biloba in the treatment of mild to moderate dementia.
| Study design (trial registration) | Patients | Interventions | Outcomes | |
|---|---|---|---|---|
| G. biloba Schneider et al. (2005) | Randomized, placebo-controlled, double-blind, parallel-group, multicenter trial | Mild to moderate dementia (n = 513) | Patients received G. biloba 120 mg or 240 mg, or placebo for 26 weeks | A-2.05 ± 2.09 reduction in 11-Point Box Scale tinnitus score vs. placebo -.21 ± 1.69 |
| G. biloba EGb 761 Herrschaft et al. (2012) | Double-blind, placebo-controlled RCT | Mild to moderate dementia with neuropsychiatric features (n = 410) | Patients received a 240 mg once-daily formulation of G. biloba extract EGb 761 or placebo for 24 weeks | No difference in 11-Point Box Scale tinnitus score between patients receiving G. biloba extract EGb 761 and those receiving placebo |
| G. bilobaEGb 761 (Napryeyenko and Borzenko, 2007) | Mild to moderate dementia with neuropsychiatric features (n = 400) | Patients received G. biloba EGb 761 or placebo for 22 weeks | A-2.11 ± 1.74 reduction in 11-Point Box Scale tinnitus score vs. placebo -.15 ± 1.01 | |
| G. biloba EGb 761 Ihl et al. (2011) | Mild to moderate dementia with neuropsychiatric features (n = 410) | Patients received 240 mg of G. biloba EGb 761 or placebo once daily for 24 weeks | A-1.11 ± 1.21 reduction in 11-Point Box Scale tinnitus score vs. placebo -.14 ± 1.92 |
Overall, these studies demonstrated that G. biloba extract benefited patients with mild to moderate dementia in terms of reducing the severity of tinnitus. However, measures of tinnitus were not assessed as a primary outcome in these trials of G. biloba extract for dementia and the results need to be interpreted with caution. In addition, depression and anxiety are prevalent in patients with tinnitus (Ziai et al., 2017). A mediation analysis showed that G. biloba extract EGb 761 directly accounted for 60% of the total effect of tinnitus severity reduction while amelioration of the symptoms of anxiety and depression and improvement in cognition contributed to 40% of the total effect (Brüggemann et al., 2021). The efficacy of G. biloba extract in reducing tinnitus severity in mild to moderate dementia patients remains to be investigated in vigorously conducted clinical trials with measures of tinnitus as the primary study end point.
Other herbal medicines for the treatment of tinnitus
Açaí
Herbal medicines other than G. biloba extract in the treatment of tinnitus are provided in Table 3. Açaí (Arecaceae; Euterpe Oleracea Martius), a fruit rich in α-tocopherols, fibers, lipids, mineral ions, and polyphenols, has been widely used for its anti-inflammatory and antioxidant properties (Benatrehina et al., 2018) It also contains flavonoids including catechin, chrysoeriol, anthocyanins and taxifolin and possesses high antioxidant capacity (de Liz et al., 2020; de Oliveira et al., 2021). Flavonoids (anthocyanins) in Açaí are suggested to be of potential activities for neurodegenerative diseases such as Parkinson’s disease (de Oliveira et al., 2019). In a double blind RCT, patients with chronic tinnitus received açai extract 100 mg or placebo (Oppitz et al., 2022). A significant reduction in THI score (-10.0 ± 6.4, p = .006) was observed in patients receiving açai extract 100 mg while no remarkable decrease in THI score was found in the placebo group (-8.8 ± 3.6, p = .093). A significant reduction in the Beck Anxiety Inventory (BAI) score was also observed in patients receiving açai extract 100 mg (p = .007). Though noted for its potent antioxidant capacity (de Liz et al., 2020; de Oliveira et al., 2021), Açai extract caused no significant changes in oxidative stress biomarkers. No other clinical evidence is currently available on the safety and efficacy of Açaí in patients with tinnitus. Additional trials are required to establish the efficacy of Açaí.
TABLE 3.
Herbal Medicines other than G. biloba in the Treatment of Tinnitus.
| Medicine herbs | Study design (trial registration) | Patients | Interventions | Outcomes |
|---|---|---|---|---|
| Açaí (Euterpe Oleracea Martius) Oppitz et al. (2022) | Double blind RCT (RBR-8z4mhq) | Chronic tinnitus (n = 30) | Patients received açai extract 100 mg or placebo | Mean difference from baseline THI -10.0 ± 6.4 and -8.8 ± 3.6 |
| Korean red ginseng Kim et al. (2015) | Open-label RCT | Chronic tinnitus (n = 61) | Patients received Korean red ginseng 1,500 mg/day or 3,000 mg/day, or G. biloba extract 160 mg/day for 4 weeks | Mean difference from baseline THI 4.38 ± 2.31, 8.05 ± 2.33, and 4.05 ± 2.22 |
| Gushen Pian Zhai et al. (2013) | Double blind RCT (Z20080046) | Non-hereditary acquired sensorineural deafness with associated tinnitus or simple tinnitus without hypoacusis (n = 120) | Patients received Gushen Pian 5 tablets (40 patients), Erlong Zuoci Pills (40 patients), or placebo (40 patients) for 4 weeks | Total effective rate, 89.2%, 74.3% and 30.8%; total relief rate, 59.5%, 57.1% and 5.1% |
| Bushen Huoxue Tongluo Zhang et al. (2022) | Assessor-blinded RCT | Chronic subjective tinnitus (n = 20) | Patients received Bushen Huoxue Tongluo granules and informative counseling, or informative counseling alone | The trial is ongoing. THI, tinnitus functional index, tinnitus sensation level, self-rated visual analogue scale on tinnitus loudness and annoyance, Pittsburgh Sleep Quality Index and adverse event |
Korean red ginseng
Korean red ginseng (Araliaceae; Panax ginseng C.A.Mey) has been used in folklore medicine for 2000 years and is currently being investigated for a variety of conditions including metabolic syndrome (Aminifard et al., 2021), inflammatory bowel disease (Kang et al., 2021) and diabetes (Liu et al., 2021). Studies have shown that Korean red ginseng has otoprotective activities including alleviation of cisplatin-induced ototoxicity in vitro and in vivo (Im et al., 2010), attenuation of noise-induced hearing loss (Mungan Durankaya et al., 2021) and amelioration of cochlear damage (Tian et al., 2013) and vestibular dysfunction (Tian et al., 2014). In an open-label RCT, patients with chronic tinnitus received Korean red ginseng 1,500 mg/day or 3,000 mg/day, or G. biloba extract 160 mg/day for 4 weeks. The authors found that only patients receiving Korean red ginseng 3,000 mg/day achieved a significant mean reduction in THI (-8.05 ± 2.33, p < .05). Notably, patients receiving Korean red ginseng 3,000 mg/day also showed significant improvement in role emotional and mental health assessed using short-form 36 (SF-36) (Kim et al., 2015). A recent meta-analysis of 36 RCTs were included with 2,761 participants showed that currently available agents including amitriptyline, acamprosate, and gabapentin, and intra-tympanic dexamethasone injection plus oral melatonin did not improve the quality of life despite demonstrable improvement in tinnitus severity and response rate of patients with tinnitus of no specific or treatable origin (Chen et al., 2021). Given that tinnitus severely impairs the quality of life of approximately 1%–2% of patients, it remains important to investigate the efficacy of Korean red ginseng and its effect on the quality of life of patients with tinnitus.
Gushen Pian
Per traditional Chinese medicine theory, chronic subjective tinnitus is mainly caused by the insufficiency of essence to maintain normal kidney function, Qiu et al. (2015) stagnation and flow of blood in the meridian through the ear. Tonification of the essence of the kidney, improvement of blood flow, and dredging of the meridian passage around the ear could alleviate chronic tinnitus (Wang and Zheng, 2011). Gushen Pian, a herbal mixture consisting of Drynaria fortunei, Danshen (Salvia miltiorrhiza Bunge [Lamiaceae; Salviae miltiorrhizae radix et rhizoma]), licorice [Leguminosae/Fabacea; Glycyrrhiza glabra L.], and Calcined Ci Shi, has been reported to increase blood circulation, remove stasis, recuperate kidney, benefit essence of life and ventilate the ear (Zhai et al., 2013) and was found to be effective in the treatment of sensorineural deafness and hearing loss (Wang et al., 1996). In a double blind RCT (Z20080046) (Zhai et al., 2013), Gushen Pian displayed statistically significant therapeutic outcomes over placebo after 4 weeks of treatment with an overall effective rate of 89.2% versus 30.8% for placebo and an overall relief rate of 59.5% versus 5.1% for placebo for tinnitus. Currently, a pilot, assessor-blinded, randomized clinical trial (ChiCTR2100046632) of a traditional Chinese medicine formula, Bushen Huoxue Tongluo, is ongoing and will provide preliminary data on THI, self-rated VAS on tinnitus loudness and annoyance (Zhang et al., 2022). However, robust multicenter RCTs involving a large population size are still lacking.
Limitations
The current review has several limitations. Despite abundant literature on herbal medicines for tinnitus, there are very few vigorously conducted clinical trials with measures of tinnitus as the primary study end point, which limits the effectiveness of the current analysis. In addition, G. bibola extract has not been standardized and different G. bibola extracts may be used in trials, making comparison across trials difficult. The conclusion of the current analysis may be constrained by the small sample size of the included trials. Furthermore, G. biloba compounds and their effects on tinnitus have not been investigated in randomized trials and further studies on the molecular mechanisms of action of G. bibola extracts should be conducted in the future.
Conclusion
Tinnitus is a highly prevalent condition and becomes increasingly common with rising ages. Multiple mechanisms are implicated in the pathogenesis of tinnitus including maladaptive neuroplasticity (Shore et al., 2016), vascular dysfunction (Farri et al., 1998), oxidative stress (Celik and Koyuncu, 2018), and genetic disposition (Celik and Koyuncu, 2018; Wells et al., 2021; Xie et al., 2021). In addition, tinnitus is often found in patients with comorbidities such as depression and anxiety which may complicate treatment as well as assessment of effect of treatment in tinnitus patients. The heterogeneous nature of tinnitus suggests that combination treatment targeting diverse pathogenetic mechanisms and active management of comorbidities are required for effective management of tinnitus. G. biloba is the most widely investigated herbal medicine for tinnitus, but clinical trials have yielded conflicting results. Lack of G . biloba extract standardization, inadequate sample size, lack of optimization of treatment dose and duration, and poor study design are some of the issues hampering the study of G. biloba extract and other herbal medicines for tinnitus. As tinnitus is multifactorial, careful selection of subjects for a clinical trial is encouraged to minimize a variable population response and identify which patient subpopulation benefits from a particular treatment. Quality of life measures should also be incorporated into future studies given the impact of tinnitus on daily functioning of the patients.
Currently available evidence on herbal medicines for tinnitus is overall of insufficient quality and the conclusions from existing RCTs are contradictory. Nevertheless, the need is clear for effective pharmacotherapy for tinnitus, it is hoped that advances in basic science research on the mechanisms of tinnitus and further phytochemical and biological characterization of herbal medicines will eventually lead to a safe and effective pharmacological treatment for tinnitus.
Author contributions
DL, YH, XM, DW, and YD contributed to the study conception and design. All authors collected the data and performed the data analysis. All authors contributed to the interpretation of the data and the completion of figures and tables. All authors contributed to the drafting of the article and final approval of the submitted version.
Funding
This work was supported by grants obtained from the National Natural Science Foundation of China (Grant No. 92168115, YD); the Science Foundation for Outstanding Young Scholars of Liaoning Province (Grant No. 2022-YQ-16, YD); 345 Talent Project of Shengjing Hospital (Grant No. M1400, YD); National Key R&D Program “Active Health and Aging Technology Response” (Grant No. 2020YFC2005200, XM), and the Project of City-University Cooperation (Grant no. 2400022047, YD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AAO-HNSF, American Academy of Otolaryngology-Head and Neck Surgery; RCTs, randomized controlled trials; THI, Tinnitus Handicap Inventory; VAS, Visual Analogue Scale; TFI, Tinnitus Functional Index; TQ, Tinnitus Questionnaire.
References
- Aminifard T., Razavi B. M., Hosseinzadeh H. (2021). The effects of ginseng on the metabolic syndrome: An updated review. Food Sci. Nutr. 9 (9), 5293–5311. Epub 2021/09/18PubMed PMID: 34532035; PubMed Central PMCID: PMCPMC8441279. 10.1002/fsn3.2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley D., McFerran D., Hall D. (2013). Tinnitus. Lancet 382 (9904), 1600–1607. Epub 2013/07/06PubMed PMID: 23827090. 10.1016/s0140-6736(13)60142-7 [DOI] [PubMed] [Google Scholar]
- Barth S. W., Lehner M. D., Dietz G. P. H., Schulze H. (2021). Pharmacologic treatments in preclinical tinnitus models with special focus on Ginkgo biloba leaf extract EGb 761®. Mol. Cell Neurosci. 116, 103669. Epub 2021/09/25PubMed PMID: 34560255. 10.1016/j.mcn.2021.103669 [DOI] [PubMed] [Google Scholar]
- Belwal T., Giri L., Bahukhandi A., Tariq M., Kewlani P., Bhatt I. D., et al. (2019). “Ginkgo biloba,” in Nonvitamin and nonmineral nutritional supplements. Editors Nabavi S. M., Silva A. S. (Amsterdam, Netherlands: Academic Press; ), 241–250. [Google Scholar]
- Benatrehina P. A., Pan L., Naman C. B., Li J., Kinghorn A. D. (2018). Usage, biological activity, and safety of selected botanical dietary supplements consumed in the United States. J. Tradit. Complement. Med. 8 (2), 267–277. Epub 2018/05/08PubMed PMID: 29736381; PubMed Central PMCID: PMCPMC5934707. 10.1016/j.jtcme.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt J. M., Lin H. W., Bhattacharyya N. (2016). Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol. Head. Neck Surg. 142 (10), 959–965. Epub 2016/10/22PubMed PMID: 27441392; PubMed Central PMCID: PMCPMC5812683. 10.1001/jamaoto.2016.1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas R., Hall D. A. (2021). Prevalence, incidence, and risk factors for tinnitus. Curr. Top. Behav. Neurosci. 51, 3–28. Epub 2020/08/26PubMed PMID: 32840860. 10.1007/7854_2020_154 [DOI] [PubMed] [Google Scholar]
- Brüggemann P., Sória M. G., Brandes-Schramm J., Mazurek B. (2021). The influence of depression, anxiety and cognition on the treatment effects of ginkgo biloba extract EGb 761(®) in patients with tinnitus and dementia: A mediation analysis. J. Clin. Med. 10 (14), 3151. Epub 2021/07/25PubMed PMID: 34300317; PubMed Central PMCID: PMCPMC8307082. 10.3390/jcm10143151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik M., Koyuncu İ. (2018). A comprehensive study of oxidative stress in tinnitus patients. Indian J. Otolaryngol. Head. Neck Surg. 70 (4), 521–526. Epub 2018/11/23PubMed PMID: 30464909; PubMed Central PMCID: PMCPMC6224822. 10.1007/s12070-018-1464-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamouton C. S., Nakamura H. Y. (2021). Profile and prevalence of people with tinnitus: A health survey. Codas 33 (6), e20200293. Epub 2021/09/30PubMed PMID: 34586328. 10.1590/2317-1782/20202020293 [DOI] [PubMed] [Google Scholar]
- Chen J. J., Chen Y. W., Zeng B. Y., Hung C. M., Zeng B. S., Stubbs B., et al. (2021). Efficacy of pharmacologic treatment in tinnitus patients without specific or treatable origin: A network meta-analysis of randomised controlled trials. EClinicalMedicine 39, 101080. Epub 2021/10/07PubMed PMID: 34611615; PubMed Central PMCID: PMCPMC8478678. 10.1016/j.eclinm.2021.101080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H. T., Liang C. S., Yeh T. C., Hu L. Y., Yang A. C., Tsai S. J., et al. (2020). Tinnitus and risk of Alzheimer's and Parkinson's disease: A retrospective nationwide population-based cohort study. Sci. Rep. 10 (1), 12134. Epub 2020/07/24PubMed PMID: 32699252; PubMed Central PMCID: PMCPMC7376045. 10.1038/s41598-020-69243-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C., Tyler R., Ji H., Rojas-Roncancio E., Witt S., Tao P., et al. (2016). Survey on the effectiveness of dietary supplements to treat tinnitus. Am. J. Audiol. 25 (3), 184–205. Epub 2016/09/30PubMed PMID: 27681261. 10.1044/2016_aja-16-0021 [DOI] [PubMed] [Google Scholar]
- Dai J. Q., Pang Y., Chen Z. Q., Wang S. J., Peng B., Xu H., et al. (2021). Epidemiological investigation of tinnitus in sichuan and chongqing. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 56 (11), 1164–1173. Epub 2021/11/10PubMed PMID: 34749455. 10.3760/cma.j.cn115330-20201019-00816 [DOI] [PubMed] [Google Scholar]
- Dawes P., Newall J., Stockdale D., Baguley D. M. (2020). Natural history of tinnitus in adults: A cross-sectional and longitudinal analysis. BMJ Open 10 (12), e041290. Epub 2020/12/12PubMed PMID: 33303456; PubMed Central PMCID: PMCPMC7733183. 10.1136/bmjopen-2020-041290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Liz S., Cardoso A. L., Copetti C. L. K., Hinnig P. F., Vieira F. G. K., da Silva E. L., et al. (2020). Açaí (Euterpe oleracea Mart.) and juçara (Euterpe edulis Mart.) juices improved HDL-c levels and antioxidant defense of healthy adults in a 4-week randomized cross-over study. Clin. Nutr. 39 (12), 3629–3636. Epub 2020/05/01PubMed PMID: 32349893. 10.1016/j.clnu.2020.04.007 [DOI] [PubMed] [Google Scholar]
- de Oliveira N. K. S., Almeida M. R. S., Pontes F. M. M., Barcelos M. P., de Paula da Silva C. H. T., Rosa J. M. C., et al. (2019). Antioxidant effect of flavonoids present in Euterpe oleracea Martius and neurodegenerative diseases: A literature review. Cent. Nerv. Syst. Agents Med. Chem. 19 (2), 75–99. Epub 2019/05/07PubMed PMID: 31057125. 10.2174/1871524919666190502105855 [DOI] [PubMed] [Google Scholar]
- de Oliveira N. K. S., Almeida M. R. S., Pontes F. M. M., Barcelos M. P., Silva G. M., de Paula da Silva C. H. T., et al. (2021). Molecular docking, physicochemical properties, pharmacokinetics and toxicity of flavonoids present in Euterpe oleracea Martius. Curr. Comput. Aided Drug Des. 17 (4), 589–617. Epub 2020/06/21PubMed PMID: 32560610. 10.2174/1573409916666200619122803 [DOI] [PubMed] [Google Scholar]
- DeKosky S. T., Williamson J. D., Fitzpatrick A. L., Kronmal R. A., Ives D. G., Saxton J. A., et al. (2008). Ginkgo biloba for prevention of dementia: A randomized controlled trial. Jama 300 (19), 2253–2262. Epub 2008/11/20PubMed PMID: 19017911; PubMed Central PMCID: PMCPMC2823569. 10.1001/jama.2008.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew S., Davies E. (2001). Effectiveness of ginkgo biloba in treating tinnitus: Double blind, placebo controlled trial. Bmj 322 (7278), 73. Epub 2001/01/13PubMed PMID: 11154618; PubMed Central PMCID: PMCPMC26593. 10.1136/bmj.322.7278.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGb (2003). EGb 761: Ginkgo biloba extract, ginkor. Drugs R. D. 4 (3):188–193. Epub 2003/05/22PubMed PMID: 12757407. 10.2165/00126839-200304030-00009 [DOI] [PubMed] [Google Scholar]
- Eisvand F., Razavi B. M., Hosseinzadeh H. (2020). The effects of ginkgo biloba on metabolic syndrome: A review. Phytother. Res. 34 (8), 1798–1811. Epub 2020/02/26PubMed PMID: 32097990. 10.1002/ptr.6646 [DOI] [PubMed] [Google Scholar]
- Enrico P., Sirca D., Mereu M. (2007). Antioxidants, minerals, vitamins, and herbal remedies in tinnitus therapy. Prog. Brain Res. 166, 323–330. Epub 2007/10/25PubMed PMID: 17956795. 10.1016/s0079-6123(07)66029-4 [DOI] [PubMed] [Google Scholar]
- Farri A., Cammarota R., Enrico A. (1998). The use of Ginkgo biloba extract associated with magnesium and arginyne in patients with tinnitus of a vascular origin. Riv. Ital. Otorinolaringol. Audiol. Foniatr. 18 (37–39). [Google Scholar]
- Guo J., Wu Y., Wang G., Wang T., Cao F. (2020). Integrated analysis of the transcriptome and metabolome in young and mature leaves of Ginkgo biloba L. Industrial Crops Prod. 143, 111906. 10.1016/j.indcrop.2019.111906 [DOI] [Google Scholar]
- Han B. I., Lee H. W., Ryu S., Kim J. S. (2021). Tinnitus update. J. Clin. Neurol. 17 (1), 1–10. Epub 2021/01/23PubMed PMID: 33480192; PubMed Central PMCID: PMCPMC7840320. 10.3988/jcn.2021.17.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S. S., Nam E. C., Won J. Y., Lee K. U., Chun W., Choi H. K., et al. (2012). Clonazepam quiets tinnitus: A randomised crossover study with ginkgo biloba. J. Neurol. Neurosurg. Psychiatry 83 (8), 821–827. Epub 2012/05/26PubMed PMID: 22626945. 10.1136/jnnp-2012-302273 [DOI] [PubMed] [Google Scholar]
- Herrschaft H., Nacu A., Likhachev S., Sholomov I., Hoerr R., Schlaefke S. (2012). Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 46 (6), 716–723. Epub 2012/03/31PubMed PMID: 22459264. 10.1016/j.jpsychires.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Hofmeister M. (2019). Do dietary factors significantly influence tinnitus? Aust. J. Gen. Pract. 48 (3), 153–157. Epub 2019/07/01PubMed PMID: 31256469. 10.31128/ajgp-07-18-4643 [DOI] [PubMed] [Google Scholar]
- Holgers K. M., Axelsson A., Pringle I. (1994). Ginkgo biloba extract for the treatment of tinnitus. Audiology 33 (2), 85–92. Epub 1994/03/01PubMed PMID: 8179518. 10.3109/00206099409071870 [DOI] [PubMed] [Google Scholar]
- Im G. J., Chang J. W., Choi J., Chae S. W., Ko E. J., Jung H. H. (2010). Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother. Res. 24 (4), 614–621. Epub 2009/12/19PubMed PMID: 20020438. 10.1002/ptr.3082 [DOI] [PubMed] [Google Scholar]
- Jafari Z., Kolb B. E., Mohajerani M. H. (2019). Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res. Rev. 56, 100963. Epub 2019/09/27PubMed PMID: 31557539. 10.1016/j.arr.2019.100963 [DOI] [PubMed] [Google Scholar]
- Kang Z., Zhonga Y., Wu T., Huang J., Zhao H., Liu D. (2021). Ginsenoside from ginseng: A promising treatment for inflammatory bowel disease. Pharmacol. Rep. 73 (3), 700–711. Epub 2021/01/20PubMed PMID: 33462754; PubMed Central PMCID: PMCPMC8180475. 10.1007/s43440-020-00213-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. S., Lee H. S., Chung J. W. (2015). The effect of Korean red ginseng on symptoms and quality of life in chronic tinnitus: A randomized, open-label pilot study. J. Audiol. Otol. 19 (2), 85–90. Epub 2015/09/29PubMed PMID: 26413574; PubMed Central PMCID: PMCPMC4582451. 10.7874/jao.2015.19.2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer F., Ortigoza Á. (2018). Ginkgo biloba for the treatment of tinnitus. Medwave 18 (6), e7295. Epub 2018/10/20PubMed PMID: 30339143. 10.5867/medwave.2018.06.7294 [DOI] [PubMed] [Google Scholar]
- Langguth B., Elgoyhen A. B., Cederroth C. R. (2019). Therapeutic approaches to the treatment of tinnitus. Annu. Rev. Pharmacol. Toxicol. 59, 291–313. Epub 2018/07/26PubMed PMID: 30044727. 10.1146/annurev-pharmtox-010818-021556 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang H., Dai X., Zhu R., Chen B., Xia B., et al. (2021). A comprehensive review on the phytochemistry, pharmacokinetics, and antidiabetic effect of Ginseng. Phytomedicine 92, 153717. Epub 2021/09/29PubMed PMID: 34583224. 10.1016/j.phymed.2021.153717 [DOI] [PubMed] [Google Scholar]
- Luetzenberg F. S., Babu S., Seidman M. D. (2020). Alternative treatments of tinnitus: Alternative medicine. Otolaryngol. Clin. North Am. 53 (4), 637–650. Epub 2020/05/05PubMed PMID: 32362562. 10.1016/j.otc.2020.03.011 [DOI] [PubMed] [Google Scholar]
- Mahmoudian-Sani M. R., Hashemzadeh-Chaleshtori M., Asadi-Samani M., Yang Q. (2017). Ginkgo biloba in the treatment of tinnitus: An updated literature review. Int. Tinnitus J. 21 (1), 58–62. Epub 2017/07/21PubMed PMID: 28723603. 10.5935/0946-5448.20170011 [DOI] [PubMed] [Google Scholar]
- Meyer B. (1986). A multicenter study of tinnitus. Epidemiology and therapy. Ann. Otolaryngol. Chir. Cervicofac. 103 (3), 185–188. Epub 1986/01/01. PubMed PMID: 3530094. [PubMed] [Google Scholar]
- Morgenstern C., Biermann E. (2002). The efficacy of Ginkgo special extract EGb 761 in patients with tinnitus. Int. J. Clin. Pharmacol. Ther. 40 (5), 188–197. Epub 2002/06/08PubMed PMID: 12051570. 10.5414/cpp40188 [DOI] [PubMed] [Google Scholar]
- Mungan Durankaya S., Olgun Y., Aktaş S., Eskicioğlu H. E., Gürkan S., Altun Z., et al. (2021). Effect of Korean red ginseng on noise-induced hearing loss. Turk Arch. Otorhinolaryngol. 59 (2), 111–117. Epub 2021/08/14PubMed PMID: 34386797; PubMed Central PMCID: PMCPMC8329393. 10.4274/tao.2021.2021-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napryeyenko O., Borzenko I. (2007). Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung 57 (1), 4–11. [DOI] [PubMed] [Google Scholar]
- National Guideline C. (2020). “NICE evidence reviews collection,” in Evidence review for assessing quality of life: Tinnitus: Assessment and management: Evidence review G (London: National Institute for Health and Care Excellence (NICE) Copyright © NICE 2020; ). [PubMed] [Google Scholar]
- Newman C. W., Jacobson G. P., Spitzer J. B. (1996). Development of the tinnitus Handicap inventory. Arch. Otolaryngol. Head. Neck Surg. 122 (2), 143–148. Epub 1996/02/01PubMed PMID: 8630207. 10.1001/archotol.1996.01890140029007 [DOI] [PubMed] [Google Scholar]
- Nishad R. K., Jain A. K., Singh M., Verma R., Jain S. (2019). Randomised controlled clinical study of injection caroverine and ginkgo biloba extract in cochlear synaptic tinnitus. Indian J. Otolaryngol. Head. Neck Surg. 71 (2), 1523–1528. Epub 2019/11/22PubMed PMID: 31750210; PubMed Central PMCID: PMCPMC6841866. 10.1007/s12070-019-01655-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak A., Kojder K., Zielonka-Brzezicka J., Wróbel J., Bosiacki M., Fabiańska M., et al. (2021). The use of ginkgo biloba L. As a neuroprotective agent in the Alzheimer's disease. Front. Pharmacol. 12, 775034. Epub 2021/11/23PubMed PMID: 34803717; PubMed Central PMCID: PMCPMC8599153. 10.3389/fphar.2021.775034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppitz S. J., Garcia M. V., Bruno R. S., Zemolin C. M., Baptista B. O., Turra B. O., et al. (2022). Supplementation with açaí (Euterpe oleracea Martius) for the treatment of chronic tinnitus: Effects on perception, anxiety levels and oxidative metabolism biomarkers. Codas 34 (4), e20210076. Epub 2022/02/03PubMed PMID: 35107519. 10.1590/2317-1782/20212021076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanski J. F., Soares A. D., de Mendonça Cruz O. L. (2016). Antioxidant therapy in the elderly with tinnitus. Braz J. Otorhinolaryngol. 82 (3), 269–274. Epub 2015/11/09PubMed PMID: 26547700. 10.1016/j.bjorl.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procházková K., Šejna I., Skutil J., Hahn A. (2018). Ginkgo biloba extract EGb 761(®) versus pentoxifylline in chronic tinnitus: A randomized, double-blind clinical trial. Int. J. Clin. Pharm. 40 (5), 1335–1341. Epub 2018/06/02PubMed PMID: 29855986; PubMed Central PMCID: PMCPMC6208604. 10.1007/s11096-018-0654-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X. D., Ma R. H., Ding M. H. (2015). Literature review (2003-2013) on traditional Chinese medicine in syndrome differentiation and treatment of tinnitus. World J. Integr. Tradit. West Med. 10 (12), 1643–1646. [Google Scholar]
- Radunz C. L., Okuyama C. E., Branco-Barreiro F. C. A., Pereira R. M. S., Diniz S. N. (2020). Clinical randomized trial study of hearing aids effectiveness in association with Ginkgo biloba extract (EGb 761) on tinnitus improvement. Braz J. Otorhinolaryngol. 86 (6), 734–742. Epub 2019/07/14PubMed PMID: 31300303. 10.1016/j.bjorl.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejali D., Sivakumar A., Balaji N. (2004). Ginkgo biloba does not benefit patients with tinnitus: A randomized placebo-controlled double-blind trial and meta-analysis of randomized trials. Clin. Otolaryngol. Allied Sci. 29 (3), 226–231. Epub 2004/05/15PubMed PMID: 15142066. 10.1111/j.1365-2273.2004.00814.x [DOI] [PubMed] [Google Scholar]
- Richard R. S. (1996). Manual of the tinnitus questionnaire. London: The Psychological Corporation. Brace & Co. [Google Scholar]
- Roland P. D., Nergård C. S. (2012). Ginkgo biloba--effect, adverse events and drug interaction. Tidsskr. Nor. Laegeforen 132 (8), 956–959. Epub 2012/05/09PubMed PMID: 22562327. 10.4045/tidsskr.11.0780 [DOI] [PubMed] [Google Scholar]
- Schneider L. S., DeKosky S. T., Farlow M. R., Tariot P. N., Hoerr R., Kieser M. (2005). A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer's type. Curr. Alzheimer Res. 2 (5), 541–551. Epub 2005/12/27PubMed PMID: 16375657. 10.2174/156720505774932287 [DOI] [PubMed] [Google Scholar]
- Schubert N. M. A., Rosmalen J. G. M., van Dijk P., Pyott S. J. (2021). A retrospective cross-sectional study on tinnitus prevalence and disease associations in the Dutch population-based cohort Lifelines. Hear Res. 411, 108355. Epub 2021/10/05PubMed PMID: 34607212. 10.1016/j.heares.2021.108355 [DOI] [PubMed] [Google Scholar]
- Sereda M., Xia J., Scutt P., Hilton M. P., El Refaie A., Hoare D. J. (2022). Ginkgo biloba for tinnitus. Cochrane Database Syst. Rev. 11 (11), Cd013514. Epub 2022/11/17PubMed PMID: 36383762; PubMed Central PMCID: PMCPMC9668350 Society of Audiology Tinnitus and Hyperacusis Special Interest Group and Associate Editor for the International Journal of Audiology and BMC Health Services Research. She is funded by the NIHR Nottingham Biomedical Research Centre. She has received tinnitus research funding from the British Tinnitus Association, the British Society of Audiology and the NIHR. Jun Xia: none known. Polly Scutt: none known. Malcolm Hilton: none known. Amr El Refaie: none known. Derek J Hoare: DJH is Editor‐at‐large for Ear and Hearing and Chair of the British Society of Audiology. He is funded by the NIHR and research lead for hearing at the NIHR Nottingham Biomedical Research Centre. He has received tinnitus research funding from the British Society of Audiology, the British Tinnitus Association, RNID, Horizon 2020, Royal British Legion, Help Musicians UK and the NIHR. 10.1002/14651858.CD013514.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore S. E., Roberts L. E., Langguth B. (2016). Maladaptive plasticity in tinnitus--triggers, mechanisms and treatment. Nat. Rev. Neurol. 12 (3), 150–160. Epub 2016/02/13PubMed PMID: 26868680; PubMed Central PMCID: PMCPMC4895692. 10.1038/nrneurol.2016.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierpina V. S., Wollschlaeger B., Blumenthal M. (2003). Ginkgo biloba. Am. Fam. Physician 68 (5), 923–926. Epub 2003/09/19. PubMed PMID: 13678141. [PubMed] [Google Scholar]
- Smith P. F., Zheng Y., Darlington C. L. (2005). Ginkgo biloba extracts for tinnitus: More hype than hope? J. Ethnopharmacol. 100 (1-2), 95–99. Epub 2005/07/07PubMed PMID: 15998570. 10.1016/j.jep.2005.05.032 [DOI] [PubMed] [Google Scholar]
- Spiegel R., Kalla R., Mantokoudis G., Maire R., Mueller H., Hoerr R., et al. (2018). Ginkgo biloba extract EGb 761(®) alleviates neurosensory symptoms in patients with dementia: A meta-analysis of treatment effects on tinnitus and dizziness in randomized, placebo-controlled trials. Clin. Interv. Aging 13, 1121–1127. Epub 2018/06/27PubMed PMID: 29942120; PubMed Central PMCID: PMCPMC6005330. 10.2147/cia.S157877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale D., McFerran D., Brazier P., Pritchard C., Kay T., Dowrick C., et al. (2017). An economic evaluation of the healthcare cost of tinnitus management in the UK. BMC Health Serv. Res. 17 (1), 577. Epub 2017/08/24PubMed PMID: 28830503; PubMed Central PMCID: PMCPMC5567641. 10.1186/s12913-017-2527-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C., Kim Y. H., Kim Y. C., Park K. T., Kim S. W., Kim Y. J., et al. (2013). Korean red ginseng ameliorates acute 3-nitropropionic acid-induced cochlear damage in mice. Neurotoxicology 34, 42–50. Epub 2012/11/21PubMed PMID: 23164932. 10.1016/j.neuro.2012.10.008 [DOI] [PubMed] [Google Scholar]
- Tian C., Kim Y. J., Lim H. J., Kim Y. S., Park H. Y., Choung Y. H. (2014). Red ginseng delays age-related hearing and vestibular dysfunction in C57BL/6 mice. Exp. Gerontol. 57, 224–232. Epub 2014/06/22PubMed PMID: 24952098. 10.1016/j.exger.2014.06.013 [DOI] [PubMed] [Google Scholar]
- Tian J., Liu Y., Chen K. (2017). Ginkgo biloba extract in vascular protection: Molecular mechanisms and clinical applications. Curr. Vasc. Pharmacol. 15 (6), 532–548. Epub 2017/07/15PubMed PMID: 28707602. 10.2174/1570161115666170713095545 [DOI] [PubMed] [Google Scholar]
- Trevis K. J., McLachlan N. M., Wilson S. J. (2018). A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin. Psychol. Rev. 60, 62–86. Epub 2018/01/26PubMed PMID: 29366511. 10.1016/j.cpr.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Trochidis I., Lugo A., Borroni E., Cederroth C. R., Cima R., Kikidis D., et al. (2021). Systematic review on healthcare and societal costs of tinnitus. Int. J. Environ. Res. Public Health 18 (13), 6881. Epub 2021/07/03PubMed PMID: 34206904; PubMed Central PMCID: PMCPMC8297244. 10.3390/ijerph18136881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger M. (2013). Pharmacokinetic drug interactions involving Ginkgo biloba. Drug Metab. Rev. 45 (3), 353–385. Epub 2013/07/20PubMed PMID: 23865865. 10.3109/03602532.2013.815200 [DOI] [PubMed] [Google Scholar]
- Veile A., Zimmermann H., Lorenz E., Becher H. (2018). Is smoking a risk factor for tinnitus? A systematic review, meta-analysis and estimation of the population attributable risk in Germany. BMJ Open 8 (2), e016589. Epub 2018/02/24PubMed PMID: 29472253; PubMed Central PMCID: PMCPMC5855477. 10.1136/bmjopen-2017-016589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boetticher A. (2011). Ginkgo biloba extract in the treatment of tinnitus: A systematic review. Neuropsychiatr. Dis. Treat. 7, 441–447. Epub 2011/08/23PubMed PMID: 21857784; PubMed Central PMCID: PMCPMC3157487. 10.2147/ndt.S22793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Gu R., Zhai S. (1996). Clinical Observation of curative effect of Shuerdan on deafness and tinnitus. J. Otolaryngology Integr. Med. 4, 127–128. [Google Scholar]
- Wang S., Zheng R. (2011). A meta-analysis on treatment of tinnitus with traditional Chinese medicine and its medication rules. J. Anhui TCM Coll. 30 (1), 13–16. [Google Scholar]
- Wells H. R. R., Abidin F. N. Z., Freidin M. B., Williams F. M. K., Dawson S. J. (2021). Genome-wide association study suggests that variation at the RCOR1 locus is associated with tinnitus in UK Biobank. Sci. Rep. 11 (1), 6470. Epub 2021/03/21PubMed PMID: 33742053; PubMed Central PMCID: PMCPMC7979698. 10.1038/s41598-021-85871-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Niu Y., Ping J., Wang Y., Yang C., Li Y., et al. (2021). Genome-wide association study identifies new loci associated with noise-induced tinnitus in Chinese populations. BMC Genom Data 22 (1), 31. Epub 2021/09/07PubMed PMID: 34482816; PubMed Central PMCID: PMCPMC8420059. 10.1186/s12863-021-00987-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S., Fang Y., Yang W., Gu R., Han D., Yang S. (2013). Clinical investigation on the beneficial effects of the Chinese medicinal herb Gushen Pian on sensorineural deafness and tinnitus. Cell Biochem. Biophys. 67 (2), 785–793. Epub 2013/03/22PubMed PMID: 23516092. 10.1007/s12013-013-9536-5 [DOI] [PubMed] [Google Scholar]
- Zhang D., Xu Q., Caimino C., Baguley D. M. (2021). The prevalence of tinnitus in China: A systematic review of the literature. J. Laryngol. Otol. 135 (1), 3–9. Epub 2021/01/23PubMed PMID: 33478606. 10.1017/s002221512000256x [DOI] [PubMed] [Google Scholar]
- Zhang H. W., Yeung K. N. K., Tong M. C. F., Lin Z. X., Chang W. W. T., Ng I. H., et al. (2022). A Chinese medicine formula (bushen Huoxue Tongluo) for the treatment of chronic subjective tinnitus: A study protocol for a pilot, assessor-blinded, randomized clinical trial. Front. Pharmacol. 13, 844730. Epub 2022/04/19PubMed PMID: 35431960; PubMed Central PMCID: PMCPMC9006145. 10.3389/fphar.2022.844730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziai K., Moshtaghi O., Mahboubi H., Djalilian H. R. (2017). Tinnitus patients suffering from anxiety and depression: A review. Int. Tinnitus J. 21 (1), 68–73. Epub 2017/07/21PubMed PMID: 28723605. 10.5935/0946-5448.20170013 [DOI] [PubMed] [Google Scholar]