Abstract
Purpose
The purpose of the study is to determine the prevalence and predictors of OSA in Chinese bariatric surgery candidates.
Materials and Methods
The clinical data were collected from 326 patients evaluated for bariatric surgery and referred for polysomnography. Multiple logistic regression was used for identifying independent predictors of presence of OSA and ROC curve analysis to determine the best cut-off value for continuous variable.
Results
Baseline BMI and age were 33.3±3.7 kg/m2 and 24.3±3.1 years. 62.9% of the patients fulfilled the diagnostic criteria for OSA; Of these, 22.7% had mild OSA; 11.3% had moderate OSA, and 28.8% had severe OSA. The prevalence was significantly higher in males (84.2%) than in females (47.3%) (P<0.001). The superobese patients and the obese patients aged older than 50 years that all of those were diagnosed with OSA. A multivariate logistic regression model displayed that increasing age, BMI and neck circumference together with presence of habitual snoring and male sex were identified as risk factors of OSA. The best cut-off values for the presence of OSA for age, BMI, neck circumference were 24.5 years, 39.45 kg/m2, 40.40 cm.
Conclusion
The prevalence of OSA is very prevalent (62.9%) in Chinese bariatric surgery candidates, especially in male patients (84%). Age, BMI and neck circumference together with presence of habitual snoring and male sex are independent predictors of OSA in these patients. As clinical predictors are not enough to be a properly screening for OSA, routine PSG testing should be recommended to bariatric surgery candidates.
Keywords: Obstructive sleep apnea, Bariatric surgery, Obesity
INTRODUCTION
Obstructive sleep apnea (OSA) is a very common and chronic sleep-related disorder that is characterized by repeated episodes of partial or complete upper airway obstruction during sleeping [1,2,3]. Repeated episodes of obstructed breathing results in repetitive hypoxemia and intermittent pauses in breathing causing oxygen desaturation, sleep fragmentation, morning headaches and excessive daytime sleepiness and some patients with OSA may be asymptomatic [4,5]. Long-term complications of OSA include pulmonary hypertension, cardiovascular disease, stroke, diabetes, dyslipidemia and sudden-death during sleep [6,7,8]. OSA prevalence ranged from 9% to 38% in the general adult population, from 13% to 33% in men and from 6% to 19% in women [9]. Although some questionnaires are available for detecting patients at high risk for OSA such as the Fatigue Severity Scale (FSS) and Epworth Sleepiness Scale (ESS), but nocturnal polysomnography (PSG) is considered as gold standard for the diagnosis of OSA [10,11]. Recently, STOP-Bang (snoring, tiredness, observed apnea, high BP-BMI, age, neck circumference and gender) questionnaires are widely used, because it was a concise, effective, and reliable OSA screening tool [12], but it can't replace PSG.
There are multifactorial etiologies for OSA, including age, male sex, smoking and alcohol intake, but obesity is recognized as a major risk factor of OSA [13,14]. Some studies have shown that 1% of increasing in BMI, the incidence of OSA increases by 1.14% [15]. Underlying mechanisms of OSA in obese people include airway narrowing caused by fat deposition in the neck and airway obstruction due to relax of tongue soft tissue and throat muscles during sleep [16,17]. Some researchers showed approximately 70% of patients with diagnosed OSA were obese [7]. The prevalence of OSA in bariatric surgery candidates ranged from 60.0% to 91.0% [18,19]. However, about 80–90% of OSA cases remain undiagnosed [20].
Patients with OSA have a greater anesthetic risk, more perioperative complications including developing cardiovascular disease and respiratory depressant, which can increase the costs of postoperative care and the length of hospital stay [21,22]. Therefore, many researches tried to develop predictive models to predict the risk of OSA [18,23,24]. However, most of previous studies were focused on OSA patients with obesity in Western countries. In fact, OSA mechanisms may vary across racial groups [25]. Asians commonly have a greater mandibular plane (MP)-hyoid distance, a longer anterior lower facial height, and retrognathia and micrognathia, which are known risk factors for OSA [26]. Therefore, the aim of the study was to determine the prevalence and predictors of OSA in Chinese bariatric surgery candidates.
MATERIALS AND METHODS
Consecutive patients who planned to undergo bariatric surgery were retrospectively collected in the Bariatric Surgery Department of the First Affiliated Hospital of Jinan University between September 2015 and March 2019. Inclusion criteria were patients aged 18 to 65 years with BMI ≥35 kg/m2 or BMI ≥27.5 kg/m2 with inadequately controlled T2DM or metabolic syndrome; The patients who evaluated for bariatric surgery agreed to be screened preoperatively for OSA by polysomnography (PSG). The exclusion criteria were alcohol or drug abuse, severe eating disorder, depression or other severe disease contraindicating bariatric surgery. Written informed consent was obtained from all participants, and this study was approved by the ethics committee of the First Affiliated Hospital of Jinan University.
Data collection included gender, age, weight, neck circumference (measured at the level of the laryngeal prominence), waist circumference (measured midway between the lower rib and the iliac crest), hip circumference (measured the horizontal circumference of the most prominent part of the hips backwards), Waist-to-hip ratio, habitual snoring (defined as a snoring frequency 4≥ days per week) [27], hypertension, diabetes, dyslipidemia. To explore the associations between the prevalence of different severity of OSA and patients' demographic data, the patients were divided into several subgroups as follows:
• Gender: men and women
• Age: 18 to <30 years, 30 to <40 years, 40 to <50 years, 50 to <65 years
• BMI: 27.5 to <34.9 kg/m2, 35 to <39.9 kg/m2, 40 to <49.9 kg/m2, and >50 kg/m2
1. Polysomnography
Sleep recordings were performed using a computerized PSG device (Compumedics E-series, Australia). The device was used to document the following parameters: oronasal airflow (using dual pressure and thermal sensors), respiratory effort (abdomen and thorax), electrocardiogram, electrooculogram, electroencephalogram, snoring using a tracheal microphone, body position and oxygen saturation (using Nonin finger probe). Apnea was defined as a complete cessation of oronasal airflow for at least 10 seconds. Hypopneas was defined by either a ≥30% decrease in oronasal airflow from baseline lasting at least 10 seconds associated with a 4% oxygen desaturation or a decrease in flow by ≥50% of baseline for at least 10s associated with either a 3% oxygen desaturation, accompanied by thoracoabdominal movement. Apneahypopnea index (AHI) was defined as a number of apnea and hypopnea events per hour (AHI less than 5 events/h was considered normal, AHI 5–15 events/h was considered as mild OSA, AHI 15–30 events/h as moderate OSA and AHI more than 30 events/h as severe OSA) [28]. An experienced sleep specialist scored manually and interpreted polysomnographic recordings in accordance with established guidelines [29].
2. Statistical analysis
Statistical analysis was performed using the Statistical Product and Service Solutions version 13.0 (SPSS 13.0, SPSS Inc. Chicago, IL, USA). Continuous data were presented as mean±standard deviation and categorical data were expressed as percentage (%). We used analysis of one-way ANOVA analyses to compare means and distributions of participants without OSA and those with mild and moderate as well as severe disease. The prevalence of OSA and OSA severity was subdivided for obesity, BMI and age subgroups. Subgroups were compared by using chi-square test or Fisher's exact test for prevalence of OSA. Variables correlated with the AHI evaluated by Pearson's correlation or Spearman's rank correlation. Multiple logistic regression analysis for OSA was employed with demographic and anthropometric characteristics. The relevant continuous variables were included in the ROC analysis. The area under the curve (AUC) and 95% confidence intervals were calculated for continuous predictor variable. The cut-off values for each significant predictor was determined, selecting the score that most closely balanced the sensitivity and specificity. P value of less than 0.05 was considered statistically significance.
RESULTS
1. Demographic, anthropometric and polysomnographic characteristics
Of those 339 patients, 13 were subsequently excluded: because of missing or incomplete data, recording failed, recording time less than 300 min. Therefore, a total of 326 patients were recruited into further study, 188 (58%) women and 138 (42%) men. Baseline BMI and age were 33.3±3.7 kg/m2 and 24.3±3.1 years. Demographic, anthropometric and polysomnographic characteristics of 326 subjects are listed in Table 1 stratified by OSA status (AHI <5, 5–14.9, 15–29.9, and ≥30). As AHI increased, there were statistically significant increases in demographic and anthropometric characteristics as well as in the proportions of subjects with hypertension, diabetes, dyslipidemia. That means that those with OSA were also more likely to be older and larger for all anthropometric characteristics. There are significant meanings between polysomnographic variables in different groups, except for stage sleep 2. SpO2 values worsened in parallel with increases in AHI (P<0.001 for mean and lowest SpO2).
Table 1. Participant demographic, anthropometric and polysomnographic variables stratified by obstructive sleep apnea status.
| Variable | AHI | P value | ||||
|---|---|---|---|---|---|---|
| <5 (n=121) | 5–14.9 (n=74) | 15–29.9 (n=37) | ≥30 (n=94) | |||
| Demographic and anthropometric variables | ||||||
| Age (years) | 25.6±8.2 | 33.8±10.8 | 32.8±10.5 | 33.1±9.5 | <0.001 | |
| Weight (kg) | 98.7±21.7 | 106.9±22.8 | 124.3±26.5 | 129.2±29.2 | <0.001 | |
| BMI (kg/m2) | 35.5±5.2 | 39.0±6.3 | 43.1±7.5 | 45.0±10.6 | <0.001 | |
| Neck circumference (cm) | 39.4±3.7 | 41.2±3.2 | 44.9±4.7 | 46.7±4.7 | <0.001 | |
| Waist circumference (cm) | 113.0±12.3 | 119.3±13.7 | 130.9±15.1 | 135.0±18.0 | <0.001 | |
| Hip circumference (cm) | 118.5±10.6 | 124.3±13.1 | 130.9±15.1 | 132.6±17.8 | <0.001 | |
| Waist-to-hip ratio | 0.95±0.07 | 0.96±0.07 | 1.00±0.06 | 1.02±0.06 | <0.001 | |
| Habitual snoring, n (%) | 88(72.7) | 59(79.7) | 32(86.5) | 90(95.7) | <0.001 | |
| Hypertension, n (%) | 9(7.4) | 26(35.1) | 21(56.8) | 68(72.3) | <0.001 | |
| Diabetes, n (%) | 17(14.0) | 17(23.0) | 13(35.1) | 27(28.7) | 0.016 | |
| Dyslipidemia, n (%) | 44(36.4) | 31(41.9) | 18(48.6) | 52(55.3) | 0.043 | |
| Polysomnographic variables | ||||||
| Total sleep time (min) | 450.2±69.2 | 416.4±91.0 | 441.8±84.0 | 419.1±78.0 | 0.007 | |
| Sleep efficiency(%) | 88.2±9.1 | 81.9±15.9 | 77.8±20.2 | 80.9±13.2 | <0.001 | |
| Sleep latency (min) | 9.5±15.8 | 14.6±26.6 | 19.6±49.7 | 6.3±8.9 | 0.014 | |
| Stage sleep 1(%) | 8.0±4.6 | 11.3±7.1 | 14.5±16.0 | 22.4±16.1 | <0.001 | |
| Stage sleep 2(%) | 45.9±8.2 | 45.7±9.2 | 45.3±10.3 | 47.4±14.4 | 0.653 | |
| Stage sleep 3–4(%) | 24.6±9.0 | 22.81±9.8 | 23.63±10.2 | 14.17±16.1 | <0.001 | |
| REM stage(%) | 21.2±5.6 | 20.3±7.9 | 19.1±7.2 | 16.6±9.4 | <0.001 | |
| Mean SaO2 (%) | 96.2±2.7 | 95.4±1.7 | 94.0±2.4 | 88.4±9.2 | <0.001 | |
| Minimum SaO2 (%) | 89.2±4.5 | 81.2±8.2 | 73.4±13.5 | 61.1±13.3 | <0.001 | |
Data presented as mean±standard deviation, numbers, with percentages in parentheses; P values refer to results of ANOVA using Tukey's HSD post hoc tests or chi-square test.
AHI = apnea-hypopnea index, BMI = body mass index, REM = rapid eye movement, SaO2 = oxygen saturation.
2. Prevalence of OSA
Based on the PSG results, 205 (62.9%) patients fulfilled the diagnostic criteria for OSA, specifically 74 (22.7%) with mild OSA, 37 (11.3%) with moderate OSA and 94 (28.8%) with severe OSA. In male patients, the prevalence was 84.1%, compared to 47.3% in female subjects (χ2=54.43, P<0.001). The prevalence of OSA (AHI ≥5) was 84.1% and 47.3% in male and female, respectively. The prevalence of OSA, by using the cut-offs of ≥15 and ≥30 was 65.2% and 50.0% in male, and 21.8% and 13.3% in female, respectively. The increase in the male/female ratio with increasing AHI cut-off value. When the cut-off was ≥5, the male/female ratio was 1.78; at ≥15, it was 2.99, and at ≥30, it was 3.76.
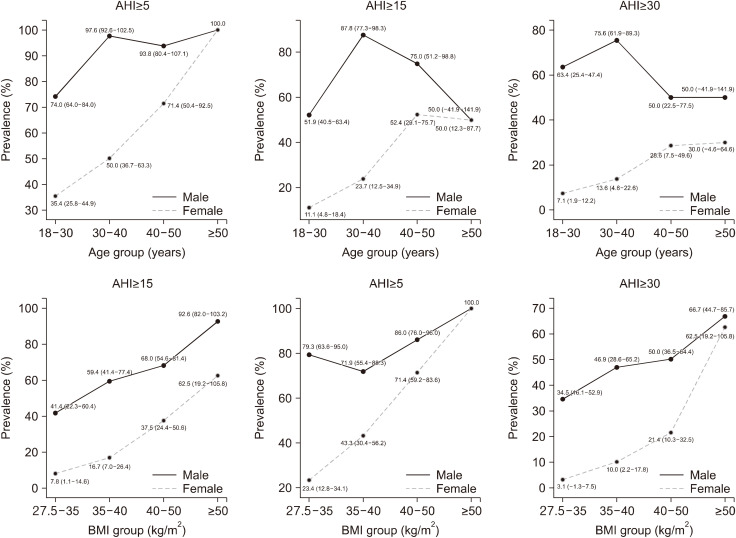
The prevalence and severity of OSA by BMI and age are shown in Table 2 and Fig. 1. In BMI groups, as BMI increased, the prevalence of OSA also increased progressively at any cut-off of AHI. All but one is a significant difference in the prevalence of OSA according to BMI at different cut-off value, the exception being AHI ≥30/h in male. All of super-obese patients with a BMI ≥50 kg/m2 were diagnosed with OSA. Furthermore, the prevalence of OSA of male subjects was higher than that of female subjects at every cut-off value of AHI.
Table 2. BMI-specific and age-specific prevalence of obstructive sleep apnea using various cutoff points for AHI in different gender.
| Male | Female | ||||||
|---|---|---|---|---|---|---|---|
| AHI≥5 | AHI≥15 | AHI≥30 | AHI≥5 | AHI≥15 | AHI≥30 | ||
| Total | 84.1 (77.9–90.2) | 65.2 (57.2–73.3) | 50.0 (41.6–58.4) | 47.3 (40.1–54.5) | 21.8 (15.9–27.8) | 13.3 (8.4–18.2) | |
| BMI (kg/m2) | |||||||
| 27.5 to <35 | 79.3 (63.6–95.0) | 41.4 (22.3–60.4) | 34.5 (16.1–52.9) | 23.4 (12.8–34.1) | 7.8 (1.1–14.6) | 3.1 (−1.3–7.5) | |
| 35 to <40 | 71.9 (55.4–88.3) | 59.4 (41.4–77.4) | 46.9 (28.6–65.2) | 43.3 (30.4–56.2) | 16.7 (7.0–26.4) | 10.0 (2.2–17.8) | |
| 40 to <50 | 86.0 (76.0–96.0) | 68.0 (54.6–81.4) | 50.0 (36.5–64.4) | 71.4 (59.2–83.6) | 37.5 (24.4–50.6) | 21.4 (10.3–32.5) | |
| ≥50 | 100.0 | 92.6 (82.0–103.2) | 66.7 (44.7–85.7) | 100.0 | 62.5 (19.2–105.8) | 62.5 (19.2–105.8) | |
| P value | 0.012 | <0.001 | 0.061 | <0.001 | <0.001 | <0.001 | |
| Age (years) | |||||||
| 18 to <30 | 74.0 (64.0–84.0) | 51.9 (40.5–63.4) | 63.4 (25.4–47.4) | 35.4 (25.8–44.9) | 11.1 (4.8–18.4) | 7.1(1.9–12.2) | |
| 30 to <40 | 97.6 (92.6–102.5) | 87.8 (77.3–98.3) | 75.6 (61.9–89.3) | 50.0 (36.7–63.3) | 23.7 (12.5–34.9) | 13.6 (4.6–22.6) | |
| 40 to <50 | 93.8 (80.4–107.1) | 75.0 (51.2–98.8) | 50.0 (22.5–77.5) | 71.4 (50.4–92.5) | 52.4 (29.1–75.7) | 28.6 (7.5–49.6) | |
| ≥50 | 100.0 | 50.0 (−41.9–141.9) | 50.0 (−41.9–141.9) | 100.0 | 50.0 (12.3–87.7) | 30.0 (−4.6–64.6) | |
| P value | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 | 0.004 | |
Data presented prevalence (95% confidence interval). Statistical analysis by chi-square test for differences in OSA prevalence.
BMI = body mass index, AHI = apnea-hypopnea index.
Fig. 1. Distribution of the OSA prevalence by sex, age, BMI.
In age groups, both male subjects and female subjects are a significant difference in the prevalence of OSA according to age at different cut-off value. Men aged 30 to 40 years in both AHI ≥5 and AHI ≥15 has the highest prevalence of OSA than any other age group. Among women, the prevalence of OSA in women increased with age at cut-off of AHI ≥5 and ≥15. For the obese patients aged older than 50 years, all of those were diagnosed with OSA.
3. Predictors of the risk factors of OSA
All variables were correlated with AHI as shown in Table 3. On the basic results, the following variables were included in the multiple logistic regression analysis based on their significant correlation with the AHI: gender, age, weight, neck circumference, waist circumference, hip circumference, waist-to-hip ratio, habitual snoring, hypertension, diabetes, dyslipidemia. Multiple logistic regression analysis confirmed that male sex, a higher BMI, a larger neck circumference, the presence of habitual snoring as predictors of OSA (Table 3).
Table 3. Predictors of obstructive sleep apnea based on univariate and multivariate logistic regression models.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| AHI | AHI≥5 | ||||
| r | P | B | OR(95% CI) | P value | |
| Sexa | 0.452 | <0.001 | 1.430 | 4.180 (2.060–8.479) | <0.001 |
| Age | 0.190 | 0.001 | 0.139 | 1.149 (1.101–1.199) | <0.001 |
| Weight | 0.459 | <0.001 | - | - | - |
| BMI | 0.453 | <0.001 | 0.148 | 1.160 (1.082–1.243) | <0.001 |
| Neck circumference | 0.581 | <0.001 | 0.178 | 1.195 (1.079–1.323) | 0.001 |
| Waist circumference | 0.506 | <0.001 | - | - | - |
| Hip circumference | 0.376 | <0.001 | - | - | - |
| Waist-to-hip ratio | 0.365 | <0.001 | - | - | - |
| Habitual snoringb | 0.223 | <0.001 | 0.831 | 2.297 (1.053–5.007) | 0.037 |
| Hypertensionc | 0.180 | 0.001 | - | - | - |
| Diabetesd | 0.160 | 0.004 | - | - | - |
| Dyslipidemiae | 0.156 | 0.005 | - | - | - |
aMale = 1, female = 2 (Spearman's correlation), bHabitual snoring = 1, no Habitual snoring = 0 (Spearman's correlation), cHypertension = 1, no hypertension = 0 (Spearman's correlation), dDiabetes = 1, no diabetes = 0 (Spearman's correlation), eDyslipidemia = 1, no dyslipidemia = 0 (Spearman's correlation),
BMI = body mass index, AHI = apnea-hypopnea index, CI = confidence interval.
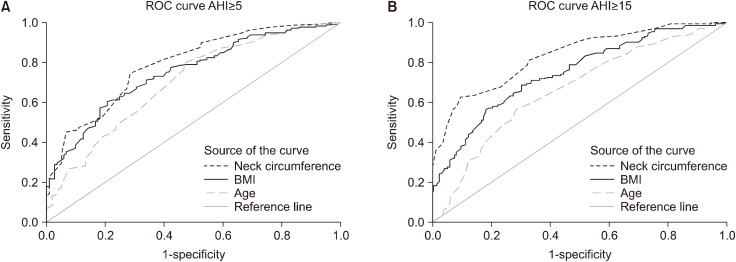
The ROC curve was analyzed the relevant continuous variables according to results of multiple logistic regression at cut-off of AHI ≥5 and ≥15 in Table 4. The cut-off values for each variable were determined to best balance between the sensitivity and specificity. In the prediction of an AHI of ≥5 , the optimal cut-off value for the presence of OSA for age, BMI, neck circumference were 24.5 years, 39.45 kg/m2, 40.40 cm, separately. In another model for the prediction of an AHI of ≥15/h, the optimal cut-off value for age, BMI, neck circumference were 30.5 years, 41.75, kg/m2, 44.75 cm, separately. As it can be seen in Fig. 2 the neck circumference was found to be the most accurate predictor of the presence of OSA at AHI of ≥5/h and AHI of ≥15/h.
Table 4. The ROC curve analyses of the relevant continuous at cutoff of AHI ≥5 and ≥15.
| Variables | AUC (95% CI) | Cutoff value | Sensitivity (%) | Specificity (%) | P value | |
|---|---|---|---|---|---|---|
| AHI≥5 | ||||||
| Age (years) | 0.702 (0.643–0.760) | 24.50 | 80.5 | 52.1 | <0.001 | |
| BMI (kg/m2) | 0.751 (0.699–0.804) | 39.45 | 60.5 | 79.3 | <0.001 | |
| Neck circumference (cm) | 0.791 (0.741–0.840) | 40.40 | 74.6 | 71.9 | <0.001 | |
| AHI≥15 | ||||||
| Age (years) | 0.663 (0.604–0.723) | 30.50 | 56.5 | 71.3 | <0.001 | |
| BMI (kg/m2) | 0.747 (0.693–0.801) | 41.75 | 56.5 | 81.5 | <0.001 | |
| Neck circumference (cm) | 0.832 (0.787–0.877) | 44.75 | 62.6 | 90.3 | <0.001 | |
BMI = body mass index, AHI = apnea-hypopnea index, CI = confidence interval, AUC = area under the curve.
Fig. 2. (A) ROC curve comparing sensitivity and specificity of age, body mass index (BMI) and neck circumference of an AHI ≥5. The mean area under the curve (AUC) for age, BMI and neck circumference were 0.702 (95% CI 0.643–0.760), 0.751 (95% CI 0.699–0.804), 0.791 (95% CI 0.741–0.840), respectively. (B) ROC curve comparing sensitivity and specificity of age, body mass index (BMI) and neck circumference of an AHI ≥15. The mean area under the curve (AUC) for age, BMI and neck circumference were 0.663 (95% CI 0.604–0.723), 0.747 (95% CI 0.693–0.801), 0.832 (95% CI 0.787–0.877), respectively.
DISCUSSION
This study described the prevalence of OSA in Chinese patients with obesity and being worked up for bariatric surgery. We examined various demographic and anthropometric data in an effort to correlate these characteristics with OSA severity. The prevalence of OSA increased with demographic and demographic data. Furthermore, the present study also revealed the prevalence of OSA in different BMI and age group. Based on the multivariate logistic regression analysis, age, BMI, male sex, neck circumference and habitual snoring remained significant predictors of OSA.
In this series of consecutive patients undergoing PSG, we found a high prevalence of OSA (62.9%) in Chinese bariatric surgery candidates. In a study, Ravesloot et al. [18] overviewed six articles that all patients were evaluated for bariatric surgery underwent a polysomnography, irrespective of history or clinical findings, and noted the prevalence of OSA ranged from 71.0% to 91.0% in bariatric surgery candidates. Unlike studies from other countries, the prevalence of OSA was lower than in the above-mentioned studies in this study, which may be contributed to the younger age and less profound obesity, compared to the western countries. Male prevalence was significantly higher than in females, and male to female ratio is 1.78:1, which was in line with a recent review reported prevalence ratios ranging from 2:1 [30].
As BMI increased, there was a trend toward higher OSA prevalence in both man and women. In a retrospective study, O'Keeffe and Patterson [31] noted that the incidence of OSA ranged from 75.9% to 86.9% across all BMI groups presenting for bariatric surgery. An interesting finding of our study is that all of super-obese patients were found to have OSA. This was higher than the previously reported data in super-obese patients ranging from 77% to 80.4% [7,18]. In comparison with Caucasian subjects, Asians appear to show a smaller maxilla, smaller and retropositioned mandible, and a shorter, steeper anterior cranial base [32]. Therefore, with the same level of BMI, the OSA level of the Chinese population is heavier. A review of studies also showed that Asians have more serious illnesses than Caucasian at a given age, gender, and BMI [33]. The prevalence of OSA in superobese patients is different from that in Western countries, which may be attributed to different ethnic and anatomical factors. Increasing prevalence of OSA with age is well-known [32]. In this study, the all obese patients aged more than 50 years old, were diagnosed with OSA, which is another interesting finding. The possible explanations were the common effect of obesity and age for OSA. High prevalence OSA among superobese patients highlights the importance of screening for OSA.
The patients with OSA often underreport their symptoms or are asymptomatic, so polysomnography remains the gold standard for diagnosing the condition. However, nocturnal polysomnography is expensive, time-consuming, and inconvenient for patients, so some patients often refuse to undergo polysomnography, which will be potential to increase perioperative risks for patients undiagnosed OSA. In fact, due to the high prevalence of OSA, many clinical models have been developed for predicting OSA in this population [18,24]. In a study by Yeh et al. [34], BMI, neck circumference, and ESS were predictors of OSA in bariatric surgery population. The recommended cut points are BMI ≥50.8, neck circumference ≥44.2 cm, and ESS score ≥10 for predicting moderate and severe OSA in bariatric patients. In another study, the cutoff value are BMI ≥45.17, neck circumference ≥40.75 cm, and age ≥41.5 years for predicting moderate and severe OSA in bariatric patients [24]. In this study, the cutoff value are BMI ≥41.75, neck circumference ≥44.75 cm, and age ≥30.5 years for predicting moderate and severe OSA.
In the present study, the prediction model showed that increasing age, BMI and neck circumference together with presence of habitual snoring and male sex were identified as independent risk factors for OSA. Our results like previous reports showed that age was also a predictor of the presence of OSA in bariatric surgery population [35]. The ROC curve showed the prediction of OSA, the optimal cut-off value for age, BMI, neck circumference were 24.5 years, 39.45 kg/m2, 40.40 cm, separately, but all of cut-off values of three predictors were not found to have a desirable sensitivity and specificity. However, the optimal cut-off value for age, BMI and neck circumference that may be useful for bariatric surgeons when patients refused to perform polysomnography. Neck circumference was found to be the strongest predictor of the presence of OSA and moderate or severe OSA, which is in line with other studies [18,20,24,36]. However, age (AUC 66.3%) was likely not to predict moderate or severe OSA. Although it is difficult to find a good model to predict OSA, our current prediction model gives us a warning. When the patients, especially male patient, appear age >30.5 years, BMI >41.75 kg/m2, neck circumference >44.75 cm or the presence of habitual snoring, bariatric surgeons should highly suspect patients with moderate to severe OSA. PSG should be given priority for those patients. We demonstrated that, by utilizing the prediction model, OSA cannot be predicted with enough certainty. Therefore, we advocate routine PSG testing for all patients that are considered for bariatric surgery.
The limitations of this study. Firstly, as a retrospective study, we are unable to collect data regarding Epworth Sleepiness Scales (ESS) and Berlin Questionnaire. Secondly, the nature of retrospective study resulted in some missing and incomplete data, which may have biased our findings. Thirdly, this is a single-center study which can cause selection bias, so our findings may have limited impact on the general population. However, because this study represents the first research study to determine the prevalence and predictors of OSA among Chinese bariatric surgery candidates, it merits attention. Furthermore, we routinely accessed bariatric surgery candidates based on full polysomnography at a sleep center, which can decrease the possibility of bias. Finally, it has investigated the relationship between the severity of OSA and demographic data, providing more detailed suggestions for clinicians when evaluating these patients.
CONCLUSION
This study suggests that the prevalence of OSA is high in Chinese bariatric surgery eligible candidates, especially in male patients. Although clinical predictors are not enough to properly screen for OSA in this population, bariatric surgeons should be highly suspicious of patients having OSA for patients with age >24.5years, BMI >39.45 kg/m2, and neck circumference >40.40 cm or the presence of habitual snoring, especially in male patients. More importantly, routine PSG testing should be recommended to bariatric surgery candidates.
ACKNOWLEDGMENTS
All authors are very thankful from patients who participated at the present study.
Footnotes
CONFLICT OF INTEREST: None of the authors have any conflict of interest.
FUNDING: No funding was obtained for this study.
References
- 1.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86:549–554. doi: 10.4065/mcp.2010.0810. quiz 554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koren D, Chirinos JA, Katz LE, et al. Interrelationships between obesity, obstructive sleep apnea syndrome and cardiovascular risk in obese adolescents. Int J Obes (Lond) 2015;39:1086–1093. doi: 10.1038/ijo.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newmarch W, Weiler M, Casserly B. Obesity cardiomyopathy: the role of obstructive sleep apnea and obesity hypoventilation syndrome. Ir J Med Sci. 2019;188:783–790. doi: 10.1007/s11845-018-01959-5. [DOI] [PubMed] [Google Scholar]
- 4.Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med. 2013;13:10. doi: 10.1186/1471-2466-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 6.Lyons OD, Ryan CM. Sleep apnea and stroke. Can J Cardiol. 2015;31:918–927. doi: 10.1016/j.cjca.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Lopez PP, Stefan B, Schulman CI, Byers PM. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74:834–838. [PubMed] [Google Scholar]
- 8.Maspero C, Giannini L, Galbiati G, Rosso G, Farronato G. Obstructive sleep apnea syndrome: a literature review. Minerva Stomatol. 2015;64:97–109. [PubMed] [Google Scholar]
- 9.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath CM, Jossen J, Kröll D, et al. Prevalence and prediction of obstructive sleep apnea prior to bariatric surgery-genderspecific performance of four sleep questionnaires. Obes Surg. 2018;28:2720–2726. doi: 10.1007/s11695-018-3222-z. [DOI] [PubMed] [Google Scholar]
- 12.Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149:631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- 13.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 14.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 15.Pannain S, Mokhlesi B. Bariatric surgery and its impact on sleep architecture, sleep-disordered breathing, and metabolism. Best Pract Res Clin Endocrinol Metab. 2010;24:745–761. doi: 10.1016/j.beem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, Zhang J, Liu Y. Roles and mechanisms of obstructive sleep apnea-hypopnea syndrome and chronic intermittent hypoxia in atherosclerosis: evidence and prospective. Oxid Med Cell Longev. 2016;2016:8215082. doi: 10.1155/2016/8215082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuck BA, Maurer JT. Recent developments in the diagnosis and treatment of obstructive sleep apnea: English version. HNO. 2017;65(Suppl 1):13–18. doi: 10.1007/s00106-016-0176-0. [DOI] [PubMed] [Google Scholar]
- 18.Ravesloot MJ, van Maanen JP, Hilgevoord AA, van Wagensveld BA, de Vries N. Obstructive sleep apnea is underrecognized and underdiagnosed in patients undergoing bariatric surgery. Eur Arch Otorhinolaryngol. 2012;269:1865–1871. doi: 10.1007/s00405-012-1948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashrafian H, le Roux CW, Rowland SP, et al. Metabolic surgery and obstructive sleep apnoea: the protective effects of bariatric procedures. Thorax. 2012;67:442–449. doi: 10.1136/thx.2010.151225. [DOI] [PubMed] [Google Scholar]
- 20.Peromaa-Haavisto P, Tuomilehto H, Kössi J, et al. Prevalence of obstructive sleep apnoea among patients admitted for bariatric surgery. A prospective multicentre trial. Obes Surg. 2016;26:1384–1390. doi: 10.1007/s11695-015-1953-7. [DOI] [PubMed] [Google Scholar]
- 21.Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–1563. doi: 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- 22.Liao P, Yegneswaran B, Vairavanathan S, Zilberman P, Chung F. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56:819–828. doi: 10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 23.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 24.Kolotkin RL, LaMonte MJ, Walker JM, Cloward TV, Davidson LE, Crosby RD. Predicting sleep apnea in bariatric surgery patients. Surg Obes Relat Dis. 2011;7:605–610. doi: 10.1016/j.soard.2011.04.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee RWW, Sutherland K, Sands SA, et al. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology. 2017;22:1015–1021. doi: 10.1111/resp.13022. [DOI] [PubMed] [Google Scholar]
- 26.Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9:419–436. doi: 10.1016/j.smrv.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170:1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 28.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 29.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester (IL): American Academy of Sleep Medicine; 2007. [Google Scholar]
- 30.Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7:1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–26. doi: 10.1381/096089204772787248. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology. 2012;17:213–222. doi: 10.1111/j.1440-1843.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 33.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh PS, Lee YC, Lee WJ, et al. Clinical predictors of obstructive sleep apnea in Asian bariatric patients. Obes Surg. 2010;20:30–35. doi: 10.1007/s11695-009-9854-2. [DOI] [PubMed] [Google Scholar]
- 35.Ahlin S, Manco M, Panunzi S, et al. A new sensitive and accurate model to predict moderate to severe obstructive sleep apnea in patients with obesity. Medicine (Baltimore) 2019;98:e16687. doi: 10.1097/MD.0000000000016687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duarte RL, Magalhães-da-Silveira FJ. Factors predictive of obstructive sleep apnea in patients undergoing pre-operative evaluation for bariatric surgery and referred to a sleep laboratory for polysomnography. J Bras Pneumol. 2015;41:440–448. doi: 10.1590/S1806-37132015000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]