Abstract
In this issue of Cell, Evavold et al. (2021) report that mTOR Complex 1 (mTORC1), a metabolic signaling complex, controls reactive oxygen species (ROS) production in mitochondria, which in turn promotes inflammatory cell death mediated by gasdermin D (GSDMD). This provides a new mechanistic connection between metabolic signaling and inflammatory cell death.
Pyroptosis is an inflammatory form of programmed cell death (Cookson and Brennan, 2001) implicated in both immune protection against infections and pathological inflammation from overexuberant immune response to infections or tissue damage (Liu et al., 2021). Gasdermin-D (GSDMD) is a key executioner of pyroptosis that drives cytolysis and inflammatory cytokine release by forming ~20 nm pores in the plasma membrane or organelle membranes (Aglietti et al., 2016; Ding et al., 2016; Sborgi et al., 2016; Xia et al., 2021). GSDMD can be activated by proteolytic processing following stimulation of the canonical and non-canonical inflammasomes, which are cytosolic signaling platforms that trigger the maturation of inflammatory caspases-1, −4, −5, −11 (Liu et al., 2021). These caspases cleave GSDMD into N- and C-terminal domains, which dissociate in the presence of phospholipids to allow the N-terminal domain of GSDMD (NT-GSDMD) to translocate to membrane, oligomerize, and form pores. The critical role of GSDMD in pyroptosis and inflammation has sparked intense interest in the upstream events that influence the protease cleavage of GSDMD, but little is known about how pyroptosis is regulated after GSDMD cleavage.
The lysosomal Ragulator complex and the associated GTPases RagA/B and RagC/D proteins are essential components of a nutrient-sensing machinery, which recruits the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) from the cytosol to the lysosome to stimulate its kinase activity (Liu and Sabatini, 2020). Activated mTORC1 increases the expression of genes that control biosynthetic pathways for macromolecules such as proteins or lipids while limiting autophagic breakdown of cellular components. Dysregulated mTOR signaling is implicated in tumorigenesis, aging, and neurodegenerative disorders (Liu and Sabatini, 2020). Even though the Ragulator-Rag-mTORC1 pathway is known to regulate cellular homeostasis, it has not been mechanistically linked to the inflammatory process involving pyroptosis. The paper by Evavold and colleagues (Evavold et al., 2021) in this issue of Cell reveals a previously unrecognized connection between the Ragulator-Rag-mTORC1 pathway and GSDMD-mediated pyroptosi, and fills a critical gap in our understanding of how pyroptosis is regulated following protease cleavage of GSDMD (Figure 1).
Figure 1. The Ragulator-Rag-mTORC1 pathway promotes GSDMD oligomerization and pore formation.
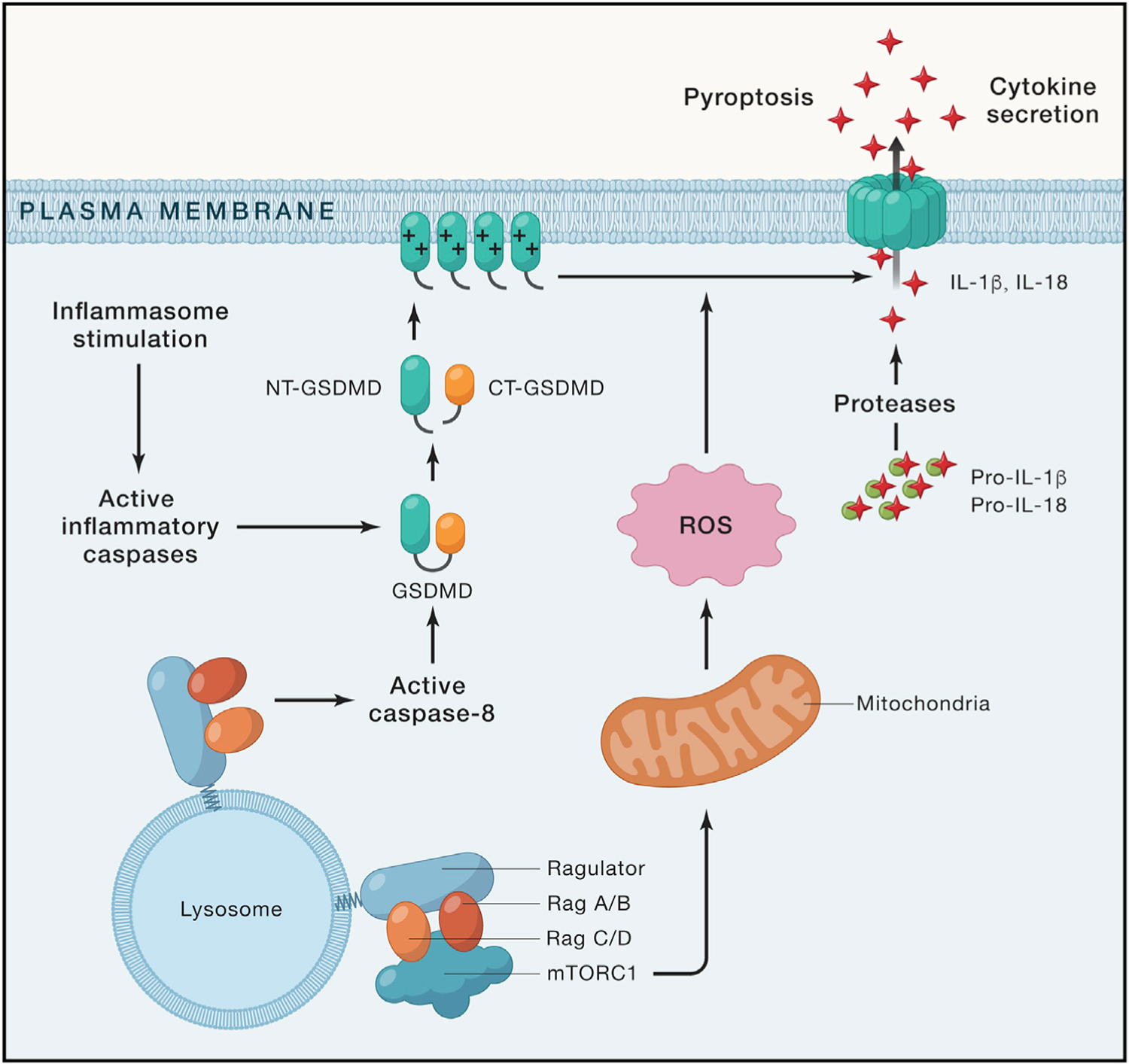
Upon inflammasome stimulation, activated inflammatory caspases process GSDMD into NT-GSDMD and CT-GSDMD. NT-GSDMD translocates to plasma membrane and oligomerizes to form pores that mediate cytokine secretion and pyroptotic cell death. The Ragulator-Rag-mTORC1 pathway at the lysosomal membrane regulates ROS production in mitochondria that promotes the oligomerization and pore formation by NT-GSDMD. Deficiency or inhibition of Rag proteins or mTORC1 components diminishes NT-GSDMD-mediated pore formation and cytolysis. The Ragulator-Rag complex also regulates pyroptosis upstream of GSDMD cleavage through caspase-8 activation.
Utilizing a genome-wide CRISPR-Cas9 screening in an inducible NT-GSD MD expression system that bypasses GSDMD cleavage, the authors identified the Ragulator-Rag complex as required for NT-GSDMD pore formation and pyroptosis. Furthermore, the mTORC1-specific component Raptor was essential for pyroptosis and cytokine release downstream of both canonical and noncanonical inflammasome pathways. This was unexpected as previous in vitro studies using purified GSDMD and liposomes have demonstrated efficient lysis of liposomes in the absence of other proteins (Aglietti et al., 2016; Ding et al., 2016; Sborgi et al., 2016). Genetic ablation of components of the Ragulator-Rag complex such as RagA or RagC resulted in defects in pore formation and pyroptosis, without impacting the cleavage of GSDMD, suggesting that the Rag proteins are required for GSDMD-mediated pyroptosis downstream of the inflammasome signaling pathways.
How might the Ragulator-Rag-mTORC1 pathway promote GSDMD-mediated pyroptosis? Previous studies have demonstrated that the ability of NT-GSDMD to oligomerize is essential for its pore-forming function (Aglietti et al., 2016; Ding et al., 2016; Sborgi et al., 2016; Xia et al., 2021). In agreement, oligomerization of NT-GSDMD was dramatically attenuated in the absence of RagA or RagC from the Ragulator-Rag complex, without defects in NT-GSDMD localization to the plasma membrane. The production of reactive oxygen species (ROS) at the mitochondria is known to be a stimulator of inflammasomes (Zhou et al., 2011) and is regulated by the Ragulator-Rag-mTORC1 pathway (Liu and Sabatini, 2020). Interestingly, ROS production rescued the NT-GSDMD oligomerization and pore formation in the RagA- or RagC-deficient cells, whereas inhibition of ROS led to reduced pore formation and cell pyroptosis without affecting GSDMD cleavage. Taken together, the Ragulator-Rag-mTORC1 signaling pathway may promote NT-GSDMD oligomerization and pore forming activities through regulation of mitochondrial ROS production, without impacting GSDMD cleavage or membrane translocation.
The new findings reveal exciting insights into the promotion of GSDMD-induced pyroptosis by ROS production, which invariably raise new questions that will require further mechanistic studies. For example, the Ragulator-Rag-mTORC1 complex is known to control mitochondrial biogenesis and oxidative function through modulation of transcriptional complexes such as yin-yang 1 (YY1)-peroxisome proliferator-activated receptor-γ (PPARγ) coactivator 1α (PGC-1α) (Liu and Sabatini, 2020). It remains to be determined whether such transcriptional complexes participate in the regulation of GSDMD oligomerization. The Ragulator-Rag complex was recently reported to function as a platform to promote receptor-interacting serine/threonine-protein kinase 1 (RIPK1)- and caspase-8-mediated pyroptosis in response to Yersinia infection (Zheng et al., 2021). This process was independent of mTORC1 activities, and the Ragulator-Rag complex functions upstream of caspase-8 activation and GSDMD cleavage. Surprisingly, in this study the canonical or noncanonical inflammasome pathways were not impacted by deficiency in the Ragulator-Rag complex. Conceivably, different components of the Ragulator-Rag complex studied by the two groups may have distinct regulatory roles in GSDMD-mediated pyroptosis.
Data from the current paper suggest that NT-GSDMD is able to translocate to the plasma membrane without pore formation, suggesting dynamic movement and assembly process within the membrane. Previous studies of GSDMD with liposomes using atomic force microscopy have revealed NT-GSDMD structures of variable sizes and shapes that may dynamically fuse together into larger ring-shaped pores (Sborgi et al., 2016). It is conceivable that ROS may promote the association of NT-GSDMD monomers or small oligomers into pores of ~20 nm in size as observed in the cryo-electron microscopy structure (Xia et al., 2021). NT-GSDMD oligomerization could be inhibited by cysteine-modifying agents and was dependent on Cys191 within the human NT-GSDMD (Liu et al., 2021). Analyzing pore formation by mutant NT-GSDMD at this Cys residue may shed light on how ROS may modulate NT-GSDMD oligomerization. This is of particular interest given that Cys191 is the target of several GSDMD inhibitors.
In summary, the current work demonstrates that the Ragulator-Rag-mTORC1 pathway is a crucial contributor of GSDMD oligomerization and pore formation preceding pyroptosis and a novel target for therapeutic interventions of infectious diseases and autoinflammatory disorders involving pyroptosis. The unexpected role of the Ragulator-Rag-mTORC1 pathway in regulating pyroptosis opens up new avenues of investigation on the metabolic regulation of inflammation.
ACKNOWLEDGMENTS
T.S.X. is supported by NIH grants R01GM127609 and P01AI141350.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, Ciferri C, Dixit VM, and Dueber EC (2016). GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl. Acad. Sci. USA 113, 7858–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson BT, and Brennan MA (2001). Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114. [DOI] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang D-C, and Shao F (2016). Poreforming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116. [DOI] [PubMed] [Google Scholar]
- Evavold CL, Hafner-Bratkovič I, Devant P, D’Andrea JM, Ngwa EM, Boršić E, Doench JG, LaFleur MW, Sharpe AH, Thiagarajah JR, and Kagan JC (2021). Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell. Published online July 14, 2021. 10.1016/j.cell.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, and Sabatini DM (2020). mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol 21, 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xia S, Zhang Z, Wu H, and Lieberman J (2021). Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov 20, 384–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Müller DJ, Broz P, and Hiller S (2016). GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 35, 1766–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM, Wang L, Fu T-M, Jacobson MP, Greka A, et al. (2021). Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Deng W, Bai Y, Miao R, Mei S, Zhang Z, Pan Y, Wang Y, Min R, Deng F, et al. (2021). The lysosomal Rag-Ragulator complex licenses RIPK1– and caspase-8–mediated pyroptosis by Yersinia. Science 372.. 10.1126/science.abg0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, and Tschopp J (2011). A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. [DOI] [PubMed] [Google Scholar]


