Abstract
Lack of interleukin-6 (IL-6) during Pseudomonas aeruginosa corneal infection leads to more severe disease with changes in neutrophil recruitment. Exogenous IL-6 leads to increased efficiency of neutrophil recruitment and reduced bacterial loads in corneal infection in both IL-6 gene knockout and wild-type mice. This may be mediated by IL-6 increasing the production of corneal macrophage inflammatory protein 2 and intercellular cell adhesion molecule 1. We conclude that effective recruitment of neutrophils into the cornea is dependent on the production of IL-6 and that early augmentation of IL-6 may be protective in corneal infection.
Pseudomonas aeruginosa is a leading cause of corneal infection, often resulting in blindness (13). The host inflammatory responses, which are orchestrated by cytokines and chemokines, are critical in determining the outcome of ocular infection with P. aeruginosa (8, 10). Our studies have shown that interleukin-6 (IL-6) is important in protecting the cornea during P. aeruginosa infection (15). Its role appears to be central to the regulation of leukocyte recruitment into the avascular cornea (15). This is consistent with findings in other infection models in IL-6 gene knockout (gko) mice (6, 7, 12). Other studies using systemic administration of IL-6 in septic shock (3) and listeriosis (14) also support the protective role of IL-6. We have examined the effects of local administration of IL-6 on the outcome of P. aeruginosa infection of the cornea in IL-6 gko and wild-type mice. Our results indicate that effective recruitment of polymorphonuclear neutrophils (PMN) into the cornea is dependent on production of IL-6 and that early augmentation of IL-6 may be protective in corneal infection.
IL-6 gko in a C57BL/6 × Sv 129 background and the corresponding wild-type mice were obtained from the Jackson Laboratory (Bar Harbour, Maine). Inbred 8-week-old mice were challenged with P. aeruginosa strain 6206 as previously described (4). Solutions of murine IL-6 for injection (2) were prepared in pyrogen-free saline and were administered as 20-μl subconjunctival injections containing 100 ng of IL-6. IL-6 was administered 4 h prior to challenge, at the time of challenge, and 6 h postchallenge. Control animals were injected subconjunctivally with 20 μl of pyrogen-free, phosphate buffered saline (PBS) on the same schedule. Mice were sacrificed 12 h postchallenge, and their eyes were collected and either formalin fixed or stored at −70°C for the enzyme-linked immunosorbent assay and the PMN and bacterial number assays. These experiments were performed on at least 12 mice per group and repeated at least twice.
Sections (5 μm) of formalin-fixed and paraffin-embedded eyes were stained with hematoxylin and eosin for histopathology. Frozen sections (8 μm) of eyes were cut using a cryostat microtome and examined for intercellular cell adhesion molecule 1 (ICAM-1) production by immunohistochemistry, as previously described (1), using rat anti-mouse ICAM-1 monoclonal antibody kindly supplied by Nicholas King. IL-6, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), macrophage inflammatory protein 2 (MIP-2), and KC were quantitated by ELISA (R & D Systems, Bioscientific, Sydney, Australia). PMN in whole eyes were quantitated by determining myeloperoxidase activity (11). Viable bacteria in whole eyes (n = 5) were quantitated as described by Cowell et al (5). Data were examined statistically using an unpaired Student t test.
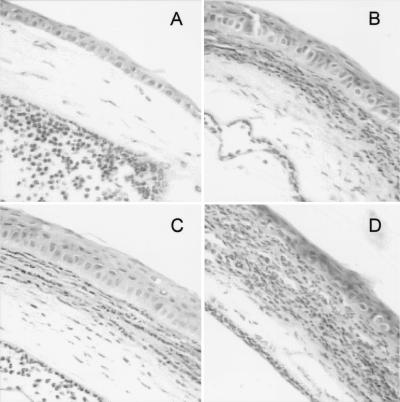
IL-6 subconjunctival injection was well tolerated by the mice, and in the absence of infection it produced only mild and transient recruitment of PMN at the site of injection (data not shown). Eyes of IL-6 gko mice injected with PBS 12 h postchallenge showed few infiltrating PMN in the cornea (Fig. 1A); however, these eyes showed numerous PMN in the anterior chamber. In contrast, eyes from similarly treated wild-type mice showed PMN penetration into the corneal stroma but few PMN in the anterior chamber (Fig. 1B). Corneas from IL-6 gko mice treated with IL-6 showed large numbers of PMN penetrating the cornea (Fig. 1C), as well as in the anterior chamber. Corneas of wild-type mice, treated with IL-6, show increased infiltrating PMN but no appreciable peratration into the anterior chamber (Fig. 1D).
FIG. 1.
Histopathology of corneas from IL-6 gko and wild-type mice 12 h after challenge with P. aeruginosa. All sections are stained with hematoxylin and eosin. Magnification of all sections, ×176. (A) Cornea of IL-6 gko mouse 12 h after challenge with P. aeruginosa after subconjunctival injection of PBS. The central cornea contains only the occasional inflammatory cell. There are numerous PMN in the anterior chamber. (B) Cornea of wild-type mouse 12 h after challenge with P. aeruginosa after subconjunctival injection of PBS. The cornea contains infiltrating inflammatory cells, predominantly PMN. (C) Cornea of IL-6 gko mouse 12 h after challenge with P. aeruginosa after subconjunctival injection of IL-6. The central cornea contains inflammatory cells, predominantly PMN. There are numerous PMN in the anterior chamber. (D) Cornea of wild-type mouse 12 h after challenge with P. aeruginosa after subconjunctival injection of IL-6. The infiltrating inflammatory cells in the cornea are more numerous than in panel B.
Significantly more IL-6 was present in the eyes from IL-6-treated mice than in the eyes from PBS-treated mice in both groups (Fig. 2C) (P < 0.05) 12 h postchallenge. The eyes from IL-6-treated wild-type mice contained approximately twice the concentration of IL-6 than did the eyes from PBS-treated mice IL-6-treated gko mice had detectable levels of IL-6 protein (approximately half that produced by wild-type mice), while the levels of IL-6 found in PBS-treated IL-6 gko mice were below the limits of detection for this assay. This indicates that the method of administration has provided effective local delivery of IL-6 to the eye and that IL-6 is stable over the time frame of this experiment.
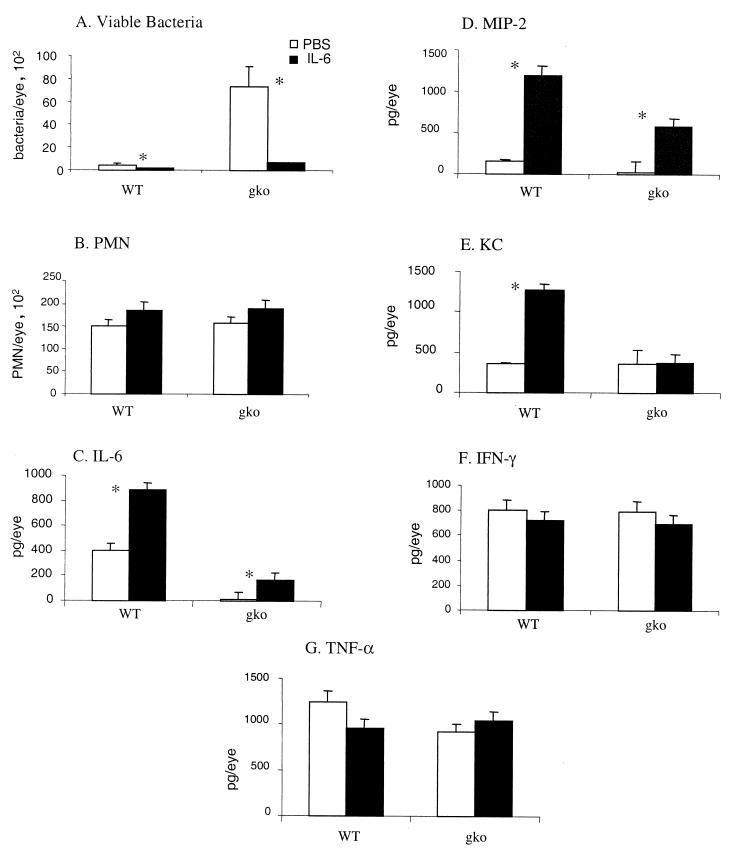
FIG. 2.
Numbers of viable bacteria and PMN and cytokine production in the eyes of IL-6 gko and wild-type mice 12 h after challenge with P. aeruginosa after treatment with either PBS or IL-6. Histograms represent the mean of observation from the experiment, and vertical bars indicate standard errors. ∗, P < 0.05. (A) Viable bacteria; (B) PMN; (C) IL-6; (D) MIP-2; (E) KC; (F) IFN-γ; (G) TNF-α.
Bacterial numbers in the eyes of IL-6 gko mice were more than 10-fold higher than in those of wild-type mice treated with PBS only (Fig. 2A). Bacterial numbers were significantly reduced (P < 0.05) in the eyes of both wild-type and gko mice treated with IL-6, by 3- and 10-fold, respectively, compared with those in the eyes of mice treated with PBS (Fig. 2A).
There was no significant difference in PMN numbers in whole eyes of IL-6 gko and wild-type mice at 12 h postinfection, although there were obviously histopathological differences between these groups (Fig. 1). This results from the differing distribution of PMN in the eyes of wild-type and gko mice. In the IL-6 gko mice, the PMN were found in the anterior chamber and did not penetrate the cornea (Fig. 1A). There was increased PMN infiltration in the eyes of both gko and wild-type mice treated with exogenous IL-6 (Fig. 1C and D).
Levels of MIP-2 and KC (murine homologues of IL-8) in the eyes of PBS-treated wild-type mice correlated well with those found by others at this time point (11). The level of MIP-2 was more than fourfold higher in the eyes of wild-type mice than in those of IL-6 gko mice in response to the challenge (Fig. 2D). More importantly, the level of MIP-2 was significantly increased (P < 0.05) by approximately 8- and 4-fold in the wild-type and gko mice, respectively, that received IL-6 treatment compared with the level in those that received PBS. This suggests that IL-6 may be involved in the up-regulation of MIP-2 in the early stages of P. aeruginosa infection of the cornea and may thereby contribute to the recruitment of neutrophils into the avascular cornea (11). No significant difference in KC was detected in the eyes of wild-type and IL-6 gko mice at 12h post-challenge (Fig. 2E). There was a 3.5-fold increase in KC in the eyes of wild-type mice receiving IL-6 treatment, but no change was detected in the gko mice treated with exogenous IL-6 (Fig. 2E). We speculate that excess IL-6 may result in increased KC production, and we are currently investigating this possibility. There was no significant difference between wild-type and gko mice or between treated and control groups in the levels of the cytokines IFN-γ (Fig. 2F) or TNF-α (Fig. 2 G) at 12 h postchallenge.
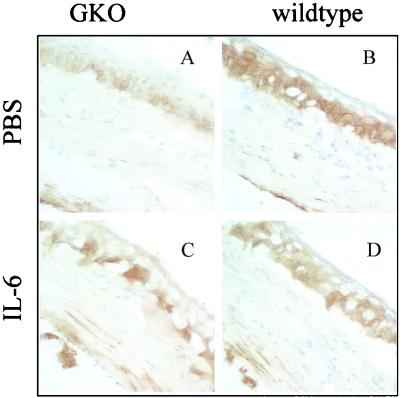
Representative examples of positive staining for ICAM-1 in IL-6 gko and wild-type mouse corneas 12 hours after challenge are shown in Fig. 3. ICAM-1 expression in the corneas of IL-6 gko mice appeared to be reduced (Fig. 3A) compared to that in the corneas of wild-type mice 12 h postinfection (Fig. 3B). This apparent reduction in ICAM-1 staining was seen particularly in the epithelium of gko mice. The epithelium was strongly stained in wild-type mice. ICAM-1 expression was increased in the corneas of IL-6 gko mice (Fig. 3C) in the presence of exogenous IL-6. This induction was seen in the epithelium and stromal keratocytes adjacent to the endothelium, whereas additional staining was seen only in keratocytes adjacent to the endothelium in the wild-type mice after treatment with IL-6 (Fig. 3D). Up-regulation of ICAM-1 may contribute locally to the recruitment of leukocytes into infected cornea (9). The above observations suggest a possible role for IL-6 in the up-regulation of adhesion molecules in the cornea.
FIG. 3.
ICAM-1 expression in the corneas of wild-type and IL-6 gko mice 12 h after challenge with P. aeruginosa. Magnification of sections, ×200. (A) Cornea of IL-6 gko mouse 12 h after challenge with P. aeruginosa after subconjunctival injection of PBS. (B) Cornea of wild-type mouse 12 h after challenge with P. aeruginosa after subconjunctival injection of PBS. (C) Cornea of IL-6 gko mouse 12 h after challenge with P. aeruginosa after subconjunctival injection of IL-6. (D) Cornea of wild-type mouse 12 h after challenge with P. aeruginosa after subconjunctival injection of IL-6.
In this study we have confirmed the role of IL-6 in corneal defense against P. aeruginosa infection and found that local administration of IL-6 during corneal infection may be protective in this disease. While several mechanisms are likely to be involved, reflecting the pleotropic nature of IL-6, the findings in IL-6 gko mice suggest that the role of IL-6 is closely linked to recruitment of PMN into the cornea. Our results indicate that the protective effects of IL-6 may in part be due to increasing production of the chemokine MIP-2 and up-regulation of the expression of ICAM-1. The data presented suggest that pharmacological augmentation of IL-6 may represent a rational approach for the development of adjunct therapies to improve patient outcome in this potentially blinding disease.
Acknowledgments
We thank Nicholas King for providing the ICAM-1 antibody, and Suzanne Fleiszig for providing P. aeruginosa strain 6206.
Financial support for this study was provided by the Australian Federal Government through the Co-Operative Research Centers Program and by an NHMRC grant.
REFERENCES
- 1.Bao S, Beagley K W, Shen J, Husband A J. Interferon-γ plays a critical role in intestinal immunity against Salmonella typhimurium infection. Immunology. 2000;99:1–12. doi: 10.1046/j.1365-2567.2000.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao S, Beagley K W, Allanson M, Husband A J. Exogenous IL-6 promotes enhanced intestinal antibody responses in vivo. Immunol Cell Biol. 1998;76:560–562. doi: 10.1046/j.1440-1711.1998.00785.x. [DOI] [PubMed] [Google Scholar]
- 3.Barton B E, Jackson J V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993;61:1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole N, Bao S, Willcox M D P, Husband A J. Expression of interleukin-6 in the cornea in response to infection with different strains of Pseudomonas aeruginosa. Infect Immun. 1999;67:2497–2502. doi: 10.1128/iai.67.5.2497-2502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowell B A, Willcox M D P, Hobden J A, Schneider R P, Tout S, Hazlett L D. An ocular strain of Pseudomonas aeruginosa is inflammatory but not virulent in a scarified mouse model. Exp Eye Res. 1998;67:347–356. doi: 10.1006/exer.1998.0524. [DOI] [PubMed] [Google Scholar]
- 6.Dalrymple S A, Lucian L A, Slattery R, McNeil T, Aud D M, Fuchino S, Lee F, Murray F. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalrymple S A, Slattery R, Aud D M, Krishna M, Lucian L A, Murray R. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect Immun. 1996;64:3231–3235. doi: 10.1128/iai.64.8.3231-3235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobden J A, Masnick S A, Barrett R P, Hazlett L D. Proinflammatory cytokine deficiency and pathogenesis of Pseudomonas aeruginosa keratitis in aged mice. Infect Immun. 1997;65:2754–2758. doi: 10.1128/iai.65.7.2754-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobden J A, Masnick-McClelland S, Barrett R P, Bark K S, Hazlett L D. Pseudomonas aeruginosa keratitis in knockout mice deficient in intracellular adhesion molecule-1. Infect Immun. 1999;67:972–975. doi: 10.1128/iai.67.2.972-975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernacki K A, Goebel D J, Poosch M S, Hazlett L D. Early cytokine and chemokine expression during Pseudomonas aeruginosa corneal infection in mice. Infect Immun. 1998;66:376–379. doi: 10.1128/iai.66.1.376-379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kernacki K A, Barrett R P, Hobden J A, Hazlett L D. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J Immunol. 2000;164:1037–1045. doi: 10.4049/jimmunol.164.2.1037. [DOI] [PubMed] [Google Scholar]
- 12.Ladel C H, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann S H E. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997;65:4843–4849. doi: 10.1128/iai.65.11.4843-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laibson P R. Pseudomonas aeruginosa. In: Fraunfelder F T, Roy F H, editors. Current ocular therapy. Philadelphia, Pa: The W. B. Saunders Co.; 1990. pp. 35–37. [Google Scholar]
- 14.Liu Z, Simpson R J, Cheers C. Recombinant interleukin-6 protects mice against experimental bacterial infection. Infect Immun. 1992;60:4402–4406. doi: 10.1128/iai.60.10.4402-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willcox M D P, Cole N, Bao S, Husband A J. IL-6 expression in the cornea is partially protective during Pseudomonas aeruginosa infection. Exp Eye Res. 1998;67:S62. [Google Scholar]