Abstract
Background
Children with rare diseases experience challenges at home and school and frequently require multi-disciplinary healthcare. We aimed to determine health service utilization by Australian children with rare diseases and barriers to accessing healthcare.
Methods
Parents completed an online survey on health professional and emergency department (ED) presentations, hospitalization, and barriers to accessing services. Potential barriers to service access included residential location (city, regional, remote) and child health-related functioning, determined using a validated, parent-completed measure-of-function tool.
Results
Parents of 462 children with over 240 rare diseases completed the survey. Compared with the general population, these children were more likely to be hospitalized [odds ratio (OR) = 17.25, 95% confidence interval (CI) = 15.50–19.20] and present to the ED (OR = 4.15, 95% CI = 3.68–4.68) or a family physician (OR = 4.14, 95% CI = 3.72–4.60). Child functional impairment was nil/mild (31%), moderate (48%) or severe (22%). Compared to children with nil/mild impairment, those with severe impairment were more likely to be hospitalized (OR = 13.39, 95% CI = 7.65–23.44) and present to the ED (OR = 11.16, 95% CI = 6.46–19.27). Most children (75%) lived in major cities, but children from regional (OR = 2.78, 95% CI = 1.72–4.55) and remote areas (OR = 9.09, 95% CI = 3.03–25.00) experienced significantly more barriers to healthcare access than children from major cities. Barriers included distance to travel, out-of-pocket costs, and lack of specialist medical and other health services.
Conclusions
Children with rare diseases, especially those with severe functional impairment have an enormous impact on health services, and better integrated multidisciplinary services with patient-centered care are needed. Access must be improved for children living in rural and remote settings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12519-022-00675-6.
Keywords: Access, Health functioning, Health service use, Rare diseases
Introduction
Rare childhood diseases (defined in Australia as having a prevalence of 1/10,000) challenge affected children, their families, health professionals and services [1]. Rare diseases are chronic, complex and disabling and require multidisciplinary care from specialist doctors, nurses, family physicians, allied health professionals, and community and disability services [1–3]. Parents often fulfil the role of healthcare coordinator and manage multiple appointments across disjointed healthcare sectors [4]. Although individual diseases are rare, people with rare diseases collectively comprise 3.5%–5.9% of the population worldwide [5] and a minimum of 2% of the Australian population [6]. Surprisingly, few studies have described the types of health services accessed by children with rare diseases, frequency of use, barriers to access, or experiences and needs of families [2, 5].
Per capita, Australians pay the highest out-of-pocket health expenses worldwide [7], despite a publicly funded, universal healthcare service (Medicare) [8] and a government-subsidized private health insurance scheme [9]. In this context, few studies have described the financial impacts on the health system of caring for children with rare diseases. Similarly, the economic impacts on families caring for children with rare diseases have seldom been described. It is assumed that the economic impacts on families are exacerbated in regional and remote Australia, where there is a recognized gap in publicly funded specialist health services and health outcomes [10]. Families from rural remote areas must travel long distances to access health care, much of which is delivered by specialists in tertiary pediatric hospitals in large cities [11].
Information is needed to support policymakers and health service providers in effectively planning and financing future services for children with rare diseases. We aimed to describe the health-related functioning of children with a wide variety of rare diseases, their use of health services, barriers encountered when accessing services, and economic impacts on families and hospital services.
Methods
Survey
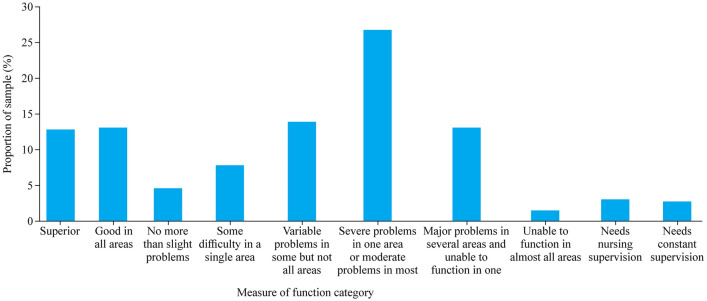
As described previously [2, 12], a multidisciplinary team developed and piloted [2] a comprehensive caregiver survey (see Supplementary file 1). A validated tool [The Royal Alexandra Hospital for Children Measure of Function (MOF)] [13] was embedded in the survey [2] and is a generic parent-reported measure of function for children aged 0–18 years [13]. The MOF scores were grouped into nil/mild (MOF 1–3, which included functioning that was superior and good in all areas, as well as no more than slight problems), moderate (MOF 4–6) and severe (MOF 7–10) (Table 1).
Table 1.
Health functioning categorized into three groups according to measure of function scores
| MOF score | Description | Severity group |
|---|---|---|
| 1 | Superior | |
| 2 | Good in all areas | Mild |
| 3 | No more than slight problems | |
| 4 | Some difficulty in one area | |
| 5 | Variable problems in some but not all areas | Moderate |
| 6 | Severe problems in one area or moderate problems in most | |
| 7 | Major problems in several areas or unable to function in one | |
| 8 | Unable to function in almost all areas | Severe |
| 9 | Needs nursing supervision | |
| 10 | Needs constant medical supervision |
MOF measure of function
We included questions on the use of supportive technology, therapy or surgery to assist mobility, eyesight, hearing/speech, pain treatments, specialist, other health professional and clinic visits, hospital admissions, emergency department (ED) presentations, access to medications and barriers to accessing health services, and out-of-pocket costs incurred (previous 12 months), including for travel, medical appointments, equipment and medications [2, 12]. Postcodes of the family residence were classified using the accessibility and remoteness index of Australia, which categorizes location according to the following Australian standard geographical classifications: major cities of Australia, inner regional Australia, outer regional Australia, remote Australia, and very remote Australia [14].
Participants
As described previously [12], families were recruited between 2013 and 2014 from lists held by our partner rare disease support organizations: the Steve Waugh Foundation (SWF), the SMILE Foundation (now part of Variety, The Children’s Charity), the Australian Genetic Alliance (formerly the Association of Genetic Support Australasia), and a large state-wide clinic [New South Wales (NSW) Genetic Metabolic Disorders Service]. The latter supports children throughout the state of NSW (population during the study period was 1,777,179, which was 32% of all Australian children) with a range of metabolic disorders, including phenylketonuria (PKU), medium chain acyl coenzyme A dehydrogenase deficiency and galactosemia. The SWF, SMILE Foundation and Australian Genetic Alliance support families nationally and are likely to include a representative group of rare diseases, but it should be noted that they are not complete databases for all children with rare diseases. Participants were invited to complete a paper copy of the survey, which was sent to them by our partner organizations and labeled with an anonymous numerical code to protect their privacy. Families consented by responding to the survey, which took less than one hour to complete, as determined from our previous pilot study[2]. Families who did not respond within three months were followed up once by the relevant organization [12].
Statistical analyses
Descriptive statistics were used for demographic and clinical characteristics of the sample. Univariate comparisons were made using the Chi-square test and odds ratios (ORs) and 95% confidence intervals (95% CIs). One-way ANOVA was used to compare group means. The alpha level of significance was P < 0.05. Data analyses were performed using SPSS version 25 (IBM SPSS® Statistics, Chicago, IL).
Population data from other sources
Data collected in this study were compared with population data for the same study period (2013–2014), which were obtained from the following sources: data on hospitalizations and ED presentations in children (< 19 years) in the general population for the study period were obtained from the Australian Institute of Health and Welfare [15, 16]; data on the number of family physician visits by children (< 15 years) in the general population were obtained from The University of Sydney Family Medicine Research Center [17]; and total numbers of children in these age groups in the general population were obtained from the Australian Bureau of Statistics [18] and used to estimate the rates of hospitalizations, ED presentations, and family physician visits in the general population. Costs for hospitalization and ED visits per patient for 2021–2022 were obtained from the Management Support and Analysis Unit at The Children’s Hospital at Westmead in Sydney, Australia.
Results
Study participants
The sample was previously described in detail [12]. Of the 1751 families invited to participate, 462 (30%) responded, with 256 (55%) male and 206 (45%) female children. The median age was 8.9 years (range: 0–18 years). Almost all children were Australian born, and 78% of families identified as Caucasian. Over 240 different rare diseases were represented (see Supplementary file 2), most commonly inborn errors of metabolism (38%), genetic syndromes/chromosomal disorders (26%) and congenital malformation syndromes (6%), and 27 (6%) had no definitive diagnosis [12].
Health functioning, needs for assistive technologies, equipment and surgery
Based on parent reports, child health functioning was classified according to the MOF (Fig. 1). When categorized by severity (Table 1), functional impairment was nil/mild (30%), moderate (48%) or severe (22%). Of the children, 93 (20%) required assistance with hearing or speech, including using sign language (15%) or communication devices (iPads®, pragmatic organization dynamic display, and picture cards) (12%), grommets (12%), and cochlear implants (2%). A third (140, 33%) of families reported that their child had surgery to help improve mobility, eyesight, hearing or speech. Almost one-third (128, 28%) of children required at least one piece of equipment to assist with mobility, including wheelchairs (28%), standing frames, walkers and/or special needs prams (12%) and leg/arm braces (6%).
Fig. 1.
Health functioning according to the measure of function among the 462 children with rare chronic and complex diseases
Health service use
Of the 462 children, 257 (56%) had at least one admission to the hospital in the previous 12 months (Table 2), and 187 (40%) had at least one ED presentation. There was a total of 1231 admissions (2.7/child/year), which is almost 18 times higher than the estimated rate of hospital admissions in the general population of children aged < 19 (0.15/child/year; OR = 17.25, 95% CI = 15.50–19.20). There was a total of 649 ED presentations in the previous 12 months (1.4/child/year), a rate four times higher than that in the general population (0.34/child/year; OR = 4.15, 95% CI = 3.68–4.68). Based on the average cost of admission (AU$7249) or ED presentation (AU$627) at The Children’s Hospital at Westmead, Sydney, Australia for the financial year 2021/2022, we estimated the minimum annual cost of hospital encounters for the 462 children in our study as AU$9,330,442 (US$6,298,048).
Table 2.
Hospital admissions and emergency department presentations in the previous 12 months and estimated costs in children (n = 462)
| Variables | Hospital admissions | ED presentations |
|---|---|---|
| Number of patients accessing at least once, n (%) | 257 (56) | 187 (40) |
| Total number of encounters | 1231 | 649 |
| Encounters per child in our cohort per annum | 2.7 | 1.4 |
| Population based data (number of encounters per child per annum)a | 0.15 | 0.34 |
| Estimated total cost for our cohortb | AU$8,923,519 | AU$406,923 |
| Total cost of hospital in-patient and ED services for our cohortb | AU$9,330,442 |
ED emergency department. aBased on Australian hospital and emergency department statistical data published by the Australian Institute for Health and Welfare [19], and population estimates published by the Australian Bureau of Statistics [20]; bbased on average per encounter costs at The Children’s Hospital at Westmead for the 2021/2022 financial year
Almost all children (387/449, 86%) visited a family physician at least once in the previous 12 months (Table 3), for a total of 3163 visits (about 10 visits/child). Children aged < 15 years (n = 370) made 5042 visits to family physicians (about 14 visits/child) compared with 3.3 visits/child/year in the general population of children aged < 15 years (OR = 4.14, 95% CI = 3.72–4.60). There were 3540 visits to specialist doctors in the previous 12 months (about 9 visits/child/year), most commonly pediatricians, geneticists, eye specialists, surgeons and neurologists (Table 3). Families recorded 10,094 visits to allied health professionals in the previous 12 months, most commonly physiotherapists, speech pathologists and occupational therapists (about 24 visits/child/year) (Table 3). Data on specialist doctor and allied health professional visits for the general Australian population were not available.
Table 3.
Visits to medical practitioners and allied health professionals in the last 12 months
| Service used | Number of patients visiting at least once, n (%) | Total number of visits for the whole cohort, n |
|---|---|---|
| Family physician | 387/449 (86) | 3163 |
| Specialists (N = 462) | ||
| Pediatrician | 284 (61) | 1131 |
| Geneticist | 197 (43) | 488 |
| Eye specialist | 163 (35) | 340 |
| Surgeon | 152 (33) | 417 |
| Neurologist | 140 (30) | 375 |
| Cardiologist | 114 (25) | 216 |
| Respiratory physician | 93 (20) | 303 |
| Pain specialist | 26 (6) | 112 |
| Psychiatrist | 24 (5) | 125 |
| Dermatologist | 18 (4) | 33 |
| Total (specialists) | 393 (85) | 3540 |
| Allied health professionals (N = 462) | ||
| Dentist | 236 (51) | 454 |
| Dietician | 226 (49) | 784 |
| Occupational therapist | 206 (45) | 1935 |
| Physiotherapist | 201 (44) | 2757 |
| Speech pathologist | 178 (39) | 2330 |
| Social worker | 94 (20) | 542 |
| Optometrist | 87 (19) | 148 |
| Specialist nurse | 78 (17) | 649 |
| Psychologist | 55 (12) | 411 |
| Genetic counselor | 45 (10) | 84 |
| Total (allied health) | 418 (91) | 10,094 |
Children were cared for by multidisciplinary teams of up to 15 different health professionals. Most children (215, 47%) had a team of 1–5 health professionals, 173 (37%) had a team of 6–10 professionals, and 56 (12%) had a team of > 10 health professionals. Most of these children had rare complex metabolic, skeletal, or neuromuscular disorders or congenital malformations. Less than half of children (n = 192, 42%) had a health professional to coordinate their care, usually a specialist doctor (n = 109, 57%) or family physician (n = 29, 15%), but included nurses, allied health professionals, child protection case managers and non-government organizations (e.g., the Cerebral Palsy Alliance Australia).
Health-related functioning and frequency of access to hospital services
The numbers of children hospitalized or presenting to the ED varied according to the MOF category (Table 1). Compared to the nil/mild group, the severe group was more likely to be admitted to the hospital in the previous 12 months (OR = 13.39, 95% CI = 7.65–23.44) and more likely to present to the ED (OR = 11.16, 95% CI = 6.46–19.27). Compared to the nil/mild group, the moderate group was more likely to be hospitalized (OR = 9.49, 95% CI = 6.42–14.03) and to present to the ED (OR = 4.52, 95% CI = 3.01–6.78) in the previous 12 months (Fig. 2).
Fig. 2.
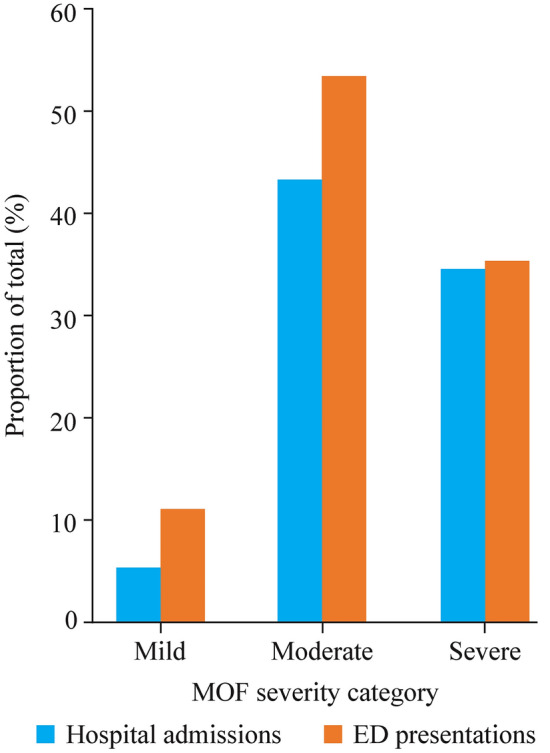
Hospital admissions and emergency department (ED) presentations by measure of function (MOF) severity category (mild, moderate, severe), as a percentage of total admissions (n = 1188) and ED presentations (n = 605), respectively (where MOF data were available) (P < 0.001)
Barriers to accessing health services
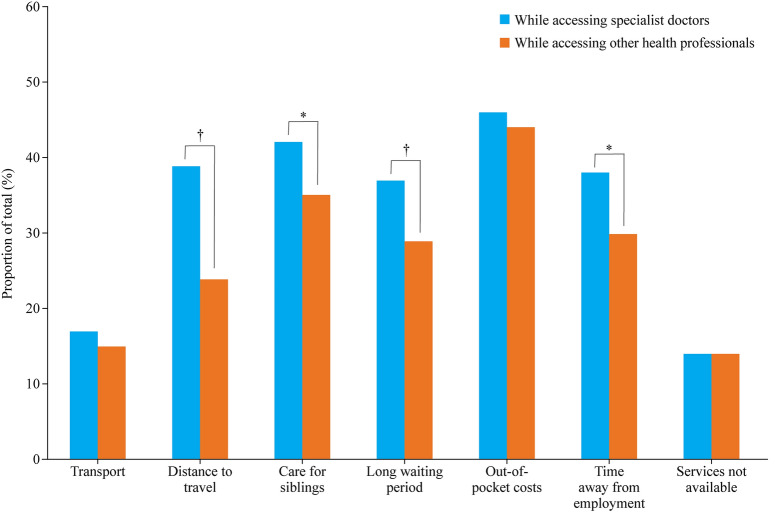
Of the 462 families, 445 provided a response about whether they had adequate access to all health services needed by their child. One-third (145/445, 33%) said their child had inadequate access to required health services, 110/445 (24%) were unsure and 192/445 (43%) had adequate access. All 462 families responded when asked about specific barriers experienced when accessing specialist doctors, and the most commonly reported barriers were personal financial cost (211/462, 46%), care for siblings (192/462, 42%), and distance to travel (180/462, 39%). Similarly, all families responded when asked about barriers when accessing family physicians and allied health professionals, the most commonly reported barriers being personal financial cost (205/462, 44%), care for siblings (164/462, 36%) and time away from employment (135/462, 29%) (Fig. 3). Families were significantly more likely to experience barriers to accessing specialist doctors than other health professionals, including distance to travel (OR = 1.97, 95% CI = 1.49–2.62), care for siblings (OR = 1.29, 95% CI = 0.99–1.69), long waiting periods for appointments (OR = 1.46, 95% CI = 1.10–1.92) and time away from employment (OR = 1.50, 95% CI = 1.14–1.98).
Fig. 3.
Barriers experienced by families when accessing care from specialist doctors compared with other health professionals. *P < 0.05, †P < 0.01
Most children (75%) lived in major cities. Families in regional and remote areas were significantly less likely to have private health insurance and/or report adequate access to health services and more likely to report three or more barriers to accessing healthcare for their child than families living in major cities (Table 4). Reported barriers were accessing specialist doctors, including distance to travel, personal financial costs, and lack of services (Table 4). When accessing other health professionals, time off work was an additional reported barrier (Table 4).
Table 4.
Barriers experienced by children while accessing health services according to Australian standard geographical classification categories of urban, regional and remote location (N = 462)
| Variables | ASGC 1a, n (%) | ASGC 2, n (%) | ASGC 3–5, n (%) | ASGC 1 vs. ASGC 2, OR (95% CI) | ASGC 1 vs. ASGC 3–5, OR (95% CI) |
|---|---|---|---|---|---|
| Number residing (n = 462) | 348 (75.3) | 87 (18.8) | 27 (5.8) | ||
| Has private health insurance (n = 235) | 193 (82.1) | 32 (13.6) | 10 (4.3) | 0.46 (0.28–0.75)* | 0.46 (0.20–1.03) |
| Adequate access to health services (n = 190) | 152 (80.0) | 29 (15.3) | 9 (4.7) | 0.52 (0.30–0.90)* | 0.37 (0.16–0.89)* |
| Barriers when accessing specialist doctors | n = 348 | n =87 | n = 27 | ||
| Transport | 52 (14.9) | 21 (24.1) | 7 (25.9) | 1.82 (1.02–3.23)* | 2.00 (0.80–5.00) |
| Distance to travel | 93 (26.7) | 63(72.4) | 24 (88.9) | 7.14 (4.17–12.50)* | 20.00 (6.25–100.00)* |
| Care for siblings | 139(39.9) | 37 (42.5) | 16 (59.3) | 1.11 (0.69–1.79) | 2.17 (0.98–4.76) |
| Waiting period | 127 (36.5) | 29 (33.3) | 14 (51.9) | 0.87 (0.53–1.43) | 1.89 (0.85–4.17) |
| Personal financial cost | 136 (39.1) | 58 (66.7) | 17 (63.0) | 3.13 (1.89–5.00)* | 2.63 (1.18–5.88)* |
| Time off work | 123 (35.3) | 41 (47.1) | 13 (48.1) | 1.64 (1.01–2.63)* | 1.69 (0.78–3.70) |
| Lack of services | 39 (11.2) | 14 (16.1) | 11 (40.7) | 1.52 (0.78–2.94) | 5.56 (2.38–12.50)* |
| Experienced ≥ 3 barriers | 136 (39.1) | 56 (64.4) | 23 (85.2) | 2.78 (1.72–4.55)* | 9.09 (3.03–25.00)* |
| Barriers when accessing other health professionals | n = 348 | n = 87 | n = 27 | ||
| Transport | 48 (13.8) | 17 (19.5) | 5 (7.1) | 1.52 (0.83–2.78) | 1.43 (0.51–4.00) |
| Distance to travel | 63 (18.1) | 35 (40.2) | 15 (55.6) | 3.03 (1.82–5.00)* | 5.56 (2.50–12.50)* |
| Care for siblings | 119 (34.2) | 30 (34.5) | 15 (55.6) | 1.01 (0.62–1.67) | 2.38 (1.09–5.26)* |
| Waiting period | 100 (28.7) | 22 (25.3) | 10 (37.0) | 0.84 (0.49–1.43) | 1.45 (0.64–3.23) |
| Personal financial cost | 143 (41.1) | 45 (51.7) | 17 (63.0) | 1.54 (0.96–2.44) | 2.44 (1.09–5.56)* |
| Time off work | 93 (26.7) | 29 (33.3) | 13 (48.1) | 1.37 (0.83–2.27) | 2.56 (1.15–5.56)* |
| Lack of services | 43 (12.4) | 13 (14.9) | 10 (37.0) | 1.25 (0.64–2.44) | 4.17 (1.79–10.00)* |
| Experienced ≥ 3 barriers | 112 (32.2) | 38 (43.7) | 18 (66.7) | 1.64 (1.01–2.63)* | 4.17 (1.82–10.00)* |
ASGC Australian standard geographical classification, OR odds ratio, CI confidence interval. aASGC 1: major cities of Australia, ASGC 2: inner regional Australia, ASGC 3: outer regional Australia, ASGC 4: remote Australia, ASGC 5: very remote Australia. *P < 0.05
Access to medications and therapies
Almost half (209, 45%) of the parents were aware that specific medications were available for their child’s condition, and of these children, 172 (82%) were receiving those medications. A quarter (172, 25%) of parents said that no effective medication was available for their child’s condition. Five families accessed medications via the Life Saving Drugs Program Australia [21], and another five families accessed medications from overseas. Barriers to accessing medication were reported by 13% of parents, the most common being lack of stock (41%), financial cost (21%), requirement for special authority prescriptions (21%), and need to travel to hospital to access medication/formulation (7%). Four families were awaiting entry into a clinical trial to access novel, expensive medications. There were no significant differences in the proportion of children experiencing barriers to accessing medications by geographic residence (29% regional and remote vs. 32% major cities; OR = 0.87, 95% CI = 0.43–1.77). Just over half the families reported that their child was receiving non-pharmaceutical treatments for their rare disease (n = 246, 54.8%), including special diets (n = 159, 64.6%), dietary supplements (n = 147, 59.8%), alternative or complementary medications (n = 18, 7.3%), and other (non-specified) treatments (n = 18, 7.3%). Furthermore, 112 (26%) families reported that their child had been recommended additional equipment, medications or services that they had been unable to access, most commonly allied health therapy (n = 39, 35%), e.g., hydrotherapy, physiotherapy and equipment (e.g., wheelchair, continuous positive airway pressure machine) (n = 33, 29%). These families also reported an inability to access specific medications that are not subsidized by the Australian government [e.g., sapropterin dihydrochloride for treatment of PKU; 9 (8%)] and treatments of unproven benefit [e.g., hyperbaric oxygen therapy; 4 (4%)].
Out-of-pocket expenses
Just over half (n = 235, 51%) of all families had private health insurance, and 79% (n = 365) received financial support from government programs. One-third of all families (153, 33%) incurred out-of-pocket expenses (not covered by Medicare) when seeking a diagnosis, and more than two-thirds of these families (101, 66%) said this caused financial stress. When seeking a diagnosis, average out-of-pocket expenses for families living in major cities were AU$2604 per family (range: AU$150-$45,000), but inner regional families spent AU$5390 (range: AU$150-$32,000), and families in rural or remote regions spent AU$9250 (range: AU$500-$20,000) (one-way ANOVA: P < 0.05). Parents were asked to estimate their health-related out-of-pocket expenses for caring for their child in the last 12 months. Out-of-pocket costs were < AU$500 for 20%, AU$500-$2000 for 40%, AU$2000–5000 for 23%, and > AU$5000 for 17%. The most common out-of-pocket expenses were travel costs (32.5%), medical appointments (30%), medications (29%), and medical and assistive equipment (27%).
Discussion
Few data are available on the impacts of rare childhood diseases on children, families and health systems. This study highlights the substantial impact on child health functioning, use of and access to healthcare services and medications, and financial burden. Of 462 children with a rare disease, 70% had moderate to severe functional impairment, and 3% required constant supervision. Children with severe impairment of health functioning were 13 times more likely to be hospitalized and 11 times more likely to present to the ED than children with mild impairment. Families of children living with rare diseases reported that their children frequently use a wide variety of primary, secondary and tertiary health services. Many depend on large, multi-disciplinary teams, and although 12% had more than 10 different health professionals, few had a care coordinator. Two-thirds of all children required surgery or specialized equipment to improve their hearing, communication or mobility.
Rates of hospitalization, ED presentation, and family physician visits in the previous 12 months were significantly higher than in the age-matched general population. These children would benefit from coordination of health care [22], streamlining of appointments, access to hospital-from-home care and telehealth care [more available since the coronavirus disease 2019 (COVID-19) pandemic] [23], integration of services across primary to tertiary health sectors [22], shared patient-held medical records (recently introduced in Australia), and disease-specific clinics. Frequent use of hospital services is associated with high system costs. A minimum estimate of the total cost for admissions and ED presentations in 286 children who used these services was AU$9 million (US$6.30 million), with an average annual cost of approximately AU$31,000 (US$21,500) per child. These findings corroborate previous reports of high health service costs for children with rare diseases [5, 19, 20, 24, 25].
We report novel data on inequitable access to necessary health services for children with rare diseases who live in regional/remote Australia. This is also a recognized problem for the general Australian population because of the relative lack of publicly funded specialist services outside major cities [26]. Families in our study who lived outside major cities also experienced other barriers to accessing services, including long travel times, transport difficulties, long waiting times to see specialist doctors and other health professionals, and the need for sibling childcare. Families from remote areas incurred higher out-of-pocket expenses in obtaining a diagnosis and were less likely to have private health insurance, limiting access to private specialist doctor services. Although over 240 rare diseases were represented in our study, 6% of children lacked a definitive diagnosis. Almost one-third of parents reported the lack of effective medications for their child’s condition, and others accessed novel treatments through Life Saving Drugs programs [21] or clinical trials.
The strengths of this study include the large sample of children with a wide range of rare conditions, use of validated instruments to assess health functioning and burden on families, and generalizability of the findings nationally and likely internationally to other high-income countries. Although children from the state of NSW are overrepresented, we do not consider this a limitation because > 30% of Australian children live in NSW and healthcare services are similar nationally. Children with inborn errors of metabolism were also overrepresented, reflecting enhanced sampling from a metabolic clinic. However, over 90% of all children in the sample had similarly complex genetic disorders. Furthermore, many rare diseases have common features [12]. The response fraction (32%) is similar to previous international surveys on rare diseases (e.g., 30% in the European Organization for Rare Diseases Care 3 survey) [27]. The retrospective nature of questions on out-of-pocket costs (previous 12 months) may be subject to recall bias. Nevertheless, our prospective study found that out-of-pocket costs incurred by families of children with rare diseases were substantial [28]. One limitation was the lack of comparative data on specialist medical and other health professional visits in the general population.
We previously called for a national, coordinated plan for Australia to help raise the profile of rare diseases and better support affected individuals and their families and carers [29]. The National Strategic Action Plan for Rare Diseases, developed with input from stakeholders, including people living with rare diseases and their families, was implemented by the Australian Government Department of Health in 2020 [30]. It emphasizes the need for awareness and education about rare diseases among health professionals and communities, evidence-based care, and research [30, 31]. Our study data show that access to healthcare is inequitable for children with rare diseases and disability. This issue was addressed in Australia’s first National Plan for Rare Diseases for Australia, published in 2020, and evaluation of the plan’s implementation will be crucial to determine whether healthcare access and outcomes improve for children with rare diseases over time. Recently, clinics for undiagnosed rare diseases have been funded [32], and a care coordination model has been proposed to reduce hospital admissions and ED presentations for children with complex medical conditions [33]. Virtual clinical care, which became widespread during the COVID-19 pandemic, promises increased support for children living in rural and remote settings [28, 34].
In conclusion, these novel data will be useful to advocate for improved service access and to inform future healthcare models that enable coordinated, multi-disciplinary care regardless of geography, disease severity and socio-economic status.
Supplementary Information
Supplementary information
Supplementary information
Acknowledgements
We thank all families who gave valuable time to complete our survey. We thank Ms Amy Phu, Ms Charmy Fernando and Mr Victor Wu from the Australian Paediatric Surveillance Unit, The University of Sydney for data entry of survey responses. We also thank representatives from our partner organizations: Steve and, Lynette Waugh from the Steve Waugh Foundation; Diane Petrie and Ayesha Wijesinghe from Genetics Alliance Australia (formerly the Association of Genetic Support Australasia); Evie Smith (the SMILE Foundation); Tam Johnston (Variety–the Children’s Charity New South Wales) and Philippa Ardlie from the Royal Australasian College of Physicians, for their assistance in recruiting families and their insightful comments on the survey questions.
Author contributions
TS and EDG contributed to data analysis, and reviewing and editing. ZY contributed to study conceptualization, methodology, data analysis and writing of the original draft. DM and DT contributed to study methodology, data validation, analysis, and reviewing and editing. JJLS contributed as a research student to study methodology (development of family survey), and reviewing and editing. CJ contributed to study conceptualization, methodology (recruitment of patients), categorizing of patient diagnoses, and reviewing and editing. LH contributed to study conceptualization, methodology, and reviewing and editing. EJE contributed to conceptualization, methodology (patient recruitment and data collection), and reviewing and editing. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
This research was supported by an Australian Research Council Linkage Project grant scheme (No. LP110200277). The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. During the period of the research, ZY held a Fellowship from the Sydney Medical School Foundation and LH was funded by a National Health and Medical Research Council of Australia Senior Research Fellowship (No. 1117105). EJE was supported by a National Health and Medical Research Council of Australia Practitioner Fellowship (No. 1021480) and a Medical Research Futures Fund Next Generation Fellowship (No. 1135959). CJ’s Chair in Genomic Medicine is supported by The Royal Children’s Hospital Foundation.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval
Ethical approval was obtained from The Children’s Hospital at Westmead (Sydney, Australia) Human Research Ethics Committee (approval number: 10/CHW/5). Participants gave consent by responding to the family survey used in this study.
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zurynski Y, Frith K, Leonard H, Elliott E. Rare childhood diseases: how should we respond? Arch Dis Child. 2008;93:1071–1074. doi: 10.1136/adc.2007.134940. [DOI] [PubMed] [Google Scholar]
- 2.Anderson M, Elliott EJ, Zurynski YA. Australian families living with rare disease: experiences of diagnosis, health services use and needs for psychosocial support. Orphanet J Rare Dis. 2013;8:22. doi: 10.1186/1750-1172-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott EJ, Zurynski YA. Rare diseases are a “common” problem for clinicians. Aust Fam Physician. 2015;44:630–633. [PubMed] [Google Scholar]
- 4.Baumbusch J, Mayer S, Sloan-Yip I. Alone in a crowd? Parents of children with rare diseases’ experiences of navigating the healthcare system. J Genet Couns. 2019;28:80–90. doi: 10.1007/s10897-018-0294-9. [DOI] [PubMed] [Google Scholar]
- 5.The Lancet Child Adolescent Health Rare diseases: clinical progress but societal stalemate. Lancet Child Adolesc Health. 2020;4:251. doi: 10.1016/S2352-4642(20)30062-6. [DOI] [PubMed] [Google Scholar]
- 6.Walker CE, Mahede T, Davis G, Miller LJ, Girschik J, Brameld K, et al. The collective impact of rare diseases in Western Australia: an estimate using a population-based cohort. Genet Med. 2017;19:546–552. doi: 10.1038/gim.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OECD Data. Health spending. 2017. https://data.oecd.org/healthres/health-spending.htm. Accessed 15 Jan 2020.
- 8.Callander EJ, Fox H, Lindsay D. Out-of-pocket healthcare expenditure in Australia: trends, inequalities and the impact on household living standards in a high-income country with a universal health care system. Health Econ Rev. 2019;9:10. doi: 10.1186/s13561-019-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Australian Taxation Office. Private health insurance rebate. https://www.ato.gov.au/Individuals/Medicare-and-private-health-insurance/Private-health-insurance-rebate/. Accessed 7 Jun 2022.
- 10.Harris B, Craike M, Dunkin R, Calder R. Is Medicare Fair? ii. The distribution of Medicare Benefits across the cities and country. Mitchell Institute Policy issues paper no.3–2019, Victoria University, Melbourne. 2019. https://vuir.vu.edu.au/40399/. Accessed 3 Jun 2022.
- 11.Thompson LM, Armfield NR, Slater A, Mattke C, Foster M, Smith AC. The availability, spatial accessibility, service utilisation and retrieval cost of paediatric intensive care services for children in rural, regional and remote Queensland: study protocol. BMC Health Serv Res. 2013;13:163. doi: 10.1186/1472-6963-13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zurynski Y, Deverell M, Dalkeith T, Johnson S, Christodoulou J, Leonard H, et al. Australian children living with rare diseases: experiences of diagnosis and perceived consequences of diagnostic delays. Orphanet J Rare Dis. 2017;12:68. doi: 10.1186/s13023-017-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dossetor DR, Liddle JL, Mellis CM. Measuring health outcome in paediatrics: development of the RAHC measure of function. J Paediatr Child Health. 1996;32:519–524. doi: 10.1111/j.1440-1754.1996.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 14.Hugo Centre for Migration and Population Research. Accessibility/remoteness index of Australia—ARIA+. Adelaide: University of Adelaide, Hugo Centre for Migration and Population Research; 2011. https://www.adelaide.edu.au/hugo-centre/services/aria. Accessed 24 Oct 2016.
- 15.Australian Institute of Health and Welfare. Admitted patient care 2013–14: Australian hospital statistics. Health services series no. 60. Cat. no. HSE 156. Canberra: AIHW; 2015. https://www.aihw.gov.au. Accessed 26 Jul 2022.
- 16.Australian Institute of Health and Welfare. Australian hospital statistics 2013–14: emergency department care. Health services series no. 58. Cat. no. HSE 153. Canberra: AIHW; 2014. https://www.aihw.gov.au. Accessed 26 Jul 2022.
- 17.Britt H, Miller GC, Henderson J, Bayram C, Valenti L, Harrison C, et al. General practice activity in Australia 2013–14. General practice series no. 36. Sydney: Sydney University Press; 2014. https://purl.library.usyd.edu.au>sup>9781743324219. Accessed 3 Aug 2022.
- 18.Australian Bureau of Statistics. Australian demographic statistics. 2014. https://www.abs.gov.au. Accessed 17 Jan 2018.
- 19.Grau J, Zöllner JP, Schubert-Bast S, Kurlemann G, Hertzberg C, Wiemer-Kruel A, et al. Direct and indirect costs and cost-driving factors of tuberous sclerosis complex in children, adolescents, and caregivers: a multicenter cohort study. Orphanet J Rare Dis. 2021;16:282. doi: 10.1186/s13023-021-01899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navarrete-Opazo AA, Singh M, Tisdale A, Cutillo CM, Garrison SR. Can you hear us now? The impact of health-care utilization by rare disease patients in the United States. Genet Med. 2021;23:2194–2201. doi: 10.1038/s41436-021-01241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The life saving drugs program within Australia. https://www.health.gov.au/initiatives-and-programs/life-saving-drugs-program. Accessed 31 Aug 2022.
- 22.Kuo DZ, Houtrow AJ, Council on Children with Disabilities. Recognition and management of medical complexity. Pediatrics. 2016;138:e20163021. [DOI] [PubMed]
- 23.Smith M, Alexander E, Marcinkute R, Dan D, Rawson M, Banka S, et al. Telemedicine strategy of the European Reference Network ITHACA for the diagnosis and management of patients with rare developmental disorders. Orphanet J Rare Dis. 2020;15:103. doi: 10.1186/s13023-020-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hime NJ, Fitzgerald D, Robinson P, Selvadurai H, Van Asperen P, Jaffé A, et al. Childhood interstitial lung disease due to surfactant protein C deficiency: frequent use and costs of hospital services for a single case in Australia. Orphanet J Rare Dis. 2014;9:36. doi: 10.1186/1750-1172-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi X, Xu J, Shan L, Li Y, Cui Y, Liu H, et al. Economic burden and health related quality of life of ultra-rare Gaucher disease in China. Orphanet J Rare Dis. 2021;16:358. doi: 10.1186/s13023-021-01963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Sullivan BG, Stoelwinder JU, McGrail MR. Specialist outreach services in regional and remote Australia: key drivers and policy implications. Med J Aust. 2017;207:98–99. doi: 10.5694/mja16.00949. [DOI] [PubMed] [Google Scholar]
- 27.European Organisation for Rare Diseases. The voice of 12,000 patients: experiences and expectations of rare disease patients on diagnosis and care in Europe. 2009. https://www.eurordis.org/publications/the-voice-of-12000-patients/. Accessed 22 Jul 2022.
- 28.Deverell M, Phu A, Elliott EJ, Teutsch SM, Eslick GD, Stuart C, et al. Health-related out-of-pocket expenses for children living with rare diseases-tuberous sclerosis and mitochondrial disorders: a prospective pilot study in Australian families. J Paediatr Child Health. 2022;58:611–617. doi: 10.1111/jpc.15784. [DOI] [PubMed] [Google Scholar]
- 29.Jaffe A, Zurynski Y, Beville L, Elliott E. Call for a national plan for rare diseases. J Paediatr Child Health. 2010;46:2–4. doi: 10.1111/j.1440-1754.2009.01608.x. [DOI] [PubMed] [Google Scholar]
- 30.The Australian Government Department of Health. National strategic action plan for rare diseases. 2020. https://www.health.gov.au/resources/publications/national-strategic-action-plan-for-rare-diseases. Accessed 31 May 2022.
- 31.Bhattacharya K, Millis N, Jaffe A, Zurynski Y. Rare diseases research and policy in Australia: on the journey to equitable care. J Paediatr Child Health. 2021;57:778–781. doi: 10.1111/jpc.15507. [DOI] [PubMed] [Google Scholar]
- 32.Government of Western Australia. New rare care centre will coordinate rare disease care at Perth Children’s Hospital. https://www.cahs.health.wa.gov.au/News/2022/02/25/Rare-diseases-centre-at-PCH. Accessed 31 Aug 2022.
- 33.Breen C, Altman L, Ging J, Deverell M, Woolfenden S, Zurynski Y. Significant reductions in tertiary hospital encounters and less travel for families after implementation of paediatric care coordination in Australia. BMC Health Serv Res. 2018;18:751. doi: 10.1186/s12913-018-3553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groft SC, Posada de la Paz M. Preparing for the future of rare diseases. Adv Exp Med Biol. 2017;1031:641–8. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.