Abstract
The role played by soluble molecules that may participate in acanthamoebal cytopathogenicity has yet to be fully characterized. We demonstrate here that Acanthamoeba castellanii trophozoites constitutively release ADP in the medium. Cell-free supernatants prepared from A. castellanii, by interaction with specific P2y2 purinoceptors expressed on the Wish cell membrane, caused a biphasic rise in [Ca2+]i, extensive cell membrane blebbing, cytoskeletal disorganization, and the breakdown of nuclei. Cell damage induced by amoebic supernatants was blocked by the P2y2 inhibitor Suramin. The same results were found in Wish cells exposed to purified ADP. These findings suggest that pathogenic free-living A. castellanii may have a cytopathic effect on human epithelial cells through ADP release, by a process that begins with a rise of cytosolic free-calcium concentration, and culminates in apoptosis.
Acanthamoeba is a genus of small free-living amoebas characterized by a life cycle of active trophozoites and dormant cysts (21, 28). Human infection due to Acanthamoeba spp. involving the brain, eyes, lungs, and skin has increased significantly during the last 10 years (10, 13, 16). Numerous in vitro studies, carried out to elucidate the virulence factors responsible for Acanthamoeba infections, have shown both contact-dependent cell killing and cell-free cytopathogenicity due to metabolites released by trophozoites (14, 15, 22), which confirms that amoebic exotoxins, enzymes, or other unidentified molecules may be involved in the interactions between the amoebas and host cells. The role played by soluble molecules that may participate in acanthamoebal cytopathogenicity has yet to be completely elucidated.
Alizadeh et al. (2, 22) have reported that corneal epithelial cells, melanoma cells, and murine neuroblastoma cells exposed to aqueous extracts of Acanthamoeba trophozoites undergo lysis by a process involving apoptosis.
We have previously shown (17) that heat-resistant and nonproteinic molecules with a low molecular weight (<10 kDa), released by viable A. castellanii trophozoites, produce a serious cytopathic effect in human epithelial Wish cells in vitro, causing a cytosolic-free-calcium ([Ca2+]i) increase, morphological changes, cytoskeletal alterations, a decrease in cell viability, and cell death. Since the increase in cytosolic free-calcium concentration was immediate in Wish cells, in this connection we also hypothesized that the [Ca2+]i rise may be the prime cause of cell death, but the identity of the molecules inducing calcium overload and the mechanisms of cell death were not characterized.
The present investigation was undertaken to identify the chemical nature of these amoebic toxic compounds and to further characterize their cytotoxic action on human epithelial Wish cells.
Our study was performed using trophozoites of A. castellanii, isolated from a case of amoebic keratitis (in Ancona, Italy), axenically grown at 25°C in PYG medium (11). The species identification of this isolate was based on cyst morphology and indirect immunofluorescence microscopy. Our previous observations have shown that this A. castellanii isolate, at 37°C, exerted contact-dependent cell killing on Wish cells.
Preparation of amoebic cell-free supernatants.
Amoebic cell-free supernatants were prepared as described previously (17). Amoebas were washed twice in phosphate-buffered saline solution (PBS) without Ca2+ and Mg2+ at pH 7.4 were resuspended (6 × 106 cells/ml) in the same buffer or in RPMI medium containing 20 mM HEPES and incubated for 2 h at 25°C. Supernatants of these cultures, obtained by centrifugation at 500 × g for 15 min, were treated at 95°C for 10 min, ultrafiltered through Centriprep-10 microconcentrators (Amicon), which have a molecular cutoff of 10 kDa, and used immediately after processing as cPBS and cRPMI, respectively. In particular, cPBS was used only in [Ca2+]i measurements, since some aromatic RPMI medium components could interfere with this assay.
Characterization of heat-resistant compounds.
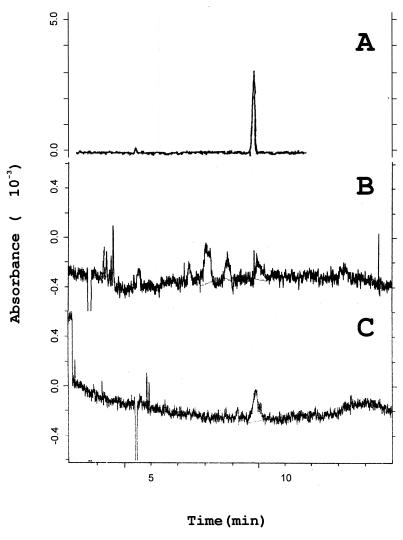
To characterize the chemical nature of the heat-resistant compounds with low molecular weight released by Acanthamoeba trophozoites we used sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and capillary electrophoresis techniques. Several peptides, such as staphylococcal aplha-toxin and delta-toxin (5), dissolving in the lipid bilayer have been shown to increase the membrane permeability to specific inorganic ions and to produce cytolysis. Tricine SDS-PAGE experiments, performed according to the method of Schagger and von Jagow (26), nevertheless excluded the presence of small peptides in both cPBS and cRPMI (data not shown). Capillary electrophoresis analysis of amoebic-cell-free supernatants was performed according to the method of Banditelli et al. (4), with a P/ACE 2100 System (Beckman Instruments) at 20 kV, using capillary tubing 50 μm in diameter and 50 cm long and a buffer of 40 mM glycine and 50 mM sodium dihydrogen phosphate (pH 9). These experiments have shown the presence of ADP in significant micromolar concentrations (Fig. 1), and the ADP concentration in both cPBS and cRPMI was calculated to be about 20 μM. Similar results were obtained when we incubated amoebae for 2 h at 37°C. Extracellular purine nucleosides and nucleotides are ubiquitous, phylogenetically ancient intercellular signals that can interact with specific cell surface receptors to mediate a variety of biological responses (20, 23). In the past decade, the cytotoxic properties of extracellular nucleotides have received a lot of attention. It has been shown, in fact, that ATP and ADP can affect the plasma membrane permeability of cultured cells (9, 27) and cause elevation of the [Ca2+]i concentration and apoptosis in certain types of cell (1, 7, 19, 25, 30). In order to establish the role of amoebic released-ADP in A. castellanii cell-free cytopathogenicity, therefore, we have compared the cytopathic effect exerted on human epithelial Wish cells, by both ultrafiltered Acanthamoeba cell-free supernatants and external ADP (ADP0).
FIG. 1.
Electropherograms of 100 μM purified ADP solution (A), heat-treated filtered cell-free RPMI (cRPMI) conditioned for 2 h by A. castellanii (6 × 106 trophozoites/ml) (B), or heat-treated filtered cell-free PBS (cPBS) conditioned for 2 h by A. castellanii (6 × 106 trophozoites/ml) (C).
[Ca2+]i measurement.
The Wish cell line was routinely maintained in RPMI 1640 medium (GIBCO BRL/Life Technologies Italy) supplemented with 10% heat-inactivated fetal calf serum (GIBCO BRL/Life Technologies Italy), 100 U of penicillin G, and 100 μg of streptomycin sulfate per ml and grown in 25-cm2 plastic flasks at 37°C in a 5% CO2 atmosphere. Intracellular calcium in Wish cells was monitored by using the fluorescent calcium probe FURA 2-AM (Sigma-Aldrich S.r.l., Milano, Italy). Dye loading was standardized by incubating cells, suspended in HEPES buffer (137 mM NaCl, 1.2 mM MgSO4, 1.5 mM CaCl2, 5 mM KCl, 15 mM glucose; pH 7.4); with 3 μM Fura 2-AM for 10 min at room temperature. Loaded cells were washed twice with the same buffer, and the assay was performed on a stirred aliquot (0.5 ml), at 37°C, with the use of a Hitachi F-2000 spectrophotometer. The excitation and emission wavelengths were 340 to 380 nm and 510 nm, respectively, detected every 500 ms, and stored in separate memories of the F-2000 spectrophotometer. A data Menager was used to monitor the fluorescence signal of Fura-2 AM-loaded cells. Basal and stimulated cytosolic calcium were quantified according to the method of Grynkiewicz et al. (12) by using the ratio technique and a Kd of 224 nM as the dissociation constant of Fura-2 AM; maximal and minimal values of fluorescence were evaluated after the addition of of 0.006% Triton X-100 and 10 mM EGTA, respectively. Hitachi F-2000 software was used for calculation.
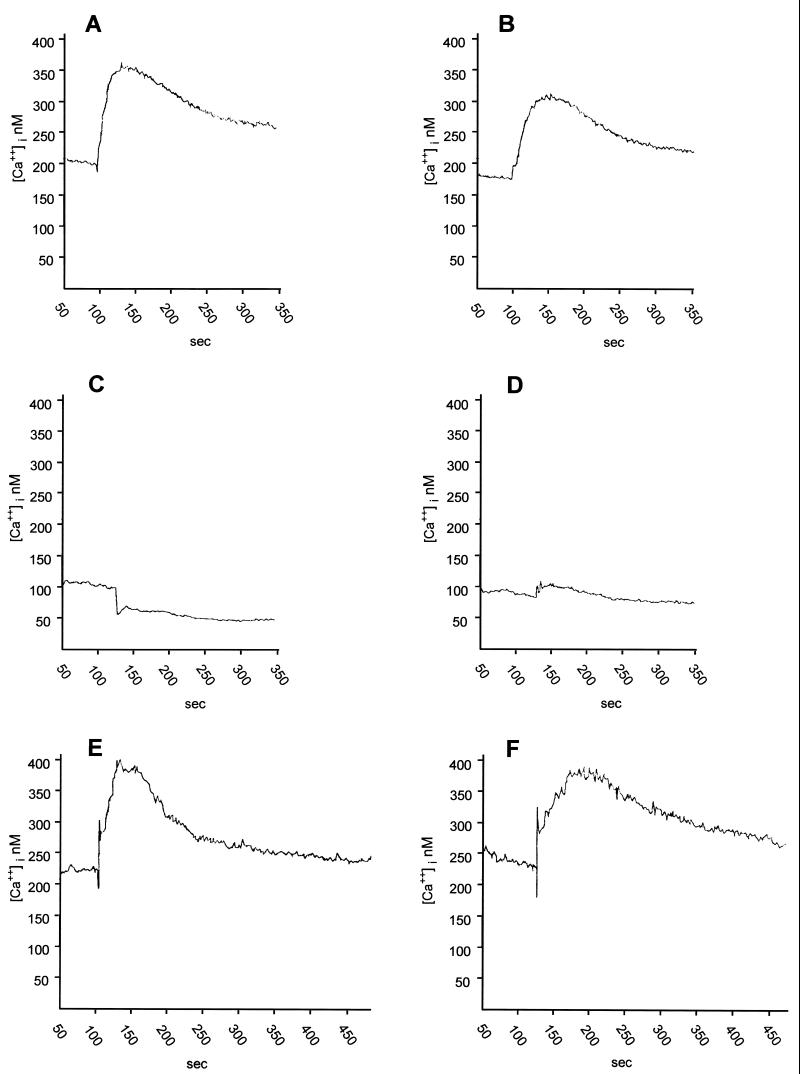
In single isolated Wish cells, the resting level of the cytosolic-free-calcium was calculated to be 196.38 ± 12.72 nM (n = 42), and the addition of PBS alone did not cause variation ([Ca2+]i = 191.96 ± 18.44; n = 12). Stimulation with 80 μl of 20 μM ADP0 (Sigma-Aldrich S.r.l.) or with 80 μl of cPBS (containing approximately the same concentration of amoebic released ADP) led to a rapid biphasic increase of [Ca2+]i, which consisted of an initial transient elevation, maintained for up to 100 s, followed by a sustained elevation at levels higher than the basal value (Fig. 2 A and B). These experiments resulted in a peak increase in [Ca2+]i of 165.82 ± 20.26 nM (n = 6) or 217.82 ± 31.95 nM (n = 6), respectively, and between these conditions any statistical difference was shown by a two-tailed Student's t test calculation. We further investigated the mechanism of ADP0 and cPBS to induce [Ca2+]i increase on Wish cells. Upon stimulation of the Wish cells, with ADP0, with external ATP (ATP0; Sigma-Aldrich S.r.l.) or with adenosine (Sigma-Aldrich S.r.l.) at micromolar concentrations (3.2, 6.4, and 12.8 μM), the potency of the different purine nucleotides in elevating the [Ca2+]i was ATP > ADP, whereas adenosine was inefficient. Both 12.8 μM ADP0 and 12.8 μM ATP0 resulted in peak increases of [Ca2+]i of 375.14 ± 20.7 nM (n = 6) and 624.70 ± 24.6 nM (n = 6), respectively. These results indicated that the response to either nucleotide was mediated by functional P2 purinoceptors, expressed on the Wish cell membrane. The classification of P2-purinergic receptors distinguishes two major classes: ionotropic P2 purinoceptors (P2x), which are ligand-gated receptors containing an intrinsic ion channel, and metabotropic P2 purinoceptors (P2y), which belong to the superfamily of G protein-coupled receptors (9). Multiple subclasses of P2y purinoceptors are well known (P2y1–2y5). Upon testing the effect of several specific antagonists, we identified and characterized the nucleotide receptor expressed in Wish cells on which ADP0 acted. Suramin (Sigma-Aldrich S.r.l.), a compound known to compete with ATP and ADP for their binding sites (P2x and P2y2 receptor antagonists), nearly abolished the effect of both ADP0 and cPBS on a [Ca2+]i increase, while the purinergic P2x receptor antagonist pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS; Sigma-Aldrich S.r.l.) was ineffective (Table 1). This clearly indicated that cPBS, like external ADP, acted on P2y2 purinergic receptors expressed in Wish cell membranes to induce calcium overload. It is known that, by means of this nucleotide receptor, micromolar concentrations of ADP are sufficient to activate Ca2+ mobilization, inositol-1,4,5-triphosphate accumulation and Ca2+ influx in most cells (24).
FIG. 2.
Time courses of the [Ca2+]i increase evoked in Wish cells, loaded with Fura 2-AM (3 μM) by 80 μl of heat-treated filtered cell-free PBS (cPBS), conditioned for 2 h by (6 × 106/ml) A. castellanii trophozoites (A) by the same volume of PBS containing 20 μM ADP0 (B); by cPBS (C) or 20 μM ADP0 (D) after chelation of extracellular Ca2+ with 5 mM EGTA; and by cPBS (E) and 20 μM ADP0 (F) after 20 min of loaded Wish cell exposure to 10 μM ryanodine.
TABLE 1.
Effect of two purinergic antagonists on the cytosolic free-calcium peak increase (Δ[Ca2+]i) induced on Wish cells stimulated with heat-treated filtered cell-free PBS–20 mM HEPES conditioned for 2 h by A. castellanii trophozoites (6 × 106/ml) (cPBS) or PBS–20 mM HEPES containing 20 μM ADP0a
| Sample | Mean Δ[Ca2+]i (nM) ± SE with:
|
|||||
|---|---|---|---|---|---|---|
| No treatment | Suramin (P2x–P2y2 antagonist) at:
|
PPADS (P2x antagonist) at:
|
||||
| 10 μM | 15 μM | 20 μM | 5 μM | 10 μM | ||
| cPBS | 217.82 ± 31.95 | 58.28 ± 3.12∗ | 38.70 ± 2.76∗ | 0 ± 0∗ | 236.91 ± 29.54† | 241.27 ± 34.18† |
| PBS-ADP (20 μM) | 165.82 ± 20.26 | 24.84 ± 1.16∗ | 20.02 ± 1.10∗ | 0 ± 0∗ | 218.49 ± 15.80† | 236.44 ± 36.04† |
Cells stimulation was obtained with 80 μl of the sample indicated. Values are means ± the standard error of at least six experiments. ∗, P < 0.01 versus the respective controls; †, not significant. The basal value of the [Ca2+]i in Wish cells is 196 ± 12.72 nM (n = 42).
To further investigate the Ca2+ signaling pathway coupled to the action exerted by ADP0 and cPBS on Wish P2y2 receptors, we removed extracellular Ca2+ by chelation with 5 mM EGTA. In this condition ADP0 and cPBS evoked solely the initial phase of [Ca2+]i peak, but the response was now transient, and no sustained plateau phase could be observed (Fig. 2C and D). To further explain whether the rise of [Ca2+]i could be dependent on ion release from intracellular calcium stores, target cells were exposed to ryanodine (Sigma-Aldrich S.r.l.) that blocks release of calcium ions from endoplasmic reticulum (18). Ryanodine treatment of Wish cells for 20 min did not abolish the [Ca2+]i increase induced by ADP0 or cPBS; however, the initial phases of the response were significantly reduced (Fig. 2E and F). These results suggested that, in response to both ADP0 and cPBS, the initial phase of the cytosolic-free-calcium increase was caused by depletion of the intracellular calcium stores, whereas the peak and the plateau phase depended on the transmembraneous influx of Ca2+. Amoebic soluble metabolites which could act directly on Wish cell calcium channels were not present in cPBS; in fact, the calcium channel blocker Diltiazem (Sigma-Aldrich S.r.l.) did not abolish the cPBS-induced [Ca2+]i increase (data not shown).
Phase-contrast microscopy, actin microfilament analysis, and assessment of apoptosis.
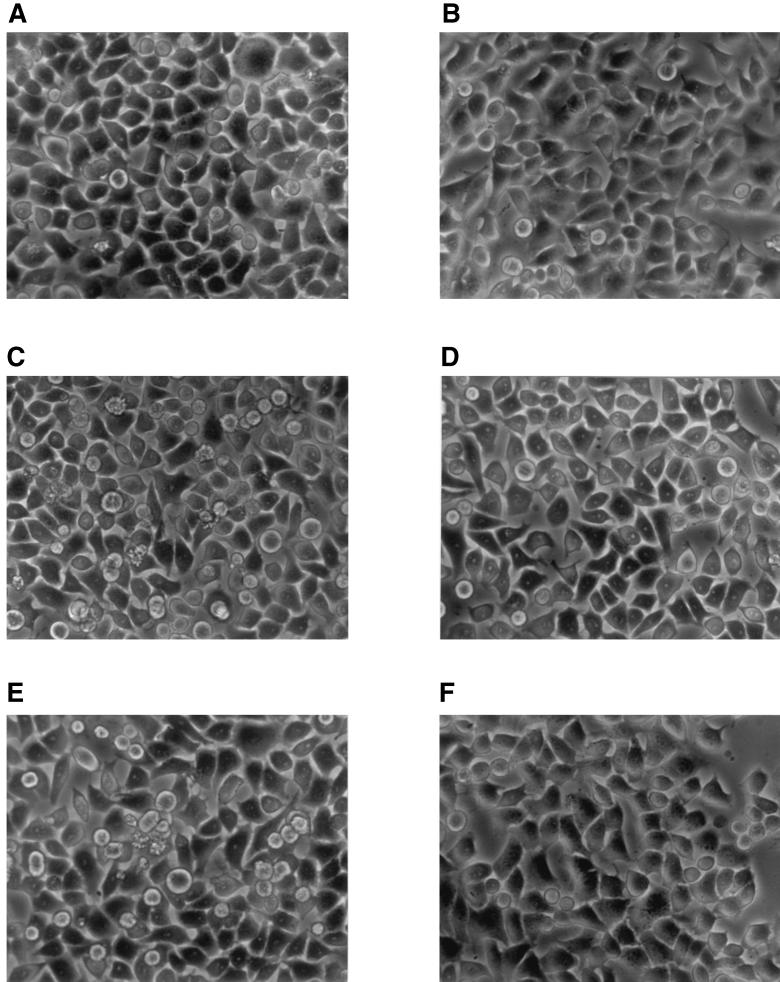
It is known that [Ca2+]i deregulation is an important link and signaling event in cells following a variety of injuries. The [Ca2+]i increase, by activation of proteases, endonucleases and phospholipase, can cause cellular prelethal changes which can occur by two principal patterns: oncosis or apoptosis (29). It has also been demonstrated that changes in cytoskeletal organization can be relevant for the cell destiny (6). Hence, to characterize the cytopathic effect induced by amoebic-cell-free supernatant and ADP0, Wish cell monolayers growing on glass coverslips, in a CO2 incubator at 37°C, were exposed for 1, 3, 6, 9, and 24 h to 0.5 ml of cRPMI (obtained as described above), 0.5 ml of RPMI containing 20 μM ADP0, or the same volume of fresh medium (cell controls). At the selected time intervals, the cell morphology was observed with a Zeiss (Tilaval 31) microscope equipped with a 40 × lens. After incubation with cRPMI or RPMI containing 20 μM ADP0, Wish cells underwent morphological changes which are typical of classical apoptosis. In both cases they developed extensive cell membrane blebs, nuclear condensation, and overall cell shrinkage (Fig. 3A, C, and E). Cell damage, on the contrary, was not observed in samples incubated for up to 9 h, with cRPMI or RPMI containing 20 μM ADP0, in the presence of 20 μM suramin (Fig. 3B, D, and F).
FIG. 3.
Phase-contrast microscopy of Wish cells incubated for 3 h at 37°C in 5% CO2 atmosphere with RPMI medium in the absence (A) or in the presence (B) of 20 μM suramin; with heat-treated filtered cRPMI, conditioned for 2 h by 6 × 106 A. castellanii trophozoites, in the absence (C) or in the presence (D) of 20 μM suramin; or with RPMI containing 20 μM ADP0 in the absence (E) or in the presence (F) of 20 μM suramin. Magnification, × 400.
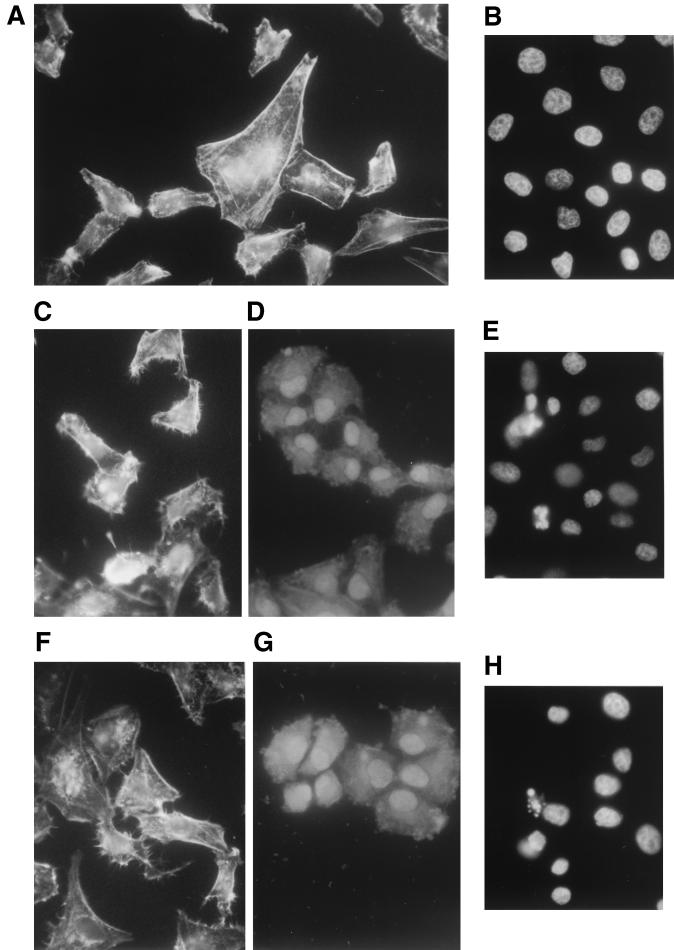
Actin microfilaments, at the same selected time intervals, were visualized by using rhodamine-conjugated phalloidin (Sigma-Aldrich S.r.l.) as we had previously described (17). In control cells, F-actin organized in stress fibers was mainly distributed at the cell periphery as a continuous thin circumferential band (Fig. 4A). The exposure to cRPMI or to RPMI containing 20 μM ADP0, in a time-dependent way, caused the cells to become round and led to the breakdown of the actin network (Fig. 4C, D, F, and G). In addition, Wish cells exposed to ADP0 or cRPMI showed the same fluorescence pattern on actin microfilaments.
FIG. 4.
Actin microfilaments of Wish cells incubated for 3 h at 37°C in 5% CO2 atmosphere with RPMI medium (A), with cRPMI for 3 h (C) and 6 h (D), or with RPMI containing 20 μM ADP0 for 3 h (F) and 6 h (G) and nuclei of Wish cells incubated for 3 h at 37°C in a 5% CO2 atmosphere with RPMI medium (B), with cRPMI (E), or with RPMI containing 20 μM ADP0 (H). Magnifications: A, C, D, F, and G, ×420; B, H, and F, ×350.
Cell monolayers growing on coverslips under the same experimental conditions as those described above were also examined by light microscopy after DAPI (4′,6′-diamidino-2-phenylindole) staining in order to visualize the nuclei. After incubation, the cells were washed with PBS, fixed in 70% ethanol at 0°C, and stained with 2× SSC buffer (1× is 0.15 M NaCl plus 0.015 M sodium citrate) containing 200 ng of DAPI(Sigma-Aldrich S.r.l.) per ml. Coverslips, repeatedly washed with 2× SSC buffer–0.05% Tween 20, were mounted in Gelvatol (Monsanto Corp.) and then examined with a Nikon Optiphot microscope. The fluorescence microscopy of nuclei showed different forms of chromatin aggregation and nuclear fragmentation that increased in proportion to the exposure time to both cRPMI and RPMI containing 20 μM ADP0. After 3 of incubation all control cells showed a normal nuclear morphology (Fig. 4B), while about 11% of nuclei were apoptotic in cells exposed to cRPMI or ADP0 (Fig. 4E and H).
Conclusions.
The present findings show that A. castellanii trophozoites constitutively release ADP in the medium and suggest that this compound might play an important role in cell-free cytopathogenicity due to A. castellanii. Our data, albeit indirectly, demonstrate that ADP is very probably the heat-resistant low-molecular-weight component of the amoebic-cell-free supernatant that causes increase in cytosolic free calcium, morphological changes, cytoskeletal damage, and death in Wish cells. In fact, purified ADP and A. castellanii culture supernatants cause a similar pattern of calcium fluxes and apoptotic cell death that are blocked by the P2y2 inhibitor suramin. It has been shown that ATP can also interact with P2y2 cell surface receptors to induce elevation of cytosolic free calcium and apoptosis in mammalian cells. In our experimental conditions, however, only ADP has been detected in the conditioned medium. We still do not know whether apoptotic cell death caused by both cRPMI and purified ADP depends directly on ADP molecules or also on ADP-metabolite generates, for example, by cleavage of the phosphate groups. Further studies are necessary to conclusively demonstrate this and to determine whether the ADP released from A. castellanii trophozoites is derived from exocytotic granules and/or vesicles or from the cytosolic ADP pool via intrinsic plasma membrane channels or pores.
Extracellular purine nucleotides are a universal and primitive system of intercellular signals that are capable of modulating several cellular functions. Some amoebae, e.g., Dictyostelium discoideum, use purines as intracellular messenger as well as for intercellular signaling (8). There is now significant evidence that extracellular ATP acts as an additional lytic mediator involved in cell-mediated cytotoxicity (24). In fact, it is released from activated cytotoxic T lymphocytes and, by interacting with cell surface purinoreceptors, induces cell death through both colloido-osmotic lysis and apoptosis (3).
Therefore, it is not surprising that Acanthamoeba may use the release of ADP as a system to kill the target cells. It is possible that, in Acanthamoeba-mediated cytolysis, released ADP may act as an extracellular messenger molecule that works together with other known or unknown secreted agents. To our knowledge, however, this is the first time that results have been presented that show purinic nucleotides as cytotoxic mediators involved in the interactions that occur between pathogenic free-living amoebae and the host cells.
Acknowledgments
This work was supported by a grant (60%) of University of Sassari, Sassari, Italy.
REFERENCES
- 1.Albertini M, Palmetshofer A, Kaczmarek E, Koziak K, Stroka D, Grey S T, Stuhlmeier K M, Robson S C. Extracellular ATP and ADP activate transcription factor NF-κB and induce endothelial cell apoptosis. Biochem Biophys Res Commun. 1998;248:822–829. doi: 10.1006/bbrc.1998.9055. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh H, Pidherney M S, McCulley J P, Niederkorn J Y. Apoptosis as a mechanism of cytolysis of tumor cells by a pathogenic free-living amoeba. Infect Immun. 1994;62:1298–1303. doi: 10.1128/iai.62.4.1298-1303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery R K, Bleier K J, Pasternack M S. Differences between ATP-mediated cytotoxicity and cell-mediated cytotoxicity. J Immunol. 1992;149:1265–1270. [PubMed] [Google Scholar]
- 4.Banditelli S, Baiocchi C, Pesi R, Allegrini S, Turriani M, Ipata P L, Camici M, Tozzi M G. The phosphotransferase activity of cytosolic 5′-nucleotidase: a purine analog phosphorylating enzyme. J Biochem Cell Biol. 1996;28:711–720. doi: 10.1016/1357-2725(95)00171-9. [DOI] [PubMed] [Google Scholar]
- 5.Bernheimer A W, Rudy B. Interactions between membranes and cytolytic peptides. Biochem Biophys Acta. 1986;864:123–128. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- 6.Bohemer R M, Scharf E, Assoian R K. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage-dependent expression of cyclin D1. Mol Biol Cell. 1996;7:101–111. doi: 10.1091/mbc.7.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawicki D D, Chatterjee D, Wyche J, Rounds S. Extracellular ATP and adenosine cause apoptosis of pulmonary artery endothelial cells. Am J Physiol. 1997;273(2 Pt. 1):L485–L494. doi: 10.1152/ajplung.1997.273.2.L485. [DOI] [PubMed] [Google Scholar]
- 8.Devroetes P N. Cyclic nucleotides and cell-cell-communication in Dictyostelium discoideum. Adv Cyclic Nucleotide Res. 1983;15:55–61. [Google Scholar]
- 9.Dubyak G R, El-Moatassim C. Signal tranduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265(3 Pt. 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 10.Ferrante A. Free-living amoebae: pathogenicity and immunity. Parasite Immunol. 1991;13:31–47. doi: 10.1111/j.1365-3024.1991.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 11.Garcia L S, Bruckner D A. Diagnostic medical parasitology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1993. pp. 601–605. [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien R J. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3451. [PubMed] [Google Scholar]
- 13.John D T. Opportunistically pathogenic free-living amebae. In: Kreier J P, Baker J R, editors. Parasitic protozoa. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1993. p. 143. [Google Scholar]
- 14.Larkin D F P, Berry M, Easty D L. In vitro corneal pathogenicity of Acanthamoeba. Eye. 1991;5:560–568. doi: 10.1038/eye.1991.98. [DOI] [PubMed] [Google Scholar]
- 15.Ma P, Visvesvara G S, Martinez A J, Theodore F H, Daggett P M, Sawyer T K. Naegleria and Acanthamoeba infections: review. Rev Infect Dis. 1990;12:490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- 16.Martinez A J, Visvesvara G S. Free-living amphizoic and opportunistic amebas. Brain Pathol. 1997;7:1583–1598. doi: 10.1111/j.1750-3639.1997.tb01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattana A, Bennardini F, Usai S, Fiori P L, Franconi F, Cappuccinelli P. Acanthamoeba castellanii metabolites increase the intracellular calcium level and cause cytotoxicity in Wish cells. Microb Pathogen. 1997;23:85–93. doi: 10.1006/mpat.1997.0138. [DOI] [PubMed] [Google Scholar]
- 18.McPherson P S, Campbell K T. The ryanodine receptor/Ca2+ release channel. J Biol Chem. 1993;268:13765–13768. [PubMed] [Google Scholar]
- 19.Murgia M, Pizzo P, Steinberg T H, Di Virgilio F. Characterization of the cytotoxic effect of extracellular ATP in J774 mouse macrophages. Biochem J. 1992;288:897–901. doi: 10.1042/bj2880897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neary J T, Rathbone M P, Cattabeni F, Abbracchio M P, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 21.Page F C. An illustrated key to freshwater and soil amoebae. Freshwater Biological Association, Scientific Publication no. 34. Ambleside, England: Freshwater Biological Association; 1976. [Google Scholar]
- 22.Pidherney M S, Alizadeh H, Stewart G L, McCulley J P, Niederkorn J Y. In vitro and in vivo tumoricidal properties of a pathogenic/free-living amoeba. Cancer Lett. 1993;72:91–98. doi: 10.1016/0304-3835(93)90016-3. [DOI] [PubMed] [Google Scholar]
- 23.Rathbone M P, Christjanson L, Deforge S, Deluca B, Gysbers J W, Hindley S, Jovetich M, Middlemiss P, Takhal S. Extracellular purine nucleosides stimulate cell division and morphogenesis: pathological and physiological implications. Med Hypoth. 1992;37:232–240. doi: 10.1016/0306-9877(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 24.Redegeld F, Filippini A, Sitikovsky M. Comparative studies of the cytotoxic T-lymphocyte-mediated cytotoxicity and the extracellular ATP-induced cell lysis: differents requirements in extracellular Mg2+ and pH. J Immunol. 1991;147:3638–3645. [PubMed] [Google Scholar]
- 25.Sage S O, Reast R, Rink T J. ADP evokes biphasic Ca2+ influx in Fura-2-loaded human platelets: evidence for Ca2+ entry regulated by the intracellular Ca2+ store. Biochem J. 1990;265:675–680. doi: 10.1042/bj2650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schagger H, von Jagow G. Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 27.Seetulsingh-Goorah S P, Stewart B W. Growth inhibition of HL-60 cells by extracellular ATP: concentration-dependent involvement of a P2 receptor and adenosine generation. Biochem Biophys Res Commun. 1998;250:390–396. doi: 10.1006/bbrc.1998.9329. [DOI] [PubMed] [Google Scholar]
- 28.Sing B N. Pathogenic and non-pathogenic amoebae. London, England: Macmillan; 1975. [Google Scholar]
- 29.Trump B F, Berezesky I K. The role of altered [Ca2+]i regulation in apoptosis, oncosis, and necrosis. Biochim Biophys Acta. 1996;1313:173–178. doi: 10.1016/0167-4889(96)00086-9. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L M, Zychlinsky A, Liu C C, Ojcius D M, Ding-E Young J. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]