Abstract
Streptococcal mitogenic exotoxin Z (SMEZ), a superantigen derived from Streptococcus pyogenes, provoked expansion of human lymphocytes expressing the Vβ 2, 4, 7 and 8 motifs of T-cell receptor. SMEZ was pyrogenic in rabbits and stimulated the expression of the T-cell activation markers CD69 and cutaneous lymphocyte-associated antigen. A variety of cytokines was released by human mononuclear leukocytes stimulated with SMEZ, which was 10-fold more active than streptococcal pyrogenic exotoxin A. Th2-derived cytokines were elicited only by superantigens and not by streptococcal cells.
Group A streptococci (Streptococcus pyogenes) provoke a wide spectrum of diseases ranging from skin infections and pharyngitis to more severe diseases such as scarlet fever, deep tissue infections, streptococcal toxic shock syndrome (STSS), and probably chronic diseases such as Kawasaki syndrome and guttate psoriasis (1, 2, 22, 23, 31, 33, 44). Several lines of evidence suggest that these diseases are at least partially mediated by extracellular mitogens that belong to the family of the superantigens (SAgs) (2, 33, 47). These effectors trigger polyclonal expansion of T lymphocytes by simultaneous binding to major histocompatibility complex class II molecules on antigen-presenting cells and T-cell receptor via its Vβ domain (37). The Vβ motifs recognized vary from one SAg to another (7). This process leads to the release of high levels of cytokines by antigen-presenting cells and lymphocytes as widely investigated for streptococcal SAgs (6, 8, 11, 12, 16, 26–28, 34, 35, 41, 42). Cytokine accumulation in vivo results in acute shock and other disorders (16, 19, 20, 41, 47). In this respect, significant levels of SAg (43) and cytokines in the biological fluids were detected in patients with STSS (6, 11, 20, 32, 36, 43).
S. pyogenes SAgs comprise the classical erythrogenic (pyrogenic) exotoxins A and C (SPEA and SPEC), encoded by bacteriophage speA and speC genes (2, 31); other novel SAgs (2, 17, 25, 33, 34, 38, 46); and the streptococcal mitogenic exotoxin Z (SMEZ) (3, 18, 38), encoded by the gene smez, which displays 24 allelic forms (39). Four newly discovered genes speG, speH, speJ, and smez-2, were identified (reference 38 and genomic database at Oklahoma University [www.genome.ou.edu/strep.html]). Their corresponding recombinant proteins were highly mitogenic for human peripheral blood mononuclear cells (PBMC). We describe here some immunological and biological properties of a potent mitogen released in the culture supernatant of an S. pyogenes strain (strain L) lacking both speA and speC genes, isolated from a French patient with STSS. This strain was selected among a number of speA- and speC-lacking isolates (40). The mitogen was identified as an SAg corresponding to SMEZ. The pyrogenic and superantigenic properties and the cytokine and skin homing antigen-inducing capacities of SMEZ were investigated in parallel with those of purified SPEA (10) used as a control. The cytokine response of PBMC challenged with heat-killed streptococci was also studied for comparative purposes.
The DNA of strain L was prepared and used for PCR with specific primers for smez, speG, and speH as previously described (38). This procedure revealed that the strain carried smez and speG but not speH. The sequence analysis of smez corresponded to allele 16 of this gene. The mitogenic material of the culture supernatant of strain L was purified by ammonium sulfate precipitation and successive chromatographic procedures as previously described (9). The fraction with the highest mitogenic activity of the final chromatographic step was submitted to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, electroblotted onto nitrocellulose (4), and stained with mouse immune serum raised against this fraction. The analysis revealed a single band corresponding to a molecular mass of ca. 25 kDa which was also observed with rabbit anti-SMEZ immune serum.
The T-cell receptor Vβ chain profile analysis was performed by reverse transcription-PCR (7) using RNA from 107 PBMC stimulated for 5 days with the purified mitogen (10 ng/ml), SPEA (10 ng/ml), or phytohemagglutinin A (1 ng/ml) as a control. The mitogen stimulated expansion of T cells expressing Vβ 2, 4, 7, and 8 motifs, which are those reported for the 24.3-kDa recombinant SMEZ (38), while SPEA expanded Vβ 2, 12, 13, 14, and 15 T-cell motifs, in accordance with the literature (45). Therefore, both electrophoretic and Vβ repertoire analyses confirm that the purified mitogen and SMEZ are identical SAgs.
Fever induction in experimental animals challenged with staphylococcal and streptococcal SAgs is one of the most important properties of these effectors (2, 15), which led to the term “pyrogenic exotoxins” for the classical erythrogenic toxins A and C (48). In this respect, a pyrogenicity test was performed in which purified SMEZ (100 ng) was injected into the marginal ear veins of New Zealand White rabbits. Rectal temperature was monitored with a mercury thermometer at hourly intervals for a period of 5 h. Preincubation of the product with polymyxin B (2 μg/ml) was done in parallel as a control to rule out a false-positive pyrogenic response caused by possible lipopolysaccharide contamination (5). The rectal temperature of the rabbits increased by about 1.5°C after 4 h and 1.8°C after 6 h (Table 1). Polymyxin B did not affect temperature elevation, confirming that the purified SAg is pyrogenic per se. To our knowledge, the pyrogenicities of other recently described SAgs such as mitogenic factor/SPEF (17, 34, 46), SSA (25), SMEZ (18), and SPEG, SPEH, and SMEZ-2 (38) have not been investigated.
TABLE 1.
Rectal temperatures of three rabbits injected with sterile nonpyrogenic saline, SMEZ, or SMEZ plus polymyxin B
| Time (h) | Mean rectal temp (°C) after injection with:
|
||
|---|---|---|---|
| Saline | SMEZ (100 ng) | SMEZ (100 ng) + polymyxin B (2 μg/ml) | |
| 0 | 38.6 | 38.5 | 38.7 |
| 1 | 38.7 | 39.0 | 39.2 |
| 2 | 38.7 | 39.3 | 39.4 |
| 3 | 38.7 | 39.9 | 39.8 |
| 4 | 38.8 | 40.2 | 40.1 |
| 6 | 38.7 | 40.3 | 40.0 |
SMEZ preparation was assessed for its ability to stimulate in vitro expression by target cells of the skin-selective lymphocyte homing receptor known as cutaneous lymphocyte-associated antigen (CLA) as already established for other staphylococcal and streptococcal SAgs (21, 49, 50). CLA is known to interact with E-selectin in an interleukin-12 (IL-12)-dependent manner. The experimental assay (49) was performed on 3 × 106 human PBMC for 5 days in the presence of SMEZ preparation (10 and 100 ng/ml), SPEA (100 ng/ml and 1 μg/ml), or RPMI 1640 medium as a control. The antibody HECA-452 (a kind gift from A. M. Duijvestijn, Maastricht, The Netherlands) was used to detect CLA. The antibody Ber-ACT8 (Dako), which recognizes CD103, the β7 chain of the integrin receptor that correlates with α4β7 expression of gut homing T cells, was used as a control. Staining was performed by indirect immunofluorescence with goat anti-mouse immunoglobulin G or goat anti-rat immunoglobulin M F(ab)2-phycoerythrin. In each sample, irrelevant monoclonal antibodies of the appropriate isotype were used as controls. Fluorocytometer analysis was performed on a FACSCalibur (Becton Dickinson, Heidelberg, Germany) using standard procedures with the CellQuest computer program. The results were expressed as percentage of antibody-reactive T cells per total T lymphocytes in a gate set on lymphocyte-sized cells. The T-cell activation marker CD69 detected by specific antibody and the skin-selective homing antigen CLA were significantly expanded in the presence of both SMEZ and SPEA. In contrast, the expression of gut homing receptor CD103 was not affected (Table 2). The SMEZ/SPEA-dependent CLA upregulation may possibly contribute to the pathogenesis of streptococcal SAg-induced skin inflammation as suggested in other studies of SAgs from gram-positive cocci (21).
TABLE 2.
CD69, CLA, and CD103 expression on SPEA- and SMEZ-stimulated T cells
| Antigen | Mean antibody-reactive T cells/total T cells (%) ± SDa
|
||||
|---|---|---|---|---|---|
| Control | SPEA
|
SMEZ
|
|||
| 100 ng | 1 μg | 10 ng | 100 ng | ||
| CD69 | 9.7 ± 4.3 | 32.5 ± 14.5* | 34.4 ± 15.4* | 36.2 ± 16.2* | 40.0 ± 17.5* |
| CLA | 10.2 ± 4.6 | 32.1 ± 14.3* | 35.4 ± 15.8* | 30.8 ± 13.8* | 35.5 ± 15.9* |
| CD103 | 3.5 ± 1.5 | 5.3 ± 2.4 | 5.6 ± 2.5 | 6.7 ± 3.0 | 6.2 ± 2.8 |
Results from five experiments. ∗, P < 0.05 for stimulated cells versus control.
The mitogenic activity of the SAgs tested was evaluated by a lymphocyte proliferation assay on human PBMC as previously described (29). Half-maximal proliferation (10,000 cpm) in response to the purified SMEZ was observed at a concentration of 100 pg/ml, in comparison to 1.8 ng/ml for SPEA (data not shown). Accordingly, SMEZ is 18-fold more potent than SPEA in the lymphocyte proliferation assay.
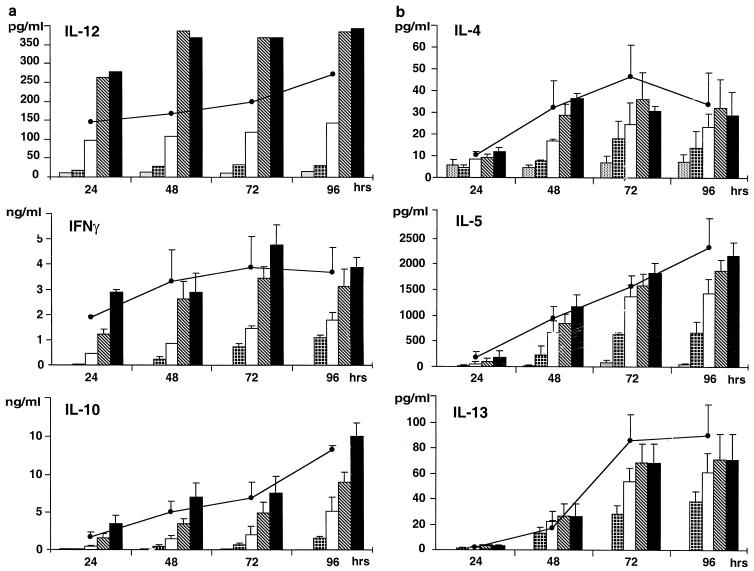
A further consequence of cell stimulation with SAg is the induction of massive cytokine release by target cells. As shown here, this is also the case with SMEZ, investigated for the first time in this respect. Cytokine release by PBMC challenged with SMEZ (0.1, 1, 10, and 100 ng/ml) was determined as reported earlier for SPEA and SPEC (26–28). PBMC were also stimulated in parallel for comparative purposes with 1 μg of SPEA per ml and 107 CFU of heat-killed (1 h at 70°C), streptococci. The release of 18 cytokines in PBMC cultures after 72 h of incubation was tested (Table 3). Except for IL-6 (27), the cytokines were assayed by enzyme-linked immunosorbent assay with the appropriate antibody kits as previously described (24, 26–28, 30). Transforming growth factor β, RANTES, and MIP-1α were immunoassayed by specific kits (R&D systems, Abington, United Kingdom). Both SMEZ and SPEA elicited the release of substantial amounts of pro- and anti-inflammatory, chemotactic, hematopoietic, and Th1- and Th2-derived cytokines (Table 3). However, the cytokine-inducing capacity of SMEZ was ca. 10-fold more potent (on a weight basis) than that of SPEA. Dose- and time-dependent production of certain cytokines in response to various concentrations of SMEZ and 1 μg of SPEA per ml was also investigated. IL-12 was produced in significant amounts starting from 24 h, and optimal release was about 400 pg/ml after 48 h in response to 10 ng of SMEZ. Gamma interferon (IFN-γ) and IL-10 release increased progressively up to 72 h for IFN-γ and 96 h for IL-10 (Fig. 1a). Similar results were found for the Th2-derived cytokines IL-4, IL-5, and IL-13 (Fig. 1b). Interestingly, striking differences in cytokine-inducing capacity were found between the SAgs tested and heat-killed streptococcal cells. The latter elicited the production of low amounts of IL-2 and did not trigger detectable tumor necrosis factor beta (TNF-β), IL-4, IL-5, and IL-13 release (Table 3). However, streptococcal cells were highly potent inducers of IFN-γ, TNF-α, IL-12 p40, and IL-12 p70, suggesting that the bacteria themselves may evoke cytokine release via their cell wall components, particularly peptidoglycan (13), and thereby could contribute with SAgs to the development of cytokine-mediated streptococcal pathological disorders.
TABLE 3.
In vitro cytokine-inducing capacities of SMEZ, SPEA, and heat-killed group A streptococci in human PBMC
| Cytokine or chemokine | No. of expts | Inductiona (mean ± SEM) by:
|
|||
|---|---|---|---|---|---|
| RPMI | SMEZ (100 ng/ml) | SPEA (1 μg/ml) | Streptococci (107 CFU/ml) | ||
| Cytokines (pg/ml) | |||||
| Pro- and anti-inflammatory | |||||
| IL-1α | 4 | 30 ± 6 | 1,497 ± 852 | 1,230 ± 674 | 2,100 ± 638 |
| IL-6 | 4 | 328 ± 163 | 3,695 ± 1,577 | 3,744 ± 1,298 | 5,900 ± 1,320 |
| TNF-α | 8 | 530 ± 198 | 8,445 ± 1,920 | 8,190 ± 2,351 | 12,832 ± 528 |
| IL-12 p40 | 6 | 8 ± 4 | 278 ± 79 | 214 ± 55 | 1,651 ± 13* |
| IL-12 p70b | 3 | 3 ± 0.3 | 21 ± 4 | 25 ± 10 | 802 ± 79* |
| IL-10 | 5 | 70 ± 32 | 7,482 ± 2,270 | 6,804 ± 2,109 | 2,526 ± 500 |
| Transforming growth factor β | 3 | 647 ± 288 | 1,102 ± 253 | 1,038 ± 275 | 905 ± 99 |
| Th1 derived | |||||
| TNF-β | 3 | 15 ± 4 | 761 ± 189 | 872 ± 243 | 25 ± 4.6* |
| IFN-γ | 6 | 4 ± 3 | 3,552 ± 698 | 2,428 ± 329 | 4,460 ± 76* |
| IL-2 | 9 | 116 ± 16 | 3,321 ± 586 | 2,720 ± 541 | 253 ± 73* |
| Th2 derived | |||||
| IL-4 | 3 | 7 ± 3 | 31 ± 3 | 47 ± 15 | 7 ± 3* |
| IL-5 | 10 | 20 ± 6 | 1,213 ± 146 | 1,075 ± 190 | 18 ± 5* |
| IL-13 | 3 | ND | 61 ± 17 | 47 ± 13 | ND |
| Hematopoietic | |||||
| IL-3 | 7 | ND | 383 ± 65 | 231 ± 65 | 22 ± 22* |
| Granulocyte-macrophage colony-stimulating factor | 4 | 12 ± 8 | 1,034 ± 150 | 1,021 ± 144 | 420 ± 358 |
| Chemokines (ng/ml) | |||||
| IL-8 | 4 | 50 ± 20 | 198 ± 40 | 156 ± 42 | 330 ± 63 |
| RANTESc | 4 | 4.2 ± 2 | 45 ± 23 | 35 ± 11 | 51 ± 22 |
| MIP-1α | 3 | 0.7 ± 0.3 | 401 ± 6.9 | 31 ± 8.2 | 29.8 ± 3.8 |
Incubation time, 72 h unless noted otherwise. ND, not detectable; * P < 0.05 between superantigens and heat-killed streptococci.
Incubation time, 24 h.
Incubation time, 48 h.
FIG. 1.
(a) Time- and dose-dependent in vitro release of IL-12, IFN-γ and IL-10 by human PBMC in response to SMEZ and SPEA.  , control (RPMI);
, control (RPMI);  , SMEZ (0.1 ng/ml); □, SMEZ (1 ng/ml);
, SMEZ (0.1 ng/ml); □, SMEZ (1 ng/ml);  , SMEZ (10 ng/ml); ■, SMEZ (100 ng/ml); ●, SPEA, (1 μg/ml). Data represent means ± standard errors for cells from five donors except for IL-12, data for which are representative of one experiment out of three. (b) Time and dose-dependent in vitro release of IL-4, IL-5, and IL-13 by human PBMC. Symbols are the same as for panel a. Data represent means ± standard errors for the cells from five donors.
, SMEZ (10 ng/ml); ■, SMEZ (100 ng/ml); ●, SPEA, (1 μg/ml). Data represent means ± standard errors for cells from five donors except for IL-12, data for which are representative of one experiment out of three. (b) Time and dose-dependent in vitro release of IL-4, IL-5, and IL-13 by human PBMC. Symbols are the same as for panel a. Data represent means ± standard errors for the cells from five donors.
A significant feature of the SAgs investigated here is their capacity to induce the release of Th2-derived cytokines as already documented (27, 28, 41). The production of these cytokines raises the question of the possible involvement of bacterial SAgs in the pathogenesis of diseases other than acute streptococcal and staphylococcal diseases, particularly in certain allergic and nonallergic diseases (14, 19, 20). The present results suggest that SMEZ is a potential pathogenicity factor of S. pyogenes that might play an important role in streptococcal diseases.
Acknowledgments
This work was supported by the Institut Pasteur de Lille, the Centre Hospitalier Regional et Universitaire de Lille (grant 96/38/9713), and Association F. Aupetit.
REFERENCES
- 1.Abe J B, Kotzin L, Jugo K, Melish M E, Glode M P, Kohsaka T, Leung D Y M. Selective expansion of T cells expressing T cell receptor variable regions Vβ2 and Vβ8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–4070. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alouf J E, Müller-Alouf H, Köhler W. Superantigenic Streptococcus pyogenes erythrogenic/pyrogenic exotoxins. In: Alouf J E, Freer J H, editors. The comprehensive sourcebook of bacterial protein toxins. London, England: Academic Press; 1999. pp. 567–588. [Google Scholar]
- 3.Arcus V L, Proft T, Sigzell J A, Baker H M, Fraser J D, Baker E N. Conservation and variation in superantigen structure and activity highlighted by the three-dimensional structures of two superantigens from Streptococcus pyogenes. J Mol Biol. 2000;299:1159–1168. doi: 10.1006/jmbi.2000.3725. [DOI] [PubMed] [Google Scholar]
- 4.Burnette W H. Western blotting: electrophoretic transfer of proteins from SDS polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 5.Cavaillon J-M, Haeffner-Cavaillon N. Polymyxin B inhibition of LPS-induced interleukin-1 secretion by human monocytes is dependent upon the LPS origin. Mol Immunol. 1986;23:965–969. doi: 10.1016/0161-5890(86)90127-6. [DOI] [PubMed] [Google Scholar]
- 6.Cavaillon J M, Müller-Alouf H, Alouf J E. Cytokines in streptococcal infections. Adv Exp Med Biol. 1997;418:929–931. doi: 10.1007/978-1-4899-1825-3_206. [DOI] [PubMed] [Google Scholar]
- 7.Champagne E, Huchenq A, Sevin J, Casteran N, Rubin B. An alternative method for T-cell receptor repertoire analysis: clustering of human V-beta subfamilies selected in response to staphylococcal enterotoxins B and E. Mol Immunol. 1993;30:877–886. doi: 10.1016/0161-5890(93)90011-y. [DOI] [PubMed] [Google Scholar]
- 8.Fast D J, Schlievert P M, Nelson R D. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect Immun. 1989;57:291–294. doi: 10.1128/iai.57.1.291-294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geoffroy-Fauvet C, Müller-Alouf H, Champagne E, Cavaillon J M, Alouf J E. Identification of a new extracellular superantigenic mitogen from group A Streptococci. Zentbl Bakteriol Suppl. 1994;24:91–92. [Google Scholar]
- 10.Gerlach D, Köhler W, Knöll H, Moràvek L, Weeks R C, Ferretti J J. Purification and characterisation of Streptococcus pyogenes erythrogenic toxin type A produced by a cloned gene in Streptococcus sanguis. Zentbl Bakteriol. 1987;266:347–358. doi: 10.1016/s0176-6724(87)80215-8. [DOI] [PubMed] [Google Scholar]
- 11.Hackett S P, Stevens D L. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J Infect Dis. 1992;165:879–885. doi: 10.1093/infdis/165.5.879. [DOI] [PubMed] [Google Scholar]
- 12.Hackett S P, Stevens D L. Superantigen associated with staphylococcal and streptococcal toxic shock syndrome are potent inducers of tumor necrosis factor-β. J Infect Dis. 1993;168:232–235. doi: 10.1093/infdis/168.1.232. [DOI] [PubMed] [Google Scholar]
- 13.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofer M F, Lester M R, Schlievert P M, Leung D Y M. Upregulation of IgE synthesis by staphylococcal toxic shock syndrome toxin-1 in peripheral blood mononuclear cells from patients with atopic dermatitis. Clin Exp Allergy. 1995;25:1218–1227. doi: 10.1111/j.1365-2222.1995.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 15.Hribalova V, Knöll H, Gerlach D, Köhler W. Purification and characterization of erythrogenic toxins. II. In vivo biological activities of erythrogenic toxin produced by Streptococcus pyogenes strain NY-5. Zentbl Bakteriol. 1980;248:314–322. [PubMed] [Google Scholar]
- 16.Imanishi K, Inada K, Akatsuda H, Gu Y, Igarishi H, Uchiyama T. Tumor necrosis factor production by human T cells stimulated with bacterial superantigens. Int J Immunopharmacol. 1995;17:841–848. doi: 10.1016/0192-0561(95)00074-c. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki M, Igarashi H, Hinuma Y, Yutsudo T. Cloning, characterization and overexpression of a Streptococcus pyogenes gene encoding a new type of mitogenic factor. FEBS Lett. 1993;331:187–192. doi: 10.1016/0014-5793(93)80323-m. [DOI] [PubMed] [Google Scholar]
- 18.Kamezawa Y, Nakahara T, Nakano S, Abe Y, Nozaki-Renard J, Isono T. Streptococcal mitogenic exotoxin Z, a novel acidic superantigenic toxin produced by a T1 strain of Streptococcus pyogenes. Infect Immun. 1997;65:3828–3833. doi: 10.1128/iai.65.9.3828-3833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.König B, Neuber K, König W. Responsiveness of peripheral blood mononulear cells from normal and atopic donors to microbial superantigens. Int Arch Allergy Immunol. 1995;106:124–133. doi: 10.1159/000236832. [DOI] [PubMed] [Google Scholar]
- 20.Kotb M. Superantigens in human diseases. Clin Microbiol Newsl. 1997;19:145–150. [Google Scholar]
- 21.Leung D Y M, Gately M, Trumble A, Ferguson-Darnell B, Schlievert P M, Pickler L. Bacterial superantigens induce T cell expression of the skin-selective homing receptor, the cutanous lymphocyte-associated antigen via stimulation of IL-12. J Exp Med. 1995;181:747–753. doi: 10.1084/jem.181.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung D Y M, Travers J B, Giorno R, Norris D A, Skinner R, Aelion J, Kazemi L V, Kim M H, Trumble A E, Kotb M, Schlievert P M. Evidence for a streptococcal superantigen-driven process in acute guttate psoriasis. J Clin Investig. 1995;96:2106–1212. doi: 10.1172/JCI118263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leung D Y M. Kawasaki syndrome: immunomodulatory benefit and potential toxin neutralization by intravenous immune globulin. Clin Exp Immunol. 1996;104(Suppl. 1):49–54. [PubMed] [Google Scholar]
- 24.Marty C, Misset B, Tamion F, Fitting C, Carlet J, Cavaillon J-M. Circulating interleukin-8 concentrations in patients with muliple organ failure of septic and non septic origin. Crit Care Med. 1994;22:673–679. doi: 10.1097/00003246-199404000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Mollick J A, Miller G G, Musser J M, Cook R G, Grossman D, Rich R R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with aminoterminal homology to staphylococcal enterotoxins B and C. J Clin Investig. 1993;92:710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller-Alouf H, Alouf J E, Gerlach D, Ozegowski J H, Fitting C, Cavaillon J-M. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigenic erythrogenic toxins, heat-killed streptococci, and lipopolysaccharide. Infect Immun. 1994;62:4915–4921. doi: 10.1128/iai.62.11.4915-4921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller-Alouf H, Alouf J E, Gerlach D, Ozegowski J H, Fitting C, Cavaillon J M. Human pro- and anti-inflammatory cytokine patterns induced by Streptococcus pyogenes erythrogenic (pyrogenic) exotoxin A and C superantigens. Infect Immun. 1996;64:1450–1453. doi: 10.1128/iai.64.4.1450-1453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller-Alouf H, Gerlach D, Desreumaux P, Leportier C, Alouf J E, Capron M. Streptococcal pyrogenic exotoxin A (SPEA) superantigen induced production of hematopoietic cytokines, IL-12 and IL-13 by human peripheral blood mononuclear cells. Microb Pathog. 1997;23:265–272. doi: 10.1006/mpat.1997.0155. [DOI] [PubMed] [Google Scholar]
- 29.Müller-Alouf H, Geoffroy C, Geslin P, Bouvet A, Felten A, Günther E, Ozegowski J-H, Alouf J E. Streptococcal pyrogenic exotoxin A, exoenzymes, serotype and biotype profiles of Streptococcus pyogenes isolates from patients with toxic shock syndrome and other severe infections. Zentbl Bakteriol. 1997;286:421–433. [PubMed] [Google Scholar]
- 30.Muñoz C, Carlet J, Fitting C, Misset B, Pleriot J-P, Cavaillon J-M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Investig. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musser J M, Hauser A R, Kim M H, Schlievert P M, Nelson K, Selander R K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadal D, Lauener R P, Bregger C P, Kaufhold A, Simma B, Lütticken R, Seger A. T cell activation and cytokine release in streptococcal toxic shock syndrome. J Pediatr. 1993;122:727–729. doi: 10.1016/s0022-3476(06)80014-4. [DOI] [PubMed] [Google Scholar]
- 33.Norgren M, Eriksson A. Streptococcal superantigens and their role in the pathogenesis of severe infections. J Toxicol Toxin Rev. 1997;16:1–32. [Google Scholar]
- 34.Norrby-Teglund A, Newton D, Kotb M, Holm S E, Norgren M. Superantigenic properties of the group A streptococcal exotoxin SpeF (MF) Infect Immun. 1994;62:5227–5233. doi: 10.1128/iai.62.12.5227-5233.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norrby-Teglund A, Lustig R, Kotb M. Differential induction of Th1 versus Th2 cytokines by group A streptococcal toxic shock syndrome isolates. Infect Immun. 1997;65:5209–5215. doi: 10.1128/iai.65.12.5209-5215.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norrby-Teglund A, Pauksens K, Norgren M, Holm S E. Correlation between serum TNF alpha and IL-6 levels and severity of group A streptococcal infections. Scand J Infect Dis. 1995;27:125. doi: 10.3109/00365549509018991. [DOI] [PubMed] [Google Scholar]
- 37.Papageorgiou A C, Collins C M, Gutman D M, Kline J B, O'Brien S M, Tranter H S, Acharya K R. Structural basis for recognition of superantigen streptococcal pyrogenic exotoxin A (SPEA1) by MHC class II molecules and T cell receptors. EMBO J. 1999;18:9–21. doi: 10.1093/emboj/18.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Proft T, Moffatt S L, Berkhahn C J, Fraser J D. Identification and characterization of novel superantigens from Streptococcus pyogenes. J Exp Med. 1999;189:89–102. doi: 10.1084/jem.189.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proft T, Moffat S L, Weller K D, Paterson A, Martin D, Fraser D. The streptococcal superantigen SMEZ exhibits wide allelic variation, mosaic structure and significant antigenic variation. J Exp Med. 2000;191:1765–1776. doi: 10.1084/jem.191.10.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichardt W, Müller-Alouf H, Alouf J E, Köhler W. Erythrogenic toxins A, B and C: occurrence of genes and exotoxin formation from clinical Streptococcus pyogenes strains associated with streptococcal toxic shock-like syndrome. FEMS Microbiol Lett. 1992;100:313–322. doi: 10.1111/j.1574-6968.1992.tb14058.x. [DOI] [PubMed] [Google Scholar]
- 41.Rink L, Luhm J, Koester M, Kirchner H. Induction of a cytokine network by superantigens with parallel TH1 and TH2 stimulation. J Interferon Cytokine Res. 1996;16:41–47. doi: 10.1089/jir.1996.16.41. [DOI] [PubMed] [Google Scholar]
- 42.Sriskandan S, Evans T J, Cohen J. Bacterial superantigen-induced human lymphocyte responses are nitric oxide dependent and mediated by IL-12 and IFNγ. J Immunol. 1996;156:2430–2435. [PubMed] [Google Scholar]
- 43.Sriskandan S, Moyes D, Cohen J. Detection of bacterial superantigen and lymphotoxin-α in patients with streptococcal toxic shock syndrome. Lancet. 1996;348:1315–1316. doi: 10.1016/s0140-6736(05)65800-x. [DOI] [PubMed] [Google Scholar]
- 44.Stevens D L, Tanner M H, Winship J, Swarts R, Ries K M, Schlievert P M, Kaplan E. Severe group A streptococci associated with toxic shock like syndrome and scarlet fever toxin A. N Engl J Med. 1989;321:1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- 45.Tomai M A, Schlievert P M, Kotb M. Distinct T-cell receptor Vβ gene usage by human T lymphocytes stimulated with the streptococcal pyrogenic exotoxins and pep M5 protein. Infect Immun. 1992;60:701–705. doi: 10.1128/iai.60.2.701-705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyosaki T, Yoshioka T, Tsuruta Y, Yutsudo T, Iwasaki M, Suzuki R. Definition of the mitogenic factor (MF) as a novel streptococcal superantigen that is different from streptococcal pyrogenic exotoxins A, B and C. Eur J Immunol. 1996;26:2693–2701. doi: 10.1002/eji.1830261122. [DOI] [PubMed] [Google Scholar]
- 47.Uchiyama T, Yan X-J, Imanishi K, Yagi J. Bacterial superantigens—mechanism of T cell activation by the superantigens and their role in the pathogenesis of infectious diseases. Microbiol Immunol. 1994;38:245–256. doi: 10.1111/j.1348-0421.1994.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 48.Watson D W. Host-parasite factors in group A streptococcal infections. Pyrogenic and other effects of immunologic distinct exotoxins related to scarlet fever toxins. J Exp Med. 1960;111:255–284. doi: 10.1084/jem.111.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zollner T M, Munk M E, Keller T, Nuber V, Boenhnke W H, Kaufmann S H E, Duijvestijn A M, Sterry W, Kaufmann R. The superantigen exfoliative toxin induces cutaneous lymphocyte-associated antigen expression in peripheral human T lymphocytes. Immunol Lett. 1996;49:111–116. doi: 10.1016/0165-2478(95)02491-3. [DOI] [PubMed] [Google Scholar]
- 50.Zollner T M, Nuber V, Duijvestijn A M, Boehnke W H, Kaufmann R. Superantigens but not mitogens are capable of inducing upregulation of E-selectin ligands on human T lymphocytes. Exp Dermatol. 1997;6:161–166. doi: 10.1111/j.1600-0625.1997.tb00200.x. [DOI] [PubMed] [Google Scholar]