OBJECTIVES:
We sought to update our 2015 work in the Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) guidelines for the diagnosis and management of pediatric acute respiratory distress syndrome (PARDS), considering new evidence and topic areas that were not previously addressed.
DESIGN:
International consensus conference series involving 52 multidisciplinary international content experts in PARDS and four methodology experts from 15 countries, using consensus conference methodology, and implementation science.
SETTING:
Not applicable.
PATIENTS:
Patients with or at risk for PARDS.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Eleven subgroups conducted systematic or scoping reviews addressing 11 topic areas: 1) definition, incidence, and epidemiology; 2) pathobiology, severity, and risk stratification; 3) ventilatory support; 4) pulmonary-specific ancillary treatment; 5) nonpulmonary treatment; 6) monitoring; 7) noninvasive respiratory support; 8) extracorporeal support; 9) morbidity and long-term outcomes; 10) clinical informatics and data science; and 11) resource-limited settings. The search included MEDLINE, EMBASE, and CINAHL Complete (EBSCOhost) and was updated in March 2022. Grading of Recommendations, Assessment, Development, and Evaluation methodology was used to summarize evidence and develop the recommendations, which were discussed and voted on by all PALICC-2 experts. There were 146 recommendations and statements, including: 34 recommendations for clinical practice; 112 consensus-based statements with 18 on PARDS definition, 55 on good practice, seven on policy, and 32 on research. All recommendations and statements had agreement greater than 80%.
CONCLUSIONS:
PALICC-2 recommendations and consensus-based statements should facilitate the implementation and adherence to the best clinical practice in patients with PARDS. These results will also inform the development of future programs of research that are crucially needed to provide stronger evidence to guide the pediatric critical care teams managing these patients.
Keywords: acute respiratory distress syndrome, best practice/evidenced-, based, guidelines, pediatric acute respiratory distress syndrome/children, pediatric critical care, systematic review
Acute respiratory distress syndrome (ARDS) is a heterogeneous clinical syndrome, which contributes to high rates of mortality and long-term morbidities (1–3). For years, pediatric practitioners relied on adult-oriented criteria to diagnose ARDS in children, until the 2015 Pediatric Acute Lung Injury Consensus Conference (PALICC) published a specific definition for pediatric ARDS (PARDS) with guidelines for management and future research (2, 4, 5). There were 744 new cases of PARDS identified by the PALICC definition over the 10 study weeks, yielding an international PARDS incidence of 3.2% (95% CI 3.0, 3.4%) amongst PICU patients and 6.1% (95% CI 5.7, 6.5%) amongst those on MV (6).
In the last years, there has been a wealth of new knowledge regarding (P)ARDS, with emerging concepts in the pathobiology, lung protection (driving pressure, mechanical power, patient self-inflicted lung injury), and use of new technologies (high-flow nasal cannula [HFNC]) and healthcare systems (informatics, clinical decision support tools) (7–9). Notable gaps exist in defining PARDS in resource-limited settings (RLS), in addition to differences in the availability of supportive therapies. Furthermore, recent literature has highlighted that implementation of 2015 PALICC recommendations varies among PICUs (6, 10), and nonadherence with recommendations is associated with higher mortality (11, 12). This motivated refinement of the PARDS definition and an update from the Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2). Two new sections focused on the use of informatics and data management strategies to help with implementation, as well as on modifications of the guidelines that may be needed in RLS.
In this executive summary, we briefly describe the methodology and present the set of recommendations and statements for the definition and the clinical management of PARDS. The full rationale and evidence-to-decision (EtD) framework for each recommendation, as well as detailed methods, are provided in accompanying articles published separately (13–24).
METHODS
The process for generation of these guidelines involved: 1) systematic literature review with identification, assessment, and synthesis of evidence; 2) stratification of recommendations into categories using Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology; 3) voting (up to three rounds) on recommendations and statements to achieve consensus; and 4) revision and harmonization of the recommendations and statements. The detailed methodology is reported in the accompanying supplement (13).
Panelists were selected based on research in specific aspects of PARDS over the preceding 10 years, with particular attention to increased diversity and international representation (Asia, Africa, South America). Final participants comprised a panel of 52 content and four methodology experts (24 women, 32 men) from multiple disciplines (52 physicians, one respiratory therapist, one nurse, one physical therapist, one PhD researcher) and geographic settings (15 countries). The panel worked with two guidelines methodologists (N.P.I., M.M.B.) along with an expert in meta-analysis (S.R.K.) and two experts in implementation science (R.P.B., K.S.).
Panelists were divided into 11 subgroups and each group was tasked with answering a key question, as listed in Table 1. In addition to their specific subgroup, a panelist from the RLS group also participated in each subgroup to ensure that RLS modifications were considered unless the group experts already included at least one expert from RLS regions.
TABLE 1.
Second Pediatric Acute Lung Injury Consensus Conference Subgroups and Related Key Questions
| Second PALICC Subgroups | Topic | Key Question |
|---|---|---|
| Section 1 | Definition, incidence, and epidemiology | How should PARDS be defined, and what are the variables that best characterize the global burden of PARDS? |
| Section 2 | Pathobiology, severity, and risk stratification | What are pediatric-specific elements of the pathobiology of PARDS, and what is the association between pathobiology and severity, and risk stratification in PARDS? |
| Section 3 | Invasive ventilatory support | What is the effectiveness and comparative effectiveness of different ventilation strategies for children with PARDS? |
| Section 4 | Ancillary pulmonary-specific treatments | What is the effectiveness and comparative effectiveness of pulmonary-specific ancillary treatments in children with PARDS? |
| Section 5 | Nonpulmonary treatments | What is the effectiveness and comparative effectiveness of nonpulmonary treatments in children with PARDS? |
| Section 6 | Monitoring | What is the role of different monitoring strategies in patients with PARDS? |
| Section 7 | Noninvasive respiratory support | What is the effectiveness of noninvasive ventilatory support in PARDS? |
| Section 8 | Extracorporeal support | What is the effectiveness of extracorporeal membrane oxygenation in children with PARDS? |
| Section 9 | Morbidity and long-term outcomes | What are the morbidity and long-term outcomes in PARDS? |
| Section 10 | Clinical informatics and data science | How can informatics, data science, and computerized decision support tools improve the diagnosis and management of PARDS? |
| Section 11 | Implementation in RLS | How should the recommendations for the diagnosis and management of PARDS be adapted to the context of RLS? |
PALICC = Pediatric Acute Lung Injury Consensus Conference, PARDS = pediatric acute respiratory distress syndrome, RLS = resource-limited settings.
Population, Intervention, Comparator, and Outcome questions were formulated based on topics addressed in the 2015 PALICC guidelines (2) as well as new key topics that were identified by each subgroup. Electronic searches were conducted by experienced medical librarians in Medline and Embase databases, as well as CINAHL, Cochrane, Scopus, and/or the Web of Science databases during November 2020–December 2020, with an update in March 2022–April 2022. For topics covered in the 2015 PALICC guidelines, searches were updated to include publications since 2013, while two new sections (i.e., key questions 10 and 11 on informatics and RLS, respectively) or new topics underwent literature review from 1980. We used a combination of medical subject heading terms and text words for concepts related to PARDS and each of the 11 sections. Search strategies are detailed in the methods article and supplemental digital files for each of the individual articles (13–24). References included in previous PALICC reviews were systematically added to the extracted evidence.
All titles and abstracts in each subgroup had single author review, and full-text article review and extraction was independently conducted by two reviewers. A third reviewer resolved differences at each stage. Risk of bias was assessed using the Cochrane risk of bias 2 (RoB2) tool for randomized clinical trials and the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool for nonrandomized studies (25, 26). Key data elements were extracted using electronic forms developed in the Research Data Electronic Capture (REDCap) browser (27) and exported into evidence tables. The protocol for the systematic reviews was registered on the International Prospective Register of Systematic Reviews (CRD42021236582).
Different types of recommendation or statements were generated (Table 2). The GRADE approach was used to summarize relevant evidence and develop recommendations for clinical practice (28, 29). Where applicable, we performed meta-analyses to obtain pooled estimates from similar studies. The methodologists independently categorized the certainty of evidence of each recommendation using the GRADE framework (30). We used the EtD framework to facilitate transition from evidence to recommendations (31). Based on the certainty of evidence and the EtD framework, clinical practice recommendations (CR) were described as “strong” (with wording “we recommend”) or “conditional” (using “we suggest”) (29). The implications of the strength of recommendations for different stakeholders are provided in Table 3. Good practice statements (GPS) are a unique category recognized by GRADE. This was used when it was abundantly clear that there is a “large net positive impact” if the recommended course of action is implemented (32). Policy statements (PS) related to healthcare delivery, education, or ethics. In making such statements, we considered the impact of the implementation of the recommended course of action on patient centered outcomes. Relevant gaps in evidence were synthesized into future research initiatives and classified as “research statements (RS).” Definition statements (DS) were offered in the context of updating the definition of pediatric ARDS. DS were primarily based on analysis of data from observational studies and clinical trials that described the impact of different variables on patient centered outcomes. Clinical attributes and indices that had a major impact on prognosis were used in formulating the definition of pediatric ARDS. GPS, PS, RS, and DS were ungraded.
TABLE 2.
Types of Recommendation and Statements Used in the Second Pediatric Acute Lung Injury Consensus Conference Guideline
| Recommendation Type | Description | Method | Label |
|---|---|---|---|
| Clinical recommendations | Recommendations on clinical interventions and diagnostic tests | GRADE framework, consensus using UCLA-RAND system | Certainty of evidence, strength of recommendation |
| Good practice statements | Absence of direct evidence but it is obvious that implementing the statement will result in a large net positive effect | GRADE framework, consensus using UCLA-RAND system | Ungraded, good practice statements |
| Research statement | Inadequate evidence after a systematic review and where the panelists believed that any recommendation would be speculative | Consensus using UCLA-RAND system | Ungraded, research statement |
| Policy statements | Position on issues that pertain to bioethics, public health policy, healthcare finance and delivery and medical education/training | Consensus using UCLA-RAND system | Ungraded, policy statements |
| Definition statement | Offered in the context of updating the definition of pediatric acute respiratory distress syndrome | Consensus using UCLA-RAND system | Ungraded, definition statement |
GRADE = Grading of Recommendations, Assessment, Development, and Evaluation, RAND = research and development.
TABLE 3.
Implications of Clinical Recommendations With Grading of Recommendations, Assessment, Development, and Evaluation Certainty of Evidence and Strength of Recommendation to Stakeholders (29)
| Stakeholder | Strong Recommendation | Conditional Recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the “recommended” course of action and only a small proportion would not | The majority of individuals in this situation would want the “suggested” course of action, but many would not |
| Clinicians | Most individuals should receive the “recommended” course of action | Recognize that different choices will be appropriate for different patients and that you must help each patient arrive at a management decision consistent with her or his values and preferences |
| Policy makers | The recommendation can be adapted as policy in most situations including for the use as performance indicators | Policy making will require substantial debates and involvement of many stakeholders. Policies are also more likely to vary between regions |
All recommendations, statements, their corresponding remarks, and their evidence were discussed by the entire panel utilizing multiple online webinars. Three co-chairs (G.E., Y.M.L.-F., R.G.K.) were responsible for leadership and coordination of the PALICC-2 meetings, oversight of the voting processes, and harmonization of recommendations/statements and manuscripts. Conflict of interest disclosures were completed by all panelists at the beginning, during the voting process, and at the time of journal submission. Three rounds of voting were conducted to achieve consensus using an online anonymous survey (Qualtrics, Provo, UT). Each recommendation/statement was scored on a 9-point Likert scale, ranging from strong disagreement (scored 1) to strong agreement (scored 9). Implementation science experts suggested revisions for recommendations/statements to each subgroup to improve clarity and to facilitate future implementation. After the first two rounds, recommendations and statements that did not meet 90% agreement (score ≥ 7) were reviewed based on comments from the full panel and resubmitted for voting. Remarks were used to provide clarification to some recommendations/statements and were included in the voting process.
The PALICC-2 Recommendations and Statements were endorsed by the World Federation of Pediatric Intensive and Critical Care Societies on June 17, 2022.
RESULTS
All the following recommendations and statements achieved the a priori specified 80% agreement threshold. There were 34 CR (one strong and 33 conditional). There were another 112 statements: 18 related to the definition of PARDS and patients at risk for PARDS, 55 GPS, seven PS, and 32 RS. The evidence tables and rationale supporting CR are presented in the corresponding subgroup manuscripts (13–24).
The revised PARDS definition is now summarized in Tables 4 and 5, and introduces key new concepts related to stratifying PARDS severity at least 4 hours after initial PARDS diagnosis for both invasive and noninvasive ventilation (NIV), allowing for the diagnosis of “possible PARDS” for children on nasal modes of support such as HFNC, and enabling modifications for RLS. Tables 6–9 summarize PALICC-2 recommendations and statements in the context of a patient’s trajectory from diagnosis to follow-up.
TABLE 4.
Diagnosis of Pediatric Acute Respiratory Distress Syndrome (Definition Statement 1.1; Definition Statement 1.7.1)
| Age (DS 1.1) | Exclude patients with perinatal lung disease | ||
| Timing (DS 1.2) | Within 7 d of known clinical insult | ||
| Origin of edema (DS 1.3) | Not fully explained by cardiac failure or fluid overload | ||
| Chest imaging (DS 1.3) | New opacities (unilateral or bilateral) consistent with acute pulmonary parenchymal disease and which are not due primarily to atelectasis or pleural effusiona | ||
| Oxygenationb (DS 1.4.1) | IMV: OI ≥ 4 or OSI ≥ 5 | ||
| NIVc: Pao2/Fio2 ≤ 300 or Spo2/Fio2 ≤ 250 | |||
| Stratification of PARDS severity: Apply ≥ 4 hr after initial diagnosis of PARDS (DS 1.4.4) | |||
| IMV-PARDS: (DS 1.4.1) | Mild/moderate: OI < 16 or OSI < 12 (DS 1.4.5) | Severe: OI ≥ 16 or OSI ≥ 12 (DS 1.4.5) | |
| NIV-PARDSc (DS 1.4.2; DS 1.4.3) | Mild/moderate NIV-PARDS: Pao2/Fio2 > 100 or Spo2/Fio2 > 150 | Severe NIV-PARDS: Pao2/Fio2 ≤ 100 or Spo2/Fio2 ≤ 150 | |
| Special populations d | |||
| Cyanotic heart disease (DS 1.6.1; DS 1.6.2) | Above criteria, with acute deterioration in oxygenation not explained by cardiac disease | ||
| Chronic lung disease (DS 1.6.3; DS 1.6.4) | Above criteria, with acute deterioration in oxygenation from baseline | ||
DS = definition statement, IMV = invasive mechanical ventilation, NIV = noninvasive ventilation, OI = oxygenation index, OSI = oxygenation saturation index, PARDS = pediatric acute respiratory distress syndrome, Spo2 = pulse oximeter oxygen saturation.
Children in resource-limited settings where imaging is not available who otherwise meet PARDS criteria are considered to have possible PARDS.
Oxygenation should be measured at steady state and not during transient desaturation episodes. When Spo2 is used, ensure that Spo2 is ≤ 97%.
OI = mean airway pressure (MAP) (cm H2O) × Fio2/Pao2 (mm Hg).
OSI = MAP (cm H2O) × Fio2/Spo2.
Diagnosis of PARDS on NIV (NIV-PARDS) requires full facemask interface with continuous airway positive pressure/positive end-expiratory pressure ≥ 5 cm H2O.
Stratification of PARDS severity does not apply to these populations.
Additional note: Possible PARDS and At-Risk for PARDS should not be diagnosed in children with respiratory failure solely from airways obstruction (e.g., critical asthma, virus-induced bronchospasm). The corresponding definition statement numbers are indicated in parentheses.
TABLE 5.
Diagnosis of Possible Pediatric Acute Respiratory Distress Syndrome and At-Risk for Pediatric Acute Respiratory Distress Syndrome (Definition Statement 1.5.3; Definition Statement 1.7.2; Definition Statement 11.2)
| Age | Exclude patients with perinatal lung disease |
| Timing | Within 7 d of known clinical insult |
| Origin of edema | Not fully explained by cardiac failure or fluid overload |
| Chest imaging (DS 1.5.2) | New opacities (unilateral or bilateral) consistent with acute pulmonary parenchymal disease and which are not due primarily to atelectasis or effusiona |
| Oxygenationb threshold to diagnose possible PARDS for children on nasal respiratory supportc (DS 1.5.1) | |
| Nasal continuous airway positive pressure/bilevel positive airway pressure or high-flow nasal cannula (≥ 1.5 L/kg/min or ≥ 30 L/min): Pao2/Fio2 ≤ 300 or Spo2/Fio2 ≤ 250 | |
| Oxygenationb threshold to diagnose at-risk for PARDS | |
| Any interface: Oxygen supplementationd to maintain Spo2 ≥ 88% but not meeting definition for PARDS or possible PARDS | |
| Special populations | |
| Cyanotic heart disease | Above criteria, with acute deterioration in oxygenation not explained by cardiac disease |
| Chronic lung disease | Above criteria, with acute deterioration in oxygenation from baseline |
DS = definition statement, PARDS = pediatric acute respiratory distress syndrome, Spo2 = pulse oximeter oxygen saturation.
Children in resource-limited environments where imaging is not available who otherwise meet possible PARDS criteria are considered to have possible PARDS.
Oxygenation should be measured at steady state and not during transient desaturation episodes. When Spo2 is used, ensure that Spo2 is ≤ 97%.
Children on nasal noninvasive ventilation (NIV) or high-flow nasal cannula are not eligible for PARDS but are considered to have possible PARDS when this oxygenation threshold is met.
Oxygen supplementation is defined as Fio2 > 21% on invasive mechanical ventilation; or Fio2 > 21% on NIV; or “oxygen flow” from a mask or cannula that exceeds these age-specific thresholds: ≥ 2 L/min (age < 1 yr), ≥ 4 L/min (age 1–5 yr), ≥ 6 L/min (age 6–10 yr), or ≥ 8 L/min (age > 10 yr). For children on a mask or cannula, oxygen flow calculated as Fio2 × flow rate (L/min) (e.g., 6 L/min flow at 0.35 Fio2 = 2.1 L/min).
Additional note: Possible PARDS and at-risk for PARDS should not be diagnosed in children with respiratory failure solely from airway obstruction (e.g., critical asthma, virus-induced bronchospasm). The corresponding definition statement numbers are indicated in parentheses.
TABLE 6.
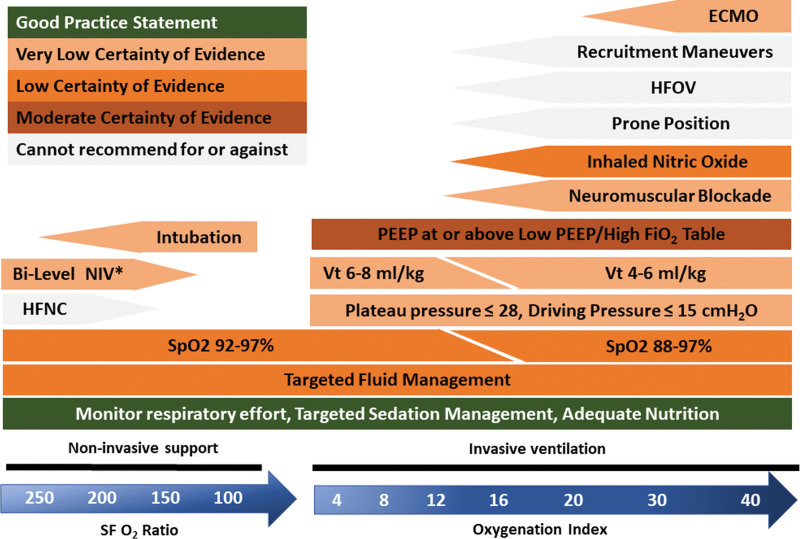
Synthesis of the Second Pediatric Acute Lung Injury Consensus Conference Clinical Recommendations and Good Practice Statements Related to the Ventilatory Support, Respiratory Monitoring, and Pulmonary Ancillary Treatment
| Topic | Recommendation | Good Practice Statement |
|---|---|---|
| Noninvasive support | ||
| Use of O2/HFNC | Worsening acute respiratory failure → time-limited trial of NIV (7.1.1) | Humidification for HFNC (7.3.3) |
| In RLS, use of HFNC/CPAP vs O2 (7.5.1) | ||
| In RLS, use of CPAP vs HFNC when available (7.5.2) | ||
| Use of NIV (CPAP or bilevel positive airway pressure) | Worsening in 0–6 hr trial → ETT (7.1.2) | Close monitoring and trained staff (7.2) |
| Humidification (7.3.3), optimal interface for synchronization (7.3.1), monitoring for complications (7.3.2) | ||
| Addition of inspiratory support if synchronized (7.3.5) | ||
| Sedation during poor tolerance of NIV (7.3.4) | ||
| Invasive ventilation | ||
| ETT | ETT: use of cuffed tubes (3.11) | |
| Maintain unobstructed airway (4.4.1) | ||
| ETT suction: nonroutine use of instilled saline (4.4.3) | ||
| MV bundle | Use of lung protective ventilation bundle (3.5) | Daily assessment for extubation readiness test and spontaneous breathing trial (6.4.1) |
| Automated monitoring of compliance with Second Pediatric Acute Lung Injury Consensus Conference lung protective strategies (10.2) | In RLS, implement locally adapted protocols (PS 11.5) | |
| Regular training and education of all staff (PS 11.6) | ||
| MV type | Cannot recommend for or against HFOV (3.8.1) | If HFOV used: lung volume optimization strategy (3.8.2) |
| Monitoring | Continuous: respiratory rate, heart rate, Spo2 (6.1.1). Intermittent: noninvasive blood pressure (6.1.1) | |
| Monitor effort of breathing (6.2.5) | ||
| Continuous monitoring of co2 during MV (6.3.3) | ||
| Calculate and monitor dead space (6.3.4) | ||
| Vt | 6–8 mL/kg (3.2) | Scale Vt and Crs to body weight (6.1.2) |
| Use of 4–6 mL/kg if needed to stay below suggested PPlat and DP (3.2) | Continuously monitor Vt (6.2.1) using compensation for circuit compliance (6.2.2) | |
| PIP and PPlat | PPlat ≤ 28 cm H2O (3.3.1) | Monitor PIP and PPlat (6.2.3) |
| PPlat ≤ 32 cm H2O if reduced chest wall compliance (3.3.1) | ||
| DP limit | DP ≤ 15 cm H2O (3.3.2) | Monitor DP (6.2.3) |
| PEEP | Titration: to O2, O2-delivery, hemodynamics, and Crs (3.4.1) | Monitor intrinsic PEEP, flow- and pressure-time curves (6.2.4) |
| Level: at or above level on Acute Respiratory Distress Syndrome Network low PEEP/Fio2 Table (3.4.2) | Titration: attend to PPlat and DP (3.4.3) | |
| Spo2 target | Mild/moderate: 92–97% strategy (3.9.1) | Avoidance of Spo2 < 88% and > 97% (3.9.3) |
| Severe: accept < 92%, with optimized PEEP (3.9.2) | Severe: when Spo2 < 92% → central venous oxygen saturation monitoring (3.9.4) | |
| pH/Paco2 target | Accept pH ≥ 7.2 to remain within PPlat, DP, and Vt ranges during permissive hypercapnia (3.10.1) | Adjust frequency of pH, Paco2 measurement to PARDS severity and stage and to noninvasive co2 monitoring (6.3.2) |
| No routine use of bicarbonate supplementation (3.10.2) | ||
| Ancillary treatment | ||
| Prone positioning | Cannot recommend for or against prone positioning (4.3) | |
| Recruitment maneuver | Cannot recommend for or against recruitment maneuver (3.6) | |
| Inhaled NO | Use of inhaled nitric oxide in selected populations only (4.1) | |
| Surfactant | Against routine use of surfactant (4.2) | |
| Corticosteroids | Use of corticosteroids in selected populations only (4.6) | |
CPAP = continuous positive airway pressure, Crs = compliance of the respiratory system, DP = driving pressure, ETT = endotracheal tube, HFNC = high-flow nasal cannula, HFOV = high-frequency oscillating ventilation, MV = mechanical ventilation, NIV = noninvasive ventilation, PEEP = positive end-expiratory pressure, PIP = peak inspiratory pressure, PPlat = plateau pressure, PS = policy statements, RLS = resource-limited settings, Spo2 = pulse oximeter oxygen saturation, Vt = tidal volume.
The corresponding definition statement numbers are indicated in parentheses.
TABLE 9.
Synthesis of the Second Pediatric Acute Lung Injury Consensus Conference Research Recommendations
| Activity | Research Neededa |
|---|---|
| Screening, diagnosis, and monitoring | |
| Diagnosis | Adult vs pediatric age-group comparison in pediatric acute respiratory distress syndrome progression and outcome (2.2) |
| Trajectory of illness using standardized minimal datasets and operational definitions (2.4.1) | |
| Diagnostic utility of Second Pediatric Acute Lung Injury Consensus Conference definition in RLS (11.3) | |
| Collaborative research networks to include RLS sites (11.4) | |
| Biomarker | Biomarker and genetic insight into pathophysiology (2.3.1) |
| Molecular phenotype and endotype studies (2.3.2) | |
| Data science | Collaborative data science (10.4) |
| Development of generalizable electronic tools (10.5) | |
| MV and cardiovascular system monitoring | |
| Endotracheal tube | Standardize spontaneous breathing trial and extubation readiness test in studies (6.4.2) |
| MV bundle | Closed vs open suctioning (4.4.2); methods and populations for airway clearance (4.5) |
| MV type | Studies of ventilator modes (3.1), studies of high-flow nasal cannula (7.4), studies needed inclusive of RLS (11.8) |
| Vt | Use of protocols and definitions in studies measuring Vt, peak inspiratory pressure, plateau pressure, positive end-expiratory pressure, mean airway pressure (2.4.2); use of mechanical power calculations (3.7); use of monitoring loops, compliance, resistance, strain-stress, etc. (6.2.6) |
| Hemodynamic | Studies of devices, approach, and tests of hemodynamics (6.6.5); studies of routine CT, lung ultrasound, electrical impedance tomography (6.5.2) |
| Other lung | |
| Other drugs | Studies of many agents (4.7; 11.9) |
| ECMO | |
| ECMO type | Studies of extracorporeal co2 removal (8.4) |
| Pain, Agitation, Neuromuscular Blockade, and Delirium in Critically Ill Pediatric Patients With Consideration of the ICU Environment and Early Mobility | |
| Assessment | Medications for prevention and/or treatment of delirium (5.2.3) |
| General approach | |
| Fluids | Validated measure of nonpulmonary organ system dysfunction (2.4.3) |
| Nutrition | Reporting of nutrition strategy in research studies (5.4.5) |
| Blood | Studies of packed RBC and alternatives (5.6.3) |
| Post-PICU follow-up | |
| Initial | Assessment of pre-PICU baseline state if follow-up is anticipated (9.4.1); practices to optimize follow-up (9.4.5) |
| Assessment | Postdischarge endpoints (9.4.2); relationship between short- and longer-term outcomes (9.4.3); factors affecting trajectory of recovery (9.4.4) |
ECMO = extracorporeal membrane oxygenation, MV = mechanical ventilation, RLS = resource-limited settings, Vt = tidal volume.
The premise of research needed statements (which is not referenced in the Table) is that everything in the Research column needs a dimension that is relevant to RLS, as well as collaboration between the spectrum of resourced settings.
The corresponding definition statement numbers are indicated in parentheses.
TABLE 7.
Synthesis of the Second Pediatric Acute Lung Injury Consensus Conference Clinical Recommendations and Good Practice Statements Related to Nonpulmonary Management
| Topic | Recommendation | Good Practice Statement |
|---|---|---|
| Diagnosis | ||
| Screening and monitoring | Use of electronic algorithms to help identify PARDS (10.1) | Policy statement: Healthcare organizations support for developing, implementing, and using electronic tools (10.3) |
| Risk stratification | Measure dead space to tidal volume ratio and/or end-tidal alveolar dead-space fraction (2.1), beside oxygenation-based stratification | Monitor Fio2, pulse oximeter oxygen saturation, Pao2, mean airway pressure, positive end-expiratory pressure (6.3.1) |
| Use of chest imaging (6.5.1) | ||
| Hemodynamic monitoring | Monitor to assess impact of MV on RV/LV (6.6.1) | |
| Arterial line for blood pressure and arterial blood gas in severe PARDS (6.6.4) | ||
| Perform cardiac ultrasound in severe PARDS or suspected RV/LV dysfunction (6.6.3) | ||
| ECMO | ||
| Failing response to treatment | Consider transfer to ECMO center (8.1.5) | |
| Evaluation | When lung protective strategies fail, and reversible cause. No strict criteria (8.1.1) | Structured evaluation by expert team (8.1.2) |
| Serial evaluations (8.1.3) | Education and competencies for ECMO clinicians (PS 8.2.1) | |
| Report data to Extracorporeal Life Support Organization (or equivalent) for benchmarking (PS 8.2.2) | ||
| Support type | Use of venovenous ECMO (8.1.4) | |
| Blood gas targets | Avoid hyperoxia (8.3.1a) | |
| Slow changes in Paco2 (8.3.1b) | ||
| MV | General lung protective strategy (8.3.2) | |
| Pain, Agitation, Neuromuscular Blockade, and Delirium in Critically Ill Pediatric Patients With Consideration of the ICU Environment and Early Mobility | ||
| Approach | Nonpharmacological multicomponent approaches (5.2.2; 5.7.1) | |
| Assessment | Use of scales (5.1.1) | |
| Daily assessment of activity and mobility goals (5.7.2) | ||
| Rehabilitation evaluation by 72 hr (5.7.3) | ||
| Daily assessment for delirium (5.2.1) | ||
| If treated ≥ 5 d assess for iatrogenic withdrawal syndrome (5.1.4) | ||
| Sedation | Titrate drugs for minimal, yet effective dose (5.1.2) | |
| Monitor and wean with goal-directed protocol (5.1.3) | ||
| NMBA | Use of NMBA, if protective ventilation is not achieved with sedation alone (5.3.1) | Monitor and titrate to goal-established (5.3.2) |
| Fluids | Optimize while preventing overload (5.5) | Monitor cumulative fluid balance (6.6.2) |
| Nutrition | Early start (< 72 hr) EN (5.4.1) | Nutrition plan (5.4.2) |
| Protein ≥ 1.5 g/kg/d (5.4.4) | EN monitoring with goal-directed protocol (5.4.3) | |
| Blood | No transfusion of pRBC for hemoglobin concentration ≥ 7 g/dL (5.6.2) | Use of pRBC for hemoglobin concentration < 5 g/dL (5.6.1) |
ECMO = extracorporeal membrane oxygenation, EN = enteral nutrition, LV = left ventricle, MV = mechanical ventilation, NMBA = neuromuscular blocking agent, PARDS = pediatric acute respiratory distress syndrome, pRBCs = packed RBCs, PS = policy statements, RV = right ventricle.
The corresponding definition statement numbers are indicated in parentheses.
TABLE 8.
Synthesis of the Second Pediatric Acute Lung Injury Consensus Conference Good Practice Statements Related to Follow-Up After Pediatric Acute Respiratory Distress Syndrome
| Assessment | Good Practice Statement |
|---|---|
| Initial approach | Primary care screening by 3 mo for post-PICU morbidities (9.1.1) |
| Stepwise addition of management, re-evaluations, referral to a specialist (9.1.2) | |
| Assessment of health-related quality of life, physical, neurocognitive, emotional, family, and social function | Evaluation within 3 mo of PICU discharge (9.3.1) |
| Additional pre-school (4–6 yr) assessment if pediatric acute respiratory distress syndrome during infancy (9.3.2) | |
| Referral for specialist help when deficits identified (9.3.3) | |
| Post-extracorporeal membrane oxygenation, short- and long-term neurodevelopment and physical function (8.5) | |
| Pulmonary assessment | Screen by 3 mo post-PICU discharge for pulmonary function abnormalities (9.2.1) |
| With spirometry in patients of sufficient age and capabilities (9.2.2) | |
| Referral to pediatrician or pediatric pulmonologist when pulmonary function deficits identified (9.2.3) |
The corresponding definition statement numbers are indicated in parentheses.
Section 1. Definition, Incidence, and Epidemiology (14)
Age
Definition statement 1.1. All patients less than 18 years old without active perinatal lung disease should be diagnosed with PARDS using PALICC-2 criteria. (Ungraded DS, 94% agreement) (14).
Remarks: Practitioners can use either the PALICC-2 or neonatal definition (Montreux NARDS) for neonates and can use either the PALICC-2 or adult definition (Berlin ARDS) for young adults.
Timing
Definition statement 1.2. Symptoms of hypoxemia and radiographic changes must occur within 7 days of a known insult to qualify for PARDS. (Ungraded DS, 96% agreement).
Imaging Findings
Definition statement 1.3. Chest imaging findings of new opacity (or opacities) consistent with acute pulmonary parenchymal disease not explained by atelectasis or effusion are necessary to diagnose PARDS. (Ungraded DS, 90% agreement).
Severity of Hypoxemia for Disease Stratification. Definition statement 1.4.1.
Oxygenation index (OI) or oxygen saturation index (OSI), in preference to Pao2/Fio2 or pulse oximeter oxygen saturation (Spo2)/Fio2, should be the primary metric of lung disease severity to define PARDS for all patients treated with invasive mechanical ventilation (IMV), with Pao2 used preferentially when available. (Ungraded DS, 90% agreement).
Definition statement 1.4.2. Pao2/Fio2 or Spo2/Fio2 should be used to diagnose PARDS and possible PARDS for patients receiving NIV or HFNC (Ungraded DS, 88% agreement).
Definition statement 1.4.3. Patients on a full-face interface of NIV (continuous positive airway pressure [CPAP] or bilevel positive airway pressure [BiPAP]) with CPAP greater than or equal to 5 cm H2O or invasively ventilated should be considered as having PARDS if they meet timing, oxygenation, etiology/risk factor, and imaging criteria (Ungraded DS, 90% agreement).
Definition statement 1.4.4. Subjects with PARDS should be stratified into severity categories after a period of at least 4 hours (Ungraded DS, 85% agreement).
Definition statement 1.4.5. When applying Spo2 criteria to diagnose PARDS, oxygen should be titrated to achieve an Spo2 between 88% and 97%. (Ungraded DS, 96% agreement).
Possible PARDS and At Risk for PARDS
Definition statement 1.5.1. Patients on a nasal interface of NIV (CPAP or BiPAP) or on HFNC greater than or equal to 1.5 L/kg/min or greater than or equal to 30 L/min should be considered as having possible PARDS if they meet timing, oxygenation, etiology/risk factor, and imaging criteria (Ungraded DS, 87% agreement).
Definition statement 1.5.2. Patients who are missing imaging due to resource limitations should be considered as having possible PARDS if they otherwise meet timing, oxygenation, and risk factor criteria. (Ungraded DS, 90% agreement).
Definition statement 1.5.3. Defining a group of patients at risk for PARDS is necessary to determine the epidemiology of disease progression and potential avenues for disease prevention. (Ungraded DS, 96% agreement).
Diagnosing PARDS in Patients With Chronic Cardiorespiratory Disease
Definition statement 1.6.1. Patients with cyanotic congenital heart disease are considered to have PARDS if they fulfill standard PARDS criteria and have an acute deterioration in oxygenation (relative to baseline) not fully explained by the underlying cardiac disease. (Ungraded DS, 98% agreement).
Definition statement 1.6.2. Patients with left ventricular (LV) dysfunction who fulfill standard PARDS criteria are considered to have PARDS if acute hypoxemia and new chest imaging changes cannot be explained solely by LV heart failure or fluid overload. (Ungraded DS, 92% agreement).
Remarks: Cardiac ultrasonography and/or left atrial pressure may be useful in identifying hydrostatic pulmonary edema.
Definition statement 1.6.3. Patients with preexisting chronic lung disease treated with supplemental oxygen, NIV, or IMV via tracheostomy are considered to have PARDS if they demonstrate acute changes that fulfill standard PARDS criteria and exhibit an acute deterioration in oxygenation from baseline that meets oxygenation criteria for PARDS. (Ungraded DS, 96% agreement).
Definition statement 1.6.4. Patients with chronic lung disease who receive IMV at baseline or have cyanotic congenital heart disease with acute onset of illness that fulfills standard PARDS criteria should not be stratified by OI or OSI risk categories. Future studies are necessary to determine PARDS risk stratification of such patients with acute-on-chronic hypoxemic respiratory failure. (Ungraded DS, 90% agreement).
Definition of PARDS, Possible PARDS, and At-Risk for PARDS.
Definition statement 1.7.1. PARDS shall be defined using Table 4. (Ungraded DS, 84% agreement).
Definition statement 1.7.2. Possible and at risk for PARDS shall be defined using Table 5. (Ungraded DS, 94% agreement).
Remarks: Clinicians should consider treating patients with possible PARDS as if they have PARDS and apply other recommendations after considering the specific risks and benefits for that specific patient.
Section 2. Pathobiology, Severity, and Risk Stratification (15)
Dead-Space Fraction for Risk Stratification. Clinical recommendation 2.1. We suggest that in PARDS patients with arterial access, dead space to tidal volume ratio and/or end-tidal alveolar dead-space fraction should be measured, compared with not measured, at the onset of PARDS to assist in the bedside assessment of severity of illness and risk stratification. (Conditional CR, low certainty of evidence, 90% agreement).
Age
Research statement 2.2. Studies should be conducted to examine whether there are differences in the progression and/or outcome of PARDS between adults and children across different age groups and between adults and children. (Ungraded RS, 96% agreement).
Biomarkers, Phenotypes, and Endotypes
Research statement 2.3.1. Biomarker and genetic studies will provide insight into the pathobiology of PARDS. Future research requires increased integration of human studies and human model systems. (Ungraded RS, 96% agreement).
Research statement 2.3.2. The heterogeneity of PARDS should be defined using combinations of biomarkers (proteins, metabolites, gene transcripts, and genetics) to define phenotypes and endotypes of patients with PARDS for prognostic and predictive enrichment in studies (Ungraded RS, 84% agreement).
Standardization for Future Research
Research statement 2.4.1. Research studies evaluating trajectory of illness and recovery should use standardized, minimal datasets with operational definitions (Ungraded RS, 90% agreement).
Research statement 2.4.2. Studies measuring variables such as tidal volume, peak and plateau pressures, positive end-expiratory pressure (PEEP), or mean airway pressure should use explicit protocols and definitions to allow for reproducibility (Ungraded RS, 100% agreement).
Research statement 2.4.3. A validated measure of nonpulmonary organ system dysfunction should be included in studies of clinical risk factors associated with outcome in patients with PARDS (Ungraded RS, 98% agreement).
Section 3. Invasive Ventilatory Support (16)
Modes of Ventilation
Research statement 3.1. We cannot make a recommendation on the specific ventilator mode preferred for PARDS patients. Future clinical studies should be conducted to assess controlled and assisted modes of ventilation on outcomes. (Ungraded RS, 94% agreement).
Remarks: There are no outcome data on the influence of mode (controlled or assisted, including airway pressure release ventilation and neurally adjusted ventilatory assist) during conventional mechanical ventilation (MV).
Tidal Volume
Clinical recommendation 3.2. We suggest the use of physiologic tidal volumes between 6 and 8 mL/kg in patients with PARDS compared with supraphysiologic tidal volumes (> 8 mL/kg). (Conditional CR, very low certainty of evidence, 98% agreement).
Remarks: Tidal volumes below 6 mL/kg should be used in patients if needed to stay below suggested plateau and driving pressure limits. Tidal volumes below 4 mL/kg should be used with caution.
Ventilation Pressure
Clinical recommendation 3.3.1. In the absence of transpulmonary pressure measurements, we suggest an inspiratory plateau pressure less than or equal to 28 cm H2O. (Conditional CR, very low certainty of evidence, 92% agreement).
Remarks: The plateau pressure may be higher (29–32 cm H2O) for patients with reduced chest wall compliance.
Clinical recommendation 3.3.2. We suggest limiting driving pressure to 15 cm H2O (as measured under static conditions) for patients with PARDS. (Conditional CR, very low certainty of evidence, 82% agreement).
Positive End-Expiratory Pressure
Clinical recommendation 3.4.1. We suggest that PEEP should be titrated to oxygenation/oxygen delivery, hemodynamics, and compliance measured under static conditions compared with other clinical parameters or any of these parameters in isolation in patients with PARDS. (Conditional CR, very low certainty of evidence, 96% agreement).
Clinical recommendation 3.4.2. We recommend that PEEP levels should typically be maintained at or above the lower PEEP/higher Fio2 table from the ARDS Network protocol (33) (Supplemental Table 1, http://links.lww.com/PCC/C287) compared with PEEP levels lower than the lower PEEP/higher Fio2 table. (Strong CR, moderate certainty of evidence, 96% agreement).
Good practice statement 3.4.3. When adjusting PEEP levels to achieve the proposed oxygen target range for PARDS, attention to avoid exceeding plateau pressure and/or driving pressure limits is warranted. (Ungraded GPS, 100% agreement).
Ventilation Bundle
Clinical recommendation 3.5. We suggest that a lung protective ventilation bundle should be used compared with no bundle when caring for ventilated patients with PARDS. (Conditional CR, low certainty of evidence, 83% agreement).
Remarks: Lung protective bundles prioritize maintaining multiple ventilator settings (tidal volume, plateau pressure, driving pressure, PEEP) within the suggested limits as well as using detection algorithms and educational programs.
Recruitment Maneuvers
Clinical recommendation 3.6. We cannot suggest for or against the use of recruitment maneuvers in patients with PARDS. (Conditional CR, low certainty of evidence, 94% agreement).
Remarks: Careful recruitment maneuvers may be applied in the attempt to improve oxygenation by slow incremental and decremental PEEP steps. Sustained inflation maneuvers cannot be recommended.
Mechanical Power
Research statement 3.7. There is insufficient evidence to suggest the use of mechanical power calculations to guide pediatric MV. Future research is needed with regards to mechanical power calculations in children before clinical use. (Ungraded RS, 90% agreement).
High-Frequency Ventilation
Clinical recommendation 3.8.1. We cannot make a recommendation as to whether high-frequency oscillatory ventilation (HFOV) should be used instead of conventional ventilation in patients with PARDS. (Conditional CR, low certainty of evidence, 90% agreement).
Remark: HFOV may be considered in patients with PARDS in whom the ventilatory goals are not achieved with a lung protective strategy on conventional MV.
Good practice statement 3.8.2. When using HFOV, the optimal lung volume should be achieved by exploration of the potential for lung recruitment with a stepwise increase and decrease of the mean airway pressure under continuous monitoring of the oxygen and co2 response and hemodynamic parameters (Ungraded GPS, 90% agreement).
Spo 2 Targets
Clinical recommendation 3.9.1. We suggest that for mild/moderate PARDS, Spo2 be maintained between 92% and 97%. (Conditional CR, low certainty of evidence, 98% agreement).
Clinical recommendation 3.9.2. We suggest that for severe PARDS, after optimizing PEEP, Spo2 less than 92% can be accepted in order to reduce Fio2 exposure. (Conditional CR, low certainty of evidence, 88% agreement).
Good practice statement 3.9.3. Prolonged exposure to hypoxemic (< 88%) or high (> 97%) Spo2 targets should be avoided while on oxygen supplementation. (Ungraded GPS, 88% agreement).
Good practice statement 3.9.4. When Spo2 is less than 92%, central venous saturation and markers of oxygen delivery/utilization should be monitored. (Ungraded GPS, 94% agreement).
pH Management
Clinical recommendation 3.10.1. We suggest allowing permissive hypercapnia (to a lower limit pH of 7.20) in patients with PARDS to remain within previously recommended pressure and tidal volume ranges. (Conditional CR, very low certainty of evidence, 100% agreement).
Remarks: Exceptions to permissive hypercapnia include, but are not limited to, intracranial hypertension, severe pulmonary hypertension, select congenital heart disease lesions, hemodynamic instability, and significant ventricular dysfunction.
Clinical recommendation 3.10.2. We suggest against the routine use of bicarbonate supplementation compared with selective use of bicarbonate in PARDS. (Conditional CR, low certainty of evidence, 96% agreement).
Remarks: Bicarbonate supplementation can be considered in situations where severe metabolic acidosis or pulmonary hypertension are adversely affecting cardiac function or hemodynamic stability.
Endotracheal Tubes
Good practice statement 3.11. Cuffed endotracheal tubes should be used when ventilating a patient with PARDS. (Ungraded GPS, 100% agreement).
Section 4. Ancillary Pulmonary Treatment (17)
Inhaled Nitric Oxide
Clinical recommendation 4.1. We suggest against the routine use of inhaled nitric oxide (iNO) in PARDS compared with selective use of iNO in PARDS. (Conditional CR, low certainty of evidence, 98% agreement).
Remarks: There may be clinical benefit in the use of iNO in some phenotypes such as patients with documented pulmonary hypertension or severe right ventricular dysfunction. In addition, the use of iNO may be considered in patients with severe PARDS as a rescue from, or bridge to, extracorporeal life support. When used, assessment of benefit should be undertaken within the first 4 hours and serially to minimize toxicity and to eliminate continued use in the absence of established effect.
Surfactant Therapy
Clinical recommendation 4.2. We suggest against the routine use of surfactant therapy in PARDS compared with selective use of surfactant. (Conditional CR, low certainty of evidence, 100% agreement).
Remarks: There may be a role for selective use of surfactant in specific populations; however, there is insufficient evidence to guide which populations may benefit.
Prone Positioning
Clinical recommendation 4.3. There are insufficient data to support or refute the use of prone positioning in patients with PARDS. (Conditional CR, low certainty of evidence, 94% agreement).
Remarks: The use of prone positioning may be considered in patients with PARDS and hypoxemia not responding to other interventions. If used, improvement in oxygenation while in the prone position should be assessed. We cannot make recommendations on the duration of prone positioning.
Endotracheal Suctioning
Good practice statement 4.4.1. In intubated patients with PARDS, an unobstructed airway should be maintained. (Ungraded GPS, 98% agreement).
Remark: Endotracheal suctioning must be performed with caution to minimize the risk of derecruitment.
Research statement 4.4.2. We cannot make a recommendation on the use of a closed versus an open suctioning system. Future research should focus on the impact of closed and open suctioning systems on outcomes. (Ungraded RS, 90% agreement).
Remarks: In severe PARDS, consideration should be given to the technique of suctioning with careful attention to minimize the potential for derecruitment.
Good practice statement 4.4.3. The routine instillation of isotonic saline prior to endotracheal suctioning should not be used in patients with PARDS. (Ungraded GPS, 94% agreement).
Remarks: The instillation of isotonic saline prior to endotracheal suctioning may be considered for lavage to remove thick tenacious secretions.
Airway Clearance
Research statement 4.5. We cannot make a recommendation on the use of specific methods of airway clearance (such as chest physiotherapy and mucolytics) in patients with PARDS. Future research should focus on the impact of specific airway clearance methods on outcomes and on specific populations likely to benefit from these methods. (Ungraded RS, 96% agreement).
Corticosteroids
Clinical recommendation 4.6. We suggest against the routine use of corticosteroids in patients with PARDS compared with selective use of steroids. (Conditional CR, low certainty of evidence, 96% agreement).
Remarks: There may be some benefit in patients with PARDS caused by severe acute respiratory syndrome coronavirus 2; however, we cannot make recommendations regarding other specific populations for use.
Other Adjunctive Therapies
Research statement 4.7. We cannot make a recommendation on the use of the following ancillary treatment in patients with PARDS: helium-oxygen mixture, inhaled or IV prostaglandins therapy, plasminogen activators, fibrinolytics or other anticoagulants, inhaled β-adrenergic receptor agonists or ipratropium, or IV N-acetylcysteine for antioxidant effects. Future research should focus on the impact of these treatments and on specific populations likely to benefit from them. (Ungraded RS, 96% agreement).
Section 5. Nonpulmonary Treatments (18)
Sedation
Good practice statement 5.1.1. Valid and reliable assessment scales for pain, sedation, delirium, and withdrawal should be used to monitor, target and titrate comfort therapies and to facilitate interprofessional communication. (Ungraded GPS, 100% agreement).
Good practice statement 5.1.2. In patients with PARDS, sedation (minimal yet effective) should be titrated to achieve the targeted MV strategy that facilitates oxygen delivery, oxygen consumption, and work of breathing goals. (Ungraded GPS, 96% agreement).
Good practice statement 5.1.3. Sedation monitoring, titration, and weaning should be managed by a goal-directed protocol with a daily sedation goal collaboratively established by the interprofessional team. (Ungraded GPS, 96% agreement).
Good practice statement 5.1.4. Patients with PARDS who are weaning from 5 or more days of sedation should be 1) assessed for symptoms of iatrogenic withdrawal syndrome using a validated instrument and 2) placed on a systematic plan that facilitates sedation weaning. (Ungraded GPS, 96% agreement).
Delirium and Sleep
Good practice statement 5.2.1. Patients with PARDS should be assessed daily for delirium using a validated pediatric delirium screening tool. (Ungraded GPS, 94% agreement).
Good practice statement 5.2.2. Patients with PARDS should receive multicomponent, nonpharmacologic interventions as first-line interventions to prevent and treat delirium through reduction of modifiable risk factors. (Ungraded GPS, 90% agreement).
Remarks: Measures include goal-directed, titrated, minimal but effective sedation, nighttime sleep promotion with noise and light optimization, augmentative and assistive communication, family engagement and activity and mobility as tolerated.
Research statement 5.2.3. We cannot make a recommendation regarding the use of typical or atypical antipsychotics, melatonin or other medications for routine prevention or treatment of delirium. Further studies are needed to evaluate the role of antipsychotic medications and melatonin in the treatment of delirium. (Ungraded RS, 98% agreement).
Neuromuscular Blockade
Clinical recommendation 5.3.1. We suggest that minimal, yet effective, neuromuscular blockade (NMB) be used in conjunction with sedation in comparison to the use of sedation alone if effective and protective MV cannot be achieved. (Conditional CR, very low certainty of evidence, 98% agreement).
Good practice statement 5.3.2. NMB should be monitored and titrated to the goal depth established by the interprofessional team. (Ungraded GPS, 94% agreement).
Remarks: Monitoring may include effective and protective ventilation, movement, and train-of-four response (if available).
Nutrition
Clinical recommendation 5.4.1. We suggest early initiation of enteral nutrition (< 72 hr) when feasible, over parenteral nutrition or delayed enteral nutrition in PARDS patients. (Conditional CR, very low certainty of evidence, 92% agreement).
Good practice statement 5.4.2. Patients with PARDS should receive a nutrition plan to facilitate their recovery, maintain their growth and meet their metabolic needs. (Ungraded GPS, 98% agreement).
Good practice statement 5.4.3. Enteral nutrition monitoring, advancement and maintenance should be managed by a goal-directed protocol that is collaboratively established by the interprofessional team. (Ungraded GPS, 96% agreement).
Clinical recommendation 5.4.4. We suggest a nutrition strategy that includes a minimum of 1.5 g/kg/d of protein compared with less than 1.5 g/kg/d of protein for PARDS patients. (Conditional CR, low certainty of evidence, 92% agreement).
Research statement 5.4.5. The reporting of the nutrition strategy, exposure and monitoring in clinical trials should be adequately explicit to allow comparison across studies (e.g., route, composition, calories delivered, time to reach nutrition goal). (Ungraded RS, 98% agreement).
Fluid Management
Clinical recommendation 5.5. We suggest that patients with PARDS should receive fluids with the daily fluid goal collaboratively established by the interprofessional team to maintain optimal oxygen delivery and preserve end organ function, while preventing fluid overload. (Conditional CR, low certainty of evidence, 98% agreement).
Transfusion
Good practice statement 5.6.1. Critically ill patients with respiratory failure who have a hemoglobin concentration less than 5 g/dL should receive a packed RBC (pRBC) transfusion. (Ungraded GPS, 96% agreement).
Remark: This statement may not be appropriate for patients with hemolytic anemia.
Clinical recommendation 5.6.2. We suggest against transfusing pRBCs in critically ill patients if the hemoglobin concentration is greater than or equal to 7 g/dL and they are hemodynamically stable and do not have chronic cyanotic condition, severe PARDS or hemolytic anemia. (Conditional CR, low certainty of evidence, 98% agreement).
Research statement 5.6.3. We cannot make a recommendation regarding optimal pRBC transfusion threshold in critically ill patients with PARDS who are hemodynamically unstable or have severe hypoxemia. Further studies are needed to determine the risks, benefits and alternatives of transfusion in PARDS patients with severe hypoxemia. (Ungraded RS, 98% agreement).
Sleep and Rehabilitation
Good practice statement 5.7.1. In patients with PARDS, day-night activity and rest patterns should be optimized with nonpharmacologic, multicomponent approaches. (Ungraded GPS, 94% agreement).
Good practice statement 5.7.2. In patients with PARDS, daily assessment and determination of activity and mobility goals based on clinical status should be made. (Ungraded GPS, 94% agreement).
Good practice statement 5.7.3. In patients with PARDS, a rehabilitation team (physiotherapy and/or occupational therapy) evaluation within 72 hours should be established for determination of baseline function, rehabilitation goals and readiness for intervention as appropriate and based on clinical status. (Ungraded GPS, 98% agreement).
Section 6. Monitoring (19)
General Monitoring
Good practice statement 6.1.1. All patients with PARDS should receive the minimum clinical monitoring of continuous respiratory frequency, heart rate, pulse oximetry, and regular intermittent noninvasive blood pressure. (Ungraded GPS, 90% agreement).
Remarks: Pulse oximetry alarms should be set to identify parameters outside of PALICC-2 recommendations.
Good practice statement 6.1.2. Metrics using lung volumes (e.g., tidal volume, compliance of the respiratory system) should be interpreted after standardization to body weight. (Ungraded GPS, 94% agreement).
Remarks: The lesser of predicted body weight or actual body weight should be used.
Respiratory System Mechanics
Good practice statement 6.2.1. During invasive ventilation in patients with PARDS, the tidal volume should be continuously monitored. (Ungraded GPS, 96% agreement).
Good practice statement 6.2.2. Tidal volumes measured at the ventilator should be adjusted using compensation for circuit compliance, either by the ventilator or manually. (Ungraded GPS, 96% agreement).
Remarks: In infants and smaller children, monitoring the exhaled tidal volumes at the end of the endotracheal tube should be considered, with caution for additive dead space due to the flow sensor.
Good practice statement 6.2.3. Ventilatory inspiratory pressures including plateau pressure and driving pressure should be monitored in patients with PARDS. (Ungraded GPS, 90% agreement).
Remarks: Plateau pressure measurement should be made under static or quasi-static conditions.
Good practice statement 6.2.4. Flow-time, and pressure-time curves and intrinsic PEEP should be monitored to assess the accuracy of respiratory timings, including detection of expiratory flow limitation and patient-ventilator asynchrony. (Ungraded GPS, 96% agreement).
Good practice statement 6.2.5. Patient effort of breathing should be monitored, at least through clinical assessment. (Ungraded GPS, 96% agreement).
Remark: other more objective methods to assess patient effort might be appropriate when available.
Research statement 6.2.6. We cannot make a recommendation on routine monitoring of the following parameters of respiratory system mechanics: Flow-volume loop, pressure-volume loop, dynamic compliance and resistance, strain, stress index, esophageal manometry and transpulmonary pressure, functional residual capacity, ventilation index, mechanical power, mechanical energy, electrical activity of diaphragm, or thoraco-abdominal asynchrony quantification by respiratory inductance plethysmography. Future research should focus on specific populations likely to benefit from routine monitoring of these parameters. (Ungraded RS, 90% agreement).
Remarks: In some subgroups of patients, monitoring these metrics may help individualize MV management.
Oxygenation Parameters, Severity Scoring, and co 2 Monitoring
Good practice statement 6.3.1. Monitoring of Fio2, Spo2, and/or Pao2, mean airway pressure, and PEEP should be used to diagnose PARDS, to assess PARDS severity, and to guide the management of oxygenation failure. (Ungraded GPS, 96% agreement).
Good practice statement 6.3.2. Blood pH and Paco2 measurement frequency should be adjusted according to PARDS severity, noninvasive monitoring data, and stage of the disease. (Ungraded GPS, 92% agreement).
Good practice statement 6.3.3. Continuous monitoring of co2 should be used in patients with PARDS during invasive MV to assess adequacy of ventilation. (Ungraded GPS, 94% agreement).
Remarks: End-tidal co2/time curves or volumetric capnography should be used in patients with invasive conventional ventilation. Transcutaneous co2 measurements should be used in patients with nonconventional ventilation therapies such as HFOV.
Good practice statement 6.3.4. Dead space should be calculated and monitored in patients with PARDS when Paco2 and either end-tidal co2 pressure or mixed-expired co2 pressure are available during invasive MV. (Ungraded GPS, 94% agreement).
Specific Weaning Considerations
Good practice statement 6.4.1. Daily assessment of predefined clinical and physiologic criteria of extubation readiness should be performed to avoid unnecessary prolonged ventilation. In patients meeting extubation readiness criteria, a spontaneous breathing trial should be performed to test extubation readiness. (Ungraded GPS, 98% agreement).
Research statement 6.4.2. Spontaneous breathing trials and extubation readiness tests should be standardized when used in clinical research. (Ungraded RS, 98% agreement).
Lung Imaging
Good practice statement 6.5.1. Chest imaging is necessary for the diagnosis of PARDS, to detect complications such as air leak or equipment displacement, and to assess severity. (Ungraded GPS, 90% agreement).
Remarks: Frequency of chest imaging should be predicated on patient clinical condition and availability.
Research statement 6.5.2. We cannot make a recommendation on the routine use of chest CT scan, lung ultrasonography, and electrical impedance tomography. Future research should focus on specific populations likely to benefit from routine use of these imaging modalities. (Ungraded RS, 94% agreement).
Hemodynamic Monitoring
Good practice statement 6.6.1. All patients with PARDS should receive hemodynamic monitoring to evaluate the impact of ventilation and disease on right and left cardiac function and to assess oxygen delivery. (Ungraded GPS, 92% agreement).
Good practice statement 6.6.2. Cumulative fluid balance should be monitored in patients with PARDS. (Ungraded GPS, 98% agreement).
Good practice statement 6.6.3. In patients with suspected cardiac dysfunction or severe PARDS, echocardiography should be performed when feasible for noninvasive evaluation of both left and right ventricular function, the preload status, and pulmonary arterial pressures. (Ungraded GPS, 94% agreement).
Remark: Frequency of assessment should be based on hemodynamic status.
Good practice statement 6.6.4. An arterial catheter should be considered in patients with severe PARDS for continuous monitoring of arterial blood pressure and arterial blood gas analysis. (Ungraded GPS, 92% agreement).
Research statement 6.6.5. We cannot make a recommendation on when to use the following hemodynamic monitoring devices: pulse contour with transpulmonary dilution technology, pulmonary artery catheters, alternative devices to monitor cardiac output (ultrasonic cardiac output monitoring, transesophageal aortic Doppler, noninvasive monitoring of cardiac output based on changes in respiratory co2 concentration caused by a brief period of rebreathing, indirect calorimetry Fick cardiac output), central venous pressure monitoring, and B-type natriuretic peptide measurements. Future research should focus on patient populations most likely to benefit from these monitoring modalities. (Ungraded RS, 100% agreement).
Section 7. Noninvasive Respiratory Support (20)
Indication for Noninvasive Ventilation
Clinical recommendation 7.1.1. We suggest that in patients with possible PARDS or at risk for PARDS on conventional oxygen therapy or HFNC who are showing signs of worsening respiratory failure, a time-limited trial of NIV (CPAP or BiPAP) should be used if there are no clear indications for intubation. (Conditional CR, very low certainty of evidence, 88% agreement).
Clinical recommendation 7.1.2. In patients on NIV who do not show clinical improvement within the first 6 hours of treatment or have signs and symptoms of worsening disease including increased respiratory/heart rate, increased work of breathing, and worsening gas exchange (Spo2/Fio2 ratio), we suggest that intubation be used in comparison to continuing NIV. (Conditional CR, very low certainty of evidence, 94% agreement).
Remark 1: This recommendation also includes patients who are at greater risk of complications from IMV (such as children with immunodeficiency).
Remark 2: We suggest that intubation be used earlier, in comparison to NIV, in patients with severe NIV PARDS or with other severe organ dysfunction.
Team and Environment
Good practice statement 7.2. NIV should be delivered in a setting with trained experienced staff and where close monitoring is available to rapidly identify and treat deterioration. (Ungraded GPS, 96% agreement).
Noninvasive Ventilation Management
Good practice statement 7.3.1. When delivering NIV, the provider should choose the interface that provides the most efficient patient-ventilator synchronization for the child. (Ungraded GPS, 100% agreement).
Good practice statement 7.3.2. Patients receiving NIV should be closely monitored for potential problems such as skin breakdown, gastric distention, barotrauma, and conjunctivitis. (Ungraded GPS, 98% agreement).
Good practice statement 7.3.3. Heated humidification should be used for NIV and HFNC in patients at risk, or with possible or confirmed PARDS. (Ungraded GPS, 96% agreement).
Good practice statement 7.3.4. For patients with poor tolerance to NIV, sedation can be used to improve tolerance. (Ungraded GPS, 92% agreement).
Remarks: The level of sedation should be adjusted to ensure adequate ventilatory drive and airway protective reflexes.
Good practice statement 7.3.5. When patients with PARDS are managed on NIV, inspiratory pressure augmentation with pressure support should be used to reduce inspiratory muscle effort. CPAP alone may be suitable for those patients who are unable to attain patient-ventilator synchrony or when using nasal interface. (Ungraded GPS, 96% agreement).
High-Flow Nasal Cannula
Research statement 7.4. We cannot make a recommendation on when to use HFNC in patients at risk of or with possible PARDS. Further studies are needed to identify clinical indications for HFNC in patients at risk or with possible PARDS. (Ungraded RS, 94% agreement).
Noninvasive Respiratory Support in Resource-Limited Settings
Clinical recommendation 7.5.1. In RLS, we suggest the use of CPAP or HFNC over standard oxygenation therapy in patients at risk for PARDS. (Conditional CR, very low certainty of evidence, 92% agreement).
Remark: The use of CPAP should be conducted under physician oversight.
Clinical recommendation 7.5.2. We suggest the use of CPAP over HFNC in RLS in patients with possible PARDS. (Conditional CR, very low certainty of evidence, 83% agreement).
Remarks: The use of CPAP should be conducted under physician oversight. Depending on availability of equipment or physician oversight, HFNC should still be preferred over standard oxygenation therapy.
Section 8. Extracorporeal Support (21)
Indications for Extracorporeal Support in PARDS
Clinical recommendation 8.1.1. We suggest that patients with a potentially reversible cause of severe PARDS should be evaluated for extracorporeal membrane oxygenation (ECMO) when lung protective strategies result in inadequate gas exchange. (Conditional CR, very low certainty of evidence, 96% agreement).
Remarks: There is no evidence to support strict criteria for the selection of patients who will benefit from ECMO in PARDS.
Good practice statement 8.1.2. Decisions to institute ECMO should be based on a structured evaluation of case history and clinical status by an established expert team. (Ungraded GPS, 94% agreement).
Clinical recommendation 8.1.3. We suggest that serial evaluation compared with a single time point of assessment be used to guide decisions about ECMO eligibility. (Conditional CR, low certainty of evidence, 98% agreement).
Clinical recommendation 8.1.4. We suggest the use of venovenous ECMO over venoarterial ECMO in patients with PARDS who have adequate cardiac function. (Conditional CR, low certainty of evidence, 94% agreement).
Good practice statement 8.1.5. Transfer to an ECMO center should be considered in patients with PARDS who are failing to stabilize with optimal non-ECMO therapies. (Ungraded GPS, 96% agreement).
Team Training and Organization
Policy statement 8.2.1. All personnel directly caring for the patient should understand the ECMO circuit and the physiologic interactions between it and the patient. Competencies for clinicians with primary patient care duties and ECMO specialists should be required. Simulation may be useful in training. (Ungraded PS, 94% agreement).
Policy statement 8.2.2. Centers providing ECMO support for PARDS should report all ECMO data to Extracorporeal Life Support Organization or a similar organization in order to benchmark outcomes of mortality and complications. (Ungraded PS, 94% agreement).
Management During ECMO
Clinical recommendation 8.3.1a. We suggest maintaining normal Pao2 compared with hyperoxia in patients with PARDS supported on ECMO. (Conditional CR, very low certainty of evidence, 96% agreement).
Clinical recommendation 8.3.1b. We suggest slow decrease in Paco2 compared with rapid decrease of Paco2 in patients with PARDS supported on ECMO, especially in the setting of hypercapnia. (Conditional CR, very low certainty of evidence, 88% agreement).
Clinical recommendation 8.3.2. In patients with PARDS supported by ECMO, we suggest mechanical ventilator pressures comply with the lung protective limits previously identified, compared with exceeding these limits, to avoid additional lung injury. (Conditional CR, low certainty of evidence, 98% agreement).
Extracorporeal Carbon Dioxide Removal
Research statement 8.4. We cannot make a recommendation on when to use extracorporeal carbon dioxide removal (ECco2R) technology in patients with PARDS. Further studies are needed to identify clinical indications for ECco2R in patients with PARDS. (Ungraded RS, 92% agreement).
Follow-Up After ECMO
Good practice statement 8.5. All pediatric ECMO survivors should receive short- and long-term neurodevelopmental and physical functioning evaluations to assess for impairment. (Ungraded GPS, 90% agreement).
Section 9. Morbidity and Long-Term Outcomes (22)
Approach to Clinical Outcome Assessment
Good practice statement 9.1.1. In patients with PARDS, the patient’s primary care providers should be advised to screen for post-ICU morbidities within 3 months of discharge from the hospital. (Ungraded GPS, 90% agreement).
Good practice statement 9.1.2. A stepwise approach to clinical evaluation of post-ICU morbidities should be used with initial screening by a primary care provider or electronic/telephonic screen. (Ungraded GPS, 96% agreement).
Remark: Full assessment, initial management, and serial re-evaluations of impairments should be made by a primary care provider if appropriate and referral to a specialist if deficits persist or are not in the scope of practice of the primary care provider. Location-specific resources should be considered, including availability of dedicated post-ICU follow-up clinics or remote consultation.
Long-Term Pulmonary Function in Patients Who Survive PARDS
Good practice statement 9.2.1. Patients with PARDS should be screened for pulmonary function abnormalities within the first three months after hospital discharge. (Ungraded GPS, 90% agreement).
Remarks: The screening should include a minimum of a respiratory symptom questionnaire, a respiratory examination, and pulse oximetry.
Good practice statement 9.2.2. Patients with PARDS who are of sufficient developmental age and capabilities should also be assessed by spirometry to screen for pulmonary function abnormalities within the first three months after discharge. (Ungraded GPS, 94% agreement).
Remarks: A follow-up assessment within the first year should be added if spirometry is abnormal.
Good practice statement 9.2.3. Patients should be referred to a specialist (pediatrician or pediatric pulmonologist) when deficits in pulmonary function are identified for further assessment, treatment, and long-term pulmonary follow-up. (Ungraded GPS, 94% agreement).
Nonpulmonary Outcomes of Patients Who Survive PARDS
Good practice statement 9.3.1. For patients who survive PARDS, health-related quality of life, physical, neurocognitive, emotional, family, and social function should be evaluated within three months of hospital discharge. (Ungraded GPS, 100% agreement).
Good practice statement 9.3.2. For infants and toddlers who survive PARDS, additional evaluation of health-related quality of life, physical, neurocognitive, emotional, family, and social function should be performed prior to entering school, for example, at 4–6 years old. (Ungraded GPS, 90% agreement).
Good practice statement 9.3.3. Patients with identified abnormalities should be treated or referred for more in-depth assessment and treatment by appropriate subspecialists and educators. (Ungraded GPS, 98% agreement).
PARDS Outcomes Research
Research statement 9.4.1. When feasible, pre-ICU baseline status for each outcome measure should be established or estimated when evaluation of post-ICU morbidity is anticipated. (Ungraded RS, 98% agreement).
Research statement 9.4.2. We cannot make a recommendation regarding the use of alternative postdischarge endpoints. Given declining mortality among patients with PARDS, additional studies are needed to evaluate potential alternative postdischarge endpoints for clinical trials. (Ungraded RS, 96% agreement).
Remarks: Potential postdischarge endpoints to evaluate may include hospital and PICU readmissions (e.g., within 30 d of discharge), unplanned health resource use, health-related quality of life, and physical, pulmonary, neurocognitive, emotional, family, and social function.
Research statement 9.4.3. Clinical studies should be designed to evaluate the association between short-term outcomes (e.g., new or progressive organ dysfunction) and longer-term postdischarge outcomes for patients with PARDS. (Ungraded RS, 100% agreement).
Research statement 9.4.4. Further studies are needed to determine factors that may affect the trajectory of recovery following PARDS. (Ungraded RS, 100% agreement).
Remarks: Additional research should include demographic characteristics, clinical factors, PICU exposures, social determinants of health, and access to care.
Research statement 9.4.5. Practices to optimize follow-up (e.g., incentives, multimodal evaluations) should be used to minimize bias due to differential loss-to-follow-up when conducting post-ICU outcomes research. (Ungraded RS, 96% agreement).
Section 10. Clinical Informatics and Data Science (23)
Clinical recommendation 10.1. We suggest that clinicians implement electronic tools to automatically screen critically ill patients to help identify those with PARDS or at significant risk of developing PARDS compared with nonelectronic screening or no standardized screening. (Conditional CR, very low certainty of evidence, 96% agreement).
Clinical recommendation 10.2. We suggest automatic monitoring of compliance with PALICC-2 clinical practice guidelines for lung protective strategies, compared with no monitoring of compliance. (Conditional CR, low certainty of evidence, 96% agreement).
Remarks: Automatic monitoring should incorporate measures of gas exchange and MV and provide feedback to clinicians in real-time with user-friendly interfaces.
Policy statement 10.3. Healthcare organizations should provide human and material resources to help clinicians develop, implement, and use electronic tools to improve the management of patients with PARDS or at significant risk of developing PARDS. (Ungraded PS, 96%).
Research statement 10.4. Collaborative networks should be developed to share clinical data and develop, test, and implement electronic tools to improve the diagnosis, management, monitoring, and prognosis of patients with PARDS. (Ungraded RS, 100% agreement).
Research statement 10.5. Electronic tools should be developed to improve the management of PARDS and designed to maximize generalizability, reproducibility, and dissemination. (Ungraded RS, 100% agreement).
Section 11. Developing Regions and Resource-Limited Settings (24)
Good practice statement 11.1. Healthcare providers working in RLS should be mindful of precipitating factors for PARDS (including dengue, malaria, measles, scrub typhus, leptospirosis) and concurrent comorbidities (e.g., HIV and associated opportunistic infections, malnutrition, chronic anemia) that are not commonly encountered in high-income countries. (Ungraded GPS, 100% agreement).
Definition statement 11.2. In RLS where all the criteria for PARDS cannot be fulfilled, the term “Possible PARDS” should be used when the patient has history and physical examination findings consistent with known precipitating factors and clinical features of PARDS. (Ungraded DS, 92% agreement).
Remarks: In these settings, the use of Spo2/Fio2 or OSI (as per PALICC-2 PARDS cut-offs) is appropriate and may be preferred over Pao2/Fio2 or OI.
Remarks: Imaging is recommended to diagnose possible PARDS and can include ultrasound or chest radiograph demonstrating parenchymal disease; however, there is no absolute need for imaging criteria to diagnose “Possible PARDS” if timing, oxygenation and risk factor criteria are present.
Research statement 11.3. Future studies conducted in RLS should apply the PALICC-2 definition to identify patients with possible PARDS or PARDS. Future studies are needed to determine the sensitivity and specificity, positive predictive value, negative predictive value, and accuracy of the PALICC-2 definition for possible PARDS or PARDS in RLS. (Ungraded RS, 96% agreement).
Research statement 11.4. Collaborative networks should engage and include sites in RLS to allow sharing of clinical data and research results related to respiratory support in PARDS. (Ungraded RS, 98% agreement).
Policy statement 11.5. All hospitals in RLS should implement locally adapted protocols for starting, maintenance, and weaning of MV and for extubation of patients with PARDS. (Ungraded PS, 94% agreement).
Policy statement 11.6. All members of the multidisciplinary team involved in the care of a patient with PARDS should be provided with regular MV training and education. (Ungraded PS, 100% agreement).
Policy statement 11.7. While using pulmonary and nonpulmonary ancillary therapies in RLS, current overall evidence (as provided by PALICC-2) as well as availability and costs of these therapies locally, should be taken into consideration. (Ungraded PS, 98% agreement).
Research statement 11.8. More research should be conducted regarding the use of NIV in patients with respiratory failure including PARDS in RLS. (Ungraded RS, 100% agreement).
Research statement 11.9. Further research is needed in RLS to determine the optimal use, applications, safety and efficacy of nonpulmonary ancillary therapies and monitoring in patients with PARDS. (Ungraded RS, 100% agreement).
Remarks: Ancillary therapies that are easily adopted within RLS and not prohibited by cost should be considered a priority for research.
Policy statement 11.10. For long-term outcomes of PARDS patients in RLS, alignment with PALICC-2 recommendations should be kept wherever possible and according to available resources. (Ungraded PS, 98% agreement).
Research statement 11.11. Research in RLS is warranted to inform development of relevant recommendations pertaining to PARDS that are appropriate to these settings. (Ungraded RS, 98% agreement).
DISCUSSION
These guidelines are the result of a large international panel’s review of the existing evidence and our interpretation of how that evidence should be applied in clinical practice. As highlighted in the recommendations and statements, the risk-benefit profile for many therapies change as a function of PARDS severity, although lack of evidence in most areas prevent clear cut points to delineate when a specific therapy should or should not be implemented. A framework highlighting several key PARDS therapies or management strategies with assessment of the balance of risk and benefits in most versus select patients with PARDS is presented in Figure 1.
Figure 1.
Schematic summary of key therapies or management strategies in pediatric acute respiratory distress syndrome based on the Second Pediatric Acute Lung Injury Consensus Conference recommendations and statements. *Continuous positive airway pressure if unable to tolerate bilevel noninvasive ventilation (NIV). ECMO = extracorporeal membrane oxygenation, HFNC = high-flow nasal cannula, HFOV = high-frequency oscillatory ventilation, PEEP = positive end-expiratory pressure, SFo2 ratio = oxygen saturation/Fio2 ratio, Spo2 = pulse oximeter oxygen saturation, Vt = tidal volume.
While most recommendations align with PALICC, the PALICC-2 process systematically considered new evidence, and applied an EtD framework for all recommendations, seeking to ensure the language of recommendations was unambiguous. There are several changes to the definition of PARDS. These include a delayed marker of oxygenation severity (at least 4 hr after initial diagnosis) to improve risk stratification. PARDS severity groups have been simplified to classify patients into one of four severity groupings based on ventilation type (invasive vs noninvasive) and oxygenation severity (mild/moderate vs severe). The definition also creates a “possible PARDS” category, to capture the increasing use of nasal modes of respiratory support such as HFNC and to be able to better capture patients who likely have PARDS in RLS. Full rationale for these changes are found in the corresponding justification manuscript for Section 1 (14).
Other substantial changes with PALICC-2 include two new sections focused on informatics and data science (23), as well as implementation in RLS (24). The informatics section provides specific recommendations on systems to screen for PARDS and monitor compliance with lung protective ventilation recommendations. The RLS section highlights how to adapt PALICC-2 recommendations for defining and treating PARDS in circumstances where availability of diagnostic testing, equipment, or training may be limited. New concepts are addressed in ventilatory management and monitoring related to mechanical power, driving pressure, and patient-self-inflicted lung injury (16). Heterogeneity of treatment effect and the importance of precision medicine approaches, which embrace biological phenotypes has also been an area of focus, with growing pediatric data that are described in section 2 (15).
In summary, the development of PALICC-2 recommendations was based on a rigorous and systematic assessment of available evidence, supported by transparent methodology using GRADE and the EtD framework. Overall, high-quality randomized studies are lacking in PARDS, making all but one of our clinical recommendations conditional. Nevertheless, we believe these clinical practice guidelines will be valuable at the bedside, as we took into consideration feasibility, safety, equity, and implementation for all recommendations. Detailed considerations for all of these recommendations can be found in the supplemental articles (13–24). These guidelines have also identified many gaps that offer opportunities for the future PARDS research agenda. Many important questions remain to be answered to improve the certainty of evidence and strength of recommendations for patients with PARDS.
ACKNOWLEDGMENTS
We thank Katie Lobner (Johns Hopkins University), Lynn Kysh (University of South California), Alix Pincivy and Philippe Dodin (Université de Montréal) for assisting with literature searches. We also thank Justin Hotz for technical support and Ariana Lane for administrative assistance.
Supplementary Material
Footnotes
The views presented are those of the author and do not represent the views of the Society of Critical Care Medicine organization.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Dr. Barbaro is currently receiving grant support (R01 HL153519; R01 HD015434) from the National Institutes of Health (NIH); he is currently Chair of the Extracorporeal Life Support Organization Registry. Dr. Bembea receives research funding to her institution from the NIH/National Institute of Neurological Disorders and Stroke (R01NS106292) and the Grifols Investigator Sponsored Research Grant. She serves as Chair of the Scientific Committee of the Pediatric Acute Lung Injury and Sepsis Investigators Research Network. Dr. Cheifetz is a medical consultant for Phillips and Medtronic. His institution receives research grant funding from the NIH. Dr. Cruces received funding from the Chilean Ministry of Sciences (Fondecyt 1220322). Dr. Curley received funding from the NIH (UH3HL141736, R01HD098269, R01HL149910, R01HL153519, R01HD104618). Dr. Dahmer received funding from the NIH (National Institute of Child Health and Human Development [NICHD], R21 HD097387; National Heart, Lung and Blood Institute [NHLBI] R01 HL149910). Dr. Dalton received funding from the Department of Defense (No. 13363072). She is a consultant for Innovative Extracorporeal Membrane Oxygenation Concepts, Hemocue, Entegrion, Medtronic, and advisory board member for Abiomed. Dr. Emeriaud’s research program is supported by the Fonds de Recherche du Québec-Santé and the Quebec Respiratory Health Network. Dr. Emeriaud is leading a study, which is financially supported by Maquet. Dr. Jouvet’s research program and salary is supported by the Fonds de Recherche du Québec-Santé and the Quebec Respiratory Health Network. Dr. Jouvet is leading studies, which is financially supported by VitalTracer, Dymedso and public financial agencies (Canadian Foundation for innovation, Institut TransMedTech, Quebec Ministry of Health, Sainte-Justine Hospital). Dr. Killien received funding from the NIH (NICHD K23HD100566). Dr. Kneyber received research funding from the NIH/NICHD (UG3 HL141736-01/U24 HL141723-01) and ZorgOnderzoek Nederland and the area Medical (848041002), Stichting Vrienden Beatrix Kinderziekenhuis, Fonds NutsOhra, University Medical Center Groningen, VU University Medical Center, and the Royal Academy of Dutch Sciences (TerMeulen stipend). Dr. Kneyber’s research program is technically supported by Vyaire, Applied Biosignals, and Timpl. Dr. Kneyber received honoraria from Vyaire. Dr. Kneyber serves as consultant for Metran and served as consultant for Vyaire. Dr. Kudchadkar received funding to her institution from the NIH/NICHD (R01HD103811 & R21HD093369) and the Donaghue Foundation. Dr. López-Fernández is funded by an academic grant from the Instituto de Salud Carlos III, Madrid, Spain (PI19/00141). Dr. Maddux received funding to her institution from the NIH/NICHD (K23HD096018). Dr. Morrow has received honoraria for Continuing Medical Education presentations from EduPro Health. Her research is part-funded by the National Research Foundation of South Africa, through the Incentive Funding for Rated Researchers program. Dr. Nadkarni receives unrestricted research grants to his institution from the NIH, U.S. Department of Defense, Agency for Healthcare Research and Quality, Laerdal Foundation, RQI Partners, Zoll Medical, Defibtech, HeartHero, and Nihon-Kohden. Dr. Nadkarni is an elected member of the Executive Committee (Council) of the Society of Critical Care Medicine. Dr. Napolitano research and consulting relationships with: Drager, Timpel, VERO-Biotech, Actuated Medical, and Philips/Respironics. Dr. Pons has been on the speaker’s bureau of Philips, ResMed and Fisher & Paykel; Hospital Sant Joan de Déu has received disposable material from these companies. Dr. Randolph receives funding from the Centers for Disease Control and Prevention, NIH (National Institute of Allergy and Infectious Diseases AI154470). Dr. Rowan receives funding from the NHLBI (NHLBI K23HL150244). Dr. Sanchez-Pinto received funding from the NIH (NICHD R01 HD105939). Dr. Sauthier research program and salary is supported by the Fonds de Recherche du Québec-Santé. Dr. Takeuchi receives funding from Japan Society for the Promotion of Science grant (KAKENHI 21K09063). Dr. Tse receives research funding to her institution from the Canadian Institute of Health Research and salary support from the Fonds de Recherche du Québec—Santé. Dr. Watson receives research funding to his institution from the NIH. Dr. Zimmerman received research funding from NIH and Biomedical Advanced Research and Development Authority; royalties from Elsevier Publishing. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This Executive Summary has been endorsed by The World Federation of Pediatric Intensive & Critical Care Societies.
The Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) group members are listed in Appendix 1 (http://links.lww.com/PCC/C287).
Contributor Information
Collaborators: Guillaume Emeriaud, Yolanda M. López-Fernández, Robinder G. Khemani, Narayan Prabhu Iyer, Melania Bembea, Steven Kwasi Korang, Katherine M. Steffen, Nadir Yehya, Lincoln Smith, Neal J. Thomas, Jerry J. Zimmerman, Simon J. Erickson, Steven L. Shein, Jocelyn R. Grunwell, Mary K. Dahmer, Anil Sapru, Michael W. Quasney, Heidi R. Flori, Analia Fernandez, Vicent Modesto i Alapont, Peter Rimensberger, Ira Cheifetz, Courtney Rowan, Adrienne G. Randolph, Martin Kneyber, Stacey Valentine, Sapna Kudchadkar, Shan Ward, Vinay Nadkarni, Martha A.Q. Curley, Anoopindar Bhalla, Florent Baudin, Muneyuki Takeuchi, Pablo Cruces, Christopher L. Carroll, Natalie Napolitano, Marti Pons-Odena, Sandrine Essouri, Jérome Rambaud, Ryan Barbaro, Duncan Macrae, Heidi Dalton, Elizabeth Killien, Aline Maddux, Sze Man Tse, Scott Watson, L. Nelson Sanchez-Pinto, Michaël Sauthier, Prakadeshwari Rajapreyar, Philippe Jouvet, Christopher Newth, Brenda Morrow, Asya Agulnik, Werther Brunow de Carvalho, Mohamod Chisti, Jan Hau Lee, Katie Lobner, Lynn Kysh, Alix Pincivy, and Philippe Dodin
REFERENCES
- 1.Force ADT, Ranieri VM, Rubenfeld GD, et al. : Acute respiratory distress syndrome: The Berlin definition. JAMA 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 2.Pediatric Acute Lung Injury Consensus Conference Group: Pediatric acute respiratory distress syndrome. Pediatr Crit Care Med 2015; 16:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quasney MW, Lopez-Fernandez YM, Santschi M, et al. : The outcomes of children with pediatric acute respiratory distress syndrome: Proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2015; 16:S118–S131 [DOI] [PubMed] [Google Scholar]
- 4.Thomas NJ, Jouvet P, Willson D: Acute lung injury in children-kids really aren´t just little adults. Pediatr Crit Care Med 2013; 14:429–432 [DOI] [PubMed] [Google Scholar]
- 5.Khemani RG, Smith LS, Zimmerman JJ, et al. : Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2015; 16:S23–S40 [DOI] [PubMed] [Google Scholar]
- 6.Khemani RG, Smith L, Lopez-Fernandez YM, et al. ; Pediatric Acute Respiratory Distress syndrome Incidence and Epidemiology (PARDIE) Investigators: Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): An international, observational study. Lancet Respir Med 2019; 7:115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amato MB, Meade MO, Slutsky AS, et al. : Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372:747–755 [DOI] [PubMed] [Google Scholar]
- 8.Brochard L, Slutsky A, Pesenti A: Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 2017; 195:438–442 [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Rehder KJ, Williford L, et al. : Use of high flow nasal cannula in critically ill infants, children, and adults: A critical review of the literature. Intensive Care Med 2013; 39:247–257 [DOI] [PubMed] [Google Scholar]
- 10.Rowan CM, Klein MJ, Hsing DD, et al. : Early use of adjunctive therapies for pediatric acute respiratory distress syndrome: A PARDIE study. Am J Respir Crit Care Med 2020; 201:1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khemani RG, Parvathaneni K, Yehya N, et al. : PEEP lower than the ARDS network protocol is associated with higher pediatric ARDS mortality. Am J Respir Crit Care Med 2018; 26:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhalla AK, Klein MJ, Emeriaud G, et al. ; Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology (PARDIE) V.2. Investigators and Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Adherence to lung-protective ventilation principles in pediatric acute respiratory distress syndrome: A pediatric acute respiratory distress syndrome incidence and epidemiology study. Crit Care Med 2021; 49:1779–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer P, Khemani R, Emeriaud G, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury And Sepsis Investigators (PALISI) Network: Methodology of the second pediatric acute lung injury consensus conference. Pediatr Crit CareMed 2023; 24 (Suppl 1):S76–S86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yehya N, Smith L, Thomas NJ, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Definition, incidence, and epidemiology of pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S87–S98 [DOI] [PubMed] [Google Scholar]
- 15.Grunwell JR, Dahmer MK, Sapru A, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Pathobiology, severity, and risk stratification of pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S12–S27 [DOI] [PubMed] [Google Scholar]
- 16.Fernández A, Modesto V, Rimensberger PC, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Invasive ventilatory support in patients with pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S61–S75 [DOI] [PubMed] [Google Scholar]
- 17.Rowan CM, Randolph AG, Iyer NP, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Pulmonary specific ancillary treatment for pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S99–S111 [DOI] [PubMed] [Google Scholar]
- 18.Valentine S, Kudchadkar S, Ward S, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Nonpulmonary treatments for pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S45–S60 [DOI] [PubMed] [Google Scholar]
- 19.Bhalla A, Baudin F, Takeuchi M, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Monitoring in pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S112–S123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll CL, Napolitano N, Pons-Òdena M, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Noninvasive respiratory support for pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S135–S147 [DOI] [PubMed] [Google Scholar]
- 21.Rambaud J, Barbaro R, Macrae DJ, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Extracorporeal membrane oxygenation in pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S124–S134 [DOI] [PubMed] [Google Scholar]
- 22.Killien EK, Tse SM, Watson S, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Long-term outcomes of children with pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S28–S44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Pinto, Sauthier M, Rajapreyar P, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Leveraging clinical informatics and data science to improve care and facilitate research in pediatric acute respiratory distress syndrome: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S1–S11 [DOI] [PubMed] [Google Scholar]
- 24.Morrow B, Agulnik A, Brunow de Carvahlo W, et al. ; Second Pediatric Acute Lung Injury Consensus Conference (PALICC-2) of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Diagnostic, management, and research considerations for pediatric acute respiratory distress syndrome in resource limited settings: From the second pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2023; 24 (Suppl 1):S148–S159 [DOI] [PubMed] [Google Scholar]
- 25.Sterne JAC, Savović J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 26.Sterne JAC, Hernán MA, Reeves BC, et al. : ROBINS-I: A tool for assesing risk of bias in non-randomized studies of interventions. BMJ 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt G, Oxman AD, Akl EA, et al. : GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64:383–394 [DOI] [PubMed] [Google Scholar]
- 29.Schunemann HJ, Brozek J, Guyatt G, Oxman AD. (Eds): The GRADE Working Group: GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. 2013. Available at: https://gdt.gradepro.org/app/handbook/handbook.html. Accessed December 20, 2022
- 30.Guyatt G, Oxman AD, Sultan S, et al. : GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013; 66:151–157 [DOI] [PubMed] [Google Scholar]
- 31.Alonso-Coello P, Oxman AD, Moberg J, et al. ; GRADE Working Group: GRADE evidence to decision (EtD) frameworks: A systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ 2016; 353:i2089. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Alonso-Coello P, Schünemann HJ, et al. : Guideline panels should seldom make good practice statements: Guidance from the GRADE working group. J Clin Epidemiol 2016; 80:3–7 [DOI] [PubMed] [Google Scholar]
- 33.Brower RG, Lanken PN, MacIntyre N, et al. ; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. National Heart, Lung, and Blood Institute ARDS Clinical TrialsNetwork: Higher versus lower positive end-expiratory pressures inpatients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–336 [DOI] [PubMed] [Google Scholar]