OBJECTIVES:
Balancing the risks of hypotension and vasopressor-associated adverse effects is a daily challenge in ICUs. We conducted a systematic review with meta-analysis to examine the effect of lower versus higher exposure to vasopressor therapy on mortality among adult ICU patients with vasodilatory hypotension.
DATA SOURCES:
We searched Ovid Medline, Embase, and the Cochrane Central Register of Controlled Trials for studies published from inception to October 15, 2021.
STUDY SELECTION:
We included randomized controlled trials of lower versus higher exposure to vasopressor therapy in adult ICU patients with vasodilatory hypotension without language or publication status limits.
DATA EXTRACTION:
The primary outcome was 90-day all-cause mortality, with seven prespecified subgroups. Secondary outcomes included shorter- and longer-term mortality, use of life-sustaining therapies, vasopressor-related complications, neurologic outcome, and quality of life at longest reported follow-up. We conducted random-effects meta-analyses to calculate summary effect measures across individual studies (risk ratio [RR] for dichotomous variables, mean difference for continuous variables, both with 95% CIs). The certainty of the evidence was assessed using Grading of Recommendations, Assessment, Development, and Evaluation. We registered this review on the International Prospective Register of Systematic Reviews (CRD42021224434).
DATA SYNTHESIS:
Of 3,403 records retrieved, 68 full-text articles were reviewed and three eligible studies included. Lower exposure to vasopressors probably lowers 90-day mortality but this is based on moderate-certainty evidence, lowered for imprecision (RR, 0.94; 95% CI, 0.87–1.02). There was no credible subgroup effect. Lower vasopressor exposure may also decrease the risk of supraventricular arrhythmia (odds ratio, 0.55; 95% CI, 0.36–0.86; low certainty).
CONCLUSIONS:
In patients with vasodilatory hypotension who are started on vasopressors, moderate-certainty evidence from three randomized trials showed that lower vasopressor exposure probably lowers mortality. However, additional trial data are needed to reach an optimal information size to detect a clinically important 10% relative reduction in mortality with this approach.
Keywords: blood pressure, mean arterial pressure, meta-analysis, systematic review, vasopressors
KEY POINTS.
Question: In patients with a vasodilatory etiology of hypotension, is lower vasopressor exposure achieved through lower blood pressure targets, compared with higher vasopressor exposure achieved through higher blood pressure targets, associated with an effect on mortality?
Findings: Three eligible studies were included in this systematic review. Lower exposure to vasopressors probably lowers 90-day mortality but this is based on moderate-certainty evidence, lowered for imprecision.
Meaning: In patients with vasodilatory hypotension who are started on vasopressors, moderate-certainty evidence from three randomized trials showed that lower vasopressor exposure probably lowers mortality.
Balancing the risks of hypotension and vasopressor-associated adverse effects is a daily challenge in ICUs, and previous studies have found benefit to resuscitation strategies that do not specifically focus on normalizing physiologic targets (1). Accordingly, clinical trials have addressed whether tolerating lower blood pressure targets to reduce vasopressor exposure in hypotensive patients may improve survival. The results of these individual trials have been inconclusive, but a mediation analysis of two earlier trials suggested that restricting vasopressor use may lead to better survival via a lower risk of supraventricular arrhythmia (2). Existing clinical practice guidelines set targets for the titration of vasopressors according to blood pressure values. In the absence of clinical trial evidence, recommendations have varied over time but have always emphasized the need to avoid hypotension (3).
Our primary objective was to determine, in patients with a vasodilatory etiology of hypotension, whether lower vasopressor exposure achieved through lower blood pressure targets, compared with higher vasopressor exposure achieved through higher blood pressure targets, was associated with an effect on mortality. In light of the publication of the 65 trial (4, 5), we updated a previous systematic review and meta-analysis of clinical trials addressing this research question (6).
MATERIALS AND METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (7). This review was registered with the international prospective register of systematic reviews (PROSPERO, CRD42021224434).
Eligibility Criteria
We included randomized controlled trials (RCTs) of lower versus higher exposure to vasopressor therapy in adult ICU patient with vasodilatory hypotension (as defined by the investigators). Trials with greater than 20% of patients with nonvasodilatory hypotension were included if data restricted to the vasodilatory hypotension group were separately reported. Causes of vasodilatory hypotension include sepsis, analgesia and sedative infusions, liver failure, and nonsepsis causes of inflammation (e.g., sterile pancreatitis). Vasopressors included epinephrine, norepinephrine, phenylephrine, ephedrine, dopamine, vasopressin and vasopressin analogs, angiotensin II, and metaraminol.
Studies predominantly focused on cardiac chronotropes or inotropes (e.g., dobutamine, milrinone) as well as physiologic studies in which experimental protocols were brief (< 24 hr) were not included. We also excluded trials in which the evaluation of blood pressure targets was, by design, combined with other interventions for shock (such as a fluid administration protocol) or accomplished by nonlicensed vasopressors (e.g., nitric oxide synthase inhibitors). We did not restrict study eligibility on the basis of publication status or language of publication.
Information Sources and Search Strategy
A health sciences librarian (R.C.) searched Ovid Medline Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid Medline Daily and Ovid Medline (1946 to October 15, 2021), Embase (1974 to October 15, 2021), and the Cochrane Central Register of Controlled Trials (on October 15, 2021). Detailed search strategies for all databases appear in the Supplemental Digital Content (http://links.lww.com/CCM/H248). We screened reference lists of included studies and personal libraries for other citations.
Study Selection
Two of four reviewers (A.R.-B., M.H., F.M., S.T.) independently assessed study eligibility based on titles and abstracts and, subsequently, on the full-text reports of studies that had not been excluded. Any disagreements were resolved by a third-party adjudicator (N.K.J.A., F.L.). Automation tools were not used in the study selection process. We captured reasons for exclusion at the full-text stage.
Data Extraction
Two reviewers independently collected data using standardized data extraction forms designed in Microsoft Excel (Office 365, Redmond, WA). Data items collected included study characteristics, study design, patient population, interventions, outcomes, and risk of bias.
We collated data on all reported outcomes and considered the primary outcome to be all-cause mortality at 90 days or the closest time point if 90-day mortality was unavailable (in descending order of preference: 60 d, hospital discharge, 28 or 30 d, ICU discharge). Secondary outcomes included longer-term mortality (time frames of 6–12 mo; > 1 to 2 yr; > 2 to 5 yr); resuscitation interventions (e.g., fluids, blood products, early [≤ 90 d] and late [> 90 d] renal replacement therapy [RRT], duration of RRT, duration of mechanical ventilation; stage 3 acute kidney injury [defined by Kidney Disease Improving Global Outcomes (8)] or similar criteria); new-onset cardiac arrhythmia; digit or limb or skin ischemia; mesenteric ischemia; myocardial ischemia; gastrointestinal bleeding; vasopressor extravasation; and neurologic outcome and health-related quality of life at longest reported follow-up. The main data sources consisted of published reports but we also contacted the primary investigators of individual studies to request access to unpublished data.
Two reviewers independently assessed risk of bias using the Cochrane risk of bias 2.0 instrument (9). The instrument addresses the following domains: randomization process, deviations from intended interventions, missing outcomes data, measurement of the outcomes, and selection of the reported results. We performed a separate risk of bias assessment for each outcome of interest and classified the overall risk of bias for each outcome as low risk (all domains judged low risk of bias), some concerns (any domain judged as having some concerns, and no domain judged at high risk of bias), or high risk of bias (any domain deemed high risk of bias). In the case of missing or unclear data regarding study characteristics, risk of bias, or outcomes, we contacted the study investigators directly. Disagreements were resolved by discussion between two review authors with a third adjudicator, as needed.
Analyses
For all outcomes, we compared patients treated in lower versus higher vasopressor exposure groups using all available trial data. We pooled data from included studies applying the DerSimonian and Laird random-effects model with inverse-variance study weighting and Wald-type CIs (10). Where events were rare (< 1% overall), we used the Peto odds ratio (OR), which is a fixed-effect method with favorable statistical properties when experimental and control groups are equal in size and when true effects are not extreme (0.5 ≤ true risk ratio [RR] < 1) (11). We displayed results in Forest plots and tables. All analyses were performed using Review Manager (Version 5.4; The Cochrane Collaboration, London, United Kingdom, 2020).
For dichotomous data and non-rare events, we generated summary effect measures pooled across individual studies, presented as RRs with 95% CIs. In case of zero events, a continuity correction of 0.5 was added to each cell. For continuous data reported using identical units in different studies (e.g., days of mechanical ventilation), summary effect measures are presented as mean differences (MDs) with 95% CIs. For outcomes reported with less familiar scales, we reported standardized MD with 95% CI (12). We reverse-coded study results, where necessary, to ensure the directionality of effect was consistent by multiplying the mean values by –1. Where only median (interquartile range) values were reported for an outcome, values were converted to mean (sd) (13).
We assessed risk of random error using trial sequential analysis (TSA) for the primary outcome, conducted using TSA software Version 09.5.10 (Copenhagen Trials Unit, Copenhagen, Denmark) (14, 15). We applied trial sequential monitoring boundaries according to an information size suggested by trial results and an a priori 20% relative risk reduction. We conducted an additional post hoc TSA to assess the optimal information size for a 10% relative risk reduction.
Assessment of Heterogeneity
We assessed clinical heterogeneity qualitatively by considering whether study populations, interventions and settings were comparable across reports. The following characteristics were considered: age, time from vasopressor initiation at randomization, presence or absence of baseline hypertension, chronic left ventricular heart failure, cause of vasodilatory hypotension, presence or absence of cointerventions (e.g., corticosteroids, fluids, inotropes), baseline disease severity, and risk of bias. We assessed statistical heterogeneity quantitatively using the I2 measure, chi-square test, and qualitatively using visual inspection of forest plots for overlapping point estimates or CIs. These assessments were then incorporated in Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) (16) summary of findings tables and in forest plots.
Subgroup Analyses
For the primary outcome, we performed the following prespecified subgroup comparisons with corresponding directions of effect: 1) age: less than 65 versus greater than or equal to 65 years, hypothesizing that lower exposure is more beneficial in older patients; 2) duration of vasopressor therapy at randomization: less than median versus greater than or equal to median time measured within studies, hypothesizing that lower exposure is more beneficial among patients randomized later; 3) presence versus absence of past medical history of hypertension, hypothesizing that hypertensive patients will benefit more from higher vasopressor exposure; 4) presence versus absence of past medical history of left ventricular heart failure, hypothesizing that lower exposure will be more beneficial among patients with heart failure; 5) sepsis versus nonsepsis, hypothesizing that lower exposure will be more beneficial among patients with sepsis; 6) disease severity: less than median versus greater than or equal to median severity score, measured within studies, hypothesizing that lower exposure will be more beneficial among more severely ill; and 7) high versus low risk of bias, hypothesizing that effects will be more pronounced in studies at high risk of bias. We planned to report the credibility of any statistically significant (p < 0.05) subgroup effects using the Instrument to assess the Credibility of Effect Modification Analyses tool (17).
Sensitivity Analyses
We planned three sensitivity analyses: 1) excluding studies published as abstracts; 2) using within-study adjusted estimates of effect, where available, rather than crude estimates of effect (for the primary outcome); and 3) evaluating the impact of loss to follow-up for 28 and 90-day mortality across studies. The latter process consisted of repeating the analyses under the following assumptions: 1) all patients lost to follow-up in the control group survived while all missing patients in the experimental group died, 2) all patients lost to follow-up in the control group died while all missing patients in the experimental group survived, and 3) patients lost to follow-up in the experimental arm had the same risk of death as other patients in the experimental arm and patients lost to follow-up in the control arm had the same risk of death as other patients in the control arm (18).
Publication Bias
We planned to evaluate the risk of publication bias graphically and statistically for each outcome if at least 10 studies were included in the meta-analysis (19).
Certainty Assessment
Teams of two independent reviewers used the GRADE framework (20) to report the overall certainty of evidence, as it relates to our confidence in estimates of effect. These recommendations consider the overall risk of bias (21), imprecision (16), inconsistency (22), indirectness (23), and, when possible, likelihood of publication bias (24) to judge the overall certainty of the evidence across studies for each individual outcome. The certainty of evidence is classified as being very low, low, moderate, or high. RCTs are initially considered to provide high-certainty evidence and rated down according to published criteria. The rating of imprecision was based on absolute risk estimates. Thresholds for important risk differences were 1% for dichotomous endpoints and 1 day for treatment durations. Narrative summaries for certainty of effect estimates adhered to published guidance (25).
The findings of this review are summarized and presented with a summary of findings table, which includes an explicit judgment on the certainty of evidence for each outcome across studies (26).
RESULTS
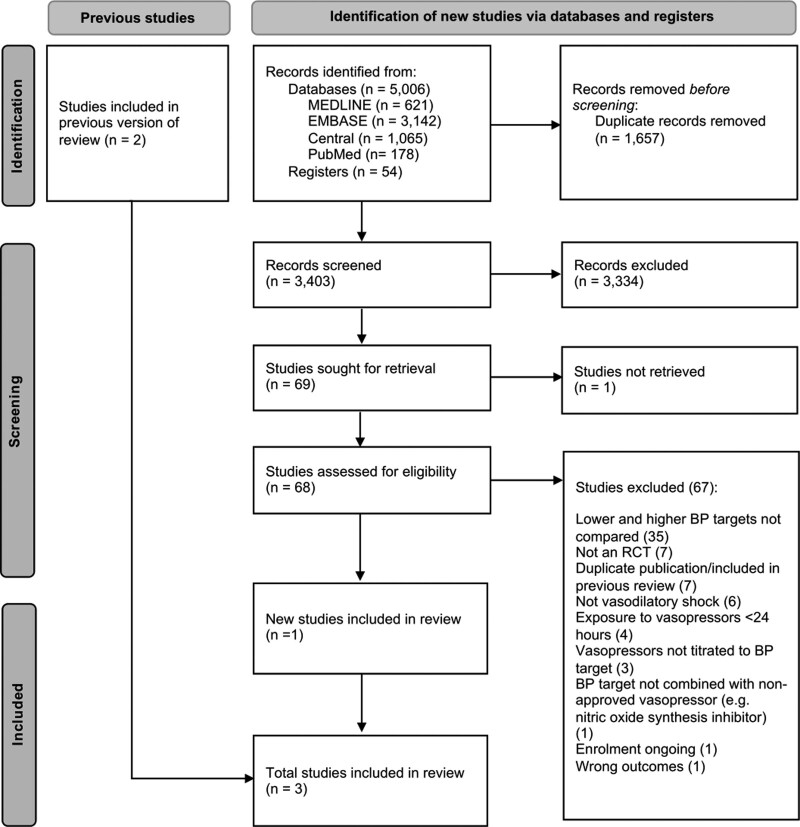
We retrieved 68 full-text articles from 3,403 citations and ultimately included three RCTs (4, 27, 28). A PRISMA flowchart illustrates the selection process (Fig. 1). Studies excluded after full-text review are listed in the Supplemental Digital Content (http://links.lww.com/CCM/H248).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 flow diagram. A list of studies not retrieved and excluded following full-text review is provided in the Supplemental Digital Content (http://links.lww.com/CCM/H248). BP = blood pressure, RCT = randomized controlled trial.
Study Characteristics, Baseline Characteristics, and Vasopressor Utilization
In total, 3,496 patients were randomized in 3 trials conducted in Canada, France, United States, and United Kingdom (Table 1). A fourth trial, Optimal VAsopressor TitraTION in patients 65 years and older (OVATION-65), will be eligible but its results are not yet available (29). In one trial, patient eligibility was restricted to septic shock (27), whereas the other trials (4, 28) enrolled patients with vasodilatory hypotension of any etiology (as defined by treating teams). Other differences in patient eligibility criteria included one trial enrolling only patients 65 years old or older (4) and two trials mandating that randomization occurred within 6 hours of commencing vasopressor therapy (4, 27).
TABLE 1.
Studies Included in the Systematic Review
| Characteristic | Asfar et al (27) | Lamontagne et al (28) | Lamontagne et al (4) |
|---|---|---|---|
| Trial registration | NCT01149278 | NCT01800877 | ISRCTN10580502 |
| Design | Parallel group RCT | Parallel group RCT | Parallel group RCT |
| Location | France | Canada and United States | United Kingdom |
| Funding source | Government | Government | Government |
| Setting | ICU | ICU | ICU |
| Sample size | 776 (97% of anticipated 800) | 120 | 2,600 |
| Recruitment years | 2010–2011 | 2013–2014 | 2017–2019 |
| Inclusion criteria | > 18 yr | > 16 yr | ≥65 yr |
| Septic shocka refractory to fluid resuscitation (requiring ≥0.1 μg/kg/min norepinephrine or epinephrine) | Receiving vasopressors for vasodilatory shock | Vasodilatory hypotension | |
| Evaluated within 6 hr of vasopressor initiation | Adequately fluid resuscitated as per treating physician | ≥1 hr of vasopressor, started within prior 6 hr (if norepinephrine, then ≥0.1 µg/kg/min) | |
| Vasopressor expected for ≥6 more hr | Adequate fluid resuscitation completed or ongoing | ||
| Vasopressor expected for ≥6 more hr | |||
| Exclusion criteria | Pregnancy | Received vasopressors for > 24 hr | Vasopressors used solely for bleeding, acute ventricular failure, or after cardiopulmonary bypass |
| Recent participation in another study with mortality as primary endpoint | Expected to die within 48 hr | Ongoing treatment for brain or spinal cord injury | |
| Decision not to resuscitate | Required vasopressor for reasons unrelated to hypotension | Death perceived as imminent | |
| Main cause of hypotension cardiogenic, hemorrhagic or neurogenic shock, or immediately after surgery | |||
| Intervention | Target MAP 65–70 mm Hg | Target MAP 60–65 mm Hg | Target MAP 60–65 mm Hg |
| Control | Target MAP 80–85 mm Hg | Target MAP 75–80 mm Hg | Usual care as per treating clinician |
| Duration of intervention period | 5 d or until weaned from vasopressor support | Entire period of vasopressor infusion, ending when MAP maintained within or above prescribed range without vasopressors, capped at 28 d | At any time vasopressors required from randomization until ICU discharge |
| Length of follow-up | 90 d | 180 d | 1 yr |
RCT = randomized clinical trial, MAP = mean arterial pressure.
The criteria for septic shock were the official criteria of the American College of Chest Physicians/Society of Critical Care Medicine, that is, sepsis plus arterial hypotension (systolic blood pressure < 90 mm Hg) refractory to fluid resuscitation (minimum 30 mL/kg within 6 hr prior to the start of catecholamines) and requiring vasopressor support.
Mean age across randomized groups was similar in two trials (27, 28) and higher in the trial that enrolled patients 65 years old or older (4). The proportion of males varied from 52% to 69% across randomized groups. In two studies, the rate of preexisting chronic hypertension was similar (just under 50% of patients) (4, 27). In the other, the rate of chronic hypertension was 57% in the lower blood pressure target group and 33% in the higher blood pressure target group (28).
The lower blood pressure target was 60–65 mm Hg in two trials (4, 28), and 65–70 mm Hg in the other (27). Higher blood pressure targets were 75–80 mm Hg (28), 80–85 mm Hg (27), and usual care (4). In all trials, blood pressure values during vasopressor therapy in the lower blood pressure groups were above the upper limit of the protocolized range. Norepinephrine was the most commonly used vasopressor. The duration of vasopressor therapy differed but was consistently shorter in the lower blood pressure target group. Patient baseline characteristics and a description of vasopressor use appear in Table 2 and eTable 1 (http://links.lww.com/CCM/H248), respectively.
TABLE 2.
Baseline Characteristics Among Included Studies
| Study, Year | Asfar et al (27) | Lamontagne et al (28) | Lamontagne et al (4) | |||
|---|---|---|---|---|---|---|
| Randomized Group | Lower MAP Target | Higher MAP Target | Lower MAP Target | Higher MAP Target | Lower MAP Target | Higher MAP Target |
| n | 388 | 388 | 60 | 58 | 1,283 | 1,300 |
| Baseline characteristics | ||||||
| Age, yr | 65 (15) | 65 (13) | 66 (13) | 63 (13) | 75.3 (6.6) | 75.2 (6.9) |
| Sex, male, n (%) | 250 (64.4) | 267 (68.8) | 31 (51.7) | 33 (56.9) | 696 (57.2) | 692 (55.9) |
| Duration of vasopressor at randomization (min) | 216 (126) | 210 (132) | 540 (180–960) | 660 (300–1,020) | 186 (102–277) (n = 1,247) | 186 (104–284) (n = 1,262) |
| Comorbidities | ||||||
| Hypertension, n (%) | 173 (44.6) | 167 (43.0) | 34 (56.7) | 19 (32.8) | 590 (46.0) | 597 (46.0) (n = 1,299) |
| Left ventricular failure, n (%) | 53 (13.7) | 59 (15.2) | 4 (6.7) | 6 (10.3) | 143 (11.1) | 143 (11.0) (n = 1,298) |
| Cause(s) of vasodilatory hypotension, n (%) | Sepsis, 388 (100) | Sepsis, 388 (100) | Sepsis, 46 (76.7) | 37 (63.8) | Sepsis, 953 (78.4) | 964 (77.8) |
| Pancreatitis, 3 (5.0) | 0 | Not sepsis, 263 (21.6) | 275 (22.2) | |||
| Overdose, 1 (1.7) | 0 | (n = 1,216) | (n = 1,239) | |||
| Pulmonary embolism, 2 (3.3) | 1 (1.7) | |||||
| Burns, 1 (1.7) | 2 (3.4) | |||||
| Other, 7 (11.7)a | 18 (31.0)b | |||||
| Illness severity | ||||||
| Sequential Organ Failure Assessment | 10.8 (3.1) | 10.7 (3.1) | — | — | — | — |
| Simplified Acute Physiology Score II score | 57.2 (16.2) | 56.1 (15.5) | — | — | — | — |
| Acute Physiology and Chronic Health Evaluation II | — | — | 24 (8) | 25 (6) | 20.9 (6.5) (n = 1,213) | 20.6 (6.1) (n = 1,239) |
MAP = mean arterial pressure.
Includes two patients in whom the admission diagnosis was unrelated to hypotension.
Includes 10 patients in whom the admission diagnosis was unrelated to hypotension.
Values are mean (sd) or median (interquartile range) as appropriate, unless otherwise specified. Dashes indicate data not reported.
Outcomes
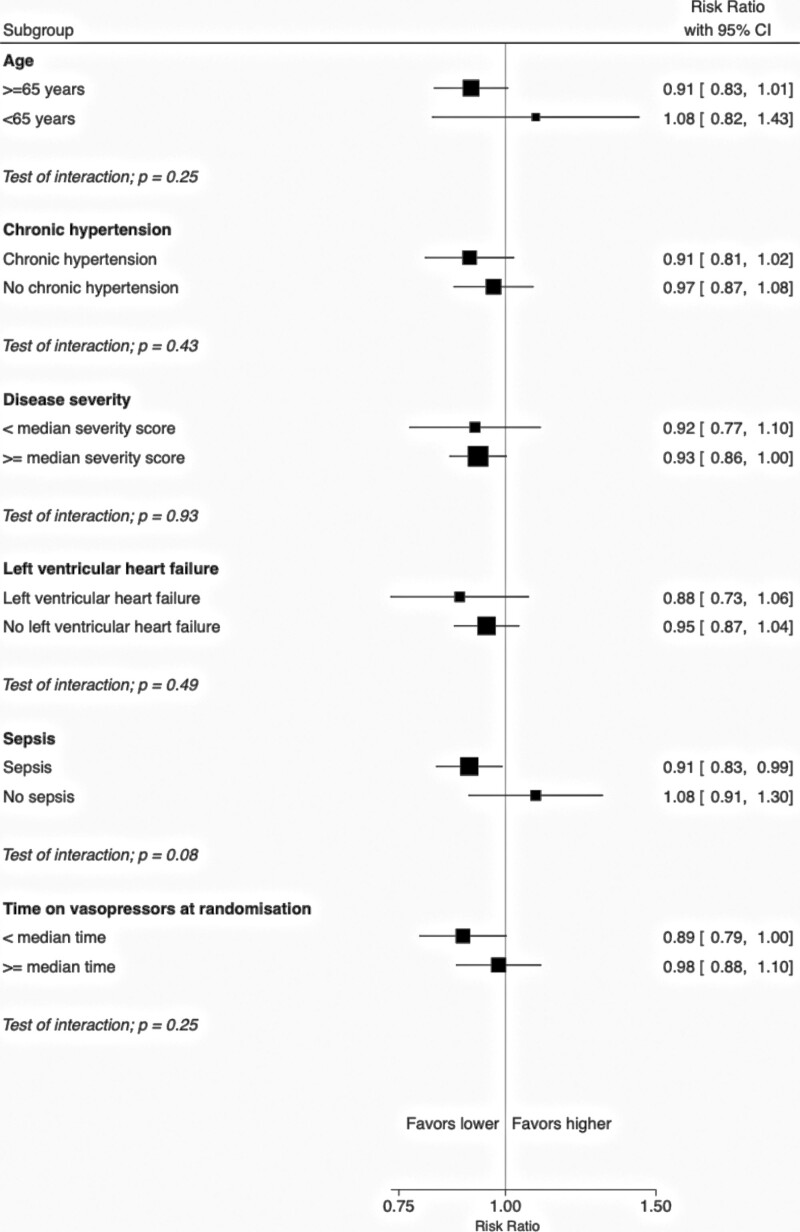
All three studies reported 90-day mortality, with a total of 1,421 events among 3,357 patients (42%). A lower vasopressor exposure probably lowers 90-day mortality (RR, 0.94; 95% CI, 0.87–1.02); however, certainty in this finding was moderate due to imprecision as the upper end of the 95% CI did not exclude the possibility of harm (Table 3; eTable 2 and eFig. 1, http://links.lww.com/CCM/H248). Age (interaction p = 0.25), sepsis (interaction p = 0.08), chronic hypertension (interaction p = 0.43), left ventricular failure (interaction p = 0.49), baseline illness severity (interaction p = 0.93), and baseline duration of vasopressor therapy (interaction p = 0.25) did not modify the effect of lower vasopressor exposure on 90-day mortality (Fig. 2).
Figure 2.
Effect of lower versus higher exposure to vasopressor therapy on 90-d all-cause mortality, by subgroups.
The TSA suggests that, assuming the effect estimates of this meta-analysis are true, a 20% decrease in relative risk has been ruled out. In contrast, a total of 5,344 participants would be required to detect a 10% decrease in relative risk (eFigs. 2 and 3, http://links.lww.com/CCM/H248).
This effect was consistent across mortality endpoints, although the effect estimate at 180 days was highly uncertain (Table 3; and eTable 2, http://links.lww.com/CCM/H248). Sensitivity analyses evaluating the impact of loss to follow-up on 28- and 90-day mortality across studies did not alter the interpretation of the primary analysis (eFigs. 4–9, http://links.lww.com/CCM/H248). Lower vasopressor exposure may also decrease the risk of supraventricular arrhythmia (OR, 0.55; 95% CI, 0.36–0.86; low certainty).
TABLE 3.
Primary and Secondary Outcomes of the Systematic Reviewa
| Outcomea | No. of Trials (Events–Patients) | Measure of Effect | Effect Estimate (95% CI) |
|---|---|---|---|
| 90-d mortality | 3 (1,421–3,357) | RR | 0.94 (0.87–1.02) |
| 90-d mortality, adjusted | 3 (1,421–3,357) | OR | 0.93 (0.85–1.01) |
| Secondary | |||
| In-ICU mortality | 3 (1,036–3,343) | RR | 0.96 (0.87–1.06) |
| In-hospital mortality | 3 (1,373–3,376) | RR | 0.95 (0.88–1.03) |
| 28-d mortality | 3 (1,215–3,357) | RR | 0.93 (0.85–1.02) |
| 60-d mortality | 2 (1,038–2,581) | RR | 0.95 (0.86–1.04) |
| 180-d mortality | 1 (46–118) | RR | 0.89 (0.56–1.39) |
| Resuscitation and interventions | |||
| Acute kidney injury (Kidney Disease Improving Global Outcomes stage 3)b | 2 (385–3,358) | RR | 1.10 (0.93–1.29) |
| Receipt of early RRTc | 3 (920–3,351) | RR | 1.02 (0.91–1.14) |
| RRT-free days (to day 28) | 3 (3,351–3,351) | MD | 1.08 (0.19–1.96) |
| Duration of RRT (d)c | 3 (3,351–3,351) | MD | –0.03 (–0.32 to 0.25) |
| ICU survivorsc | 3 (2,307–2,307) | MD | –0.00 (–0.33 to 0.32) |
| ICU nonsurvivorsc | 3 (1,036–1,036) | MD | 0.27 (–0.81 to 1.35) |
| Duration of mechanical ventilation (d) | 3 (3,329–3,329) | MD | –0.09 (–0.70 to 0.53) |
| ICU survivors | 3 (2,296–2,296) | MD | –0.33 (–1.06 to 0.41) |
| ICU nonsurvivors | 2 (1,025–1,025) | MD | 0.16 (–0.91 to 1.24) |
| Ventilator-free days (to day 28) | 3 (3,351–3,351) | MD | 0.71 (–0.13 to 1.54) |
| Proportion receiving blood products | 2 (459–893) | RR | 0.89 (0.57–1.38) |
| Blood products quantity/volumed | 2 (893–893) | SMD | 0.11 (–0.02 to 0.24) |
| Cumulative fluid balancee | 3 (3,408–3,408) | SMD | –0.01 (–0.16 to 0.13) |
| New-onset supraventricular arrhythmia | 3 (90–3,476) | OR | 0.55 (0.36–0.86) |
| New-onset ventricular arrhythmia | 3 (61–3,476) | OR | 0.97 (0.58–1.61) |
| Mesenteric ischemia | 3 (44–3,476) | OR | 0.91 (0.50–1.66) |
| Digit or limb or skin ischemia | 3 (30–3,476) | OR | 1.15 (0.56–2.36) |
| Myocardial ischemia | 3 (49–3,476) | OR | 0.73 (0.41–1.31) |
| Gastrointestinal bleeding | 1 (73–776) | OR | 1.39 (0.86–2.26) |
| Vasopressor extravasation | 1 (0–118) | OR | Not estimable (zero events) |
| Quality of life | 1 (494–494) | MD | –0.01 (–0.05 to 0.03) |
| Neurologic outcomef | 2 (559–559) | SMD | –0.15 (–0.32 to 0.02) |
MD = mean difference, OR = odds ratio, RR = risk ratio, RRT = renal replacement therapy, SMD = standardized mean difference.
The following prespecified secondary outcomes were not available: Longer-term mortality (i.e., > 1 to 2 yr; > 2 to 5 yr) and proportion receiving late RRT.
In Asfar et al (27), this was defined as a doubling of plasma creatinine levels. In Lamontagne et al (4), this was defined as severe acute renal failure in ICU reported as a serious adverse event.
In Asfar et al (27), RRT follow-up time period varied (0–7 d for all participants and up to 28 d for some; the best overall estimate is presented here). In Lamontagne et al (28) and Lamontagne et al (4), RRT was measured to ICU discharge.
In Asfar et al (27), the number of packed RBC units transfused was reported. In Lamontagne et al (28), blood products requirement by volume while in ICU (mL) was reported.
In contrast, the intervention was not associated with important differences in ventricular arrhythmia, mesenteric ischemia, digit ischemia, myocardial ischemia, duration of RRT or mechanical ventilation, ventilator-free days, volume of transfused blood products, or cumulative fluid balance (low- to moderate-certainty evidence). The effect on the need for blood products, gastrointestinal bleeding, stage 3 acute kidney injury, and need for RRT was highly uncertain (Table 3; and eTable 2, http://links.lww.com/CCM/H248).
Two studies reported neurologic outcome; one using the Functional Independence Measure (30) at 6 months and the other, the Short Form of the Informant Questionnaire on Cognitive Decline in the Elderly (31) at 1 year after randomization. Lower vasopressor exposure may not be associated with differences in neurologic outcome at longest available follow-up (standardized mean difference, –0.15; 95% CI, –0.32 to 0.02; low certainty) or quality of life (low certainty)—with the latter reported in only one study. Forest plots for outcomes are in eFigures 10–41 (http://links.lww.com/CCM/H248).
Summary of Findings
eTable 2 (http://links.lww.com/CCM/H248) summarizes how assessors rated the certainty of the evidence, as well as the best estimates of effects in relative and absolute terms. There were no serious concerns regarding risk of bias for mortality at any time point, but certainty was downgraded due to imprecision because the CI does not exclude possible harm. There were serious concerns regarding risk of bias, due to lack of blinding, for all other outcomes. In addition, certainty was downgraded due to imprecision for new-onset supraventricular arrhythmia, gastrointestinal bleeding, stage 3 acute kidney injury, RRT and mechanical ventilation-free days, blood product requirements, and cognitive outcomes. Certainty was further downgraded for statistical inconsistency for blood product requirements. All renal outcomes were downgraded for indirectness because follow-up and ascertainment varied considerably across studies and because short-term renal outcomes are indirectly associated with other renal outcomes that are more important to patients.
DISCUSSION
The results of this systematic review provide evidence that, in patients with vasodilatory shock, lowering exposure to vasopressors by tolerating lower blood pressures probably reduces the risk of death. However, uncertainty persists due to imprecision and the applicability of this conclusion is limited to the range of blood pressure values achieved in the trials, rather than what was prescribed, which defines the limits for the signals of efficacy and safety.
Importantly, such restriction in vasopressor exposure constitutes a significant departure from usual practices. Previous observational studies and data from the usual care control group in the 65 trial consistently show that even though clinical guidelines have recommended mean arterial pressure (MAP) targets of 65 mm Hg, such targets translate into vasopressor-induced MAP values between 70 and 75 mm Hg (25% of the patients in the usual care group of the 65 trial had an average MAP greater than 77 mm Hg during vasopressor therapy) (4, 32). Although the protocols followed in the included trials were different, it was sensible to pool the results of these trials because they all compared lower versus higher exposures to vasopressors obtained via lower versus higher blood pressure targets. The separation between the randomized groups in terms of blood pressure, vasopressor dose, and/or duration was consistent across all included trials (eTable 1, http://links.lww.com/CCM/H248) as was the effect estimates, as indicated by the absence of statistical heterogeneity (I2 = 0). This approach is comparable to meta-analyses of trials using different protocols to achieve higher versus lower levels of positive end-expiratory pressure levels in acute respiratory distress syndrome (33). However, this study does not allow to establish the optimal blood pressure target for vasopressor therapy in patients with vasodilatory hypotension and the specific blood pressure value below which it would be harmful to withhold vasopressor therapy remains unclear. Given the consistent effect estimates across all trials, we surmise that the optimal MAP target is equal or lower than the lowest blood pressure tolerated in the 65 trial, which was the most restrictive of the three included trials. Accordingly, two lines of inquiry are now emerging: 1) how to safely apply the intervention in the clinical setting without increasing the risk of prolonged episodes of MAP below 60 mm Hg and 2) whether there is incremental benefit to further reducing exposure to vasopressors, in a research setting, by tolerating even lower blood pressure values.
This review increases the number of patients exposed to a vasopressor lowering strategy four-fold and thereby considerably increases the confidence that tolerating lower blood pressure values than those observed under usual care improves survival (6). Other strengths include access to unpublished data from original studies, explicit and prespecified eligibility criteria, a comprehensive literature search, duplicate adjudication of eligibility, data extraction and risk of bias assessment, prespecified analyses and subgroups, and use of GRADE to assess and communicate confidence in the effect estimates.
However, we also acknowledge the following limitations. Uncertainty persists because the three included trials collectively enrolled 2,000 patients fewer than required to rule out a 10% relative risk reduction from a baseline risk of 40% (4% absolute risk reduction or a number needed to treat of 25) (34, 35). In all included trials, actual blood pressure values were consistently above the upper limit of the protocolized target range in the lower blood pressure target arms. Accordingly, whether increased protocol adherence would be associated with greater benefit, or increased harm, remains unclear. The applicability of these findings is limited to monitored patients in highly resourced ICUs. Therefore, it is also unclear whether the safety profile of strategies designed to reduce vasopressor exposure would be similar in other settings. Different blood pressure targets were used across included trials—but between-group separation in the dose and duration of vasopressors was achieved in all trials, with lower blood pressure targets leading to reduced exposure. Our estimates of the effect of this intervention, including in clinically important subgroups such as patients with sepsis, are likely to gain certainty with completion of additional trials.
CONCLUSIONS
In patients with vasodilatory hypotension who are started on vasopressors, moderate-certainty evidence from three randomized trials showed that lower vasopressor exposure probably lowers mortality. However, an additional large trial is needed to reach an optimal information size to detect a clinically important 10% relative reduction in mortality with this approach.
ACKNOWLEDGMENTS
We thank Karen Thomas and Kathy Rowan from Intensive Care National Audit & Research Centre for their support in completing this review.
Supplementary Material
Footnotes
*See also p. 326.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Adhikari and Lamontagne contributed equally as joint corresponding authors.
REFERENCES
- 1.Lamontagne F, Marshall JC, Adhikari NKJ: Permissive hypotension during shock resuscitation: Equipoise in all patients? Intensive Care Med. 2018; 44:87–90 [DOI] [PubMed] [Google Scholar]
- 2.Walkey AJ, Adhikari NK, Day AG, et al. : Mediation analysis of high blood pressure targets, arrhythmias, and shock mortality. Am J Respir Crit Care Med. 2019; 199:802–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, et al. : Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017; 43:304–377 [DOI] [PubMed] [Google Scholar]
- 4.Lamontagne F, Richards-Belle A, Thomas K, et al. : Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: A randomized clinical trial. JAMA. 2020; 323:938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouncey PR, Richards-Belle A, Thomas K, et al. : Reduced exposure to vasopressors through permissive hypotension to reduce mortality in critically ill people aged 65 and over: The 65 RCT. Health Technol Assess. 2021; 25:1–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hylands M, Moller MH, Asfar P, et al. : A systematic review of vasopressor blood pressure targets in critically ill adults with hypotension. Can J Anaesth. 2017; 64:703–715 [DOI] [PubMed] [Google Scholar]
- 7.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012; 120:c179–c184 [DOI] [PubMed] [Google Scholar]
- 9.Sterne JAC, Savovic J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 11.Bradburn MJ, Deeks JJ, Berlin JA, et al. : Much ado about nothing: A comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007; 26:53–77 [DOI] [PubMed] [Google Scholar]
- 12.Thorlund K, Walter SD, Johnston BC, et al. : Pooling health-related quality of life outcomes in meta-analysis—a tutorial and review of methods for enhancing interpretability. Res Synth Methods. 2011; 2:188–203 [DOI] [PubMed] [Google Scholar]
- 13.Wan X, Wang W, Liu J, et al. : Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wetterslev J, Thorlund K, Brok J, et al. : Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008; 61:64–75 [DOI] [PubMed] [Google Scholar]
- 15.Wetterslev J, Thorlund K, Brok J, et al. : Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. 2009; 9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Oxman AD, Kunz R, et al. : GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol. 2011; 64:1283–1293 [DOI] [PubMed] [Google Scholar]
- 17.Schandelmaier S, Briel M, Varadhan R, et al. : Development of a new Instrument to assess the Credibility of Effect Modification ANalyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ. 2020; 192:E901–E906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akl EA, Johnston BC, Alonso-Coello P, et al. : Addressing dichotomous data for participants excluded from trial analysis: A guide for systematic reviewers. PLoS One. 2013; 8:e57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JA, Sutton AJ, Ioannidis JP, et al. : Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011; 343:d4002. [DOI] [PubMed] [Google Scholar]
- 20.Balshem H, Helfand M, Schünemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011; 64:401–406 [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist G, et al. : GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011; 64:407–415 [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Kunz R, et al. : GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011; 64:1294–1302 [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Kunz R, et al. : GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol. 2011; 64:1303–1310 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Montori V, et al. : GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011; 64:1277–1282 [DOI] [PubMed] [Google Scholar]
- 25.Santesso N, Glenton C, Dahm P, et al. : GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020; 119:126–135 [DOI] [PubMed] [Google Scholar]
- 26.Guyatt G, Oxman AD, Akl EA, et al. : GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64:383–394 [DOI] [PubMed] [Google Scholar]
- 27.Asfar P, Meziani F, Hamel JF, et al. : High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014; 370:1583–1593 [DOI] [PubMed] [Google Scholar]
- 28.Lamontagne F, Meade MO, Hebert PC, et al. : Higher versus lower blood pressure targets for vasopressor therapy in shock: A multicentre pilot randomized controlled trial. Intensive Care Med. 2016; 42:542–550 [DOI] [PubMed] [Google Scholar]
- 29.Masse MH, Battista MC, Wilcox ME, et al. : Optimal VAsopressor TitraTION in patients 65 years and older (OVATION-65): Protocol and statistical analysis plan for a randomised clinical trial. BMJ Open. 2020; 10:e037947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinkle JL, McClaran J, Davies J, et al. : Reliability and validity of the adult alpha functional independence measure instrument in England. J Neurosci Nurs. 2010; 42:12–18 [DOI] [PubMed] [Google Scholar]
- 31.Jorm AF: A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Devel opment and cross-validation. Psychol Med. 1994; 24:145–153 [DOI] [PubMed] [Google Scholar]
- 32.Lamontagne F, Cook DJ, Meade MO, et al. : Vasopressor use for severe hypotension-a multicentre prospective observational study. PLoS One. 2017; 12:e0167840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briel M, Meade M, Mercat A, et al. : Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome systematic review and meta-analysis. JAMA. 2010; 303:865–873 [DOI] [PubMed] [Google Scholar]
- 34.Young PJ, Nickson CP, Perner A: When should clinicians act on non-statistically significant results from clinical trials? JAMA. 2020; 323:2256–2257 [DOI] [PubMed] [Google Scholar]
- 35.Hultcrantz M, Rind D, Akl EA, et al. : The GRADE working group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017; 87:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]