Abstract
Objectives
To investigate the feasibility of Zucker fatty rats as a model for obesity-associated erectile dysfunction (OAED) research and to determine the effect of low intensity extracorporeal shock wave therapy (Li-ESWT) on penile tissue and function in these rats.
Materials and methods
Eight newborn male Zucker Lean (ZL group) rats (ZUC-Leprfa 186) and 16 newborn male Zucker Fatty (ZF) rats (ZUC-Leprfa 185) were injected with 5-ethynyl-2’-deoxyuridine (EdU) at birth to identify and monitor endogenous stem cells. Insulin tolerance testing was performed at 10 weeks of age. Beginning at 12 weeks of age, 8 ZF rats were kept as controls, and the remaining 8 ZF rats were treated with Li-ESWT (0.02 mJ/mm2, 3 Hz, 500 pulses; ZF + SW group) twice a week for 4 weeks. Following a 1-week washout period, erectile function was evaluated by measuring intracavernosal pressure (ICP) and mean arterial pressure (MAP). Penile tissues were then harvested for histological study to assess smooth muscle/collagen content and endothelium content in the corpora cavernosum. LipidTOX staining was used to evaluate lipid accumulation. EdU as a marker of cell activation and phosphorylated histone 3 (H3P) as a marker of cell mitosis were also assessed.
Results
The ICP/MAP indicated that erectile function was severely impaired in the ZF group as compared with the ZL group. In the ZF + Li-SW group, erectile function was significantly improved (P<0.05). Muscle atrophy was observed in the ZF group while Li-ESWT increased the muscle content in ZF + SW group. Moreover, the penile endothelium was damaged in the ZF group, and Li-ESWT enhanced the regeneration of endothelial cells (P<0.01) in the ZF + SW group. Lipid accumulation was observed in the penile tissue of ZF rats. Li-ESWT significantly reduced both the amount and the distribution pattern of LipidTOX suggesting decreased overall lipid infiltration. Furthermore, Li-ESWT increased EdU+ cells and markedly enhanced the phosphorylation level of H3P at Ser-10 in the ZF + SW group. Most H3P positive cells were located within smooth muscle cells with some located in the endothelium suggesting that these tissues are the reservoirs of penile stem/progenitor cells.
Conclusion
ZF rats can serve as an animal model in which to study OAED. This study reveals that obesity impairs erectile function by causing smooth muscle atrophy, endothelial dysfunction, and lipid accumulation in the corpus cavernosum. Li-ESWT restored penile hemodynamic parameters in the ZF rats by restoring smooth muscle and endothelium content and reducing lipid accumulation. The underlying mechanism of Li-ESWT appears to be activation of stem/progenitor cells which prompts cellular proliferation and accelerates penile tissue regeneration. Our findings are of interest not just as a validation of this emerging treatment for erectile dysfunction but also as a novel and potentially significant method to modulate endogenous stem/progenitor cells in other disease processes.
Keywords: obesity-associated erectile dysfunction, Li-ESWT, Zucker Fatty rats, endogenous stem cells, histone 3
Introduction
The number of obese people in both developed and developing countries has increased continuously during the past 30 years [1]. In the United States alone, more than 78 million people are suffering from obesity. Obese men in particular are at high risk of developing erectile dysfunction (ED) [2, 3]. The prevalence of obesity in men reporting symptoms of ED may be as high as 79% [4], although vascular risk factors commonly associated with obesity also may play a crucial role. Patients with obesity-associated erectile dysfunction (OAED) often have a poor response to the first-line therapy [5], phosphodiesterase type 5 (PDE5) inhibitors, therefore novel therapeutic modalities for OAED are in high demand.
Although it is clear that obesity and ED are tightly linked, little is known about the mechanisms of OAED. Previous studies have utilized animal models of diet-induced obesity to investigate OAED [6, 7]. This is not an ideal animal model as most of the diet-induced animals develop diabetes mellitus, which itself may impair erectile function. Zucker fatty (ZF) rats, which feature a leptin receptor gene mutation, develop glucose intolerance without diabetes mellitus and are utilized to study genetic obesity, insulin resistance, glucose intolerance, and metabolic syndrome. Therefore, the ZF rat is conceivably a superior animal model for the study of OAED. Several studies have demonstrated ED in these animals though the underlined mechanisms are far from well understood [8, 9].
Low-intensity extracorporeal shock wave therapy (Li-ESWT) has been used to treat musculoskeletal disorders for decades [10]. Previous studies have shown that Li-ESWT has been expanded to address neurovascular ED [11]. Li-ESWT has the ability to mobilize stem cells to the site of injury, to promote nerve regeneration, and to reverse muscle content reduction. Furthermore, our recent study indicates that Li-ESWT activates penile endogenous stem cells in normal rats [12]. To the best of our knowledge, the utility of Li-ESWT for potentiation of erectile function in an animal model of obesity has not yet been explored.
In this study, we treated ZF rats with Li-ESWT. After treatment, the rats underwent functional testing of erectile hemodynamics as well as histological and molecular assessment of corpus cavernosum tissues. Our goals were to elucidate how obesity affects the structure and function of the penis in a rat model of genetic obesity and to investigate the feasibility and underlying mechanisms of the therapeutic effect of Li-ESWT in OAED.
Material and Methods
Animals
Experiments were approved by the Institutional Animal Care and Use Committee at the University of California, San Francisco. Eight newborn male Zucker Lean (ZL group) rats (ZUC-Leprfa 186) and 16 Zucker Fatty (ZF) rats (ZUC-Leprfa 185) were used in this study. In order to track putative endogenous stem cells, each pup received an intraperitoneal injection of 5-ethynyl-2-deoxyuridine (EdU, Invitrogen, Carlsbad, CA, USA) (150 mg/kg) at birth as previously described [13]. Body weight was monitored every 2 weeks. At the age of 10 weeks, all rats were subjected to insulin tolerance testing. At 12 weeks, the remaining 8 ZF rats were treated with Li-ESWT (0.02 mJ/mm2, 3 Hz, 500 pulses; ZF + SW group) twice a week for 4 weeks. The rest of the ZF rats were used as the ZF control (ZF group). Following a 1-week washout period, the erectile function was evaluated by measuring intracavernosal pressure (ICP) and mean arterial pressure (MAP). After surgery, animals were sacrificed, and penile tissues were harvested for histological study.
Intraperitoneal Insulin Tolerance Test
All rats were fasted for 6 hours with free access to drinking water. Basal blood glucose levels were measured before insulin injection. Fast-acting insulin (Sigma-Aldrich, St. Louis, MO, USA) was given at a dose of 0.5 IU/kg by intraperitoneal injection. Blood was taken by tail puncture at 30, 60, 90, and 120 min post-insulin administration and a blood glucose meter (ACCU-CHEK Active; Roche Diagnostics, Indianapolis, IN, USA) was used to measure blood glucose.
Erectile Function Evaluation
The ICP was measured as previously described [14]. Briefly, under proper anesthesia (ketamine and midazolam, 100 mg/kg and 5 mg/kg, respectively), the major pelvic ganglion (MPG) and cavernous nerve (CN) were exposed via midline laparotomy. A 25-gauge butterfly needle was inserted into the left penile crus to monitor ICP. A PE-50 tube filled with heparinized saline (200 IU/mL) was cannulated into the left common carotid artery and connected to a pressure transducer (Utah Medical Products, Midvale, UT, USA) to continuously monitor MAP. Erectile response was elicited by electrical stimulation using a bipolar electrode hooked to the cavernous nerve. Stimulation parameters were 1.5 mA, 20 Hz, pulse width 0.2 ms, and duration 50 s. The maximum increase of ICP for three stimuli on each side for each rat was used for statistical analysis. The ratios between increase ICP and MAP (ICP/MAP) were calculated to normalize for variations in systemic blood pressure. Total ICP was determined by the area under the curve (AUC) during stimulation.
Immunofluorescence and Lipid Droplet Staining
Cryosections of penile tissue samples were prepared according to a previously described protocol [15]. Tissue sections were incubated with primary antibodies, including anti-actin, α-smooth muscle (α-SMA) antibody (1:1000; Sigma-Aldrich), mouse anti rat RECA-1 antibody clone HIS52 (1: 500; Bio-Rad, Hercules, CA, USA) and anti-phospho-histone H3 (Ser10) antibody (H3P, 1:100; Merck Millipore, Billerica, MA, USA). Smooth muscle was stained by Alexa-488-conjugated phalloidin (1:500; Invitrogen). Sections were assessed for EdU labeling using the Click-iT reaction cocktail (Invitrogen), which contained Alexa Fluor 594 (1:500), for 30 minutes at room temperature, followed by staining with 4’, 6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 μg/ml, Invitrogen).
For identifying the location of lipid deposition in the penile tissue, LipidTOX and phalloidin double staining was used as previously described [2]. Following phalloidin staining, LipidTOX neutral lipid stain (1:200; Invitrogen) was applied to the slides at room temperature for 30 minutes. Nuclear staining was performed by incubation with DAPI.
Masson’s Trichrome Staining
Penile tissue sections were prepared as above. Masson’s trichrome staining was carried out according to the standard protocol. Semiquantitative analysis was performed to evaluate intensity using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA).
Image Analysis and Quantification
For image analysis, four fields per tissue per animal in each treatment group were randomly selected, photographed, and recorded using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY, USA). To evaluate penile lipid accumulation, five fields in the sections were photographed at 200x magnification. For quantifying lipid accumulation, the LipidTOX-positive area (in red) was measured and expressed as percentage of total area.
Statistical Analysis
Results were analyzed using GraphPad Prism Version 6.02 (GraphPad Software, San Diego, CA, USA) and expressed as means ± standard deviations. Statistical analyses were performed using one-way analysis of variance followed by the Tukey’s multiple comparisons test. Differences among groups were considered significant at P < 0.05.
Results
Metabolic Variables
The body weight of ZF rats was higher than that of ZL rats starting at 6 weeks old (P < 0.05, Figure S1). During the insulin tolerance test, the initial blood glucose was higher in the ZF rats compared with the ZL rats (P < 0.05). The blood glucose levels dropped sharply in the ZL rats after insulin administration but declined gently and even rebounded at 60 minutes in the ZF rats.
At 12 weeks, ZF rats had a higher body weight compared to ZL rats. At both the week 12 randomization and after the 4-week treatment, ZF and ZF + SW groups did not differ with respect to body weight (Figure 1B).
Figure 1.
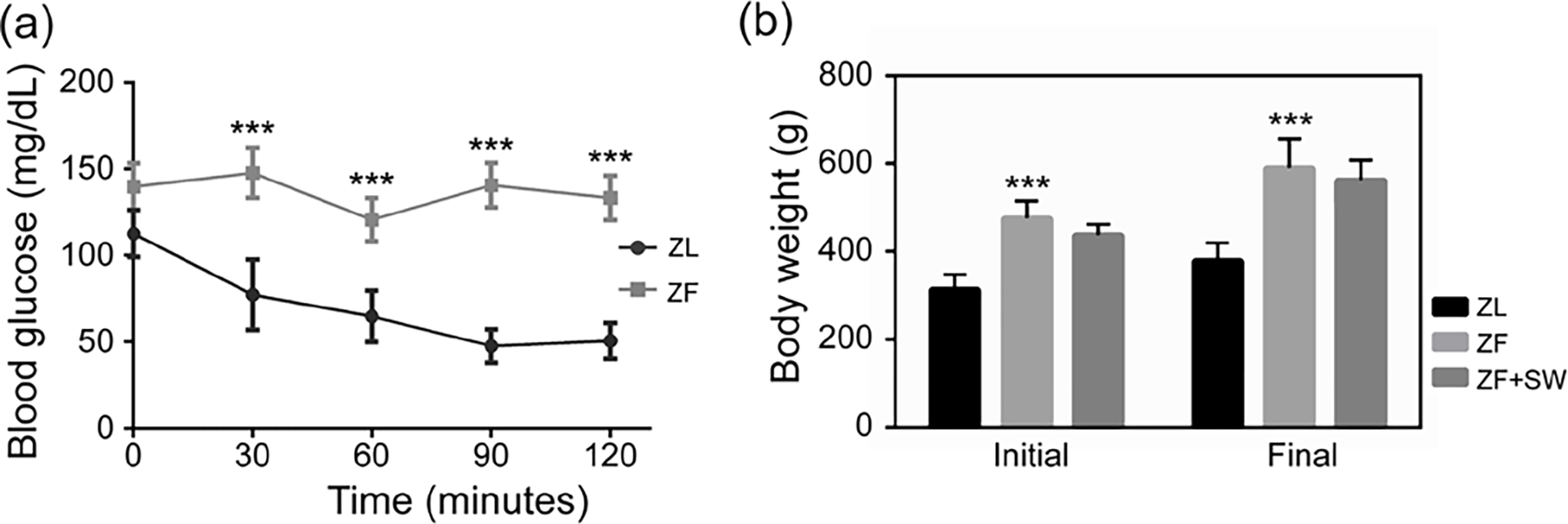
Insulin tolerance test and body weight changes. (a) Intraperitoneal insulin tolerance at 10 weeks of age. (b) Body weight in each group at 12 weeks of age and before sacrifice. *** P < 0.01 compared to the ZL group
Li-ESWT Ameliorates Impaired Erectile Function in ZF Rats
As illustrated in Figure 2, the ZL group exhibited a normal ICP curve and high ICP/MAP ratios. ZF rats had a lower ICP curve, and the curve fluctuated at its plateau (0.78 ± 0.07 versus 0.42 ± 0.11, P < 0.001). Li-ESWT significantly increased the ICP/MAP ratio in response to cavernous nerve electrical stimulus (0.73 ± 0.16, P < 0.01).
Figure 2.
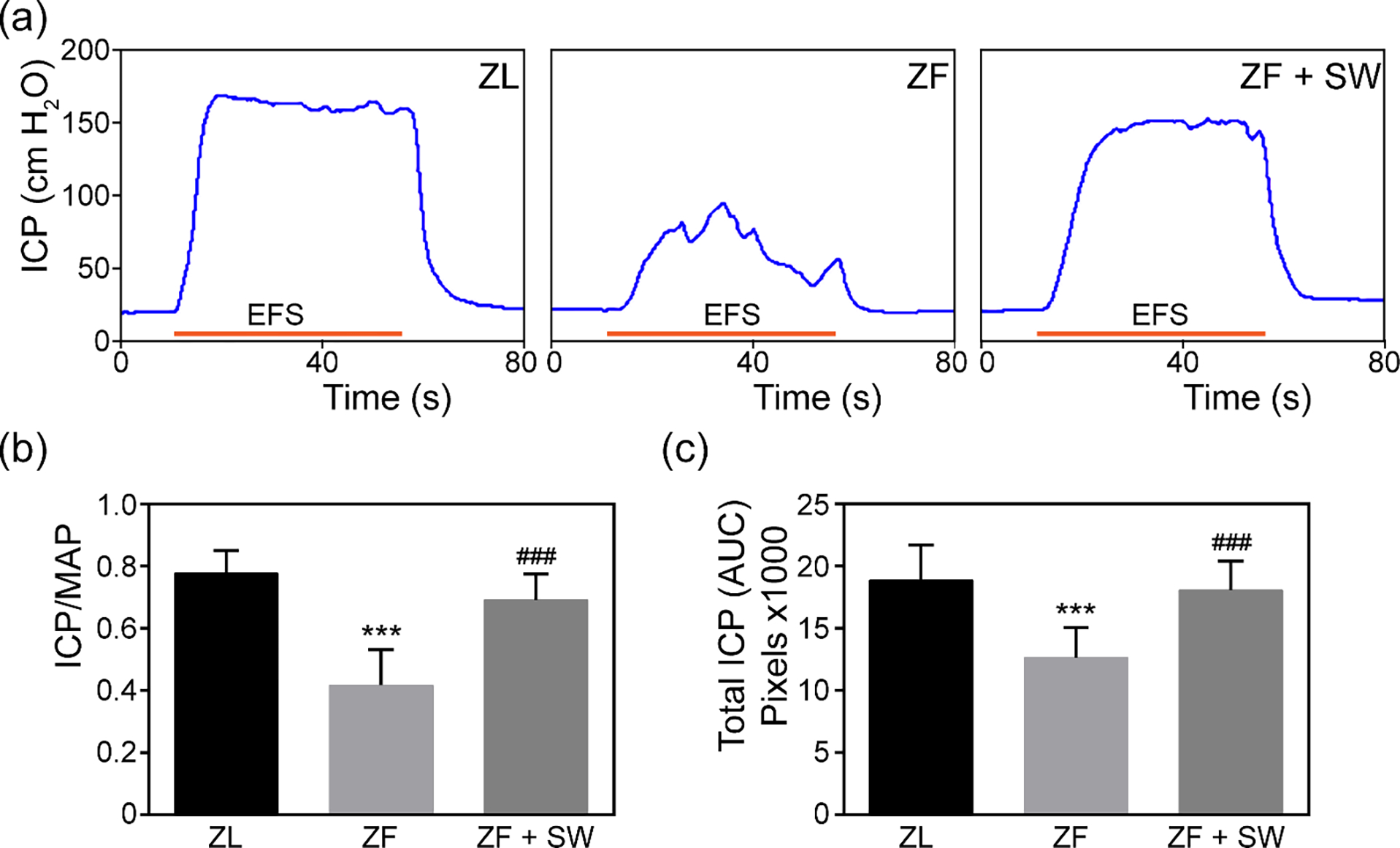
Li-ESWT improved erectile function upon cavernous nerve electrostimulation in ZF rats. (a) Representative tracings of intracavernosal pressure for ZL, ZF, and ZF + SW groups. (b, c) Erectile function0 is presented as intracavernosal pressure/mean arterial pressure and total intracavernosal pressure (area under the curve) in each group. *** P < 0.01 compared to ZL group; ### P < 0.01 compared with ZF group
Li-ESWT Improves Smooth Muscle Content and the Ratio of Smooth Muscle to Collagen in the Corpus Cavernosum
As evidenced by the significant decrease in smooth muscle content in penile tissue of ZF rats as compared to ZL rats, obesity provoked cavernous smooth muscle atrophy. After Li-ESWT, the ZF + SW group showed significant regeneration of smooth muscle content. In addition, as shown in Figure 3, Li-ESWT reduced cavernous fibrosis in ZF + SW rats. In the ZL group, the smooth muscle/ collagen ratio was 0.220 ± 0.035, while the ratio in penile tissue from ZF rats was strikingly decreased (0.140 ± 0.017). Li-ESWT almost fully preserved the ratio (0.217 ± 0.033).
Figure 3.
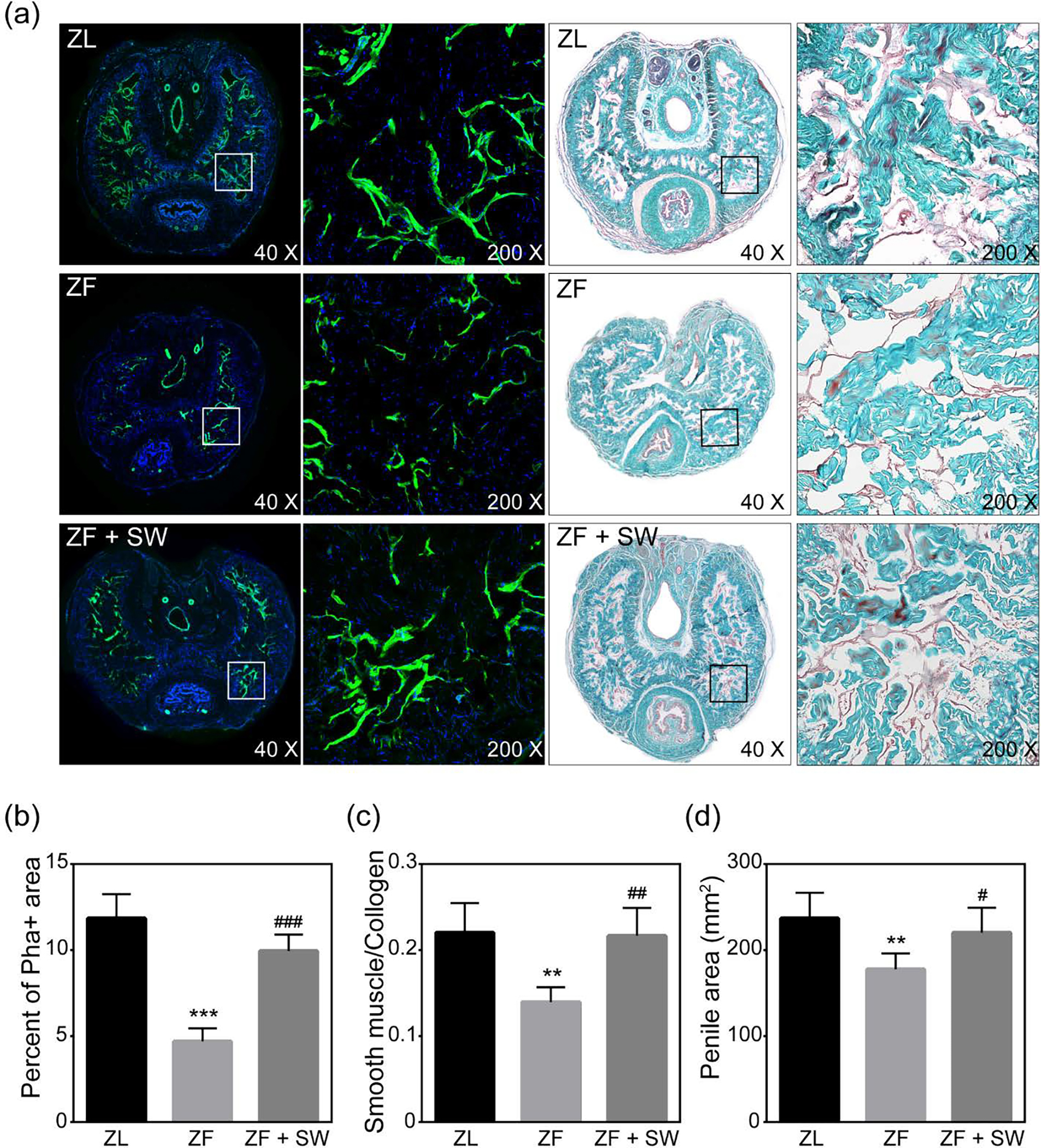
Phalloidin staining and Masson’s trichrome staining of penile mid-shaft specimens. (a) Representative images of smooth muscle stained with Alexa Fluor 488 phalloidin (green) and Masson’s trichrome staining for ZL, ZF, and ZF + SW groups. The rectangle indicates the area selected for amplification. Original magnification, X40 and X200. (b) Smooth muscle content as a percentage of total intra-tunical area. (c) Effect of Li-ESWT treatment on the ratio of smooth muscle to collagen in the corpus cavernosum. (d) Area of the penile tissue cross section for the three groups. * P <0.05; ** 0.01 < P <0.05; *** P <0.01 compared with the ZL group; # P <0.05; ### P < 0.01 compared with the ZF group.
Li-ESWT Reverses Endothelium Damage in ZF rats
As shown in Figure 4, RECA-1 (a specific marker for endothelium in rats) markedly decreased in the ZF group as compared to that of the ZL group. Endothelial cells form a complete lining of the sinusoids within the erectile tissue. Disruption of the integrity of the endothelial monolayer was observed in the ZF group but not the ZL group. Li-ESWT preserved the integrity of the sinusoidal lining in the ZF + SW group.
Figure 4.
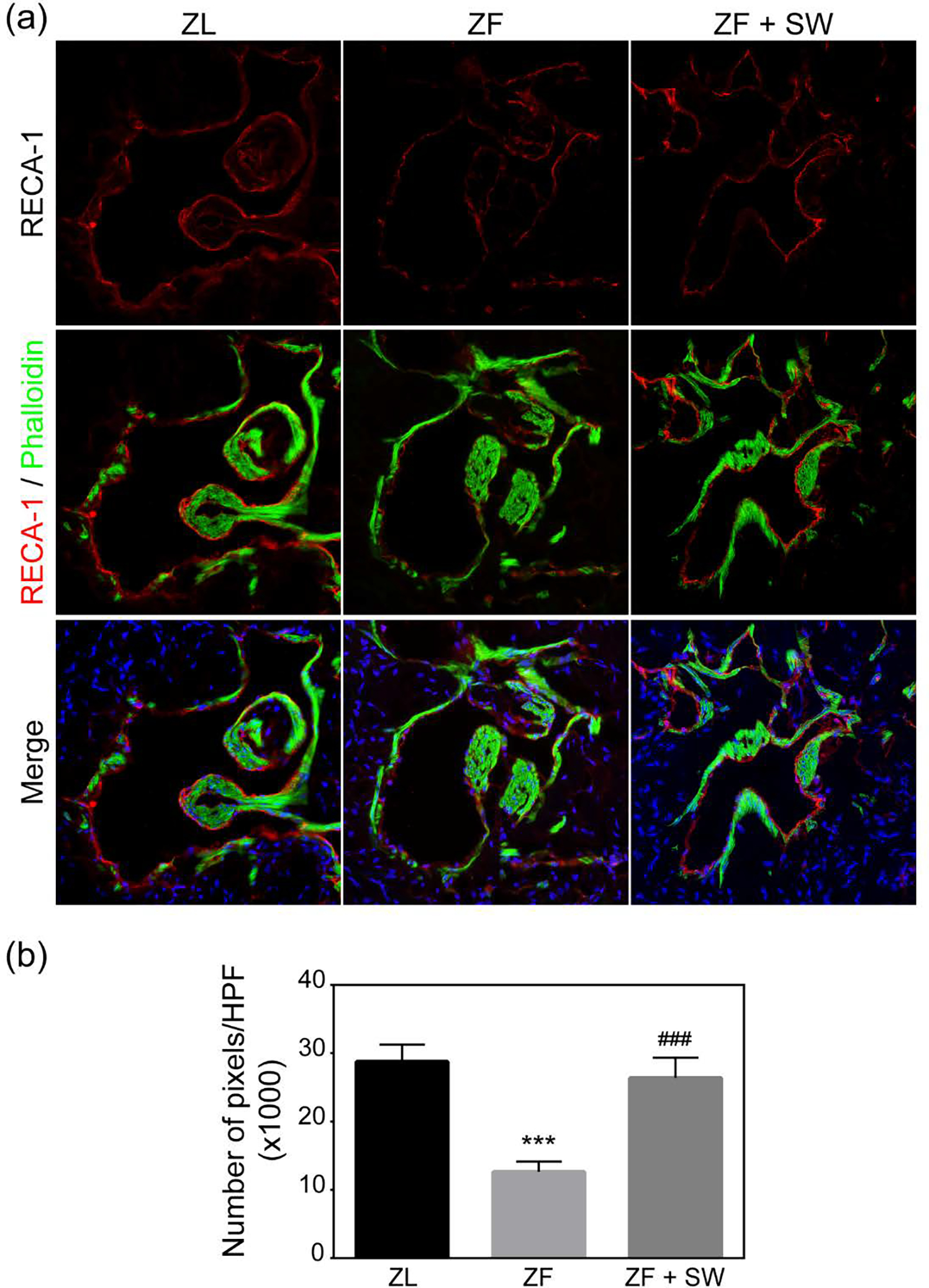
RECA-1 staining of the cavernosal sinusoids. (a) Representative images of the endothelial lining of sinusoids. Rat penile tissues stained with Alexa Fluor 488 phalloidin and with Alexa Fluor 594 anti-RECA-1 antibody. (b) Quantification of RECA-1 staining in pixels/HPF. *** P <0.01 compared to the ZL group; ### P < 0.01 compared to the ZF group.
Li-ESWT Suppresses Lipid Droplet Accumulation in the Corpus Cavernosum of ZF Rats
Abundant lipid droplets accumulated within the corpus cavernosum of the ZF rats. Compared with ZL rats, ZF rats had a higher percentage of LipidTOX- positive area in the penile tissue (4.85 ± 0.64 vs 25.62 ± 2.48, p < 0.05). This is one possible mechanism to account for OAED. Few lipid droplets were observed in the corpus cavernosum of the ZL rats. Figure 5 shows that lipid droplets infiltrated the cavernous sinusoids in the ZF group but that Li-ESWT treatment reduced the total amount of lipid droplets in the ZF + SW group’s cavernous tissue (6.99 ± 1.38).
Figure 5.
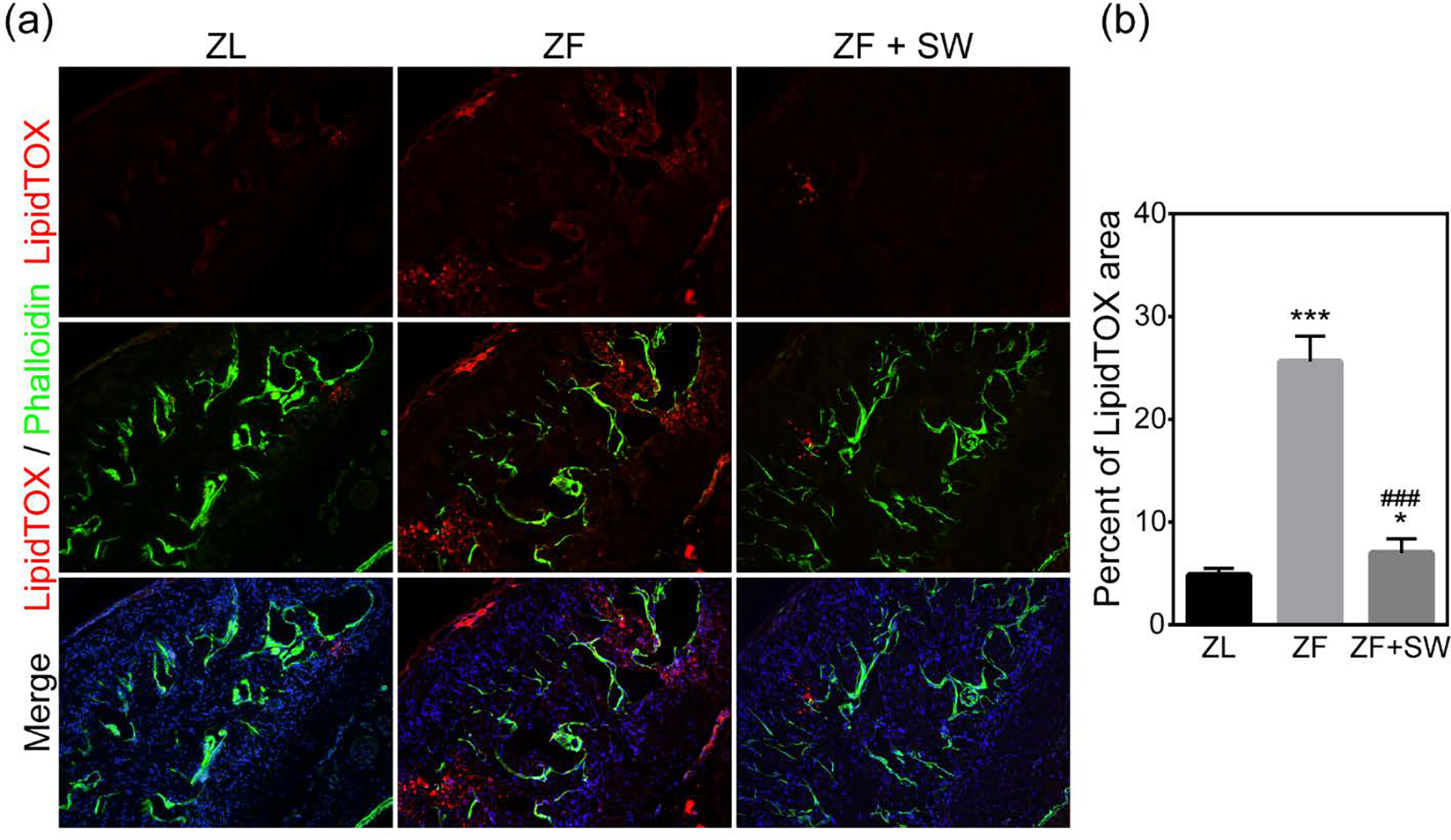
Lipid accumulation in the corpus cavernosum for ZL, ZF, and ZF + SW groups. (a) Representative images of LipidTOX (red) and smooth muscle stained with phalloidin (green). Original magnification, X100. (b) Quantification of LipidTOX in the whole section. * P < 0.05, *** P < 0.01 compared to the ZL group; ### P < 0.01 compared to the ZF group.
Li-ESWT Activates EdU+ Progenitor/Stem Cells in the Corpus Cavernosum
Progenitor cells or cells with stem properties are characterized by the ability to retain nucleotide analogs, such as BrdU or EdU, for a prolonged period of time. Therefore, we examined EdU+ cells in the penile cavernous tissue to identify and monitor penile progenitor cells. The total number of EdU-+ cells in the ZF group showed a conspicuous decrease (P < 0.05) in comparison to the ZL group. Moreover, after Li-ESWT treatment, the total number of EdU+ cells increased dramatically in the ZF + SW group.
Li-ESWT Promotes Endogenous Stem/Progenitor Cells Proliferation in ZF Rats
To test whether the increase in EdU+ cells was the result of resident stem cell proliferation, we assessed the number of cells staining positive for H3P, a nuclear protein indicative of cell proliferation. We noted no significant difference between the ZL and ZF groups, but there was an obvious increase in the number of H3P+ cells in the ZF + SW group as compared to the ZL and ZF control groups (Figures 7a and 7b).
Figure 7.
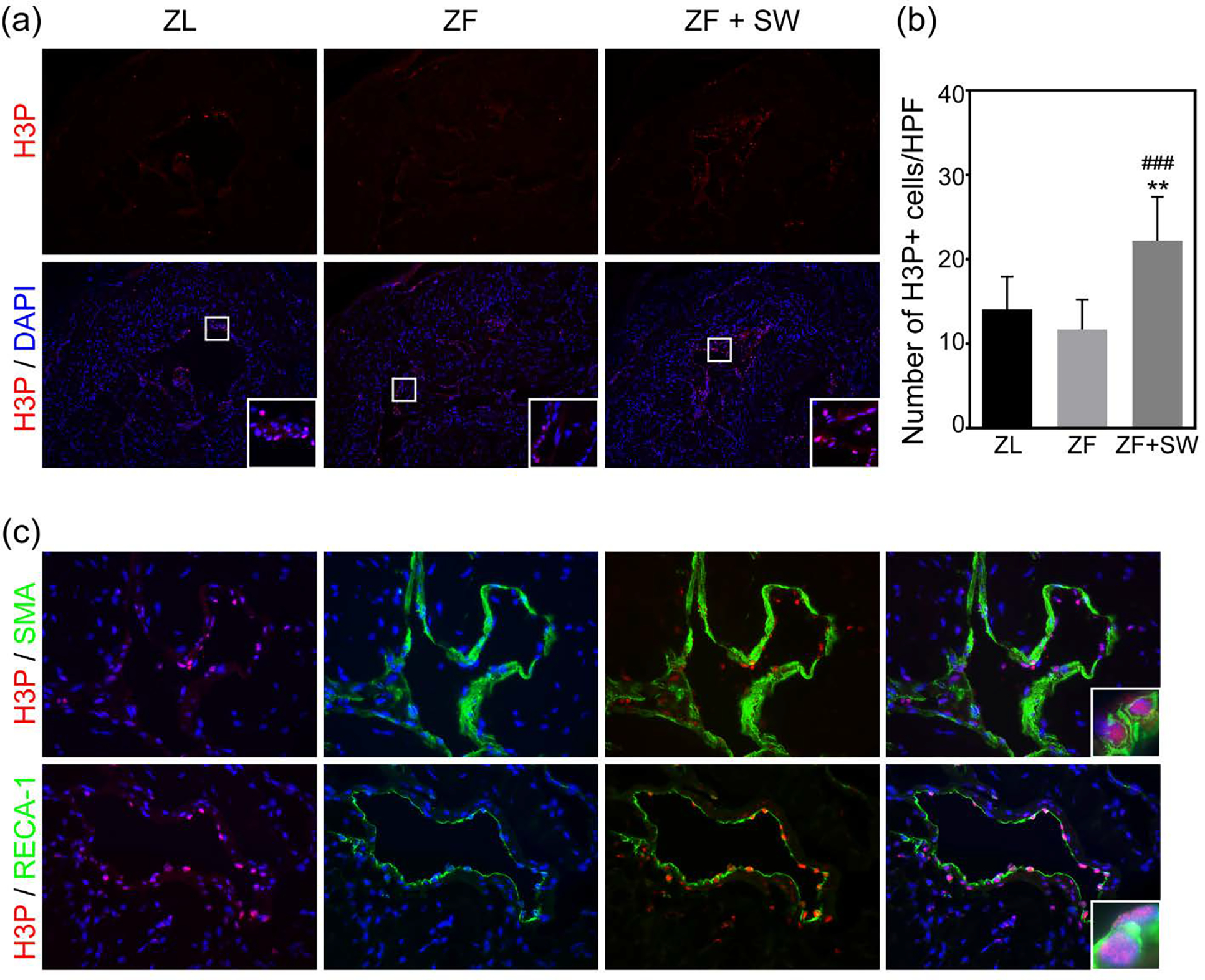
Li-ESWT promoted endogenous stem/progenitor cell proliferation and differentiation. (a) Representative images of H3P staining (red) in the corpus cavernosum. Original magnification, X100. (b) Quantification of H3P+ cells/HPF. (c) Immunofluorescence staining of cavernous tissue using H3P (red) and SMA (green) /RECA-1 (green), original magnification, X400. *** P < 0.01 compared to the ZL group; ### P < 0.01 compared to the ZF group.
Activated H3P+ cells were tracked by staining with cell markers for differentiated cells. Dividing cells (H3P+) co-expressing ether α-SMA or RECA-1 were found in the cavernosal sinusoids and sinusoidal endothelial lining, respectively (Figure 7c).
Discussion
In this study, we utilized a genetic obesity animal model to demonstrate that obesity alone significantly impairs erectile function by inducing penile smooth muscle atrophy, endothelial dysfunction, and lipid accumulation. Li-ESWT ameliorated erectile dysfunction in this animal model by activating penile endogenous stem cells and stimulating these cells to proliferate and differentiate into functional cells in the corpus cavernosum.
Obesity is associated with cardiovascular disease, hypertension, type 2 diabetes mellitus, hyperlipidemia, and stroke. Erectile dysfunction (ED) is regarded as a benchmark for the aforementioned diseases. However, the relationship between obesity and ED is controversial. In a retrospective study, Chung et al. investigated erectile capacity in men with ED according to their relative weights and found that obesity in itself is not an underlying risk factor for impotence [16]. Several epidemiologic studies suggest that obesity is independently related to the likelihood of ED [17]. In addition, treating obesity has been proven to restore erectile function in OAED patients [4, 18]. In a recent study, Villalba and his colleagues used an insulin-resistant obese rat model to investigate penile artery changes and reported a reduction in the internal diameter of the penile artery as well as a reduction in the vasodilatory response to the PDE5 inhibitors [8]. These findings indicate that obesity may influence male sexual function via direct and/or indirect mechanisms.
Our current study investigated the erectile function in obese rats in a pre-diabetic state and revealed that the fatty rats had impaired erectile function versus lean rats of the same age. Insulin resistance and lipid accumulation in the corpus cavernosum of the ZF rats resulted in penile smooth muscle atrophy and endothelial damage. These histologic changes may account for the decreased sexual function in the ZF rats. From 6 weeks of age, the obesity phenotype in the ZF rats began to emerge. At 10 weeks of age, the ZF rats exhibited marked insulin-resistance. In obese subjects, adipose tissue secretes a number of cytokines, including leptin, tumor necrosis factor alpha (TNFα), interleukin (IL)-6, IL-1β, and plasminogen activator inhibitor-1 (PAI-1), in abnormal proportions [19]. These adipokines can trigger insulin resistance by modulating insulin signaling. JUN N-terminal kinase 1 (JNK1) is a protein kinase of the MAPK family that is activated by many inflammatory stimuli including TNFα and IL-6 [20]. Activation of JUK1 leads to serine phosphorylation of insulin receptor substrate-1 (IRS-1) which induces insulin resistance. In addition, free fatty acids (FFAs) have been proposed to have an important role in the pathogenesis of obesity-related insulin resistance [21]. Plasma FFA are commonly elevated in obese individuals due to the expansion in fat mass. FFAs serve as signaling molecules that activate protein kinase C (PKC), which can also result in phosphorylation of IRS-1. Furthermore, increases in FFAs promote fatty acid oxidation and storage and inhibit glucose oxidation, leading to further deleterious effects on insulin sensitivity [22].
As evidenced by significant LipidTOX staining in the penile tissues of ZF rats in the current study, obesity-associated increases in FFAs and other lipids result in the storage of ectopic fat as lipid droplets in penile tissues. Accumulation of intramyocellular lipids (IMCLs) is associated with deterioration of insulin sensitivity. In the current study, the majority of lipids in the ZF rat penis were extramyocellular lipids (EMCLs), possibly contributing to an impairment of veno-occlusion needed for erection [23]. Collectively, chronic systemic inflammation and elevated FFAs initiate insulin resistance as well as lipid accumulation in obesity. This results in cavernosal smooth muscle atrophy, endothelial dysfunction, and significantly decreases erectile function in ZF rats.
Li-ESWT has recently gained favor in the treatment of ED and may modify the underlying pathophysiology of ED [11, 24, 25]. In 2013, our group first reported the application of Li-ESWT in a diabetic ED rat model. Since then several researchers from different groups have investigated the efficacy of Li-ESWT in various pathologic subtypes of ED [11, 25, 26]. In several clinical trials Li-ESWT treatment achieved promising results with improvements in cavernosal hemodynamics and the potential to enable penile rehabilitation without drug therapy [27, 28]. In these studies, researchers suggested that Li-ESWT ameliorated erectile dysfunction via restoring endothelial and smooth muscle content, promoting angiogenesis. However, the mechanisms underlying the penile regeneration were not elucidated. To address how Li-ESWT restores the cavernosal architecture, we used EdU to track penile endogenous stem/progenitor cells in the current study. Consistent with previous studies, Li-ESWT treatment restored erectile function and dramatically reversed the obesity-associated loss of smooth muscle and endothelium content. Moreover, since EdU was administrated immediately after birth, label-retaining cells (LRCs) are regarded as stem cells within the penile tissues. In obese individuals, the number of LRCs was significantly decreased, and with Li-ESWT treatment more LRCs were observed in the corpus cavernosum. Our recent study indicates that Li-ESWT activates endogenous penile stem cells in situ in both young and middle-aged Sprague-Dawley rats [12]. Our current data confirms that in the pathological state of obesity, Li-ESWT activates penile stem cells and promotes penile regeneration.
As we previously reported, there are various kinds of cells in the penis including terminally differentiated cells and stem/progenitor cells [13]. Most cavernous smooth muscle cells and fibroblasts are terminally differentiated and cannot be activated to proliferate [12]. Histone 3 phosphorylation at Ser10 is a conserved event that occurs during mitosis and serves as a specific marker for mitotically active cells [29]. To figure out the fate of activated endogenous stem cells, we performed H3P staining and staining for H3P+ cells co-expressing α-SMA or RECA-1. We found that the number of H3P+ cells was significantly higher in the ZF + SW penis following Li-ESWT treatment compared with the ZF group, indicating more stem/progenitor cells were activated to proliferation. More importantly, these cells committed to differentiate into functional cells, as evidenced by co-expression of α-SMA/RECA-1 and H3P in ZF rats treated with Li-ESWT. Taken together, Li-ESWT activated endogenous stem/progenitor cells in the penile tissues, and these activated cells underwent differentiation toward smooth muscle cells and endothelial cells, participating in penile tissue repair and regeneration.
Another possible mechanism for the recovery of erectile function in ZF + SW rats is the reduction of lipid accumulation caused by Li-ESWT. It has been demonstrated that Li-ESWT could induce adenosine 5′-triphosphate (ATP) release [30]. FFAs serve as the major energy substrate in obesity [22], thus we speculate that Li-ESWT might accelerate FFA consumption while releasing ATP. On the other hand, ATP has been identified as a trigger of the biological effects of shock wave treatment, which can activate p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase 1/2 (ErK1/2) [31]. These signaling pathways are involved in cell proliferation and proper differentiation and could participate in penile tissue regeneration.
In summary, the current study showed that obesity is associated with ED and pathophysiological consequences including loss of smooth muscle, endothelial dysfunction, and lipid accumulation. Li-ESWT was able to partially but significantly restore erectile function and diminish these pathological changes in ZF rats. In addition, we showed that the beneficial effects of Li-ESWT appeared to involve enhanced endogenous stem/progenitor cell proliferation and differentiation. However, it should be cautioned that that present study has limitations and the findings require further validation. Our data did not investigate the exact mechanisms of interaction between Li-EWST and tissue. In future studies, physicists, biologists, and clinicians should collaborate to delve deeper into the basis of Li-ESWT’s biological effects. Moreover, because chronic systemic inflammation is thought to play a vital role in obesity-related diseases, future research addressing the effects of Li-ESWT on macrophages is warranted.
Supplementary Material
Figure 6.
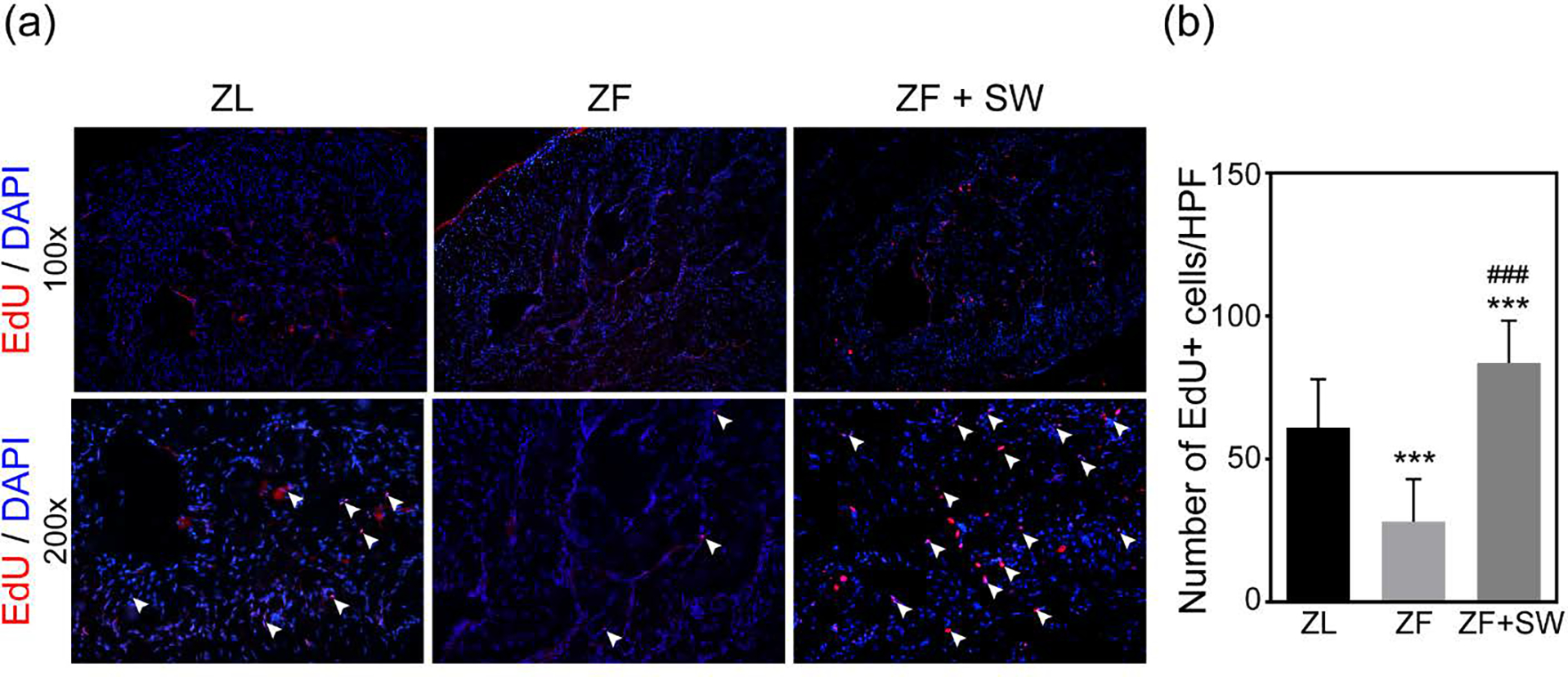
Evaluation of label-retaining cells (LRCs). (a) Representative images of EdU+ cells (red) in the corpus cavernosum for ZL, ZF, and ZF + SW groups. Original magnification, X100 and X200. (b) Total number of EdU+ cells/HPF in all groups. *** P < 0.01 compared to the ZL group; ### P < 0.01 compared to the ZF group.
Acknowledgements
This work is supported in part by the China Scholarship Council under the grant CSC 20160616110 and a grant from the SMSNA P0522946.
Abbreviations
- OAED
Obesity-associated erectile dysfunction
- PDE5
Phosphodiesterase type 5
- Li-ESWT
Low-intensity extracorporeal shock wave therapy
- ZF/ZL
Zucker fatty/lean rats
- ICP
Intracavernosal pressure
- MAP
Mean arterial pressure
Footnotes
Conflicts of Interest
None declared.
References
- 1.Arroyo-Johnson C, Mincey KD. Obesity Epidemiology Worldwide. Gastroenterol Clin North Am. 2016; 45:571–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Lin G, Lee YC, et al. Transgenic animal model for studying the mechanism of obesity-associated stress urinary incontinence. BJU Int. 2017; 119:317–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013; 381:153–65 [DOI] [PubMed] [Google Scholar]
- 4.Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004; 291:2978–84 [DOI] [PubMed] [Google Scholar]
- 5.Park NC, Kim TN, Park HJ. Treatment Strategy for Non-Responders to PDE5 Inhibitors. World J Mens Health. 2013; 31:31–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang H, Bae WJ, Kim SJ, et al. The herbal formula KH-204 is protective against erectile dysfunction by minimizing oxidative stress and improving lipid profiles in a rat model of erectile dysfunction induced by hypercholesterolaemia. Bmc Complem Altern M. 2017; 17:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahman NU, Phonsombat S, Bochinski D, Carrion RE, Nunes L, Lue TF. An animal model to study lower urinary tract symptoms and erectile dysfunction: the hyperlipidaemic rat. BJU Int. 2007; 100:658–63 [DOI] [PubMed] [Google Scholar]
- 8.Villalba N, Martinez P, Briones AM, et al. Differential structural and functional changes in penile and coronary arteries from obese Zucker rats. Am J Physiol Heart Circ Physiol. 2009; 297:H696–707 [DOI] [PubMed] [Google Scholar]
- 9.Sanchez A, Contreras C, Martinez P, et al. Endothelin A (ET(A)) receptors are involved in augmented adrenergic vasoconstriction and blunted nitric oxide-mediated relaxation of penile arteries from insulin-resistant obese zucker rats. J Sex Med. 2014; 11:1463–74 [DOI] [PubMed] [Google Scholar]
- 10.Ioppolo F, Rompe JD, Furia JP, Cacchio A. Clinical application of shock wave therapy (SWT) in musculoskeletal disorders. Eur J Phys Rehabil Med. 2014; 50:217–30 [PubMed] [Google Scholar]
- 11.Li H, Matheu MP, Sun F, et al. Low-energy Shock Wave Therapy Ameliorates Erectile Dysfunction in a Pelvic Neurovascular Injuries Rat Model. J Sex Med. 2016; 13:22–32 [DOI] [PubMed] [Google Scholar]
- 12.Lin G, Reed-Maldonado AB, Wang B, et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. J Sex Med. 2017; 14:493–501 [DOI] [PubMed] [Google Scholar]
- 13.Lin G, Alwaal A, Zhang X, et al. Presence of stem/progenitor cells in the rat penis. Stem Cells Dev. 2015; 24:264–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan Y, Li M, Wang T, et al. Taurine Supplementation Improves Erectile Function in Rats with Streptozotocin-induced Type 1 Diabetes via Amelioration of Penile Fibrosis and Endothelial Dysfunction. J Sex Med. 2016; 13:778–85 [DOI] [PubMed] [Google Scholar]
- 15.Breyer BN, Fandel TM, Alwaal A, et al. Comparison of spinal cord contusion and transection: functional and histological changes in the rat urinary bladder. BJU Int. 2017; 119:333–41 [DOI] [PubMed] [Google Scholar]
- 16.Chung WS, Sohn JH, Park YY. Is obesity an underlying factor in erectile dysfunction? European urology. 1999; 36:68–70 [DOI] [PubMed] [Google Scholar]
- 17.Tsai AG, Sarwer D. Obesity and Erectile Dysfunction. Obesity and Weight Management 2009; 5:183–5 [Google Scholar]
- 18.Corona G, Rastrelli G, Filippi S, Vignozzi L, Mannucci E, Maggi M. Erectile dysfunction and central obesity: an Italian perspective. Asian J Androl. 2014; 16:581–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traish AM, Feeley RJ, Guay A. Mechanisms of obesity and related pathologies: androgen deficiency and endothelial dysfunction may be the link between obesity and erectile dysfunction. FEBS J. 2009; 276:5755–67 [DOI] [PubMed] [Google Scholar]
- 20.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007; 21:1443–55 [DOI] [PubMed] [Google Scholar]
- 21.Frohnert BI, Jacobs DR Jr., Steinberger J, Moran A, Steffen LM, Sinaiko AR. Relation between serum free fatty acids and adiposity, insulin resistance, and cardiovascular risk factors from adolescence to adulthood. Diabetes. 2013; 62:3163–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007; 10:142–8 [DOI] [PubMed] [Google Scholar]
- 23.Alwaal A, Wang L, Zaid UB, Lin G, Lue TF. Case series of lipid accumulation in the human corpus cavernosum. Medicine (Baltimore). 2015; 94:e550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013; 10:738–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Assaly-Kaddoum R, Giuliano F, Laurin M, et al. Low Intensity Extracorporeal Shock Wave Therapy Improves Erectile Function in a Model of Type II Diabetes Independently of NO/cGMP Pathway. J Urol. 2016; 196:950–6 [DOI] [PubMed] [Google Scholar]
- 26.Jeon SH, Shrestha KR, Kim RY, et al. Combination Therapy Using Human Adipose-derived Stem Cells on the Cavernous Nerve and Low-energy Shockwaves on the Corpus Cavernosum in a Rat Model of Post-prostatectomy Erectile Dysfunction. Urology. 2016; 88:226 e1–9 [DOI] [PubMed] [Google Scholar]
- 27.Chung E, Cartmill R. Evaluation of clinical efficacy, safety and patient satisfaction rate after low-intensity extracorporeal shockwave therapy for the treatment of male erectile dysfunction: an Australian first open-label single-arm prospective clinical trial. BJU Int. 2015; 115 Suppl 5:46–9 [DOI] [PubMed] [Google Scholar]
- 28.Tsai CC, Wang CJ, Lee YC, et al. Low-Intensity Extracorporeal Shockwave Therapy Can Improve Erectile Function in Patients Who Failed to Respond to Phosphodiesterase Type 5 Inhibitors. Am J Mens Health. 2017:1557988317721643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin GN, Choi MJ, Kim WJ, et al. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc Natl Acad Sci U S A. 2014; 111:E2731–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D, Junger WG, Yuan C, et al. Shockwaves induce osteogenic differentiation of human mesenchymal stem cells through ATP release and activation of P2X7 receptors. Stem Cells. 2013; 31:1170–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weihs AM, Fuchs C, Teuschl AH, et al. Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation. J Biol Chem. 2014; 289:27090–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


