ABSTRACT
Introduction
Due to the anatomical proximity and functional connection, vestibular symptoms (VS) are common in temporomandibular disorders (TMD). However, it is not known whether the degree of severity of TMD affect the report of associated vestibular symptoms.
Objective
To evaluate associations of demographic, clinical and functional factors, as well as report of VS, with the severity of TMD.
Method
Cross-sectional study carried out at a higher education institution in Salvador, Bahia, Brazil. After approval by the Ethics Committee of Hospital Santo Antônio (CAAE 81517317.2.0000.0047), the collection team applied the Dizziness Handicap Inventory (DHI), the Fonseca Anamnestic Questionnaire (QAF) and the Research Diagnostic Criteria for Temporomandibular Disorders axis II questions (RDC/TMD) in employees, teachers and students. Statistical tests of association with the Chi-square, t test for independent samples and ANOVA (alpha 5% and 80% power) were performed.
Results
The sample of 623 participants identified 333 (63.7%) people with TMD and 418 (79.9%) with VS. Females were associated with both temporomandibular dysfunction and vestibular symptoms. The degree of severity of the TMD showed a strong correlation with VS (p < 0.001).
Conclusion
In the studied sample, it was observed that the greater the degree of severity of the TMD, the greater the VS, which confirms the need to evaluate both systems in symptomatic patients for each of the clinical conditions.
KEYWORDS: Vestibulopathies, temporomandibular disorders, prevalence, dizziness
Introduction
Temporomandibular disorder (TMD) designates a group of health conditions that affect the temporomandibular joint (TMJ), masticatory muscles and associated structures [1]. The prevalence of TMD in adults affects about 50% of the world population [2]. Its etiology is complex and multifactorial, and may be associated with a combination of psychological, physiological, structural, postural and genetic factors [3,4]; and with morphofunctional, neuromuscular, traumatic, degenerative and sociodemographic aspects.
Individuals with TMD may present orofacial pain in the preauricular region and masticatory muscles, in addition to headaches mostly classified as tension headaches, cervicogenic, and migraine [5,6]. Vestibular disorders are also prevalent in the general population, affecting 20–30% of adults [7]. Vestibular Symptoms (VS) include, vertigo, tinnitus and imbalance, ear pain or earache, a sensation of ear fullness, reduced hearing acuity or hearing loss, tinnitus, dizziness and vertigo [5]. These symptoms mentioned above are often related to disorders of the vestibular system, whose function is to promote spatial orientation and balance of the human body [5].
The prevalence of otologic symptoms in the world population ranges from 10 to 31%, but it increases to 85% in patients with TMD [8]. Due to the anatomical proximity and functional connection, some signs and symptoms are common in temporomandibular and vestibular disorders [6]. Both TMD and isolated VS generate symptoms that affect the quality of life of those affected [5,9]. The presence of both these conditions can, therefore, potentiate symptoms and have a more intense impact on quality of life [9].
Despite these epidemiological findings, it is not known whether the degree of TMD in persons influences the report of VS. Their identification can help create preventive, therapeutic and health rehabilitation strategies for people who suffer from these problems. Therefore, this study aimed to test the hypothesis that the greater the degree of severity of TMD, the greater the associated vestibular signs and symptoms.
Methods
A cross-sectional observational study was conducted according to the STROBE checklist for sectional studies (available https://www.strobe-statement.org/checklists/) at a higher education institution in Salvador, Bahia, Brazil. It is a private institution with 3,100 university students, 322 professors and 259 technical-administrative employees.
The research was approved by the Research Ethics Committee of the Hospital Santo Antônio, Obras Sociales Irmã Dulce (CAAE 81517317.2.0000.0047; opinion 3,017,541), and followed all the recommendations of the National Health Council of Brazil and the Declaration of Helsinki for research involving beings humans.
Volunteers over 18 years of age, male and female gender, with a regular relationship with the institution (students with active enrollments and employees registered in the human resources department) and who signed an informed consent form, were included. Individuals with difficulties in understanding the instruments applied, expressed in more than 10% of the answers to the blank questionnaires, were excluded. Answers were left blank after repeating the same question three times by the examiner.
Between March and August 2018, the previously trained collection team applied a sociodemographic questionnaire developed by the authors, the Dizziness Handicap Inventory (DHI) (α = 0.89) [10], the Fonseca Anamnestic Questionnaire (QAF) and Axis II questions of the Research Diagnostic Criteria for Temporomandibular Disorders (DC/TMD).
The Brazilian version of the Dizziness Handicap Inventory (DHI) [11] aims to assess the influence of dizziness on the quality of life, considering the physical, functional and emotional aspects of each individual. In this instrument, the final score will be obtained by the sum of the points obtained in all aspects, with a minimum score of 0 and a maximum of 100 points. The option ‘yes’ (4 points) should be marked by the presence of severe symptoms and difficulties, while the options ‘sometimes’ (2 points) and ‘no’ (0 points) should be marked, respectively, by the occasional presence. and absence of these same symptoms and difficulties.
The QAF proposes to characterize the severity of TMD symptoms, classifying them into four categories: no TMD (0 to 15 points), mild TMD (20 to 40 points), moderate (45 to 65 points) or severe (70 to 100 points) [12]. The QAF was tested in patients with TMD, thoroughly and obtained a correlation of 95% with the clinical index of Helkimo, which was one of the pioneers in the evaluation of questionnaires for TMD. Questions number 23 to number 30 of the Brazilian version of the DC/TMD were used to indicate the presence of TMD signs and symptoms.
The criteria used to identify participants with vestibular symptoms were the presence of at least one symptom, such as dizziness, imbalance, tinnitus or nystagmus. Temporomandibular symptoms, on the other hand, were defined by the presence of at least two classic signs and/or symptoms of TMD, being essential the symptom of orofacial pain in the last month or the presence of a joint noise.
The data obtained with the applied instruments were tabulated and analyzed using the Statistical Package for Social Sciences (SPSS) version 26.0 for Windows. Statistical tests of association with the Chi-square, t-test for independent samples and ANOVA (alpha 5% and 80% power) were performed.
To examine the association between the sociodemographic profile of the sample regarding age, gender, marital status, self-reported skin color and function at the institution with the degrees of severity of the temporomandibular disorder (Fonseca anamnestic questionnaire), a chi-square test was used. To test the sociodemographic differences in total scores and physical, functional and emotional subscales obtained using the Brazilian version of the Dizziness Handicap Inventory (DHI), independent sample t-test or ANOVA (depending on groups size) were used. ANOVA was also used to compare the differences of the severity degrees of temporomandibular disorders (Fonseca anamnestic questionnaire) among the subscales of the Brazilian version of the Dizziness Handicap Inventory. Concerning missing values, pairwise deletion was chosen to handle missing data since its probability on the dependent variable is unrelated to other independent variables as well as the dependent variable itself [13].
The sample size was calculated using the Lee calculator (Epidemiology and Statistics Laboratory of the University of São Paulo) and determined a minimum number of 339 participants. For this, a study was used the whose prevalence of dizziness was 85% in a population of 20 university students with TMD [14], with a margin of error and absolute estimation precision of 5% and a confidence level of 99%.
Results
623 volunteers were interviewed, of which 267 (52.9%) were students, 87 (17.2%) were teachers, 93 (18.4%) were administrative staff and 58 (11.5%) were general service assistants. The average age of the sample was 28.6 ± 9.6 years in which women (61.7 %), brown (43.5%), black (36.9%) and single (76.3%) prevailed. The characterization of the sample is described in Table 1.
Table 1.
Characterization of the sample of a higher education institution in Salvador, Bahia, Brazil.
| Sample Features N % | |||
|---|---|---|---|
| Age range (n = 492) | |||
| 18y to 21y | 135 | 27.4 | |
| 22y to 25y | 124 | 25.2 | |
| 26y to 34y | 115 | 23.4 | |
| 35y to 65y | 118 | 24.0 | |
| Gender (n = 509) | |||
| Male | 195 | 38.3 | |
| Feminine | 314 | 61.7 | |
| Marital status (n = 502) | |||
| Not married | 383 | 76.3 | |
| Married | 102 | 20.3 | |
| Separated/Divorced | 17 | 3.4 | |
| Skin Color (n = 501) | |||
| White | 79 | 15.8 | |
| Yellow | 17 | 3.4 | |
| Red | 2 | 0.4 | |
| Dun | 218 | 43.5 | |
| Black | 185 | 36.9 | |
| University Function (n = 505) | |||
| Student | 267 | 52.9 | |
| Teacher | 87 | 17.2 | |
| Administrative Technician | 93 | 18.4 | |
| General Services | 58 | 11.5 | |
| TMD* (n = 332) | |||
| Without TMJ | 89 | 26.8 | |
| Light TMJ | 167 | 50.3 | |
| Moderate TMJ | 57 | 17.2 | |
| Severe TMJ | 19 | 5.7 | |
| DHI**(n = 505) | Average | Standard deviation | |
| Total | 10.2 | 14.4 | |
| Physical aspects | 3.6 | 4.7 | |
| Emotional aspects | 4.0 | 6.3 | |
| Functional aspects | 2.0 | 4.0 | |
*Temporomandibular Dysfunction; **Dizziness Handicap Inventory; Legend: Physical aspects – questions 1, 4, 8, 11, 13, 17 and 25; Functional aspects – questions 3, 5, 6, 7, 12, 14, 16, 19 and 24; Emotional aspects – questions 2, 9, 10, 15, 18, 20, 21, 22 and 23.
Of the 523 study participants, 332 (63.4%) were classified as having TMD by the QAF, most of them with light TMD (50.3%). As for vestibular signs and symptoms, 418 (79.9%) participants reported dizziness, tinnitus or nystagmus, alone or in the association. The association between VS and QoL was then analyzed, with no significant association (p > 0.05) according to the DHI. Crossing sociodemographic variables with VS, using the Chi-square test (bivariate analysis), associations were identified between VS of head movement with female gender (p < 0.001), with young adults aged between 18 and 25 years (p < 0.001) and in students (p < 0.001). The presence of tinnitus was associated with singles and separated (p < 0.001).
When performing an analysis on the association between degrees of TMD and sociodemographic variables (Table 2), an association with gender (p = 0.002) and with brown and black skin color (p = 0.006) was confirmed.
Table 2.
Analysis of the association of sociodemographic variables (age group, gender, marital status, skin color and function) (%) with the degrees of severity of the temporomandibular disorder (N = 333).
| Without TMD | Light TMJ | Moderate TMJ | Severe TMJ | pa | ||
|---|---|---|---|---|---|---|
| Gender | Male | 49.4 | 32.9 | 28.1 | 10.5 | 0.002 |
| Feminine | 50.6 | 67.1 | 71.8 | 89.5 | ||
| Age Range | 18–21 | 20.7 | 34.0 | 30.2 | 33.3 | 0.251 |
| 22–25 | 23.0 | 27.2 | 32.1 | 38.9 | ||
| 26–34 | 32.2 | 20.4 | 22.6 | 16.7 | ||
| 35–65 | 24.1 | 18.5 | 15.1 | 11.1 | ||
| Marital status | Not married | 75.9 | 83.6 | 82.5 | 72.2 | 0.172 |
| Married | 20.7 | 16.4 | 14.0 | 27.8 | ||
| Divorced | 3.4 | 0.0 | 3.5 | 0.0 | ||
| Skin Color | White | 10.2 | 12.8 | 10.7 | 22.2 | 0.006 |
| Yellow | 2.3 | 3.0 | 8.9 | 5.6 | ||
| Dun | 52.3 | 43.3 | 35.7 | 44.4 | ||
| Black | 35.2 | 40.9 | 44.6 | 22.2 | ||
| Red | 0.0 | 0.0 | 0.0 | 5.6 | ||
| Occupation | Student | 43.8 | 61.2 | 66.1 | 63.2 | 0.175 |
| Teacher | 9.0 | 5.5 | 5.4 | 10.5 | ||
| Administrative | 32.6 | 20.6 | 21.4 | 10.5 | ||
| General Services | 14.6 | 12.7 | 7.1 | 15.8 |
a = chi-square test; TMD = temporomandibular disorder
When comparing the DHI scores with the sociodemographic variables, associations of the total score and the physical and functional domains with female gender were identified; in addition to the teaching function with all domains (physical, functional and emotional) and with the total score (Table 3).
Table 3.
Analysis of the association of independent variables (age group, gender, marital status, skin color, function) through mean values, the standard deviation of total scores and physical, functional and emotional subscales obtained using the Brazilian version of the Dizziness Handicap Inventory (DHI).
| DHI score | Physical DHI | Functional DHI | Emotional DHI | ||
|---|---|---|---|---|---|
| M± DP | M± DP | M± DP | M± DP | ||
| Gender | Male | 7.64 ± 12.0 | 2.47 ± 4.09 | 3.21 ± 5.35 | 1.23 ± 0.92 |
| Feminine | 11.8 ± 15.6 | 4.30 ± 4.84 | 4.45 ± 6.79 | 2.40 ± 0.83 | |
| pa | 0.001 | <0.001 | 0.032 | 0.372 | |
| Marital status | Not married | 7.90 ± 8.91 | 3.80 ± 3.32 | 4.20 ± 5.61 | 2.20 ± 3.58 |
| Married | 6.74 ± 7.76 | 2.75 ± 2.61 | 1.75 ± 2.49 | 1.14 ± 2.69 | |
| Divorced | 11.25 ± 10.99 | 2.33 ± 3.20 | 5.00 ± 7.56 | 1.47 ± 3.03 | |
| pb | 0.221 | 0.615 | 0.493 | 0.793 | |
| Skin Color | White | 7.80 ± 8.05 | 3.64 ± 3.56 | 4.91 ± 6.22 | 1.64 ± 2.34 |
| Yellow | 5.25 ± 5.43 | 4.00 ± 1.74 | 4.00 ± 1.12 | 6.00 ± 1.42 | |
| Dun | 7.65 ± 8.80 | 2.89 ± 2.84 | 3.11 ± 5.11 | 1.75 ± 4.20 | |
| Black | 8.13 ± 9.44 | 1.33 ± 1.16 | NA | 0.67 ± 1.16 | |
| Red | 11.00 ± 4.24 | NA | NA | NA | |
| pb | 0.764 | 0.705 | 0.579 | 0.530 | |
| Occupation | Student | 12.1 ± 15.4 | 4.39 ± 4.91 | 4.56 ± 6.74 | 2.32 ± 4.20 |
| Teacher | 5.52 ± 10.1 | 2.21 ± 3.59 | 2.02 ± 4.52 | 0.92 ± 2.35 | |
| Administrative | 8.61 ± 13.1 | 3.00 ± 4.50 | 3.23 ± 5.35 | 1.89 ± 3.91 | |
| General Services | 10.5 ± 15.9 | 2.85 ± 4.47 | 5.26 ± 7.37 | 2.59 ± 5.25 | |
| pb | 0.002 | <0.001 | 0.003 | .0.028 |
a = t-test for independent samples; b = Anova; NA = not applicable since there was no response to these items; DHI = Dizziness Handicap Inventory.
When comparing TMD degrees by QAF with DHI scores, it was found that all domains were significantly associated with TMD severity (Figure 1).
Figure 1.
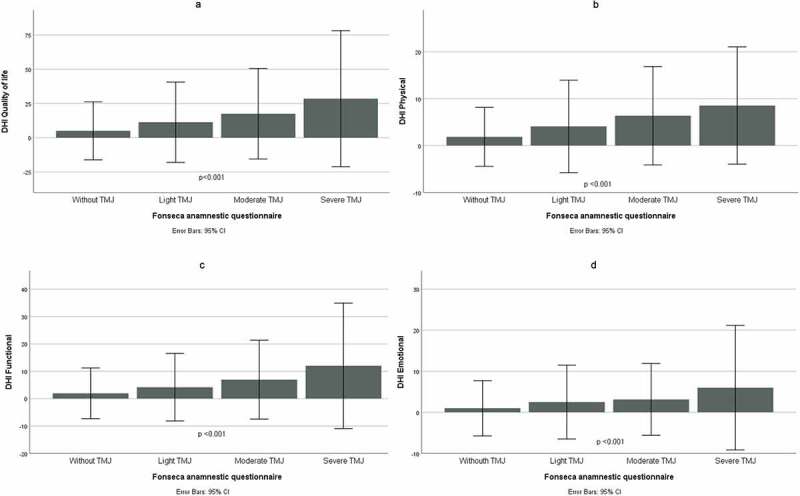
Comparison of the severity degrees of temporomandibular disorders (Fonseca anamnestic questionnaire), with the Brazilian version of the Dizziness Handicap Inventory (n = 418) in quality of life (A), physical (B), functional (C) and emotional (D) dimensions.
Discussion
This cross-sectional study confirmed the hypothesis that the greater the degree of TMD severity, the greater the number of reported VS. In a sample of adults from a higher education institution, it was found that both in students, as well as in professors and employees who attend TMD, symptoms of dizziness, vertigo, tinnitus and balance problems are found, and vice versa.
The prevalence of TMD (63.7%) and VS (79.9%) can be considered high in this sample. This result is similar to that found in the study by Machado et al. [14] who observed a prevalence of 85% of TMD and 75% of otological symptoms in a sample of 20 women. Corroborating the conclusions of Silveira et al. [15], our findings confirm that more than 50% of the population has at least one sign and/or symptom of TMD. Regarding dizziness, the results of several studies reveal a high prevalence in the world population, which agrees with our findings [16–18]. Also, Paulino et al. [19] found, in a sample similar to the present study, that 89.8% of the participants presents some level of TDM, of which 50.2% had mild TMD, 33.0% moderate and only 6.6% severe.
Despite being highly prevalent, vestibular symptoms did not significantly affect the quality of life of the participants. It worth to remark that dizziness can be a symptom of vestibular dysfunction as well as many other conditions However, the literature describes that many people with dizziness restrict their daily and leisure activities, to reduce the risk of this unpleasant and frightening symptom, as well as to avoid the social embarrassment and stigma they can cause [20]. In addition, it generates physical and emotional problems, which can cause an incapacity to perform professional, social and domestic activities [20,21]. A sample with so many university students may have influenced this result, demanding caution in its interpretation.
The highest prevalence of TMD in females found in the present study coincides with previous data [1,22–24]. This finding can be explained by the sum of anatomical and psychosomatic factors [23]. Still, on sociodemographic data, the mean age of participants with TMD was 28.6 years and agrees with authors who reported that the prevalence of TMD is higher between 20 and 45 years, that is, among young people and adults [17,23].
VS in this sample, although more present in women, in agreement with previous data [25,26], affected more young adults. It is noteworthy that the convenience sample of a population involving university students limits the extrapolation of results, requiring that future population-based studies confirm this finding.
The greater the degree of severity of the TMD, both assessed by the QAF and by the RDC/TMD questions, the more symptoms of involvement of the vestibular system were found, which is aligned with a recent review conducted by Hernández-Nuño de la Rosa et al. [27]. This finding confirms several results of previous studies that reveal that patients with pathognomonic signs of TMD are at increased risk of hearing complaints [15,28,29]. However, Machado et al. [14] bring results that do not show statistical correlations between VS and TMD. The exclusive sample of 20 women with otoneurological symptoms likely failed to reveal this association that our findings and other previous studies have revealed.
Considering the social and functional limitations that can be caused by VS, with unpleasant debilitating sensations and causes of social isolation [18], it is possible that living with TMD can potentiate the symptoms and make the therapeutic approach difficult. Since there is no specific treatment, a review shows that conservative or reversible TMD therapy can provide relief, but with low evidence. However, the close relationship of a multi-professional team is essential to achieve the best recovery of the patient [30,31]. For this reason, it is necessary to investigate these symptoms from an early and insignificant stage.
Limitations of the study
These results demonstrate important findings for clinical practice and research, confirming that VS and TMD can coexist. However, the nonuse of a validated questionnaire for the diagnosis of TMD, such as the RDC/TMD, but only some of its questions, made it impossible to obtain a score for analysis and may have been a limiting factor for this work. When data were collected, this instrument was not used, as it is an extensive questionnaire that would make the application even more difficult, prolonging the response time, and increasing the risk of fatigue and indisposition for the participants. However, as a review of an existing database was carried out, it was not possible to have access to individuals to apply the questionnaire in full and perform a physical analysis.
Another point was to use the Fonseca Anamnestic Index, which has only been validated in Brazil but is a simple, easy-to-understand questionnaire, which favors its use in studies, especially in population epidemiological studies, to classify the degree of severity of the disease, signs and symptoms of TMD, but not the diagnosis of this dysfunction. In addition, there are not many questionnaires that assess the impact of TMD severity on patients’ complaints.
Additional research is needed to further study the otological symptoms in patients with TMD, to assess the specific etiology of the combination of vestibular and temporomandibular symptoms, encompassing the probable causes of each condition, which may be isolated or simultaneously.
Conclusion
It can be concluded from the results of this study that the greater the degree of severity of the TMD, the greater number of reported VS. This underscores the importance of adding examination of the vestibular system in patients with TMD, as well as the examination of the temporomandibular function in people with vestibular disorders. This also points to the need for multidimensional prevention, treatment and rehabilitation. Observational population-based studies and clinical trials are necessary to strengthen the conclusion of these results. In addition, it is essential to empower the population about signs and symptoms of TMD and vestibular disorders.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Okeson JP. Management of temporomandibular disorders and occlusion. 7th ed. United States: Elsevier - Health Sciences Division; 2012. p. 504. St Louis. [Google Scholar]
- [2].Taucci RA, Bianchini EMG. Verificação da interferência das disfunções temporomandibulares na articulação da fala: queixas e caracterização dos movimentos mandibulares. Rev da Soc Bras Fonoaudiol. 2007;12(4):274–280. [Google Scholar]
- [3].Chisnoiu AM, Picos AM, Popa S, et al. Factors involved in the etiology of temporomandibular disorders - a literature review. Clujul Med. 2015;88(4):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].National Academies of Sciences . Engineering and M. temporomandibular disorders: priorities for research and care. Washington (DC): The National Academies Press; 2020. [PubMed] [Google Scholar]
- [5].Donnarumma MDC, Muzilli CA, Ferreira C, et al. Disfunções temporomandibulares: sinais, sintomas e abordagem multidisciplinar. Rev CEFAC. 2010;12(5):788–794. [Google Scholar]
- [6].Gauer RL, Semidey MJ. Diagnosis and treatment of temporomandibular disorders. Am Fam Physician. 2015. Mar;91(6):378–386. [PubMed] [Google Scholar]
- [7].Lopes AL, Lemos SMA, Chagas CA, et al. Evidências científicas da reabilitação vestibular na atenção primária à saúde: uma revisão sistemática. Audiol - Commun Res. 2018. 23;23. DOI: 10.1590/2317-6431-2018-2032 [DOI] [Google Scholar]
- [8].Kusdra PM, Stechman-Neto J, De Leão BLC, et al. Relationship between otological symptoms and TMD. Int Tinnitus J. 2018;22(1):30–34. [DOI] [PubMed] [Google Scholar]
- [9].Socher DD, Socher JA, Azzi VJB. Evaluation of quality of life pre- and post-vestibular rehabilitation in patients with benign paroxysmal positional vertigo associated with Meniere’s disease. Int Arch Otorhinolaryngol. 2012;16(4):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Neck Surg. 1990;116(4):424–427. [DOI] [PubMed] [Google Scholar]
- [11].de Castro ASO, Gazzola JM, Natour J, et al. Brazilian version of the Dizziness Handicap Inventory. Pro-Fono. 2007;19(1):97–104. [DOI] [PubMed] [Google Scholar]
- [12].Paulino CA, Prezotto AO, Frias AC, et al. Sintomas de estresse e tontura em estudantes de pós-graduação. Rev Equilíbrio Corpor e Saúde. 2010;2(1):15–26. [Google Scholar]
- [13].Peugh JL, Enders CK. Missing data in educational research: a review of reporting practices and suggestions for improvement. Rev Educ Res. 2004;74(4):525–556. [Google Scholar]
- [14].Machado IM, Pialarissi PR, Minici TD, et al. Relação dos sintomas otológicos nas disfunções temporomandibulares. Arq Int Otorrinolaringol. 2010;14(3):274–279. [Google Scholar]
- [15].Silveira AM, Feltrin PP, Zanetti RV, et al. Prevalência de portadores de DTM em pacientes avaliados no setor de otorrinolaringologia. Rev Bras Otorrinolaringol. 2007;73(4):528–532. [Google Scholar]
- [16].Rosa TSM, de Moraes AB, Dos Santos Filha VAV. The institutionalized elderly: sociodemographic and clinical-functional profiles related to dizziness. Braz J Otorhinolaryngol. 2016;82(2):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bittar RSM, Oiticica J, Bottino MA, et al. Population epidemiological study on the prevalence of dizziness in the city of São Paulo. Braz J Otorhinolaryngol. 2013;79(6):688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hannaford PC, Simpson JA, Bisset AF, et al. The prevalence of ear, nose and throat problems in the community: results from a national cross-sectional postal survey in Scotland. Fam Pract. 2005;22(3):227–233. [DOI] [PubMed] [Google Scholar]
- [19].Paulino MR, Moreira VG, Lemos GA, et al. Prevalência de sinais e sintomas de disfunção temporomandibular em estudantes pré-vestibulandos: associação de fatores emocionais, hábitos parafuncionais e impacto na qualidade de vida. Cienc e Saude Coletiva. 2018;23(1):173–186. [DOI] [PubMed] [Google Scholar]
- [20].Ganança FF, Castro ASO, Branco FC, et al. Impact of dizziness on the quality of life in patients with peripheral vestibular dysfunction. Rev Bras Otorrinolaringol. 2004;70(1):94–101. [Google Scholar]
- [21].Scherer S, Lisboa HRK, Pasqualotti A. Tontura em idosos: diagnóstico otoneurológico e interferência na qualidade de vida. Rev da Soc Bras Fonoaudiol. 2012;17(2):142–150. [Google Scholar]
- [22].Menezes MS, Bussadori SK, Fernandes KPS, et al. Correlação entre cefaléia e disfunção temporomandibular. Fisioter e Pesqui. 2008;15(2):183–187. [Google Scholar]
- [23].de CJH, Sousa A, Oliveira Bf de LX, et al. Disfunção temporomandibular: revisão sistematizada. Arch Heal Investig. 2020;9(6):570–575. [Google Scholar]
- [24].de MSP, Batista AUD, Forte FDS. Prevalence of temporomandibular dysfunction symptoms and oral parafunctional habits in university students. RGO - Rev Gaúcha Odontol. 2011;59(2):201–208. [Google Scholar]
- [25].Tsukamoto HF, de Sp CV, Júnior Ra da S, et al. Influência do tratamento com fármacos antivertiginosos sobre o equilíbrio postural e qualidade de vida de indivíduos com queixas de tontura. Rev CEFAC. 2015;17(5):1394–1402. [Google Scholar]
- [26].Moreira DA, Bohlsen YA, Momensohn-Santos TM, et al. Study of the handicap caused by dizziness in patients associated or not with tinnitus complaint. Arq Int Otorrinolaringol/Intl Arch Otorhinolaryngol. 2006;10(4):270–277. [Google Scholar]
- [27].de la Rosa Mf H-N, Keith DA, Siegel NS, et al. Is there an association between otologic symptoms and temporomandibular disorders?: an evidence-based review. J Am Dent Assoc. 2021. DOI: 10.1016/j.adaj.2021.07.029 [DOI] [PubMed] [Google Scholar]
- [28].Jacob LCBR, Maio Campêlo T, Mocelin Aguiar R, et al. Hearing symptoms and analysis of transient evoked otoacoustic emissions in individuals suffering from temporomandibular joint dysfunction. Distúrbios da Comun. 2005;17(2):173–182. [Google Scholar]
- [29].Pita MS, Ribeiro AB, Zuim PRJ, et al. Hearing Symptoms and Temporomandibular Disorders. Rev Odontológica Araçatuba. 2010;31(1):38–45. [Google Scholar]
- [30].Manni A, Brunori P, Giuliani M, et al. I sintomi otovestibolari nei pazienti con disfunzioni dell’articolazione temporomandibolare. Studio elettromiografico Minerva Stomatol. 1996;45(1–2):1–7. [PubMed] [Google Scholar]
- [31].Ramirez LM, Ballesteros LE, Sandoval GP. Topical review: temporomandibular disorders in an integral otic symptom model. Int J Audiol. 2008;47(4):215–227. [DOI] [PubMed] [Google Scholar]


