Abstract
Epicoccum nigrum is a saprophytic or endophytic fungus that is found worldwide. Because of the antagonist effects of E. nigrum on many plant pathogens, current studies on E. nigrum have focused on the development of biological control agents and the utilization of its various metabolites. In this study, E. nigrum was collected from a wheat field, and its genetic diversity was analyzed. Phylogenetic analyses identified 63 isolates of E. nigrum divided into seven groups, indicating a wide genetic diversity. Isolates antagonized the wheat pathogen Fusarium graminearum, and reduced disease symptoms caused by F. graminearum in wheat coleoptiles. Moreover, pretreatment of wheat coleoptiles with E. nigrum induced the upregulation of pathogen-related (PR) genes, PR1, PR2, PR3, PR5, PR9, and PR10 in wheat coleoptiles responding to F. graminearum invasion. Overall, this study indicates that E. nigrum isolates can be used as biological pathogen inhibitors applied in wheat fields.
Keywords: Antagonism, Epiccocum nigrum, Fusarium graminearum, genetic diversity
1. Introduction
Epicoccum nigrum is a saprophytic and endophytic ascomycete fungus that is found worldwide and exerts antagonistic effects on various plant pathogens (i.e., Sclerotinia sclerotiorum, Pythium irregulare, and Monilinia spp.) [1–4]. Because of the antagonist effects of E. nigrum, the development of biological control agents from E. nigrum and the utilization of various metabolites generated by E. nigrum is well underway [5,6]. Similar to other prevalent fungal genera, E. nigrum is usually isolated from the inner tissues of several plants (i.e., sugarcane, peach, and wheat) [6–8].
Fusarium graminearum (teleomorph Gibberella zeae) also has a high frequency of wheat colonization [9,10]. It is a pathogen that causes Fusarium head blight (FHB) in wheat and results in serious damage to crop production worldwide [11,12]. Cereals with FHB are usually contaminated with mycotoxins, such as zearalenone and trichothecenes that result in mycotoxicosis in humans [13,14]. Previous in vitro studies have shown that endophytic E. nigrum can inhibit the growth and mycotoxin production of F. graminearum [2,15,16]. However, there is still insufficient on researches whether E. nigrum effectively prevents F. graminearum infection in host plants, as well as on the diversity of E. nigrum populations.
Many studies have shown that endophytic fungi have plant growth promoting (PGP) traits that can improve plant growth and production. Fusarium petersiae and Penicillium chrysogenum isolated from Cheilanthes vellea produce high levels of indole-3-acetic acid (IAA) and siderophore that promote wheat growth [17]. Pestalotiopsis microspore is derived from important medicinal plants and induces pathogen resistance in tomato plants while also improving fruit weight [18]. Epiccocum nigrum is also considered to have PGP properties and the treatment of E. nigrum can improve the emergence rate of sorghum seeds, increase shoot and root length, and increase the weight of sorghum [19]. However, interactions between E. nigrum and host plants are not well studied. Genetic diversity is also not well studied and there is no clear standard for its classification [1]. Therefore, it is necessary to investigate the morphological, genetic, and biological characteristics of E. nigrum to investigate its genetic diversity and species classification.
The objectives of this study were to analyze the genetic diversity of E. nigrum in a Korean wheat field and to identify wheat isolates that carry antagonistic activity against F. graminearum. More specifically, we tested the PGP effects of E. nigrum to determine whether it has the possibility of being epiphytic or endogenous as well as whether it can affect plant growth. This study showed the antagonist effects of E. nigrum against F. graminearum in wheat plants and the influence of E. nigrum on wheat immunity.
2. Materials and methods
2.1. Isolation of fungal isolates from a wheat field
Pentachloronitrobenzene (PCNB) medium [13] was used to collect E. nigrum from a wheat field at Dong-A Research Farm in Gimhae, Korea (35.29°N, 127.75°E) as previously described [20]. Plates with PCNB medium were placed at both wheat height and ground level for 30 min. The plates were then incubated at 25 °C for 2 days, and fungal isolates with the colony morphology of E. nigrum were transferred to a complete medium (CM). Sample collection was carried out in both 2019 and 2020. The isolates used in this experiment were stored in 20% glycerol at −80 °C.
2.2. Identification of fungal isolates
Previously described primers for highly conserved fungal rRNA genes (i.e., ITS1 and ITS4) were used to amplify fungal DNA [21,22]. Amplified PCR products were confirmed using electrophoresis and were sequenced at Macrogen (Seoul, Korea). After manually checking the obtained sequences and correcting any errors, the nucleotide sequences were assembled using the SeqMan (DNASTAR, Madison, WI, USA) program. Finally, a basic local alignment searching tool (i.e., BLASTN) was used to identify assembled nucleotide sequences at the National Center for Biotechnology Information. Primers used in this study are listed in Table 1.
Table 1.
Primers used in this study.
| Primer name | Sequence (5′→3′) |
|---|---|
| For identification [23,24] | |
| ITS1 | TCCGTAGGTGAACCTGCGG |
| ITS4 | TCCTCCGCTTATTGATATGC |
| EF_F | GCYCCYGGHCAYCGTGAYTTYAT |
| EF_R | ATGACACCRACRGCRACRGTYTG |
| LSU_F | ACCCGCTGAACTTAAGC |
| LSU_R | CGCCAGTTCTGCTTACC |
| SSU_F | GTAGTCATATGCTTGTCTC |
| SSU_R | CTTCCGTCAATTCCTTTAAG |
| For qRT-PCR [25] | |
| β-actin_F | AAATCTGGCATCACACTTTCTAC |
| β-actin_R | GTCTCAAACATAATCTGGGTCATC |
| PR1_F | CTGGAGCACGAAGCTGCAG |
| PR1_R | CGAGTGCTGGAGCTTGCAGT |
| PR2_F | CTCGACATCGGTAACGACCAG |
| PR2_R | GCGGCGATGTACTTGATGTTC |
| PR3_F | AGAGATAAGCAAGGCCACGTC |
| PR3_R | GGTTGCTCACCAGGTCCTTC |
| PR5_F | ACAGCTACGCCAAGGACGAC |
| PR5_R | CGCGTCCTAATCTAAGGGCAG |
| PR9_F | GAGATTCCACAGATGCAAACGAG |
| PR9_R | GGAGGCCCTTGTTTCTGAATG |
| PR10_F | TTAAACCAGCACGAGAAACATCAG |
| PR10_R | ATCCTCCCTCGATTATTCTCACG |
2.3. Observation of colony and conidia morphology
The mycelial growth of fungal isolates was measured on potato dextrose agar (PDA) after cultivation for 7 days at 25 °C in the dark. For conidia production, each isolate was inoculated onto CM agar and was incubated under near-ultraviolet light (i.e., 20 W and 50 lux) for 7 days at 25 °C. Mycelia were subsequently gathered using 10 ml of distilled water (DW) and were filtered using sterilized gauze. Conidia were then observed using an Olympus BX50 microscope (Olympus, Tokyo, Japan).
2.4. Phylogenetic analyses
Phylogenetic analyses of E. nigrum isolates were performed using an internal transcribed spacer (ITS), the large (LSU) and small subunit (SSU) of rDNA, and translation elongation factor 1 alpha (EFα) [23]. Fusarium graminearum and Petrakia echinata (GenBank Accession No. KR047057.1 and MN310550.1) were used as outgroup taxa. Sequences of the ITS, LSU, SSU, and EFα regions, as well as concatenated sequences, were aligned using the MEGA X software; a phylogram was generated using MEGA X, based on the maximum likelihood (ML) method. Support for branches was evaluated using 1000 bootstrap values [26,27].
2.5. Detached leaf assay
Wheat seeds were soaked in 70% ethanol for 1 min soaked in 5% sodium hypochlorite for 3 min and were washed twice with DW. Sterilized wheat seeds were germinated at 25 °C for 1 day, and were transferred to the growth chamber with 25 °C, 60% moisture, and 12 h each of light and darkness. After 14-day cultivation, wheat leaves were cut to ∼8 cm, and the front of the leaves was placed on a 1% agar medium containing 0.5 mM benzimidazole [28]. A 1 mm incision was made at the center of the wheat leaf, and a 5 mm mycelium fragment was placed in the cut. The wheat leaves were then placed in the growth chamber and mycelium fragments were removed after 3 days. Finally, the Fiji (http://fiji.sc/) program was used to measure the lesion area of wheat leaves after an additional 3-day incubation. Fusarium graminearum and Fusarium oxysporum were used as positive and negative controls, respectively.
2.6. Determination of the in vitro antagonistic effects of E. nigrum against F. graminearum
The antagonistic effects of E. nigrum isolate on F. graminearum were tested on CM agar. Isolates were inoculated in the center of CM agar and were cultured for 4 days at 25 °C. Fusarium graminearum was then inoculated at the edge of CM agar and was cultured at 25 °C. Mycelial growth of F. graminearum was measured at 7 days.
2.7. Antagonistic effects of E. nigrum against F. graminearum in wheat
Each isolate was inoculated into wheat coleoptiles and seeds [29,30]. For coleoptile inoculation, sterilized wheat seeds were cultivated at 25 °C for 1 day. Germinated wheat seeds were then transferred to the growth chamber at 25 °C for 12 h each of light and darkness. After 24 h, 1 mm of the tops of wheat coleoptiles was cut off, and 1 µl of the conidia suspensions (1 × 106 conidia/ml in 0.01% Tween-20) of each isolate was inoculated. After an additional 1 day of incubation, 1 mm from the top of the treated coleoptile was removed and reinoculated with 1 µl of the F. graminearum conidia suspension (1 × 106 conidia/ml in 0.01% Tween-20). Coleoptiles were then cultured in the growth chamber for 7 days.
For F. graminearum inoculation on wheat seeds, 5 g of sterilized wheat seeds were immersed in E. nigrum conidia suspension (1 × 106 conidia/ml in 0.01% Tween-20) and shaken using a rocking shaker at 50 rpm for 24 h in room temperature. Wheat seeds were placed in the growth chamber for 2 days at 25 °C under 12 h each of light and darkness. The top 1 mm of wheat coleoptiles were then removed and 1 µl of F. graminearum conidia suspension (1 × 106 conidia/ml in 0.01% Tween-20) was inoculated. Coleoptiles were then placed in the growth chamber for 7 days and disease lesions on the stems were measured.
2.8. Quantitative real-time PCR (qRT-PCR)
To validate differently expressed genes related to pathogen-related (PR) proteins, qRT-PCR was performed. The top 1 mm of 2-day-old wheat coleoptiles were cut off, inoculated with 1 µl of E. nigrum conidia suspension (1 × 106 conidia/ml in 0.01% Tween-20), and then cultured in a growth chamber for 48 h. Coleoptiles were subsequently harvested at 0, 4, 8, 12, 24, and 48 h after inoculation and were ground under liquid nitrogen. Total RNA was extracted from each sample using the Easy-spin Total RNA Extraction Kit (iNtRON Biotechnology, Seongnam, Korea), in accordance with manufacturer’s protocol. cDNA was generated using ReverTra Ace® qPCR RT Kit (Toyobo, Osaka, Japan) according to the manufacturer’s instructions. Synthesized cDNA was diluted to 100 ng/µl, of which 2 µl was used for qRT-PCR. Relative transcription levels were normalized using an internal reference gene, β-actin [31]. In addition, 1 µl of the F. graminearum conidia suspension (1 × 106 conidia/ml in 0.01% Tween-20) was inoculated into wheat coleoptiles 24 h after E. nigrum was inoculated.
2.9. Production of IAA
Potato dextrose broth (PDB), supplemented with 5 mM L-tryptophan, was used to test the IAA production of each isolate [32]. Inoculated flasks were incubated at 200 rpm for 5 days at 25 °C. After incubation, 1 ml of the culture medium was centrifuged at 13,000 rpm for 10 min; 100 µl of the supernatant was combined with 200 µl of Salkowski’s reagent (i.e., 1 ml of 0.5 M FeCl3 and 49 ml of 35% perchloric acid) and incubated for 20 min at room temperature. IAA production was determined using a spectrophotometer at 530 nm. Colletotrichum gloeosporioides [33] and F. graminearum were used as controls.
3. Results
3.1. Identification of fungal isolates
A total of 63 strains were identified as E. nigrum. The isolates had white or yellow-brown colonies on PDA, and 31 of them showed yellow or dark pigments on PDA (Figure 1(A)). Epiccocum nigrum generally produces dark brown spherical conidia, which are difficult to induce under normal culture conditions [1,34]. In CM and under near-UV, 52 of the 63 isolates produced conidia successfully, whereas 11 isolates did not produce conidia at all (Figure 1(B)).
Figure 1.
Colonial and conidial morphology of Epicoccum nigrum isolates. (A) Isolates were cultivated on PDA for 7 days at 25 °C; (B) Isolates were incubated on CM under near UV (20 W and 50 lux) for 7 days at 25 °C. Scale bar = 10 µm.
3.2. Phylogenetic analyses
Phylogenetic analyses were performed on the ITS, LSU, SSU, and EFα regions with a length of 528, 969, 1004, and 858 bp, respectively, as well as the outgroup sequences with the same length as each region. Although most strains of isolates were divided into a similar pattern, there were certain differences among analyses of four different rDNA regions (Figures S1–S4). To compromise these differences, we conducted an additional combined phylogenetic analysis, and finally, the isolates were divided into seven groups (Figure 2).
Figure 2.
Phylogenetic analysis of Epicoccum nigrum isolates. The phylogenetic tree was inferred using concatenated ITS, LSU, SSU, and EFα sequences of 63 isolates. Sequences were aligned using Clustal W in MEGA X, which was used to perform 1000 bootstrap phylogenetic analyses using maximum likelihood.
3.3. Antagonist effects of E. nigrum isolates on F. graminearum
Before confirming the antagonist effects of E. nigrum, we first confirmed the virulence of E. nigrum isolates in wheat, which showed that isolates did not cause disease in wheat (Figure 3). In the dual culture of E. nigrum and F. graminearum, the isolates showed antagonism to F. graminearum, excluding certain strains (i.e., A20, A25, A26, A28, A34, A35, S17, S19, S21, S25, and S30) (Figure 4). When wheat was treated with isolates, the lengths of lesions were significantly shorter than those in the control that was not treated with E. nigrum. Strains A33, A39, and S11 showed the strongest inhibition in the experiment (Figure 5).
Figure 3.
Pathogenicity of Epicoccum nigrum isolates on wheat leaves. Mycelia plugs of each strain were placed on the middle of wheat leaves and were cultivated for 3 days at 25 °C. The plugs were then removed and wheat leaves were cultivated for another 3 days. Lesion areas were measured using the Fiji program. The error bar represents a standard deviation from five replicates. Fg, Fusarium graminearum; Fo, F. oxysporum.
Figure 4.
Antagonistic activity of Epicoccum nigrum isolates against Fusarium graminearum. Each isolate was inoculated in the center of the CM and was cultured for 4 days at 25 °C. Fusarium graminearum was then inoculated at the edge of the CM and cultured for another 7 days.
Figure 5.
Disease control activity of Epicoccum nigrum isolates against Fusarium graminearum in wheat coleoptiles. (A) Tops of the 2-day-old wheat coleoptiles (i.e., 1 mm) were cut off and conidia suspensions of each isolate was injected. Coleoptiles were cultured in the growth chamber at 25 °C for 24 h, after which the tops of coleoptiles were removed again and the conidia suspension of F. graminearum was inoculated. Lesion length was measured 24 h after F. graminearum inoculation; (B) Surface sterilized wheat seeds were immersed in conidia suspensions of each isolate and were shaken using a rocking shaker for 24 h. Wheat seeds were then transferred into the growth chamber and were cultured for 2 days. The top 1 mm of wheat coleoptiles were removed and the conidia suspension of F. graminearum was inoculated. Lesion length was measured 24 h after F. graminearum inoculation. Error bar represents standard error and statistical analyses were performed via t-test.
3.4. Effects of E. nigrum on PR gene expression
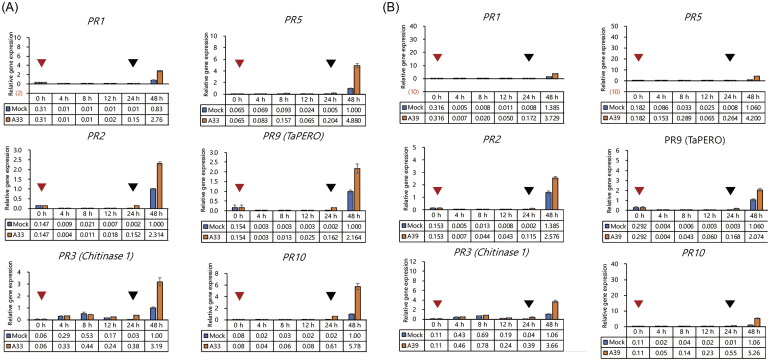
We inoculated the coleoptiles with isolates of A33 and A39 that showed high antagonistic effects on F. graminearum in the coleoptile test and inoculated with F. graminearum after 24 h to confirm the differential expression of PR genes in wheat coleoptiles. Results of the qRT-PCR showed that PR genes in wheat coleoptiles inoculated with only E. nigrum were not differentially transcribed with the control group that was not inoculated with E. nigrum. However, the transcription of PR genes in coleoptiles treated with E. nigrum was upregulated 24 h after F. graminearum inoculation, as compared to the control groups without E. nigrum inoculation (Figure 6).
Figure 6.
Effect of Epicoccum nigrum isolates on wheat PR gene expression. Tops of the 2-day-old wheat coleoptiles were cut off and the conidia suspensions of E. nigrum A33 (A) and E. nigrum A39 (B) were injected into them. Coleoptiles were then cultured at 25 °C in the growth chamber for 24 h. The tops of the coleoptiles were removed again and the conidia suspension of Fusarium graminearum was inoculated. Coleoptile were then cultured at 25 °C for another 24 h. The red arrow represents the injection time point of the isolate and the black arrow represents the inoculation time point of F. graminearum. Gene expression levels were normalized to β-actin gene expression.
3.5. IAA production of isolates
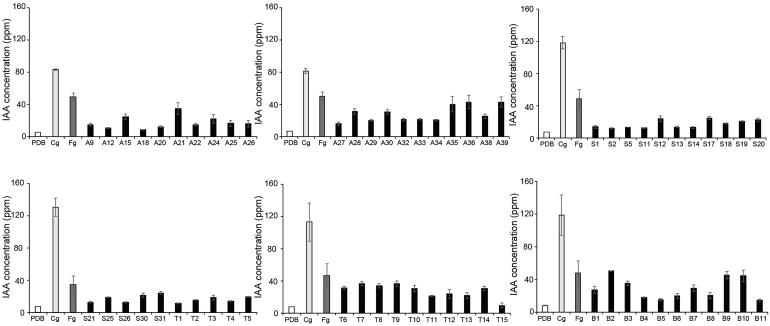
All 63 isolates produced IAA (Figure 7) and most produced 21-41 mg/l IAA. None of those produced more IAA than C. gloeosporioides and F. graminearum also produced higher IAA than most E. nigrum isolates tested in this study (Table 2).
Figure 7.
IAA production of Epicoccum nigrum isolates. Each isolate was inoculated in PDB supplied with 5 mM L-tryptophan and was cultivated for 5 days at 25 °C. Following this, 1 ml of each culture medium was used to determine the production of IAA using a spectrophotometer at 530 mm. Error bar represents the standard deviation from three replicates. Fg, Fusarium graminearum; Cg, C. gloeosporioides.
Table 2.
IAA production of isolates.
| IAA production (mg/l) | Isolate code | No. of E. nigrum isolates |
|---|---|---|
| 1–11 | A18, T15, A12 | 3 |
| 11–21 | T1, S2, A20, S11, S26, S21, S5, S14, S13, S1, T4, A22, B11, A9, B5, T2, A26, A27, A25, S18, B4, T3, S25, T5, B6, A29, A34, B8, S19, T11, A32, S30 | 32 |
| 21–31 | A33, T13, A24, S20, T12, S12, S31, S17, A15, A38, B1, B7, T10, T14, A30 | 15 |
| 31–41 | A28, T6, T8, A21, B3, T9, T7, A35 | 8 |
| 41–51 | F. graminearum, A36, A39, B10, B9, B2 | 5 |
| >51 | C. gloeosporioides | 0 |
4. Discussion
Epicoccum nigrum is distributed in different types of soils and host plants. Although E. nigrum has been described as a weak plant pathogen in a range of plants, the species is considered a saprophytic fungus and can exhibit an endophytic lifestyle [7,35]. Epiccocum nigrum has also been used as a biocontrol agent against certain pathogens in peaches, nectarines, sunflowers, and other plants [36–39].
Although E. nigrum was only collected at a single location in this study, the isolates showed diverse morphological differences and were divided into multiple groups. The isolates belonging to groups 2–7 showed similar mycelial growth and conidial morphology and isolates belonging to group 1 showed diverse morphological characteristics, which might indicate that group 1 can be further divided into more groups; these results suggest that E. nigrum has high genetic diversity.
Previous studies on E. nigrum primarily focused on its biological control properties. This fungus produces antimicrobial agents such as 2-methyl-3-nonyl prodiginine and Bis (2-ethylhexyl) phthalate against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Candida albicans [40]. Additionally, E. nigrum isolated from maize can inhibit the mycelial growth of F. graminearum, and also reduce the yield of trichothecenes and zearalenone produced by F. graminearum in maize [16]. Similarly, most E. nigrum isolates collected during this study showed antagonistic effects against F. graminearum (Figure 4). Moreover, the pretreatment of wheat seeds with isolated strains resulted in the inhibition of disease progression by F. graminearum (Figure 5).
Plants resist pathogen infection through a series of defense mechanisms, such as pattern-triggered immunity and effector-triggered immunity [41,42]. Effector-triggered immunity is caused by effector proteins that are secreted by pathogenic fungi and these effector proteins are key components of fungal virulence in plants [43,44]. The efficiency of PR proteins in plant–fungal pathogen interactions has been widely recognized and there is a growing list of pathogen-effector proteins that directly interact with PR proteins during infection [45,46]. The expression levels of PR genes were also verified in this study. Consistent with the result that E. nigrum isolates had no pathogenicity toward wheat leaves (Figure 3), E. nigrum treatment to wheat coleoptiles did not trigger the transcription of PR genes (Figure 6). However, pretreatment of E. nigrum to wheat coleoptiles resulted in faster transcription of PR genes responding to F. graminearum inoculation (Figure 6), suggesting that endogenous E. nigrum may interact with host plants and induce upregulation of PR gene expression to protect against F. graminearum (Figure 5(B)).
In conclusion, this study suggests that E. nigrum collected from wheat fields has high genetic diversity. All isolates showed F. graminearum inhibition in the medium, and isolates (i.e., A33, A39, and S11) showed strong F. graminearum inhibition in the host plants. This study also showed that pretreatment of E. nigrum could induce the upregulation of PR gene expression in host plants responding to F. graminearum infection. Similar to the results of previous studies [19], the isolates in this study were also found to produce IAA that exerted positive promoting effects on plant growth (Figure 7). This study not only reveals the genetic diversity of E. nigrum in a wheat field but also provides a new strategy for protecting wheat from F. graminearum infection. Future studies should focus on determining the mechanisms by which endogenous E. nigrum induces the upregulation of PR gene expression in host plants.
Supplementary Material
Funding Statement
This work was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education [2020R1A2C2013617, 2020R1A6A1A03047729] and the Green Fusion Technology Program funded by the Ministry of Environment, Republic of Korea.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Favaro LCL, Melo FL, Aguilar-Vildoso CI, et al. Polyphasic analysis of intraspecific diversity in Epicoccum nigrum warrants reclassification into separate species. PLOS One. 2011;6(8):e14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taguiam JD, Evallo E, Balendres MA.. Epicoccum species: ubiquitous plant pathogens and effective biological control agents. Eur J Plant Pathol. 2021;159(4):713–725. [Google Scholar]
- 3.Zhou T, Reeleder RD.. Application of Epicoccum purpurascens spores to control white mold of snap bean. Plant Dis. 1989;73(8):639–642. [Google Scholar]
- 4.Koutb M, Ali EH.. Potential of Epicoccum purpurascens strain 5615 AUMC as a biocontrol agent of Pythium irregulare root rot in three leguminous plants. Mycobiology. 2010;38(4):286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braga RM, Padilla G, Araujo WL.. The biotechnological potential of Epicoccum spp.: diversity of secondary metabolites. Crit Rev Microbiol. 2018;44(6):759–778. [DOI] [PubMed] [Google Scholar]
- 6.Fávaro LCL, Sebastianes FLS, Araújo WL.. Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLOS One. 2012;7(6):e36826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold AE. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biol Rev. 2007;21(2–3):51–66. [Google Scholar]
- 8.Perello A, Simon MR, Arambarri AM.. Interactions between foliar pathogens and the saprophytic microflora of the wheat (Triticum aestivum L.) phylloplane. J Phytopathol. 2002;150(4–5):232–243. [Google Scholar]
- 9.Larran S, Perello A, Simon MR, et al. The endophytic fungi from wheat (Triticum aestivum L.). World J Microbiol Biotechnol. 2007;23(4):565–572. [Google Scholar]
- 10.Boedi S, Berger H, Sieber C, et al. Comparison of Fusarium graminearum transcriptomes on living or dead wheat differentiates substrate-responsive and defense-responsive genes. Front Microbiol. 2016;7:1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang F, Jacobsen S, Jorgensen HJ, et al. Fusarium graminearum and its interactions with cereal heads: studies in the proteomics era. Front Plant Sci. 2013;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanal R, Choo TM, Xue AG, et al. Response of barley genotypes to Fusarium head blight under natural infection and artificial inoculation conditions. Plant Pathol J. 2021;37(5):455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leslie JF, Summerell BA, Bullock S.. The Fusarium laboratory manual. Ames (IA): Blackwell Publising; 2006. [Google Scholar]
- 14.Yang J-W, Kim J-Y, Lee M-R, et al. Identification and chemotype profiling of Fusarium head blight disease in triticale. Res Plant Dis. 2021;27(4):172–179. [Google Scholar]
- 15.Jensen BD, Knorr K, Nicolaisen M.. In vitro competition between Fusarium graminearum and Epicoccum nigrum on media and wheat grains. Eur J Plant Pathol. 2016;146(3):657–670. [Google Scholar]
- 16.Abdallah MF, De Boevre M, Landschoot S, et al. Fungal endophytes control Fusarium graminearum and reduce trichothecenes and zearalenone in maize. Toxins. 2018;10(12):493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed AH, Abd El-Megeed FH, Hassanein NM, et al. Native rhizospheric and endophytic fungi as sustainable sources of plant growth promoting traits to improve wheat growth under low nitrogen input. J Fungi. 2022;8(2):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sujatha HS, Murali M, Amruthesh KN.. Fungal endophytes as growth promoters and inducers of resistance in tomato (Lycopersicon esculentum Mill.) against Alternaria solani. Int J Life Sci Pharma Res. 2021;11:227–235. [Google Scholar]
- 19.Rajini SB, Nandhini M, Udayashankar AC, et al. Diversity, plant growth‐promoting traits, and biocontrol potential of fungal endophytes of Sorghum bicolor. Plant Pathol. 2020;69(4):642–654. [Google Scholar]
- 20.Larena I, Melgarejo P.. Development of a new strategy for monitoring Epicoccum nigrum 282, a biological control agent used against brown rot caused by Monilinia spp. in peaches. Postharvest Biol Tec. 2009;54(2):63–71. [Google Scholar]
- 21.Sulaiman IM, Jacobs E, Simpson S.. Application of ribosomal internal transcribed spacer 1, internal transcribed spacer 2, and large-subunit D1–D2 regions as the genetic markers to identify fungi isolated from different environmental samples: a molecular surveillance study of public health importance. J AOAC Int. 2020;103(3):843–850. [DOI] [PubMed] [Google Scholar]
- 22.Mwamula AO, Kim Y, Kim YH, et al. Molecular characterization of Filenchus cylindricus (Thorne & Malek, 1968) Niblack & Bernard, 1985 (Tylenchida: Tylenchidae) from Korea, with comments on its morphology. Plant Pathol J. 2022;38(4):323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raja HA, Miller AN, Pearce CJ, et al. Fungal identification using molecular tools: a primer for the natural products research community. J Nat Prod. 2017;80(3):756–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Zhang YJ, Liu XZ, et al. On the reliability of DNA sequences of Ophiocordyceps sinensis in public databases. J Ind Microbiol Biotechnol. 2013;40(3–4):365–378. [DOI] [PubMed] [Google Scholar]
- 25.Desmond OJ, Edgar CI, Manners JM, et al. Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol Mol Plant Pathol. 2005;67(3–5):171–179. [Google Scholar]
- 26.Tamura K, Nei M.. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10(3):512–526. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perochon A, Doohan FM.. Assessment of wheat resistance to Fusarium graminearum by automated image analysis of detached leaves assay. Bioprotocol. 2016;6:e2065–e2065. [Google Scholar]
- 29.Jia LJ, Wang WQ, Tang WH.. Wheat coleoptile inoculation by Fusarium graminearum for large-scale phenotypic analysis. Bio Protoc. 2017;7(15):e2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin S, Kim KH, Kang CS, et al. A simple method for the assessment of Fusarium head blight resistance in Korean wheat seedlings inoculated with Fusarium graminearum. Plant Pathol J. 2014;30(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paudel B, Zhuang Y, Galla A, et al. WFhb1-1 plays an important role in resistance against Fusarium head blight in wheat. Sci Rep. 2020;10(1):7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman MT, Gunatilaka MK, Wijeratne K, et al. Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte. PLOS One. 2013;8(9):e73132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maor R, Haskin S, Levi-Kedmi H, et al. In planta production of indole-3-acetic acid by Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol. 2004;70(3):1852–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larena I, De Cal A, Melgarejo P.. Solid substrate production of Epicoccum nigrum conidia for biological control of brown rot on stone fruits. Int J Food Microbiol. 2004;94(2):161–167. [DOI] [PubMed] [Google Scholar]
- 35.Schulz B, Boyle C.. The endophytic continuum. Mycol Res. 2005;109(6):661–686. [DOI] [PubMed] [Google Scholar]
- 36.De Cal A, Larena I, Linan M, et al. Population dynamics of Epicoccum nigrum, a biocontrol agent against brown rot in stone fruit. J Appl Microbiol. 2009;106(2):592–605. [DOI] [PubMed] [Google Scholar]
- 37.Larena I, Torres R, De Cal A, et al. Biological control of postharvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biol Control. 2005;32(2):305–310. [Google Scholar]
- 38.Mari M, Torres R, Casalini L, et al. Control of post‐harvest brown rot on nectarine by Epicoccum nigrum and physico‐chemical treatments. J Sci Food Agric. 2007;87(7):1271–1277. [Google Scholar]
- 39.Pieckenstain FL, Bazzalo ME, Roberts AMI, et al. Epicoccum purpurascens for biocontrol of Sclerotinia head rot of sunflower. Mycol Res. 2001;105(1):77–84. [Google Scholar]
- 40.Perveen I, Raza MA, Iqbal T, et al. Isolation of anticancer and antimicrobial metabolites from Epicoccum nigrum; endophyte of Ferula sumbul. Microb Pathog. 2017;110:214–224. [DOI] [PubMed] [Google Scholar]
- 41.Roux F, Voisin D, Badet T, et al. Resistance to phytopathogens e tutti quanti: placing plant quantitative disease resistance on the map. Mol Plant Pathol. 2014;15(5):427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones JD, Dangl JL.. The plant immune system. Nature. 2006;444(7117):323–329. [DOI] [PubMed] [Google Scholar]
- 43.Dangl JL, Jones JD.. Plant pathogens and integrated defence responses to infection. Nature. 2001;411(6839):826–833. [DOI] [PubMed] [Google Scholar]
- 44.Sonah H, Zhang X, Deshmukh RK, et al. Comparative transcriptomic analysis of virulence factors in Leptosphaeria maculans during compatible and incompatible interactions with canola. Front Plant Sci. 2016;7:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breen S, Williams SJ, Outram M, et al. Emerging insights into the functions of pathogenesis-related protein 1. Trends Plant Sci. 2017;22(10):871–879. [DOI] [PubMed] [Google Scholar]
- 46.Ali S, Ganai BA, Kamili AN, et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol Res. 2018;212:29–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.