ABSTRACT
Background: Dialectical Behaviour Therapy for Posttraumatic Stress Disorder (DBT-PTSD) is a phase-based treatment for PTSD. The DBT-PTSD treatment programme’s efficacy has not been tested during standard operation, outside of laboratory outcome studies.
Objective: The present pilot study investigated the transportability of the DBT-PTSD treatment to a real word clinical setting in a residential mental health centre.
Methods: The DBT-PTSD treatment was compared to a treatment as usual (TAU) condition in a non-randomized study. Overall, 156 patients from a residential mental health centre were included. Propensity score matching was used to match participants in the two treatment arms based on baseline characteristics. Primary and secondary outcomes (PTSD and other symptoms) were assessed at the time of admission and at the time of discharge.
Results: The DBT-PTSD treatment outperformed the TAU condition in the improvement of all primary outcomes, as indicated by a significant time and group interaction. There were notable differences in the effect sizes between the unmatched and matched sample as well as between the available and the intent-to-treat (ITT) data analyses. The effect sizes in the ITT data analyses were much lower. Both treatment groups showed similar improvements in secondary outcomes.
Conclusions: This study provides initial evidence for the transportability of the DBT-PTSD treatment to a naturalistic clinical care setting, but with considerably lower effect sizes than in previously published laboratory RCTs. The higher efficacy of DBT-PTSD compared to TAU may largely depend on patient’s adherence to treatment.
KEYWORDS: Dialectical behaviour therapy, posttraumatic stress disorder, DBT-PTSD, transportability, dissemination
HIGHLIGHTS
The objective of the present study was to investigate the transportability of the DBT-PTSD programme to a real word clinical setting in a residential mental health centre.
The DBT-PTSD treatment outperformed the TAU condition in the reduction of trauma-related symptoms, dissociative symptoms and DSO related but with lower effect sizes compared to previously published RCTs.
The study results indicate the influence of treatment adherence on estimates of treatment effects and stress the necessity to routinely monitor the symptoms of patients who are at high risk of dropout or deterioration.
Abstract
Antecedentes: La Terapia Dialéctica Conductual para el Trastorno de Estrés Postraumático (DBT-PTSD) es un tratamiento basado en fases para el TEPT. La eficacia del programa de tratamiento DBT-PTSD no se ha probado durante el funcionamiento estándar, fuera de los estudios de resultados de laboratorio.
Objetivo: El presente estudio piloto investigó la transportabilidad del tratamiento DBT-PTSD a un entorno clínico de mundo real en un centro residencial de salud mental.
Métodos: El tratamiento DBT-PTSD se comparó con una condición de tratamiento habitual (TAU) en un estudio no aleatorizado. En total, se incluyeron 156 pacientes de un centro residencial de salud mental. Se utilizó el emparejamiento por puntuación de propensión para emparejar a los participantes en los dos brazos de tratamiento en función de las características basales. Los resultados primarios y secundarios (TEPT y otros síntomas) se evaluaron en el momento del ingreso y en el momento del alta.
Resultados: El tratamiento DBT-PTSD superó a la condición TAU en la mejora de todos los resultados primarios, como indica una interacción significativa de tiempo y grupo. Hubo diferencias notables en los tamaños del efecto entre la muestra no emparejada y la emparejada, así como entre los análisis de datos disponibles y los de intención de tratar (ITT, siglas en inglés). Los tamaños del efecto en los análisis de datos ITT fueron mucho menores. Ambos grupos de tratamiento mostraron mejoras similares en los resultados secundarios.
Conclusiones: Este estudio proporciona evidencia inicial de la transportabilidad del tratamiento DBT-PTSD a un entorno de atención clínica naturalista, pero con tamaños del efecto considerablemente más bajos que en los ECA de laboratorio publicados anteriormente. La mayor eficacia de la DBT-TEPT en comparación con la TAU puede depender en gran medida de la adherencia del paciente al tratamiento.
PALABRAS CLAVE: Terapia dialéctica conductual, trastorno de estrés postraumático, DBT-PTSD, Transportabilidad, Diseminación
Abstract
抽象背景:创伤后应激障碍辩证行为疗法 (DBT-PTSD) 是一种阶段性的 PTSD 治疗方法。 DBT-PTSD 治疗计划的疗效尚未在实验室结果研究之外的标准操作期间被检验。
目的:本试点研究考查了 DBT-PTSD 治疗在居民心理健康中心真实临床环境中的可推广性。
方法:在一项非随机研究中,将 DBT-PTSD 治疗与常规治疗 (TAU) 条件进行比较。 总共纳入了156名来自居民心理健康中心的患者。根据基线特征使用倾向得分匹配两个治疗组的参与者。 在入院时和出院时评估了主要和次要结果(PTSD 和其他症状)。
结果:DBT-PTSD 治疗在所有主要结果的改善上优于 TAU 条件,如显著的时间和组相互作用所示。 不匹配样本和匹配样本之间以及可用性和意向性 (ITT) 数据分析之间,效应量存在显著差异。 ITT 数据分析中的效应量低得多。 两个治疗组在次要结果上均表现出相似的改善。
结论:本研究为 DBT-PTSD 治疗在自然临床护理环境中的可推广性提供了初步证据,但其效应量远低于先前发表的实验室 RCT。 与 TAU 相比,DBT-PTSD 的更高疗效可能在很大程度上取决于患者对治疗的依从性。
关键词: 辩证行为疗法, 创伤后应激障碍, DBT-PTSD, 可推广性, 传播
1. Introduction
A Childhood trauma is consistently associated with a risk for mental disorders. This is true for posttraumatic stress disorder (PTSD) as well as other disorders (D’Andrea et al., 2012; van Der Kolk et al., 2019). Given the extend nature of PTSD symptoms an independent diagnostic category has been postulated (Brewin, 2020): Complex Posttraumatic Stress Disorder (CPTSD) has been included in the latest revision of the International Classification of Diseases (ICD-11) (World Health Organization, 2019) as a disorder that may develop following exposure to extremely threatening or horrifying events from which escape is difficult or impossible, most commonly prolonged or repeated events including childhood sexual or physical abuse. In addition to the three core symptoms of classical PTSD, i.e. re-experiencing of traumatic events in the present, avoidance of traumatic reminders and a sense of current threat, survivors of childhood abuse and other individuals with CPTSD experience three additional symptom groups summarized as disturbances in self-organization (DSO), i.e. emotional regulation difficulties, relationship difficulties, and negative self-concept (Maercker, 2021). In an effort to recognize the heterogeneity of symptoms among various trauma survivors, the latest edition of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013) added a symptom cluster of negative alterations in mood and cognitions, similar to the DSO symptom groups in the ICD-11 (Cloitre, 2020).
Despite the growing research on psychological interventions for adults with CPTSD symptoms, the evidence for their efficacy is limited to PTSD and depression symptoms. More systematic research is needed for the DSO symptom outcomes, considering the high heterogeneity and various types and combinations of trauma histories and life circumstances leading to a CPTSD (Ford, 2021). Thus, Ford (2021) argues for more specific interventions to appropriately target CPTSD outcomes and to tailor treatment components to individual expressions of CPTSD.
At least four relevant systematic reviews and meta-analysis specifically address the efficacy of psychological therapies for adult survivors of childhood abuse and other individuals with CPTSD. Trauma-focused interventions, such as Cognitive Behavioural Therapy with or without exposure (CBT), exposure therapy alone (EA) and Eye Movement Desensitization and Reprocessing (EMDR) (Karatzias et al., 2019) as well as trauma memory processing therapies (TMP), i.e. interventions with exposure to traumatic memories (imaginal or in vivo) as well as cognitive restructuring through discussion of traumatic memories and their associated faulty appraisal (Mahoney et al., 2019) are effective treatments for PTSD symptoms. Another recent meta-analysis of 94 randomized controlled trials (RCTs) of psychological and pharmacological interventions for PTSD following complex traumatic events (Coventry et al., 2020), adopted a non-diagnostic approach, including all studies of adults with complex trauma (including childhood abuse). The results from this meta-analysis indicate that phase-based psychological interventions that include skills-based strategies along with trauma-focused strategies are most effective in reducing PTSD symptoms, including DSO symptoms such as emotion dysregulation and interpersonal problems in adults with complex trauma.
Dialectical Behaviour Therapy (DBT; Linehan et al., 2001) was originally developed to treat individuals with a high risk for suicide. DBT is typically used for patients with a borderline personality disorder (BPD) and self-injurious behaviour (SIB) and does not specifically target PTSD symptoms. The DBT Prolonged Exposure (DBT PE) protocol extended DBT treatment to patients with PTSD. Besides basic DBT treatment elements, DBT-PE includes also front-line evidence-based treatment components for PTSD, such as prolonged exposure (PE), treatment of behavioural dyscontrol and fostering coping skills (Harned et al., 2012). The efficacy of DBT-PE has been demonstrated in a pilot outpatient (Harned et al., 2021) and randomized-controlled setting (Harned et al., 2014). Dialectical Behavioural Therapy for Posttraumatic Stress Disorder (DBT-PTSD; Bohus & Priebe, 2018) is another phase-based psychological intervention programme, similar to DBT-PE. Thus far, the DBT-PTSD treatment programme has been tested primarily with female adults with complex trauma childhood sexual or physical abuse respectively. DBT-PTSD combines individual as well as group sessions. It is comprised of a pre-treatment phase and seven consecutive thematic treatment phases. It combines fundamental DBT skills-based elements (Linehan et al., 2001) with trauma-focused cognitive and exposure-based elements (Ehlers et al., 2005; Foa et al., 2019) as well as elements of Compassion-Focus Therapy (CFT; Gilbert, 2014) and Acceptance and Commitment Therapy (Hayes et al., 2013). In a residential setting the DBT-PTSD treatment programme extends over a period of 12 weeks.
Two RCTs supported the efficacy of DBT-PTSD (Bohus et al., 2013, 2020). The first RCT tested the efficacy of the DBT-PTSD treatment programme in an inpatient sample of 74 women with childhood sexual abuse (CSA) related PTSD. Participants were randomized to either 12-week residential DBT-PTSD treatment or treatment-as-usual wait list (TAU-WL). Enrolment was restricted to women, aged 17–65 years, meeting a DSM-IV diagnosis (American Psychiatric Association, 2013) of PTSD related to CSA (i.e. being sexually assaulted under the age of 18). The DBT-PTSD treatment was delivered by four clinical psychologists (graduates and post-graduates) who were all involved in the treatment development and who had previously conducted at least four training cases. The mean change in PTSD symptoms between pre-treatment and 12-week post-discharge was greater in the DBT-PTSD group than in the TAU-WL group, with a controlled effect size (ES) of Hedges’ g = 1.60 for clinician-rated PTSD symptoms and of Hedges’ g = 0.98 for self-rated PTSD symptoms. This study also compared treatment outcomes between patients with and without BPD (i.e. meeting either ≥5 or <5 DSM-5 BPD criteria). Both subgroups showed significant PTSD symptom improvement in comparison with the TAU-WL group, with similar effect sizes. Results for the secondary outcomes in this study were mixed. The DBT-PTSD group showed significantly more improvement in depressive symptoms and global functioning than the TAU-WL group. However, there was no significant group difference in respect to improvements in BPD symptoms, dissociative symptoms, and general symptom distress. Thus, the results from this study confirm that with specialized therapists, supervised by the developers of the DBT-PTSD programme, the DBT-PTSD programme outperforms TAU-WL, in a selective sample of female inpatients with CSA-related PTSD. In a second RCT Bohus et al. (2020) compared the efficacy of DBT-PTSD against cognitive processing therapy (CPT) in an outpatient sample of 193 women (mean age = 36.3, SD = 11.1) with childhood sexual or physical abuse-related PTSD, who additionally met 3 or more DSM-5 criteria for BPD (including affective instability). Participants, recruited from three university outpatient clinics, were randomly assigned to either 45 weekly sessions of DBT-PTSD or CPT over one year, followed by a booster phase of 3 monthly sessions. Treatment included individual therapy session, plus homework, and telephone consultation as needed. Participating therapists were trained in either DBT-PTSD or CPT in 4-day workshops led by the respective treatment developers. The primary outcomes were clinician-rated PTSD symptoms and the secondary outcomes were patient-rated PTSD symptoms, BPD symptoms, dissociative symptoms, depressive symptoms, and global functioning. Clinician and patient-rated PTSD symptoms significantly improved in both treatment groups from pre- to post-treatment, with a small but significant advantage of the DBT-PTSD treatment. The findings for other secondary outcomes were mixed. There were small but significant differences between treatment groups regarding BPD symptoms, dissociative symptoms, and depressive symptoms, with greater improvement in the DBT-PTSD treatment group. However, there was no significant group difference in respect to global functioning. The results from this study confirmed the efficacy of the DBT-PTSD programme in an outpatient setting, when it is delivered by specialized therapists, supervised by the developers of the DBT-PTSD programme, in a selective sample of female survivors of childhood abuse-related PTSD and symptoms of BPD.
A vastly understudied key aspect of research on the efficacy of a novel intervention to its dissemination is transportability (Lincoln et al., 2003; McEvoy et al., 2012; Southam-Gerow et al., 2008). The efficacy of the DBT-PTSD treatment programme, as most other treatment programmes, has previously only been tested in laboratory outcome studies conducted in specialized training clinics by specialized therapists and mentors. Since the research therapy in laboratory outcome studies differs from everyday clinical care it is important to study the transportability of efficacious treatments to usual clinical care settings. The objective of the present study was to investigate the transportability and implementation of the DBT-PTSD programme in a non-randomized pilot study with patients of a residential mental health centre. In order to test the efficacy and transportability of the DBT-PTSD programme to every day clinical care we compared it to a treatment as usual condition (i.e. an eclectic trauma-focused treatment) and generalized the findings to a broader sample also including male patients. Based on the available evidence we hypothesize that the DBT-PTSD treatment will outperform treatment as usual in an everyday clinical care setting.
2. Materials and methods
2.1. Treatment setting
The study was conducted at a single site, at the University Hospital for Psychosomatic Medicine in Austria. The residential mental health centre is part of the Austrian public health care system and treatments are covered by the Austrian public health insurance. Treatment outcomes are routinely monitored for all patients. The clinic only serves adult patients (18+ years) and does not accept patients with acute suicidal behaviour or current substance abuse. However, patients with a past history of suicidal behaviour and past and current self-injurious behaviour (SIB) are eligible for treatment at the residential mental health centre. The Computer-based Health Evaluation System (CHES) is used for the routine clinical outcome monitoring (Holzner et al., 2012), including assessments at preadmission, at the beginning and the end of the inpatient treatment.
Since the study has been conducted in a naturalistic setting analysing retrospective data collected as part of routine clinical care, no information about treatment adherence or treatment fidelity was available.
2.1.1. Study participants
The present study is a non-randomized trial. All patients were self-referred or referred by mental health professionals to the University Hospital. Psychological outcome data were included from all patients treated in one of the three PTSD treatment units between February 26th, 2020, and April 14th, 2021. Patients were assigned to either DBT-PTSD treatment (Bohus et al., 2013) or treatment-as-usual (TAU) by an administrator (a psychiatrist) according to the case history, an unstructured interview and the respective capacity at one of the three units. Since the diagnoses of PTSD was not established with a structured clinical interview, baseline (admission) results from the Posttraumatic Stress Disorder Checklist- Civilian Version (PCL-C; Weathers et al., 1994), a self-report measure of PTSD symptoms were used to validate the diagnosis of PTSD. Accordingly, only the data from patients with a cut-off score of 45 or higher in the PCL-C were included in the present analyses (McDonald & Calhoun, 2010). Further, other self-report measures were used to rate PTSD-related psychological symptoms.
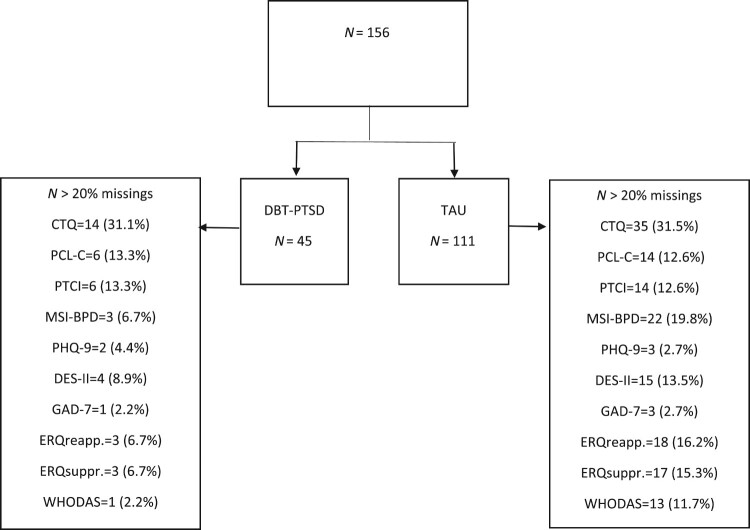
Overall, 156 patients were included in the present study. 111 patients (43.0 mean age, 81.1% female) were in the TAU group, and 45 patients (47.2 mean age, 86.7% female) in the DBT-PTSD group. The flow of participants and percentage of missing data is displayed in Figure 1. Groups differed significantly regarding the variables age (SMD = 0.42, p = .020), treatment duration (SMD = −0.31, p = .080), number of diagnosis (SMD = 0.64, p < .001) and experienced physical, sexual, or emotional childhood trauma level (CTQ; SMD = −0.48, p = .027). Patients in the two groups also differed significantly in many symptom levels at admission (T1): PCL-C total score (SMD = −0.39, p = .040), DES-II (SMD = −0.58, p = .002), MSI-BPD (SMD = −0.41, p = .030), and ERQ subscale suppression (SMD = −0.39, p = .037) (Table 1). Thirty-one (34.8%) patients of the TAU group and 21 (50%) of the DBT-PTSD group fulfilled at least seven criteria of the MSI-BPD screening for BPD.
Figure 1.
Patient flow and missings.
Table 1.
Sample baseline characteristics (after imputation).
| Unmatched (n = 156) | Matched (n = 32) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DBT-PTSD (n = 45) | TAU (n = 111) | DBT-PTSD (n = 16) | TAU (n = 16) | ||||||||||||
| N | M or % | SD | N | M or % | SD | p | SMDa | Missing (%) | M or % | SD | M or % | SD | p | SMDa | |
| Age | 45 | 47.2 | 9.49 | 111 | 43.0 | 11.1 | .020 | 0.42 | 0 | 44.9 | 9.24 | 47.9 | 10.2 | .399 | 0.30 |
| Sex (female) | 45 | 86.7 | 111 | 81.1 | .403 | 0.13 | 0 | 81.3 | 87.5 | .500 | 0.17 | ||||
| Education (> secondary education) | 45 | 37.8 | 111 | 44.1 | .466 | 0.12 | 5.8 | 37.5 | 31.3 | .710 | 0.13 | ||||
| Employment | 45 | 11.1 | 111 | 7.2 | .523 | 0.13 | 7.1 | 18.8 | 12.5 | .626 | 0.17 | ||||
| Treatment duration | 45 | 60.8 | 21.8 | 111 | 55.1 | 16.4 | .080 | −0.31 | 0 | 58.4 | 19.2 | 61.1 | 15.3 | .664 | 0.16 |
| No. of times in clinic | 45 | 1.40 | 0.84 | 111 | 1.71 | 1.15 | .100 | 0.29 | 6.4 | 1.56 | 0.96 | 1.75 | 1.06 | .605 | 0.19 |
| No. diagnoses | 45 | 3.56 | 2.04 | 111 | 4.86 | 2.02 | <.001 | 0.64 | 0 | 3.94 | 1.57 | 3.88 | 1.59 | .912 | −0.04 |
| Chronicity (> 2 years) | 45 | 97.8 | 111 | 99.1 | .495 | 0.11 | 6.4 | 100 | 100 | 0 | |||||
| CTQtotal | 31 | 75.4 | 10.5 | 76 | 69.9 | 11.7 | .027 | −0.48 | 4.71 | 71.4 | 10.6 | 69.2 | 13.3 | .613 | −0.18 |
Standardized mean difference = difference in mean or proportions divided by the standard error; imbalance between groups is defined as absolute value greater than 0.20 (corresponding to a small effect size).
Note: DBT-PTSD = Dialectical Behaviour Therapy – Posttraumatic Stress Disorder, TAU = Treatment as usual, CTQ = Childhood Trauma Questionnaire.
2.2. Treatment
2.2.1. DBT-PTSD
DBT-PTSD is a phase-based psychological intervention programme based on standard DBT treatment supplemented by treatment elements from trauma-focused cognitive–behavioural therapy, compassion-focused therapy and acceptance and commitment therapy (Bohus & Priebe, 2018). DBT-PTSD consists of seven treatment phases. To specifically address the diverse symptom constellation of individuals with childhood abuse-related PTSD each treatment phase comprises obligatory and optional treatment modules and manualized ‘if–then rules’ indicate suitable modules. The DBT-PTSD programme was spread over 12 weeks and was delivered by a multi-professional team. Therapists delivering the DBT-PTSD treatment were both licensed clinical psychologists, with 6 respectively 2 years of experience working as a clinical psychologist. One of the two therapists was also licensed as a (cognitive) behavioural (psycho)therapists, with 1 year experience working as a CBT therapist.
All therapists of the patients in the DBT-PTSD group were trained in the DBT-PTSD programme at the Central Institute in Mannheim coordinated by Martin Bohus and are supervised according to the DBT-PTSD treatment guidelines (Bohus & Priebe, 2018). The DBT-PTSD programme is conducted within the multidisciplinary therapy setting of the University Hospital including physical activity group training (Á 45 min twice a week), biofeedback (group training Á 80 min once a week), art therapy (Á 90 min once a week) and music therapy (Á 60 min once a week).
2.2.2. TAU
Trauma-specific therapy takes place in a combined individual and group setting. The mandatory basic groups include an open-topic group, knowledge transfer, mindfulness, and skills training (Á 50 min 5 times per week). Additional therapeutic group therapies include art therapy (Á 90 min once a week), music therapy (Á 60 min once a week), group biofeedback (Á 75 min once a week) and animal-assisted therapy (Á 90 min once a week). Individual sessions with trauma therapists are offered two times per week. Individual therapists were free in deciding on specific techniques and their timing to achieve sufficient patients’ improvement according to the commitment of the University Hospital to apply evidence-based methods. All trauma therapists are female and have at least three years experience working as (psycho)therapists. The therapeutic coordinators of the TAU group have a clinical and health psychology education, between 12- and 14-year experience working as (psycho)therapists, with additional training in behavioural or cognitive therapy techniques and education in Eye Movement Desensitization and Reprocessing (EMDR). Depending on the patient and type of trauma, different interventions were used: EMDR and screening techniques, Imagery Rescripting & Reprocessing Therapy (IRRT), Trauma-Focused Cognitive Behavioural Therapy (TF-CBT), Imagery Rehearsal Therapy (IRT), Narrative Exposure Therapy (NET), Concept of Structural Dissociation (Enactive Trauma Therapy), Somatic Experiencing, Neuroaffective Relational Model (NARM), Schema Therapy. Further stabilization techniques, reorientation techniques, hypnotherapeutic interventions, imagination exercises and trauma sensitive yoga were also applied. Besides the high variety of trauma-focused interventions, no PE-related therapy approaches such as DBT-PE have been used.
2.3. Measures
2.3.1. Childhood trauma questionnaire
The Childhood Trauma questionnaire CTQ is a retrospective measure of childhood trauma that has been psychometrically assessed in diverse populations (Bernstein et al., 2003; Häuser et al., 2011). The CTQ contains 5 subscales, emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect.
2.3.2. Posttraumatic stress disorder checklist – civilian version
The Posttraumatic Stress Disorder Checklist – Civilian Version (PCL-C) is a well validated 17-items questionnaire to measure the core symptoms of PTSD (Weathers et al., 1994). Three core areas of the PTSD symptomatology, (a) intrusive thoughts and recollections of the traumatic event (b) emotional blunting and overexcitement (hyperarousal), and (c) avoidance of places, people, or situations associated with the traumatic event, are assessed. The overall PCL-C score can have a range from 17 to 85, diagnostic relevant scores for PTSD patients under psychological or psychiatric treatment are from 45 to 50 (Wilkins et al., 2011).
2.3.3. Dissociative experience scale
The Dissociative Experience Scale (DES-II) surveys dissociative symptoms with a total of 28 questions asking about the frequency (i.e. what percentage of the time) of various dissociative experiences in everyday life (Bernstein & Putnam, 1986). The overall score of the DES-II can have a range between 0 and 100. The cut-off for clinical relevant symptoms is defined with ≥ 30 (Carlson & Putnam, 1993; van Ijzendoorn & Schuengel, 1996).
2.3.4. Posttraumatic cognitions inventory
The Posttraumatic Cognitions Inventory (PTCI) asks a total of 33 questions about negative cognitions as a result of traumatic experiences. Further, PTSD symptoms and negative cognitions about the self, the world, and one's own attribution of guilt regarding the trauma are assessed (Beck et al., 2004; Foa et al., 1999). The subscales as well as the total score were found to correlate highly with measures of PTSD (Foa et al., 1999).
2.3.5. Mclean screening instrument for borderline personality disorder
The McLean Screening Instrument for Borderline Personality Disorder (MSI-BP) is a dichotomous 10-item self-reported scale assessing the diagnostic criteria according to the Diagnostic and Statistical Manual 5th Edition (American Psychiatric Association, 2013). The initial validation study recommended a cutoff score of 7 as providing for an optimal sensitivity (81%) and specificity (89%) (Zanarini et al., 2003).
2.3.6. Patient health questionnaire
The Patient Health Questionnaire-9 (PHQ-9) uses a total of nine items to determine the expression of depressive symptoms such as lack of interest, depressed mood, sleep problems, decreased self-esteem, difficulty concentrating, suicidal thoughts, altered appetite, and how these symptoms interfere with work, home, and interactions with people. Scores range from 0 to 27, with a cut-off score of ≥10 for a moderate depression severity (Beard et al., 2016; Kroenke et al., 2001; Kroenke & Spitzer, 2002).
2.3.7. Brief measure for assessing generalized anxiety disorder
The Brief Measure for assessing generalized anxiety disorder (GAD-7) assesses various anxiety disorder symptoms with a total of 7 questions, by querying the expression of anxious thoughts in the last 2 weeks, e.g. nervousness, excessive worry and inability to relax (Gyani et al., 2013; Kroenke et al., 2010; Spitzer et al., 2006).
2.3.8. Emotion regulation questionnaire
The Emotion Regulation Questionnaire (ERQ) is a 10-item self-report scale to measure the habitual use of two commonly used emotion regulation strategies: reappraisal and suppression. Cognitive reappraisal involves thinking differently about a situation to change its meaning related to the persons’ emotional experience. Expressive suppression involves decreasing the outward expression of emotion (Gross & John, 2003).
2.3.9. WHO disability assessment schedule
The WHO Disability Assessment Schedule (WHODAS) is 12-item self-reported scale measuring limitations in functioning and participation associated with current health status in the areas of: comprehension and communication, mobility, self-care, interaction with other people, activities of daily living, participation in social life in the last 30 days (Gold, 2014).
2.4. Outcome analysis
Repeated measures analysis of variance (ANOVA) were used to investigate differences in primary and secondary treatment outcomes at admission (Tbaseline) and discharge (Tpost-treatment) between both treatment groups for both available (completers) data and intent-to-treat (ITT) data. Since the ANOVA is robust in cases of violation of the normality assumption (Schmider et al., 2010), we did not use parameter-free procedures for not normally distributed treatment outcomes. Effect sizes are expressed in Cohen’s d which was computed using pooled standard deviations of scores from Tbaseline and Tpost-treatment for intragroup differences, and pooled standard deviation of scores from Tpost-treatment between the DBT-PTSD and TAU group. Missing data in treatment outcomes were imputed if ≤20% of the questionnaire items were missing. For the ITT data, primary and secondary outcomes of the Tbaseline assessment were carried forward for the Tpost-treatment values according to the last observation carried forward (LOCF) approach which is the most common for ITT analysis in the literature on treatment outcomes (Liu, 2016).
2.5. Supplementary analysis
Because of the significant group differences in the baseline scores, we conducted a supplementary analysis using the propensity score matching (PSM) method to match participants in the two treatment arms based on sociodemographic characteristics (age, gender, education, employment), pre-treatment variables (treatment duration, number of times in clinic, number of diagnosis and chronicity of the mental illness) and baseline scores on primary and secondary treatment outcomes. The propensity score has been defined by Rosenbaum and Rubin (Rosenbaum & Rubin, 1983, 1985) to be the probability of treatment assignment dependent on observed baseline covariates: ei = Pr(Zi = 1|Xi). Conditional on the propensity score, the distribution of measured baseline covariates can be matched between two treatment groups. The propensity score can be also estimated using a logistic regression model, in which treatment status is regressed on observed baseline characteristics (Austin, 2011). In the present study, the IBM-SPSS software program (Version 27) was used to create propensity scores, using the PSM command.
Given the vastly different baseline characteristics in the two groups, the PSM yielded only 32 patients (16 per group), with matching baseline characteristics. There were no significant differences in baseline characteristics between matched groups, except for the variable age (Table 2).
Table 2.
Sample baseline characteristics: Supplementary (matched) data set.
| Matched (n = 32) | ||||||
|---|---|---|---|---|---|---|
| DBT-PTSD (n = 16) | TAU (n = 16) | |||||
| M or % | SD | M or % | SD | p | SMDa | |
| Age | 44.9 | 9.24 | 47.9 | 10.2 | .399 | 0.30 |
| Sex (female) | 81.3 | 87.5 | .500 | 0.17 | ||
| Education (>secondary education) | 37.5 | 31.3 | .710 | 0.13 | ||
| Employment | 18.8 | 12.5 | .626 | 0.17 | ||
| Treatment duration | 58.4 | 19.2 | 61.1 | 15.3 | .664 | 0.16 |
| No. of times in clinic | 1.56 | 0.96 | 1.75 | 1.06 | .605 | 0.19 |
| No. diagnoses | 3.94 | 1.57 | 3.88 | 1.59 | .912 | −0.04 |
| Chronicity (>2 years) | 100 | 100 | 0 | |||
| CTQtotal | 71.4 | 10.6 | 69.2 | 13.3 | .613 | −0.18 |
Standardized mean difference = difference in mean or proportions divided by the standard error; imbalance between groups is defined as absolute value greater than 0.20 (corresponding to a small effect size).
Note: DBT-PTSD = Dialectical Behaviour Therapy – Posttraumatic Stress Disorder, TAU = Treatment as usual, CTQ = Childhood Trauma Questionnaire.
In the matched data, patients of the DBT-PTSD group had a baseline PCL-C score of 65.4 (SD = 7.48) and patients of the TAU-group had a baseline PCL-C score of 66.8 (SD = 7.86). In the DBT-PTSD group, 81.3% of patients were female, with a mean age of 44.9 (SD = 9.24) and 1.56 (SD = 0.96) mean number of previous inpatient stays in the clinic. 87.5% of the patients in the TAU group were female, had a mean age of 47.9 (SD = 10.2) and a mean number of 1.75 (SD = 1.06) previous stays in the clinic. In both groups patients had experienced their psychological symptoms for two years before admission to the current inpatient treatment.
Moreover, an analysis of covariance (ANCOVA) was done, treating sex, age, the related baseline outcome score, and the treatment group factor (DBT-PTSD or TAU) as covariates for the treatment change ( = difference from Tbaseline to Tpost-treatment) of primary treatment outcomes (Supplemental Table 1).
3. Results
3.1. Main analyses
3.1.1. Primary outcomes
The PCL-C total score, in both the DBT-PTSD group (d = 1.38, p < .001) and the TAU group (d = 0.70, p ≤ .001) improved significantly from admission to discharge, with the largest effect size on all primary outcome variables for the TAU group (Table 3). In the ITT data set, the DBT-PTSD group (d = 0.71, p = .05) and the TAU group (d = 0.50, p < .001) have medium effect sizes, group differences are not significant (F(1,134) = [0.172], p = .679) but both the factor time (F(1,134) = [55.01], p < .001) and the interaction between group and time are significant (F(1,134) = [11.54], p = <.001).
Table 3.
Treatment outcomes in different treatment groups after imputation: Primary outcomes.
| Available data | Intent to treat data | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tbaseline | Tpost-treatment | Tpost-treatment | ||||||||||||
| Measure | Group | N | M(SD) | N | M(SD) | da | p | N | M(SD) | da | p | ANOVAb | Factor | p |
| PCL-C Total score | DBT-PTSD | 39 | 65.6 (7.59) | 20 | 48.7 (13.5) | 1.38 | <.001 | 39 | 55.8 (13.3) | 0.71 | <.001 | F(1,134) = 55.01 | Time | <.001 |
| TAU | 97 | 61.8 (10.5) | 60 | 56.1 (11.3) | 0.70 | <.001 | 97 | 58.2 (11.4) | 0.50 | <.001 | F(1,134) = 0.172 | Group | .679 | |
| dc | 0.49 | .05 | 0.19 | .32 | F(1,134) = 11.54 | Gr. x ti. | <.001 | |||||||
| PCL-C Re-experiencing | DBT-PTSD | 39 | 3.81 (0.74) | 20 | 2.95 (0.99) | 0.93 | <.001 | 39 | 3.32 (0.97) | 0.56 | .001 | F(1,134) = 20.15 | Time | <.001 |
| TAU | 97 | 3.72 (0.93) | 60 | 3.59 (0.84) | 0.21 | .12 | 97 | 3.62 (0.89) | 0.16 | .12 | F(1,134) = 0.490 | Group | .485 | |
| dc | 0.61 | .02 | 0.34 | .08 | F(1,134) = 9.07 | Gr. x ti. | .003 | |||||||
| PCL-C Numbing/avoidance | DBT-PTSD | 39 | 5.18 (0.73) | 20 | 3.73 (1.19) | 1.50 | <.001 | 39 | 4.31 (1.20) | 0.74 | <.001 | F(1,134) = 20.15 | Time | <.001 |
| TAU | 97 | 4.67 (0.94) | 60 | 4.41 (0.89) | 0.41 | .002 | 97 | 4.49 (1.02) | 0.32 | .002 | F(1,134) = 0.490 | Group | .485 | |
| dc | 0.50 | .05 | 0.17 | .37 | F(1,134) = 9.07 | Gr. x ti. | .003 | |||||||
| PCL-C Hyperarousal | DBT-PTSD | 39 | 4.27 (0.78) | 20 | 3.13 (1.15) | 1.29 | <.001 | 39 | 3.63 (1.14) | 0.66 | <.001 | F(1,134) = 49.39 | Time | <.001 |
| TAU | 97 | 4.17 (0.81) | 60 | 3.78 (0.89) | 0.68 | <.001 | 97 | 3.92 (0.87) | 0.49 | <.001 | F(1,134) = 0.430 | Group | .513 | |
| dc | 0.56 | .03 | 0.32 | .10 | F(1,134) = 10.30 | Gr. x ti. | .002 | |||||||
| DES-II | DBT-PTSD | 41 | 42.1 (20.6) | 20 | 31.3 (17.9) | 0.98 | <.001 | 41 | 34.7 (20.1) | 0.56 | <.001 | F(1,135) = 14.01 | Time | <.001 |
| TAU | 96 | 31.5 (17.4) | 59 | 30.3 (18.0) | 0.06 | .632 | 96 | 31.0 (18.4) | 0.05 | .631 | F(1,135) = 4.71 | Group | .032 | |
| dc | 0.05 | .853 | 0.20 | .291 | F(1,135) = 10.59 | Gr. x ti. | .001 | |||||||
| PTCI total score | DBT-PTSD | 39 | 148.0 (29.8) | 20 | 115.4 (45.3) | 1.31 | <.001 | 39 | 124.6 (40.8) | 0.69 | <.001 | F(1,134) = 39.76 | Time | <.001 |
| TAU | 97 | 139.4 (31.4) | 60 | 133.8 (34.9) | 0.32 | .02 | 97 | 135.1 (32.2) | 0.25 | <.001 | F(1,34) = 0.026 | Group | .871 | |
| dc | 0.60 | .02 | 0.30 | .11 | F(1,134) = 19.19 | Gr. x ti. | <.001 | |||||||
Cohen’s d was computed using the pooled standard deviation of scores at admission (t1) and discharge (t2).
Repeated measures analysis of variance with intent to treat data.
Cohen’s d was computed using the pooled standard deviation of scores at discharge (t2) from the DBT-PTSD and TAU groups.
Similar results are found concerning the PCL-C numbing/avoidance subscale: the DBT-PTSD group (d = 1.50, p < .001) shows the largest effect size amongst primary outcome variables and the TAU group (d = 0.41, p = .002) has only a small effect size. The large effect size diminishes in the ITT data set, with medium effect size for the DBT-PTSD group (d = 0.74, p < .001), a small effect size for the TAU group (d = 0.32, p = .002) and no significant group difference (F(1,134) = [0.490], p = .485) but significant effect of time (F(1,134) = [20.15], p = <.001) and interaction of group and time (F(1,134) = [9.07], p = .003).
Treatment in the DBT-PTSD group (d = 0.93, p < .001) has a large effect for the PCL-C re-experiencing subscale and in the TAU group (d = 0.21, p = .12) a small effect. For the PCL-C scale hyperarousal a large effect is observed for the DBT-PTSD group (d = 1.29, p < 0.001) and a medium effect for the TAU group (d = 0.68, p < .001).
Concerning the dissociative symptoms measured with the DES-II, the treatment in the TAU group (d = 0.06, p = .632) has a negligible effect on the symptoms at discharge compared to a large effect in the DBT-group (d = 0.98, p < .001). Results are very similar in the ITT data set for the TAU group (d = 0.05, p = .631), and in the DBT-group (d = 0.56, p < .001) a medium effect can be found, with significant differences for the time variable (F(1,134) = [14.01], p = .67 < .001), group variable (F(1,134) = [4.71], p = .032) and interaction of group and time (F(1,134) = [10.59], p = .001).
For the posttraumatic cognitions assessed with the PTCI scale, treatment in the DBT-PTSD group has large effects (d = 1.31, p < .001) and small effects in the TAU group (d = 0.32, p = .02). In the ITT data set the respective effect sizes are lower, with medium effects in the DBT-PTSD group (d = 0.69, p < .001) and small effects in the TAU group (d = 0.25, p < .001). The ANOVA reveals significant differences for the factor time (F(1,134) = [39.76], p = <.001) and the interaction between group and time (F(1,134) = [19.19], p = <.001).
Main results concerning the effect sizes of both groups for available and ITT data are summarized in Figure 2, demonstrating the large effect sizes for the treatment in the DBT-PTSD group compared to the TAU group.
Figure 2.
Effect sizes (Cohen’s d) of primary outcomes for available data and ITT data of the DBT-PTSD and TAU group.
3.2. ANCOVA
An analysis of covariance (ANCOVA) controlling for sex, age, the related baseline outcome score, and the treatment group factor (DBT-PTSD or TAU) showed a significant influence of the factor treatment group with a partial eta squared of 0.196 (F(1,175) = [18.32], p = < .001) for the treatment change in the PCL-C total score in the available data. The factor treatment group was significant for all other primary treatment outcomes except the PTCI total score with medium to large effect sizes (Supplemental Table 1).
3.2.1. Secondary outcomes
For the secondary outcome variables, the highest effect sizes can be found in the TAU group (d = 1.28, p < .001) for depressive symptoms assessed with the PHQ-9 (Table 4), comparable to the DBT-PTSD group (d = 1.18, p < .001). In the ITT data set lower effect sizes are found for both the DBT-PTSD group (d = 0.65, p < .001) and for the TAU group (d = 0.78, p < .001), with significant differences for the factor time (F(1,149) = [68.60], p < .001).
Table 4.
Treatment outcomes in different treatment groups after imputation: Secondary outcomes.
| Available data | Intent to treat data | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tbaseline | Tpost-treatment | Tpost-treatment | ||||||||||||
| Measure | Group | N | M(SD) | N | M(SD) | da | p | N | M(SD) | da | p | ANOVAb | Factor | p |
| MSI-BPD | DBT-PTSD | 42 | 6.47 (2.19) | 21 | 5.24 (3.05) | 0.65 | .007 | 42 | 5.63 (2.78) | 0.42 | .009 | F(1,129) = 17.18 | Time | <.001 |
| TAU | 89 | 5.54 (2.30) | 53 | 4.27 (2.57) | 0.48 | <.001 | 89 | 4.83 (2,53) | 0.36 | .001 | F(1,129) = 4.311 | Group | .032 | |
| dc | 0.36 | .170 | 0.31 | .102 | F(1,129) = 0.121 | Gr. x ti. | .728 | |||||||
| PHQ-9 | DBT-PTSD | 43 | 17.7 (4.41) | 22 | 11.5(4.96) | 1.18 | <.001 | 43 | 14.3 (5.51) | 0.65 | <.001 | F(1,149) = 68.60 | Time | <.001 |
| TAU | 108 | 16.9 (4.86) | 66 | 12.5 (4.81) | 1.28 | <.001 | 108 | 13.8 (5.04) | 0.78 | <.001 | F(1,149) = 0.559 | Group | .456 | |
| dc | 0.19 | .441 | 0.09 | .637 | F(1,149) = 0.169 | Gr. x ti. | .681 | |||||||
| GAD-7 | DBT-PTSD | 44 | 13. (3.81) | 22 | 9.27 (5.28) | 1.04 | <.001 | 44 | 11.2 (5.01) | 0.59 | <.001 | F(1,150) = 46.96 | Time | <.001 |
| TAU | 108 | 14.2 (4.24) | 66 | 10.6 (4.73) | 0.89 | <.001 | 108 | 11.8 (4.85) | 0.61 | <.001 | F(1,150) = 0.432 | Group | .512 | |
| dc | 0.26 | .291 | 0.11 | .524 | F(1,150) = 0.053 | Gr. x ti. | .818 | |||||||
| ERQ Reappraisal | DBT-PTSD | 42 | 22.5 (7.81) | 21 | 28.8 (5.98) | 1.01 | <.001 | 42 | 26.2 (7.74) | 0.58 | <.001 | F(1,133) = 26.19 | Time | <.001 |
| TAU | 93 | 22.5 (7.77) | 55 | 26.1 (6.46) | 0.48 | <.001 | 93 | 24.7 (6.95) | 0.36 | <.001 | F(1,133) = 0.35 | Group | .556 | |
| dc | 0.44 | .094 | 0.21 | .260 | F(1,133) = 1.79 | Gr. x ti. | .183 | |||||||
| ERQ Suppression | DBT-PTSD | 42 | 19.1 (5.09) | 21 | 16.5 (4.93) | 0.70 | .004 | 42 | 17.3 (4.73) | 0.45 | .006 | F(1,134) = 5.26 | Time | .023 |
| TAU | 94 | 17.0 (5.53) | 56 | 17.2 (4.48) | 0.01 | .921 | 94 | 17.0 (4.81) | 0.01 | .921 | F(1.134) = 1.91 | Group | .169 | |
| dc | 0.14 | .590 | 0.06 | .748 | F(1.134) = 5.78 | Gr. x ti. | .018 | |||||||
| WHODAS | DBT-PTSD | 44 | 22.8 (8.05) | 22 | 18.4 (8.57) | 0.99 | <.001 | 44 | 19.8 (7.95) | 0.58 | <.001 | F(1,140) = 38.6 | Time | <.001 |
| TAU | 98 | 22.9 (8.07) | 62 | 18.6 (8.26) | 0.76 | <.001 | 98 | 20.0 (7.97) | 0.55 | <.001 | F(1,140) = 0.01 | Group | .928 | |
| dc | 0.03 | .907 | 0.02 | .901 | F(1,140) = 0.01 | Gr. x ti. | .909 | |||||||
Note: DBT-PTSD = Dialectical Behavioural Therapy – Posttraumatic Stress Disorder, TAU = Treatment as usual, MSI-BPD = McLean Screening Instrument for Borderline Personality Disorder, PHQ-9 = Patient Health Questionnaire-9, GAD-7 = Brief Measure for assessing General Anxiety Disorder, ERQ = Emotion Regulation Questionnaire, WHODAS = WHO Disability Assessment Schedule.
Cohen’s d was computed using the pooled standard deviation of scores at admission (t1) and discharge (t2).
Repeated measures analysis of variance with intent to treat data.
Cohen’s d was computed using the pooled standard deviation of scores at discharge (t2) from the DBT-PTSD and TAU groups.
Secondly, anxiety symptoms could be reduced with a high effect size for both treatment groups: DBT-PTSD group (d = 1.04, p < .001), TAU group (d = 0.89, p < .001), with very similar effect sizes in the ITT data set: DBT-PTSD group (d = 0.59, p < .001), TAU group (d = 0.61, p < .001) and significant differences for the factor time (F(1,150) = [46.96], p < .001).
Borderline symptoms measured with the MSI-BPD improve with a medium effect for the DBT-PTSD group (d = 0.65, p = .007) and small effect for the TAU group (d = 0.48, p < .001), with a significant group difference (F(1,129) = [4.311], p = .032).
Concerning the emotional regulation strategy ‘suppression’ assessed with the ERQ, treatment in the TAU group (d = 0.01, p = .921) had a negligible effect, whereas treatment in the DBT-PTSD group (d = 0.70, p = .004) had a medium effect. In the ERQ reappraisal subscale large effects could be found for the DBT-PTSD group (d = 1.01, p < .001) and small effects for the TAU group (d = 0.48, p < .001). These effects are considerably higher in the ITT data set for the DBT-group (d = 0.58, p < .001) and somewhat higher for the TAU group (d = 0.36, p < .001), with significant differences only for the factor time (F(1,133) = [26.19], p < .001).
Effects were large in the DBT-PTSD group (d = 0.99, p < .001) for the WHODAS limitations in functioning and participation scale and medium for the TAU group (d = 0.76, p < .001). There was no significant group difference but a significant effect of the factor time (F(1,140) = [38.6], p = <.001) in the ITT data set.
4. Supplementary analyses
4.1. Primary outcomes
Both treatments have a large effect for the PCL-C total score in the matched data set (Table 5), DBT-PTSD (d = 1.39, p = .019); TAU group (d = 1.27, p = .003) and PCL-C re-experiencing subscale: DBT-PTSD group (d = 0.95, p = .067); TAU group (d = 0.82 p = .039). For the PCL-C numbing scale treatment in the DBT-PTSD group has a large effect (d = 1.53, p = .013) and a medium effect in the TAU group (d = 0.39, p = .252). Effect sizes for the PCL-C hyperarousal scale are large in the DBT-PTSD group (d = 1.44, p = .017) and in the TAU-group (d = 0.98, p = .013). Treatment in the DBT-PTSD group has large effect sizes in the DES-II scale (d = 1.42, p = .018) and the PTCI scale (d = 1.39, p = .019). In the TAU group a small effect can be shown for the DES-II scale (d = 0.20, p = .548) and a medium effect for the PTCI scale (d = 0.50, p = .150).
Table 5.
Supplementary analysis (matched data) of treatment outcomes in different treatment groups: Primary outcomes.
| Available data | Intent to treat data (n = 16) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tbaseline | Tpost-treatment | Tpost-treatment | |||||||||||
| Measure | Group | N | M(SD) | N | M(SD) | da | p | M(SD) | da | p | ANOVAb | Factor | p |
| PCL-C Total score | DBT-PTSD | 16 | 65.4 (7.48) | 6 | 50.7 (12.6) | 1.39 | .019 | 59.1 (11.6) | 0.58 | .036 | F(1,30) = 13.46 | Time | <.001 |
| TAU | 16 | 66.8 (7.86) | 10 | 61.5 (7.17) | 1.27 | .003 | 61.2 (6.89) | 0.79 | .006 | F(1,30) = 0.456 | Group | .848 | |
| dc | 1.14 | .044 | 0.22 | .544 | F(1,30) = 0.037 | Gr. x ti. | .505 | ||||||
| PCL-C Re-experiencing | DBT-PTSD | 16 | 3.51 (0.89) | 6 | 2.83 (1.08) | 0.95 | .067 | 3.21 (0.98) | 0.48 | .075 | F(1,30) = 8.117 | Time | .008 |
| TAU | 16 | 4.13 (0.78) | 10 | 4.10 (0.40) | 0.82 | .039 | 3.90 (0.71) | 0.58 | .034 | F(1,30) = 5.244 | Group | .029 | |
| dc | 1.75 | .004 | 0.81 | .030 | F(1,30) = 0.166 | Gr. x ti. | .687 | ||||||
| PCL-C Numbing/ avoidance | DBT-PTSD | 16 | 4.99 (0.62) | 6 | 3.67 (0.98) | 1.53 | .013 | 4.42 (0.97) | 0.60 | .030 | F(1,30) = 7.173 | Time | .012 |
| TAU | 16 | 4.87 (1,04) | 10 | 4.79 (0.82) | 0.39 | .252 | 4.70 (0.95) | 0.30 | .244 | F(1.30) = 0.068 | Group | .796 | |
| dc | 1.27 | .027 | 0.29 | .422 | F(1,30) = 2.108 | Gr. x ti. | .157 | ||||||
| PCL-C Hyperarousal | DBT-PTSD | 16 | 4.30 (0.76) | 6 | 4.50 (0.96) | 1.44 | .017 | 3.83 (0.90) | 0.58 | .034 | F(1,30) = 11.29 | Time | .002 |
| TAU | 16 | 4.35 (0.68) | 10 | 3.96 (0.87) | 0.98 | .013 | 4.04 (0.80) | 0.67 | .017 | F(1,30) = 0.270 | Group | .607 | |
| dc | 0.79 | .149 | 0.25 | .484 | F(1,30) = 0.481 | Gr. x ti. | .493 | ||||||
| DES-II | DBT-PTSD | 16 | 36.9 (19.6) | 6 | 29.4 (14.2) | 1.42 | .018 | 32.3 (18.5) | 0.58 | .035 | F(1,30) = 3.551 | Time | .069 |
| TAU | 16 | 37.5 (14.8) | 10 | 39.9 (15.5) | 0.20 | .548 | 35.8 (15.1) | 0.16 | .537 | F(1,30) = 0.129 | Group | .722 | |
| dc | 0.70 | .198 | 0.21 | .557 | F(1,30) = 0.750 | Gr. x ti. | .393 | ||||||
| PTCI total score | DBT-PTSD | 16 | 144.4 (24.2) | 6 | 112.7 (31.6) | 1.39 | .019 | 128.5 (28.9) | 0.58 | .036 | F(1,30) = 7.66 | Time | .010 |
| TAU | 16 | 147.3 (35.9) | 10 | 145.4 (32.9) | 0.50 | .150 | 139.7 (32.1) | 0.38 | .147 | F(1,30) = 0.500 | Group | .485 | |
| dc | 1.01 | .071 | 0.37 | .306 | F(1,30) = 0.984 | Gr. x ti. | .329 | ||||||
Note: DBT-PTSD = Dialectical Behavioural Therapy – Posttraumatic Stress Disorder, TAU = Treatment as usual, PCL-C = Posttraumatic Stress Disorder Checklist-Civilian Version, DES-II = Dissosciative Experience Scale, PTCI = Posttraumatic Cognitions Inventory.
Cohen’s d was computed using the pooled standard deviation of scores at admission (t1) and discharge (t2).
Repeated measures analysis of variance with intent to treat data.
Cohen’s d was computed using the pooled standard deviation of scores at discharge (t2) from the DBT-PTSD and TAU groups.
Compared with the ITT data (Table 5), no significant interaction effects with the factors group and time could be found. There are significant differences for all relevant primary outcome scales for the factor time but only one significant group difference for the PCL-C Re-experiencing scale (F(1,30) = [5.244], p = .029). Compared with the ITT data, treatment effects for the matched data set are small to medium.
4.2. Secondary outcomes
Treatment in the DBT-PTSD (d = 1.79, p = .00) group had a large effect on borderline-related symptoms assessed with the MSI-BPD and medium effects for the TAU group (d = 0.63, p = .079) (Table 6). Large effects can be observed for depressive symptoms in both groups (Table 6): DBT-PTSD group (d = 1.47, p = .015), TAU group (d = 1.21, p = .002). Anxiety symptoms are improved with medium effects in both groups: DBT-PTSD group (d = 0.79, p = .112), TAU group (d = 0.67, p = .049). Compared with the ITT data, there were no significant treatment effects for the factor time for the ERQ Reappraisal (F(1,30) = [2.380], p = .139) and ERQ Suppression scale (F(1,30) = [0.132], p = .719). There were no significant treatment effects for the factor group or the interaction of group and time.
Table 6.
Supplementary analysis (matched data) of treatment outcomes in different treatment groups: Secondary outcomes.
| Available data | Intent to treat data (n = 16) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tbaseline | Tpost-treatment | Tpost-treatment | |||||||||||
| Measure | Group | N | M(SD) | N | M(SD) | da | p | M(SD) | da | p | ANOVAb | Factor | p |
| MSI-BPD | DBT-PTSD | 16 | 6.90 (1.85) | 6 | 4.17 (1.60) | 1.79 | .007 | 5.78 (2.22) | 0.63 | .023 | F(1,30) = 9.211 | Time | .005 |
| TAU | 16 | 6.88 (1.15) | 10 | 5.00 (2.62) | 0.63 | .079 | 5.81 (2.40) | 0.47 | .080 | F(1,30) = 0.0001 | Group | .995 | |
| dc | 0.36 | .497 | 0.015 | .966 | F(1,30) = 0.008 | Gr. x ti. | .931 | ||||||
| PHQ-9 | DBT-PTSD | 16 | 17.7 (4.92) | 6 | 11.7 (3.56) | 1.47 | .015 | 14.9 (4.94) | 0.59 | .032 | F(1,30) = 16.38 | Time | <.001 |
| TAU | 16 | 18.1 (4.36) | 11 | 13.5 (4.72) | 1.21 | .002 | 13.8 (4.31) | 0.83 | .005 | F(1,30) = 0.051 | Group | .823 | |
| dc | 0.41 | .432 | 0.23 | .522 | F(1,30) = 0.726 | Gr. x ti. | .401 | ||||||
| GAD-7 | DBT-PTSD | 16 | 14.3 (4.44) | 6 | 9.33 (4.50) | 0.79 | .112 | 12.9 (5.23) | 0.42 | .112 | F(1,30) = 7.298 | Time | .011 |
| TAU | 16 | 14.1 (3.94) | 11 | 11.4 (3.67) | 0.67 | .049 | 12.0 (3.88) | 0.53 | .052 | F(1,30) = 0.233 | Group | .633 | |
| dc | 0.51 | .329 | 0.19 | .595 | F(1,30) = 0.158 | Gr. x ti. | .633 | ||||||
| ERQ Reappraisal | DBT-PTSD | 16 | 22.9 (7.04) | 6 | 24.7 (7.00) | 0.16 | .714 | 23.2 (6.92) | 0.10 | .690 | F(1,30) = 2.308 | Time | .139 |
| TAU | 16 | 21.7 (8.36) | 10 | 24.5 (8.00) | 0.48 | .161 | 24.1 (7.50) | 0.37 | .157 | F(1,30) = 0.004 | Group | .950 | |
| dc | 0.02 | .967 | 0.12 | .734 | F(1,30) = 1.359 | Gr. x ti. | .253 | ||||||
| ERQ Suppression | DBT-PTSD | 16 | 17.3 (6.19) | 6 | 15.7 (5.57) | 0.61 | .199 | 16.4 (5.12) | 0.35 | .184 | F(1,30) = 0.132 | Time | .719 |
| TAU | 16 | 18.1 (6.56) | 10 | 18.1 (4.28) | 0.08 | .798 | 18.4 (5.01) | 0.07 | .791 | F(1,30) = 0.557 | Group | .461 | |
| dc | 0.12 | .742 | 0.41 | .259 | F(1,30) = 0.719 | Gr. x ti. | .403 | ||||||
| WHODAS | DBT-PTSD | 16 | 23.9 (9.18) | 6 | 21.1 (10.7) | 1.11 | .042 | 22.4 (9.01) | 0.52 | .055 | F(1,30) = 9.767 | Time | .004 |
| TAU | 16 | 23.6 (6.83) | 11 | 20.3 (8.23) | 0.80 | .024 | 20.4 (6.97) | 0.61 | .028 | F(1,30) = 0.181 | Group | .674 | |
| dc | 0.08 | 0.880 | 0.26 | .474 | F(1,30) = 1.384 | Gr. x ti. | .249 | ||||||
Note: DBT-PTSD = Dialectical Behavioural Therapy – Posttraumatic Stress Disorder, TAU = Treatment as usual, MSI-BPD = McLean Screening Instrument for Borderline Personality Disorder, PHQ-9 = Patient Health Questionnaire-9, GAD-7 = Brief Measure for assessing General Anxiety Disorder, ERQ = Emotion Regulation Questionnaire, WHODAS = WHO Disability Assessment Schedule.
Cohen’s d was computed using the pooled standard deviation of scores at admission (t1) and discharge (t2).
Repeated measures analysis of variance with intent to treat data.
Cohen’s d was computed using the pooled standard deviation of scores at discharge (t2) from the DBT-PTSD and TAU groups.
5. Discussion
The present study was conducted during standard operation of an inpatient mental health centre with patients treated with either the DBT-PTSD treatment (Bohus et al., 2013) or TAU. Primary and secondary PTSD-related treatment outcomes were analysed before and after both treatments. The DBT-PTSD group shows better primary treatment outcomes than the TAU group as shown by an interaction of time and group. However, the observed effect sizes (ES) were lower than in previously published RCT studies (Bohus et al., 2013, 2020). The ITT data analyses demonstrated low to medium effects for both treatment groups with significant differences in outcomes between the two time points and a significant interaction of time and group. However, the ITT analyses yielded considerably lower ES than available data (completers) analysis, which indicates that adherence to treatment may have influenced ES in this and previously published studies (Bohus et al., 2013, 2020).
For secondary treatment outcomes there was no interaction of time and group. The DBT-PTSD and the TAU group showed comparable improvements in all secondary treatment outcomes, except for the maladaptive emotion regulation strategy suppression which was better improved in the DBT-PTSD group.
The study results demonstrate that particularly in the DBT-PTSD group, treatment adherence (i.e. treatment completion) can bias accurate estimation of the treatment effects. Since the TAU does not use mandatory exposure for all patients, patients’ adherence to the exposure in the DBT-PTSD group seems to be an essential treatment element contributing to the efficacy of the treatment.
The results from the present study are comparable with previously published RCTs on the DBT-PTSD treatment protocol by Bohus et al. (2020; Hayes et al., 2013), although previous studies only included female patients with childhood physical or sexual abuse. The effects of the DBT-PTSD treatment for PTSD-related symptoms were large, although not as large as in the previous RCT study (Bohus et al., 2020) with outpatients (d = 2.34; Posttraumatic-Stress Disorder Checklist for DSM-V). In the present study effect sizes for BPD-related symptoms (d = 1.4; Borderline Symptom List-23) and depression (d = 1.37; Beck Depression Inventory-II) were comparable to previous studies.
In a supplementary analysis, we have used propensity score matching to match patients with similar baseline scores on primary and secondary treatment outcomes as well as sociodemographic data from both treatment groups. Treatment effects in both the DBT-PTSD group and the TAU group were large for the PCL-C, the main symptom scale for PTSD. Further, higher ES were observed for both treatment groups in the matched sample, which suggests that a selective sampling bias could have inflated ES in the main analysis of the data. Nevertheless, the sample sizes for the matched data are small and effect sizes are limited interpretable.
Since research on treatment outcomes is often based on RCT studies in laboratory research settings it is possible that treatments only work under strictly controlled conditions. Thus, treatment protocols tend to be less effective under representative clinical conditions (Kendall, 1998; McEvoy et al., 2012; Nathan, 1998; Shadish et al., 2000). However, a meta-analysis by Shadish et al. (2000) suggests that psychological treatments can be similarly effective in laboratory research-settings and in clinically representative settings. Besides, Lambert (2017) highlights the need of patient feedback and monitoring of treatment response and particularly identifying patients at risk for deterioration or dropout.
Our study was conducted in a naturalistic setting using observational data from routine clinical care to maximize the degree of clinical representativeness and to assess the implementation of an established treatment in a mental health centre setting. Participants of the study were patients not only diagnosed with a PTSD but also with other comorbid diagnosis further enhancing the external face validity of routine care conditions and representativeness of patients with trauma specific disorders. Moreover, therapists in the TAU group and to a somewhat lesser extend also in the DBT-PTSD group, were able to independently determine the content and intensity of treatment and were not restricted to a specific study protocol.
The naturalistic setting representing routine clinical care conditions in an inpatient mental health setting is a major strength of this pilot study. Further, state-of-the-art psychological instruments were used for outcome assessments. One limitation of the present study is that no structured diagnostic interviews were used to determine diagnosis. Another limitation of the study in respect to its external validity is that patients with current suicidal behaviour were not accepted for treatment, even though the DBT and DBT PE protocols as well as the DBT-PTSD modification by Bohus and Priebe (2018) have been developed as an effective psychological intervention for this particular group of patients. This restriction can be found in many RCT studies focusing on patients with BPD or PTSD which usually exclude patients with suicidal and self-injurious behaviour or other psychiatric comorbidities (Harned et al., 2012). Finally, the results from the present study are limited to immediate treatment effects, since follow-up data were not assessed.
In summary, the present study confirms that the DBT-PTSD treatment is also effective in a routine clinical care setting, but with lower effect sizes compared to previously published RCTs. The DBT-PTSD treatment outperformed the TAU treatment in the reduction of trauma-related symptoms, dissociative symptoms and DSO-related symptoms. The notable differences in effect sizes between the available data and the ITT data analyses suggest that selective sampling may have inflated effect sizes in previously published RCTs. Moreover, the much lower effect sizes in the ITT data analyses indicate the influence of treatment adherence on estimates of treatment effects. Thus, it is important to routinely monitor the symptoms of patients who are at high risk of dropout or deterioration and to append relevant strategies in the treatment protocols to prevent or reduce patient dropout respectively to increase patient’s adherence to treatment.
Supplementary Material
Acknowledgements
The authors thank all patients and therapists from the trauma treatment units at the University Hospital for Psychosomatic Medicine Eggenburg as well as Elisabeth Busta and Thomas Schütt who conducted the routine clinical outcome monitoring. The authors also like to thank Friedrich Riffer who made this study possible through his lobbying for the establishment of the endowed professorship in clinical psychology at the Karl Landsteiner University and his advocacy for the implementation of the DBT-PTSD treatment programme at the University Hospital for Psychosomatic Medicine Eggenburg. Author Contributions: CO, MS were involved in designing and conceptualization of the research study. CO, MS, SG and JB were responsible for the data analysis and data curation. CO and MS prepared the original draft and were responsible for revisions and prepared the final version. SG and JB were involved in the review and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding Statement
We gratefully acknowledge the financial support of the Society for Research Promotion Lower Austria [Gesellschaft für Forschungsförderung Niederösterreich (GFF)] as part of the endowed professorship in clinical psychology (MS, JB, SG). We also thank the VAMED for funding the position held by CO. We further acknowledge support by Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria. These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets presented in this article are not readily available because of the vulnerability of the study sample. Participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Statement of ethics
The present study is a retrospective analysis of data collected during routine clinical care. The analysis was approved by the ethics commission of the Karl Landsteiner University of Health Sciences (Nr: 1020/2021). Written informed consent was obtained from all study participants and the study follows the ethical standards proposed in the Declaration of Helsinki (World Medical Association, 2013). All participants consented to the use of their data.
References
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed). [Google Scholar]
- Austin, P. C. (2011). An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research, 46(3), 399–424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard, C., Hsu, K. J., Rifkin, L. S., Busch, A. B., & Björgvinsson, T. (2016). Validation of the PHQ-9 in a psychiatric sample. Journal of Affective Disorders, 193, 267–273. 10.1016/j.jad.2015.12.075 [DOI] [PubMed] [Google Scholar]
- Beck, J. G., Coffey, S. F., Palyo, S. A., Gudmundsdottir, B., Miller, L. M., & Colder, C. R. (2004). Psychometric properties of the posttraumatic cognitions inventory (PTCI): A replication with motor vehicle accident survivors. Psychological Assessment, 16(3), 289–298. 10.1037/1040-3590.16.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, D. P., Stein, J. A., Newcomb, M. D., Walker, E., Pogge, D., Ahluvalia, T., Stokes, J., Handelsman, L., Medrano, M., Desmond, D., & Zule, W. (2003). Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse & Neglect, 27(2), 169–190. 10.1016/S0145-2134(02)00541-0 [DOI] [PubMed] [Google Scholar]
- Bernstein, E. M., & Putnam, F. W. (1986). Development, reliability, and validity of a dissociation scale. The Journal of Nervous and Mental Disease, 174(12), 727–735. 10.1097/00005053-198612000-00004 [DOI] [PubMed] [Google Scholar]
- Bohus, M., Dyer, A. S., Priebe, K., Krüger, A., Kleindienst, N., Schmahl, C., Niedtfeld, I., & Steil, R. (2013). Dialectical behaviour therapy for post-traumatic stress disorder after childhood sexual abuse in patients with and without borderline personality disorder: A randomised controlled trial. Psychotherapy and Psychosomatics, 82(4), 221–233. 10.1159/000348451 [DOI] [PubMed] [Google Scholar]
- Bohus, M., Kleindienst, N., Hahn, C., Müller-Engelmann, M., Ludäscher, P., Steil, R., Fydrich, T., Kuehner, C., Resick, P. A., Stiglmayr, C., Schmahl, C., & Priebe, K. (2020). Dialectical behavior therapy for posttraumatic stress disorder (DBT-PTSD) compared with cognitive processing therapy (CPT) in complex presentations of PTSD in women survivors of childhood abuse: A randomized clinical trial. JAMA Psychiatry, 77(12), 1235–1245. 10.1001/jamapsychiatry.2020.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohus, M., & Priebe, K. (2018). DBT–PTSD: A treatment programme for complex PTSD after childhood abuse. In Swales M. A. (Ed.), The Oxford handbook of dialectical behaviour therapy (pp. 814–828). Oxford University Press. Retrieved January 10, 2022, from http://oxfordhandbooks.com/view/10.1093oxfordhb/9780198758723.001.0001/oxfordhb-9780198758723-e-48. [Google Scholar]
- Brewin, C. R. (2020). Complex post-traumatic stress disorder: A new diagnosis in ICD-11. BJPsych Advances, 26(3), 145–152. 10.1192/bja.2019.48 [DOI] [Google Scholar]
- Carlson, E. B., & Putnam, F. W. (1993). An update on the dissociative experiences scale. Dissociation: Progress in the Dissociative Disorders, 6(1), 16–27. [Google Scholar]
- Cloitre, M. (2020). ICD-11 complex post-traumatic stress disorder: Simplifying diagnosis in trauma populations. The British Journal of Psychiatry. 216(3), 129–131. 10.1192/bjp.2020.43 [DOI] [PubMed] [Google Scholar]
- Coventry, P. A., Meader, N., Melton, H., Temple, M., Dale, H., Wright, K., Cloitre, M., Karatzias, T., Bisson, J., Roberts, N. P., Brown, J. V. E., Barbui, C., Churchill, R., Lovell, K., McMillan, D., & Gilbody, S. (2020). Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: Systematic review and component network meta-analysis. PLOS Medicine, 17(8): e1003262. 10.1371/journal.pmed.1003262. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7446790/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea, W., Ford, J., Stolbach, B., Spinazzola, J., & van der Kolk, B. A. (2012). Understanding interpersonal trauma in children: Why we need a developmentally appropriate trauma diagnosis. American Journal of Orthopsychiatry, 82(2), 187–200. 10.1111/j.1939-0025.2012.01154.x [DOI] [PubMed] [Google Scholar]
- Ehlers, A., Clark, D. M., Hackmann, A., McManus, F., & Fennell, M. (2005). Cognitive therapy for post-traumatic stress disorder: Development and evaluation. Behaviour Research and Therapy, 43(4), 413–431. 10.1016/j.brat.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Foa, E. B., Ehlers, A., Clark, D. M., Tolin, D. F., & Orsillo, S. M. (1999). The posttraumatic cognitions inventory (PTCI): Development and validation. Psychological Assessment, 11(3), 303–314. 10.1037/1040-3590.11.3.303 [DOI] [Google Scholar]
- Foa, E., Hembree, E. A., Rothbaum, B. O., & Rauch, S. (2019). Prolonged exposure therapy for PTSDEmotional processing of traumatic experiences - therapist guide: Emotional processing of traumatic experiences - therapist guide. Oxford University Press. https://www.oxfordclinicalpsych.com/view/10.1093med-psych/9780190926939.001.0001/med-9780190926939. [Google Scholar]
- Ford, J. D. (2021). Progress and limitations in the treatment of complex PTSD and developmental trauma disorder. Current Treatment Options in Psychiatry, 8(1), 1–17. 10.1007/s40501-020-00236-6 [DOI] [Google Scholar]
- Gilbert, P. (2014). The origins and nature of compassion focused therapy. British Journal of Clinical Psychology, 53(1), 6–41. 10.1111/bjc.12043 [DOI] [PubMed] [Google Scholar]
- Gold, L. H. (2014). DSM-5 and the assessment of functioning: The World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0). Journal of the American Academy of Psychiatry and the Law Online, 42(2), 173–181. [PubMed] [Google Scholar]
- Gross, J. J., & John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–362. 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Gyani, A., Shafran, R., Layard, R., & Clark, D. M. (2013). Enhancing recovery rates: Lessons from year one of IAPT. Behaviour Research and Therapy, 51(9), 597–606. 10.1016/j.brat.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harned, M. S., Korslund, K. E., Foa, E. B., & Linehan, M. M. (2012). Treating PTSD in suicidal and self-injuring women with borderline personality disorder: Development and preliminary evaluation of a dialectical behavior therapy prolonged exposure protocol. Behaviour Research and Therapy, 50(6), 381–386. 10.1016/j.brat.2012.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harned, M. S., Korslund, K. E., & Linehan, M. M. (2014). A pilot randomized controlled trial of dialectical behavior therapy with and without the dialectical behavior therapy prolonged exposure protocol for suicidal and self-injuring women with borderline personality disorder and PTSD. Behaviour Research and Therapy, 55, 7–17. 10.1016/j.brat.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harned, M. S., Schmidt, S. C., Korslund, K. E., & Gallop, R. J. (2021). Does adding the dialectical behavior therapy prolonged exposure (DBT PE) protocol for PTSD to DBT improve outcomes in public mental health settings? A pilot nonrandomized effectiveness trial with benchmarking. Behavior Therapy, 52(3), 639–655. 10.1016/j.beth.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, S. C., Levin, M. E., Plumb-Vilardaga, J., Villatte, J. L., & Pistorello, J. (2013). Acceptance and commitment therapy and contextual behavioral science: Examining the progress of a distinctive model of behavioral and cognitive therapy. Behavior Therapy, 44(2), 180–198. 10.1016/j.beth.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häuser, W., Schmutzer, G., Brähler, E., & Glaesmer, H. (2011). Maltreatment in childhood and adolescence: Results from a survey of a representative sample of the German population. Deutsches Arzteblatt international, 108(17), 287–294. 10.3238/arztebl.2011.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzner, B., Giesinger, J. M., Pinggera, J., Zugal, S., Schöpf, F., Oberguggenberger, A. S., Gamper, E. M., Zabernigg, A., Weber, B., & Rumpold, G. (2012). The computer-based health evaluation software (CHES): A software for electronic patient-reported outcome monitoring. BMC Medical Informatics and Decision Making, 12(1), Article 126. 10.1186/1472-6947-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzias, T., Murphy, P., Cloitre, M., Bisson, J., Roberts, N., Shevlin, M., Hyland, P., Maercker, A., Ben-Ezra, M., Coventry, P., Mason-Roberts, S., Bradley, A., & Hutton, P. (2019). Psychological interventions for ICD-11 complex PTSD symptoms: Systematic review and meta-analysis. Psychological Medicine, 49(11), 1761–1775. 10.1017/S0033291719000436 [DOI] [PubMed] [Google Scholar]
- Kendall, P. C. (1998). Empirically supported psychological therapies. Journal of Consulting and Clinical Psychology, 66(1), 3–6. 10.1037/0022-006X.66.1.3 [DOI] [PubMed] [Google Scholar]
- Kroenke, K., & Spitzer, R. L. (2002). The PHQ-9: A new depression diagnostic and severity measure. Psychiatric Annals, 32(9), 509–515. 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- Kroenke, K., Spitzer, R. L., & Williams, J. B. (2001). The PHQ-9. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K., Spitzer, R. L., Williams, J. B. W., & Löwe, B. (2010). The patient health questionnaire somatic, anxiety, and depressive symptom scales: A systematic review. General Hospital Psychiatry, 32(4), 345–359. 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Lambert, M. J. (2017). Maximizing psychotherapy outcome beyond evidence-based medicine. PPS, 86(2), 80–89. 10.1159/000455170 [DOI] [PubMed] [Google Scholar]
- Lincoln, T. M., Rief, W., Hahlweg, K., Frank, M., von Witzleben, I., Schroeder, B., & Fiegenbaum, W. (2003). Effectiveness of an empirically supported treatment for social phobia in the field. Behaviour Research and Therapy, 41(11), 1251–1269. 10.1016/S0005-7967(03)00038-X [DOI] [PubMed] [Google Scholar]
- Linehan, M. M., Cochran, B. N., & Kehrer, C. A. (2001). Dialectical behavior therapy for borderline personality disorder. In D. H. Barlow (Ed.), Clinical handbook of psychological disorders: A step-by-step treatment manual (3rd ed., pp. 470–522). The Guilford Press. [Google Scholar]
- Liu, X. (2016). Methods for handling missing data. In X. Liu (Ed.), Methods and applications of longitudinal data analysis (pp. 441–473). Elsevier. Retrieved March 15, 2022 from https://linkinghub.elsevier.com/retrieve/pii/B9780128013427000149. [Google Scholar]
- Maercker, A. (2021). Development of the new CPTSD diagnosis for ICD-11. Borderline Personality Disorder and Emotion Dysregulation, 8(1), Article 7. 10.1186/s40479-021-00148-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney, A., Karatzias, T., & Hutton, P. (2019). A systematic review and meta-analysis of group treatments for adults with symptoms associated with complex post-traumatic stress disorder. Journal of Affective Disorders, 243, 305–321. 10.1016/j.jad.2018.09.059 [DOI] [PubMed] [Google Scholar]
- McDonald, S. D., & Calhoun, P. S. (2010). The diagnostic accuracy of the PTSD checklist: A critical review. Clinical Psychology Review, 30(8), 976–987. 10.1016/j.cpr.2010.06.012 [DOI] [PubMed] [Google Scholar]
- McEvoy, P. M., Nathan, P., Rapee, R. M., & Campbell, B. N. C. (2012). Cognitive behavioural group therapy for social phobia: Evidence of transportability to community clinics. Behaviour Research and Therapy, 50(4), 258–265. 10.1016/j.brat.2012.01.009 [DOI] [PubMed] [Google Scholar]
- Nathan, P. E. (1998). Practice guidelines: Not yet ideal. American Psychologist, 53(3), 290–299. 10.1037/0003-066X.53.3.290 [DOI] [Google Scholar]
- Rosenbaum, P. R., & Rubin, D. B. (1983). The central role of the propensity score in observational studies for causal effects. Biometrika, 70(1), 41–55. 10.1093/biomet/70.1.41 [DOI] [Google Scholar]
- Rosenbaum, P. R., & Rubin, D. B. (1985). Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. The American Statistician, 39(1), 33–38. 10.2307/2683903 [DOI] [Google Scholar]
- Schmider, E., Ziegler, M., Danay, E., Beyer, L., & Bühner, M. (2010). Is it really robust?: Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodology, 6(4), 147–151. 10.1027/1614-2241/a000016 [DOI] [Google Scholar]
- Shadish, W. R., Matt, G. E., Navarro, A. M., & Phillips, G. (2000). The effects of psychological therapies under clinically representative conditions: A meta-analysis. Psychological Bulletin, 126(4), 512–529. 10.1037/0033-2909.126.4.512 [DOI] [PubMed] [Google Scholar]
- Southam-Gerow, M. A., Austin, A. A., & Marder, A. M. (2008). Transportability and dissemination of psychological treatments. In D. McKay (Ed.), Handbook of research methods in abnormal and clinical psychology (pp. 203). The SAGE publications. [Google Scholar]
- Spitzer, R. L., Kroenke, K., Williams, J. B. W., & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- van Der Kolk, B., Ford, J. D., & Spinazzola, J. (2019). Comorbidity of developmental trauma disorder (DTD) and post-traumatic stress disorder: Findings from the DTD field trial. European Journal of Psychotraumatology, 10(1), Article 1562841. 10.1080/20008198.2018.1562841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn, M. H., & Schuengel, C. (1996). The measurement of dissociation in normal and clinical populations: Meta-analytic validation of the dissociative experiences scale (DES). Clinical Psychology Review, 16(5), 365–382. 10.1016/0272-7358(96)00006-2 [DOI] [Google Scholar]
- Weathers, F. W., Litz, B. T., Herman, D., Huska, J., & Keane, T. (1994). The PTSD Checklist-Civilian Version (PCL-C) (p. 10). National Center for PTSD. [Google Scholar]
- Wilkins, K. C., Lang, A. J., & Norman, S. B. (2011). Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depression and Anxiety, 28(7), 596–606. 10.1002/da.20837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2019). International classification of diseases for mortality and morbidity statistics (11th Revision). https://icd.who.int/browse11/l-m/en [Google Scholar]
- World Medical Association . (2013). World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- Zanarini, M. C., Vujanovic, A. A., Parachini, E. A., Boulanger, J. L., Frankenburg, F. R., & Hennen, J. (2003). A screening measure for BPD: The McLean screening instrument for borderline personality disorder (MSI-BPD). Journal of Personality Disorders, 17(6), 568–573. 10.1521/pedi.17.6.568.25355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because of the vulnerability of the study sample. Participants of this study did not agree for their data to be shared publicly, so supporting data is not available.