ABSTRACT
A systematic review (Prospero CRD42017075562) including articles published between 1 January 1990 and 31 October 2021 was performed to synthesize evidence on the effect of integrating tuberculosis (TB) and diabetes mellitus (DM) healthcare on screening coverage and treatment loss to follow-up as compared to non-integrated care services for TB and DM in low- to middle-income countries (LMICs). Searches were performed in PubMed, Web of Science, WHO Global Index Medicus, and Cochrane Central Library. This review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and we adopted Cochrane data collection form for Randomized Controlled Trials (RCTs) and non-RCTs. Due to heterogeneity and limited data of studies included, meta-analysis was not performed. Of 6902 abstracts, 10 studies from South America, Asia, and Africa were included. One study from Zimbabwe showed 57% increase in DM screening among TB patients in integrated care as compared to non-integrated care; 95% CI: 54.1, 59.8. Seven studies with before-after comparison groups reported increased screening coverage during implementation of integrated healthcare that ranged from 10.1% in Mexico to 99.1% in China. Three studies reported reduction in loss to follow-up among TB patients in integrated care; two in China showed 9.2%, 95% CI: −16.7, –1.7, and −9.5%, 95% CI: −18.4, −0.7 differences, while a study from Mexico showed −5.3% reduction, 95% CI: −9.8, -0.9.
With few and heterogenous included studies, the synthesized evidence is weak to establish effect of TB/DM integrated care. Therefore, further robust studies such as randomized clinical trials and well-designed observational studies are needed.
KEYWORDS: Tuberculosis, diabetes mellitus, bidirectional screening, integrated care, treatment outcomes
Introduction
With the global increase of non-communicable diseases (NCDs) prevalence, many low- to middle-income countries (LMICs) are facing a double burden of both communicable diseases and non-communicable diseases (NCDs) [1,2]. For instance, global diabetes mellitus (DM) prevalence was estimated 9.3% (463 million people) in 2019, and the prevalence is expected to reach 10.9% (700 million people) by 2045 [3]. The World Health Organization (WHO) reported that approximately 1.5 million deaths in 2012 were directly caused by DM globally, while an estimated 2.2 million deaths were attributed to high blood glucose [4]. Global prevalence of DM is expected to increase particularly rapidly in LMICs, where 80% of the global disease burden occurs [2,5,6].
Studies have found that the risk of developing active tuberculosis (TB) is 2–3 times higher in persons with DM (PWDs) than in persons without DM [7,8]. It is estimated that 15% of TB cases are attributed to DM [9] worldwide, and more than 1 million TB and DM comorbid cases are estimated globally [2,10,11]. Evidence shows that poorly controlled DM impairs TB treatment response with increased risk of poor TB treatment outcomes and TB-related mortality [12,13]. According to systematic reviews that have been previously performed [14,15], prevalence of DM among TB patients was 9.0%, 95% CI: 6.0, 12.0 in Sub-Saharan Africa (SSA), while global DM prevalence among TB patients was 15.3%, 95% CI: 2.5, 36.1; I2 = 99.8%. However, global TB prevalence among PWDs is estimated at 4.7%, 95% CI: 3.6, 5.8, with prevalence in Africa estimated at 5.13%, 95% CI: 4.34, 5.92 [16]. An observational study that was conducted in India found that 13% of TB patients were living with DM, while a survey that was conducted in Uganda found that 2.3% of the TB patients were living with DM [17,18]. The observed association between TB and DM has led to an effort in LMICs to start implementing integrated healthcare programs, as encouraged by WHO, The International Union against Tuberculosis and Lung Diseases (IUATLD), and The International Diabetes Federation (IDF) [19,20]. Systematic reviews and clinical trials have shown consistent evidence of the association between TB and DM, and the impact of the rapidly increasing prevalence of DM on the management of TB control programs [7,21–26]. Several systematic reviews that have been conducted, and synthesized evidence highlights the strength of association between DM and TB and its effect on the efforts in fighting TB and DM, especially in LMICs [12,27]. Further reviews have been performed to synthesize the evidence on effects of increasing NCDs on service delivery models and healthcare systems in primary healthcare settings [28], especially in tropical countries where bidirectional screening and integrated management approaches are highly recommended [21,29–31]. Since many LMICs are facing, among others, financial and governance challenges in their respective health sectors, several studies aim to understand the impacts of NCDs on the weak health systems in LMICs [32–34]. However, none of the currently available systematic reviews like those listed in Table 1 have assessed the evidence on the effects of DM and TB integration on utilization of healthcare services and treatment outcomes for TB and DM in LMICs with a focus on bidirectional screening and treatment loss to follow-up. The information on the effects of DM and TB integrated healthcare, however, is important when prioritizing programmatic interventions in LMICs, where the vulnerability of healthcare systems may mean that a surplus of new programmatic measures may endanger the running of well-established and rather strong vertical programmes such as Malaria, TB, and HIV and AIDS control programs. Therefore, in this systematic review, we aimed to assess available evidence on how integrated healthcare for TB and DM affects utilization of services and treatment outcomes for both TB and DM among TB patients and PWDs in integrated healthcare settings as compared to non-integrated healthcare settings in LMICs. We have defined integrated healthcare, explained target population, setting, and specific outcomes assessed in the sections on eligibility criteria and objectives, respectively.
Table 1.
Describing related systematics reviews and their findings on effects of TB on NCDs especially DM care and effects of NCDs and DM on TB treatment outcomes in low- to middle-income countries.
| Title of the Systematic Review, first author, and publication year. | Brief findings and discussions of the Review |
| Diabetes among tuberculosis patients and its impact on tuberculosis treatment in South Asia: a systematic review and meta-analysis (Gautam, S, et al 2021). | This review found that TB-DM comorbid patients are at high risk of treatment failure and high risk of mortality, and that the prevalence of DM among TB patients is high in Asia (21%, 95% CI: 18.0, 23.0). Furthermore, authors recommend strategic planning and implementation of screening for DM among TB patients for early identification and starting management at early stage of both diseases. |
| Elements and Performance Indicators of Integrated Healthcare Programmes on Chronic Diseases in Six Countries in the Asia-Pacific Region: A Scoping Review (Pinter, KA, et al 2021). | In this scoping review, integration elements to healthcare, barriers and facilitators were identified. Authors found that many integration programmes are being implemented in Asia-Pacific region; however, most of these programmes lack evaluation plans and or reports to assess the effects and implementation indicators of the programmes. |
| Co-prevalence of type 2 diabetes mellitus and tuberculosis in low-income and middle-income countries: A systematic review (McMurry, H S, et al 2019). | Like the systematic review that was performed in South Asia, the results in this review showed a high prevalence of DM among TB patients with most studies reporting between 10% and 30%, while that of TB and PWDs ranged from 0.1% to 6.0%. With these findings, authors encourage more research on bidirectional screening and integrated chronic diseases management. Main limitations highlighted by the authors were huge diversity of study designs and diagnostic methods |
| Association between diabetes mellitus and multi-drug-resistant tuberculosis: evidence from a systematic review and meta-analysis (Tegegne, BS et al. 2018). | This systematic review and meta-analysis demonstrated consistent evidence of a substantially increased risk of TB disease among people with DM (PWDs). This evidence was based on data from studies using different designs and reported from six continents. PWDs with uncontrolled blood glucose (measured by higher FBG or HbA1c) appeared to be at higher risk of active TB than patients with controlled DM. |
| The impact of diabetes on tuberculosis treatment outcomes: A systematic review (Baker MA, et al. 2011). | The study findings suggest that diabetes increases the risk of failure and death combined, death, and relapse among patients with tuberculosis. This study highlights a need for increased attention to treatment of tuberculosis in people with diabetes (PWDs), which may include testing for suspected diabetes, improved glucose control, and increased clinical and therapeutic monitoring. |
| Bidirectional screening for tuberculosis and diabetes: a systematic review (Jeon CY, et al. 2010). | The findings support the evidence global high prevalence of TB among PWDs and vice versa. |
| Is there an effect of glucose-lowering treatment on incidence and prognosis of tuberculosis? A systematic review(Jørgensen & Faurholt-Jepsen 2014). | Authors in this review concluded that although numerous studies have reported adverse treatment response; that is morbidity, mortality, in patients with hyperglycemia and/or diabetes; there is still no evidence on the effect of glucose-lowering drugs on tuberculosis incidence or during anti-tuberculosis treatment. Despite this, the WHO and the International Union Against Tuberculosis and Lung Diseases state that “Although there are no published trials assessing if improved glucose control reduces the risk of adverse TB treatment outcomes, the existing evidence indirectly suggests that optimized management of diabetes in TB patients, including early diagnosis, optimized treatment and health education, and clinical and therapeutic monitoring, would improve TB treatment outcomes and reduce the risk of recurrent TB.” |
| Epidemiology and interaction of diabetes mellitus and tuberculosis and challenges for care: a review (Harries AD, et al. 2013). | This review reports that in LMICs, and especially in Asia, the DM epidemic is growing rapidly. There is now strong evidence that there is an important association between DM and TB and that this association results in poor TB treatment outcomes. Undiagnosed, inadequately treated and poorly controlled DM appears to be a much greater threat to TB care and prevention than previously realized, and this needs to be tackled. The situation is critical in poor countries. |
| Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts (Marais BJ, et al. 2013). | Authors in the review conclude that destructive competition between communicable and non-communicable diseases must be avoided for the limited funds available for health services. Integrated solutions should seek to unlock potential commonalities and synergies and optimize scarce resources. Efficient integration of tuberculosis services with those for non-communicable and other communicable diseases should be a priority while maintaining some vertical elements to secure essential functions, such as drug supply, monitoring and assessment, and national surveillance, to a point at which a small central team could oversee the national tuberculosis programme. |
Abbreviations: NCDs: Non-communicable Diseases, DM: Diabetes mellitus, TB: Tuberculosis
Review objectives
This systematic review was conducted to synthesize evidence of effects of programmatic TB and DM integrated healthcare on bidirectional screening and treatment loss to follow-up of both TB patients and PWDs in LMICs by comparing studies with TB/DM integrated healthcare to settings without integration of DM and TB in LMICs. We specifically extracted data and synthesized evidence on screening proportions in both TB patients and PWDs, and treatment loss to follow-up among TB patients and PWDs. Our secondary aims were to assess the impact on cure rate among TB patients and blood glucose control among PWDs in LMICs.
Methods
The systematic review was performed by following the reporting guidelines of Meta-Analysis of Observational Studies in Epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions [35,36]. This review was registered in 2017 with Prospero ID: CRD42017075562. We described ranges of effects of studies comparing changes in screening coverage, treatment loss to follow-up, and cure rate among PWDs and TB patients after implementation of integrated healthcare measures.
Eligibility criteria
We targeted studies in which integration of TB and DM care interventions enable participants to access services under one consultation or at one delivery point during a visit or TB /DM integrated screening and joint case management in LMICs, or both. In this review, the following definition of integration of healthcare was adopted; “a variety of managerial or operational changes to health systems to bring together inputs, delivery, management and organisation of particular service functions” [37]. These integrated care services aim to improve the service in relation to efficiency and quality, thereby maximizing use of resources and opportunities for patients to access care for more health conditions. In integrated care, a patient receives a complete care for two or more health problems or diseases at one service point [37,38]. Furthermore, we considered integration at the service delivery level as different kinds of DM and TB services, or operational programs or activities joined together to ensure and perhaps maximize collective outcomes for both PWDs and TB patients. The comparators in the review were non-integrated healthcare interventions or vertical delivery of services for TB and DM services where services users, for instance, make separate appointments or attend separate clinic days or where there is no TB/DM integrated screening and joint case management, or both. Only studies that were done in LMICs were included, and specifically studies that assessed the effects on screening coverage and treatment loss to follow-up. According to WHO [16], coverage was defined as“ the proportion of people with a need for health services who actually receive such services within a given time, while TB treatment loss to follow-up is defined as a TB patient who did not start treatment or whose treatment was interrupted for two consecutive months or more.” The reference list of LMICs was adopted from World bank website [39]. In addition to integration status and location, we targeted studies that were focusing on people aged ≥15 years, and only English publications were included.
Information sources and search strategy
As shown in Table 2, we searched PubMed, Web of Science (WoS), WHO Global Index Medicus (WHO-GIM), and Cochrane Central Library (CCL). Studies published in the English language between 1 January 1990 and 31 October 2021 were considered for inclusion. The searches were initially performed by combining the search terms; “Diabetes” OR “Diabetes Melitus” AND “Tuberculosis” AND “Integrated care,” into the selected databases; however, in final searches performed on 31 October 2021, additional search terms; “joint” and “combine,” related terms to integrated care were included. We searched for both Randomized Control Trials (RCTs) and Observational studies that compared DM and TB integrated care to DM and TB non-integrated care in LMICs. The applied search strategy was adopted from Tegegne (2017) et al. [40], and details of the search strategies and the key words that we entered to find the suitable articles are shown in Table 2.
Table 2.
Search strategies, abstracts and final hits found that were screened during both initial and updated searches that were performed from July 2017 to October 2021.
| NO | DATA BASE | DESCRIPTION (search terms used were tuberculosis, diabetes, integrate, combine, joint) | #FINAL HITS |
|---|---|---|---|
| 1 | Medline (PubMed) | Initial Search: (“tuberculosis”[MeSH Terms] OR “tuberculosis”[All Fields]) AND ((“diabetes mellitus”[MeSH Terms] OR (“diabetes”[All Fields] AND “mellitus”[All Fields]) OR “diabetes mellitus”[All Fields]) OR (“diabetes mellitus”[MeSH Terms] OR (“diabetes”[All Fields] AND “mellitus”[All Fields]) OR “diabetes mellitus”[All Fields] OR “diabetes”[All Fields] OR “diabetes insipidus”[MeSH Terms] OR (“diabetes”[All Fields] AND “insipidus”[All Fields]) OR “diabetes insipidus”[All Fields])) AND ((“1990/01/01”[PDAT]: “2017/12/31”[PDAT]) AND “humans”[MeSH Terms] | 2178 |
| Updated Search (31/10/2021): ((diabetes) AND (tuberculosis) AND (English[Filter])) AND (integrate) (“diabetes”[All Fields] OR “diabetes mellitus”[MeSH Terms] OR (“diabetes”[All Fields] AND “mellitus”[All Fields]) OR “diabetes mellitus”[All Fields] OR “diabetes”[All Fields] OR “diabetes insipidus”[MeSH Terms] OR (“diabetes”[All Fields] AND “insipidus”[All Fields]) OR “diabetes insipidus”[All Fields] OR “diabetic”[All Fields] OR “diabetics”[All Fields] OR “diabetes”[All Fields]) AND (“tuberculosis”[All Fields] OR “tuberculosis”[MeSH Terms] OR “tuberculosis”[All Fields] OR “tuberculosis”[All Fields] OR “tuberculosis s”[All Fields]) AND “English”[Language] AND (“integrability”[All Fields] OR “integrable”[All Fields] OR “integral”[All Fields] OR “integrally”[All Fields] OR “integrals”[All Fields] OR “integrant”[All Fields] OR “integrates”[All Fields] OR “integrate”[All Fields] OR “integrated”[All Fields] OR “integrates”[All Fields] OR “integrating”[All Fields] OR “integration”[All Fields] OR “integrational”[All Fields] OR “integrations”[All Fields] OR “integrative”[All Fields] OR “integrative”[All Fields] OR “integrator”[All Fields] OR “integrators”[All Fields]) Search: ((diabetes) AND (tuberculosis) AND (English[Filter])) AND (combine) (“diabetes”[All Fields] OR “diabetes mellitus”[MeSH Terms] OR (“diabetes”[All Fields] AND “mellitus”[All Fields]) OR “diabetes mellitus”[All Fields] OR “diabetes”[All Fields] OR “diabetes insipidus”[MeSH Terms] OR (“diabetes”[All Fields] AND “insipidus”[All Fields]) OR “diabetes insipidus”[All Fields] OR “diabetic”[All Fields] OR “diabetics”[All Fields] OR “diabetes”[All Fields]) AND (“tuberculosis”[All Fields] OR “tuberculosis”[MeSH Terms] OR “tuberculosis”[All Fields] OR “tuberculoses”[All Fields] OR “tuberculosis s”[All Fields]) AND “English”[Language] AND (“combinable”[All Fields] OR “combinated”[All Fields] OR “combination”[All Fields] OR “combinational”[All Fields] OR “combinations”[All Fields] OR “combinative”[All Fields] OR “combine”[All Fields] OR “combined”[All Fields] OR “combines”[All Fields] OR “combining”[All Fields]) Search: ((diabetes) AND (tuberculosis) AND (English[Filter])) AND (joint) (“diabetes”[All Fields] OR “diabetes mellitus”[MeSH Terms] OR (“diabetes”[All Fields] AND “mellitus”[All Fields]) OR “diabetes mellitus”[All Fields] OR “diabetes”[All Fields] OR “diabetes insipidus”[MeSH Terms] OR (“diabetes”[All Fields] AND “insipidus”[All Fields]) OR “diabetes insipidus”[All Fields] OR “diabetic”[All Fields] OR “diabetics”[All Fields] OR “diabetes”[All Fields]) AND (“tuberculosis”[All Fields] OR “tuberculosis”[MeSH Terms] OR “tuberculosis”[All Fields] OR “tuberculoses”[All Fields] OR “tuberculosis s”[All Fields]) AND “English”[Language] AND (“joint s”[All Fields] OR “joints”[MeSH Terms] OR “joints”[All Fields] OR “joint”[All Fields]) |
460 | ||
| 2 | Web of Science | initial Search: TOPIC: (Tuberculosis), TOPIC: (Diabetes) OR TOPIC: (Diabetes Mellitus)#2 AND #1 Indexes = SCI-EXPANDED, SSCI, CPCI-SSH Timespan = All years, #2 AND #1 Refined by: LANGUAGES: (ENGLISH) AND [excluding] PUBLICATION YEARS: (1983 OR 1980 OR 1975 OR 1974 OR 1970 OR 1969 OR 1965 OR 1968 OR 1963 OR 1961 OR 1959 OR 1956 OR 1958 OR 1954 OR 1957 OR 1953 OR 1955 OR 1950 OR 1949 OR 1948)Indexes = SCI-EXPANDED, SSCI, CPCI-SSH Timespan = All years | 1407 |
| Updated Search (31/10/2021): #1 = Tuberculosis (All Fields), #2 = Diabetes (All Fields), #3 = (#2) AND #1 and English (Languages), #4 = integrate (All Fields), #5 = (#3) AND #4, #6 = combine (All Fields), #7 = joint (All Fields), #8 = (#3) AND #6, #9 = (#3) AND #7. (((TS = (Tuberculosis)) AND TS = (Diabetes)) AND TS = (integrate)) AND (LA = = (“ENGLISH”)), (((TS = (Tuberculosis)) AND TS = (Diabetes)) AND TS = (combine)) AND (LA = = (“ENGLISH”)), and (((TS = (Tuberculosis)) AND TS = (Diabetes)) AND TS = (joint)) AND (LA = = (“ENGLISH”)), | 517 | ||
| 3 | Cochrane Library | “diabetes”: ti,ab,kw, or Diabetes Mellitus (Word variations have been searched) Initial Search. “diabetes”: ti,ab,kw, or Diabetes Mellitus (Word variations have been searched) AND “tuberculosis”:ti,ab,kw (Word variations have been searched) #1 and #2. (Cochrane reviews) |
|
| 108 | |||
| Updated Search (31/10/2021): “diabetes” in All Text AND “tuberculosis” in All Text (Trials) | 228 | ||
| 4 | WHO Global Index Medicus Database | Initial Search: Tuberculosis AND Diabetes, (tw:((tw:(tuberculosis)) AND (instance:”ghl”) AND (limit:(“humans”)))) AND (tw:((tw:(diabetes)) OR (tw:(diabetes mellitus)) AND (instance:”ghl”) AND (limit:(“humans”)))) AND (instance:”ghl”) | 1734 |
| Updated Search (/10/2021): Tuberculosis AND Diabetes, tw:((tw:(tuberculosis*)) AND (tw:(diabetes*))) AND (la:(“en”)) AND (year cluster:[1990 TO 2021]) | 270 | ||
| 5 | Total Final Hits | 6902 |
Data extraction
In this review, we adopted PRSIMA guidelines and the Cochrane data collection form for RCTs and non-RCTs [41,42] to conform to the standards and information required for reporting a systematic review. We used EndNote X8.2 to screen the identified studies, and both screening and extraction were conducted by two independent researchers (JN and AB) in parallel, while the other two senior researchers (BL and DW) were involved to solve any discrepancies. The researchers used a standardized extraction scheme where information on study outcomes were extracted. Furthermore, heterogeneity and risks of bias assessment were checked for compatibility and quality of included studies. Heterogeneity was physically assessed as the studies were more diverse in many aspects, such as those of the study participants, study period, comparison groups, designs, and setting. On risk of bias assessment for observational studies, we focused on confounding, selection of participants, interventions and deviation from intended interventions, missing data, measurement of outcome, and reporting of results, while blinding and randomization were additional domains that were included for RCTs.
Data analysis
Data were summarized to describe effects of integrated healthcare on the predefined outcomes; screening coverage and treatment loss to follow-up in both PWDs and TB patients. Studies that were included reported on screening coverage or treatment outcomes of TB or DM patients either as a before to after comparison or compared to a standard program setting. We performed descriptive statistics to describe proportions and risk differences (RD) reported in studies that reported changes in screening coverage and treatment loss to follow-up among PWDs and TB patients after measures of TB and DM program integration. Furthermore, we described cure rate and blood glucose controls as additional outcomes that were assessed. The included studies demonstrated significant clinical as well as methodological diversities in terms of designs, risks of bias, and methods [43]. Therefore, we neither perform random effects nor fixed-effects meta-analyses for change in screening coverage and treatment loss to follow-up because of heterogeneity and limited number of studies included.
Results
Abstract screening and overview of full texts included in the review
As shown in Figure 1 and in Table 3, 6902 abstracts were retrieved, and 10 papers were included in this systematic review. Nine included studies [18–20,44–49] were observational studies, while one was a RCT [50]. Of the 10 studies included in the review, one was conducted in Mexico, three in China, two were conducted in India, one in Indonesia, one in Zimbabwe, one in Angola, and one in Uganda. In eight included studies, screening was performed to assess the proportion of either TB in DM cases or DM among TB cases. Only a study from Indonesia was a RCT [50]. In the included studies, effects of TB and DM integrated healthcare on screening coverage, treatment loss to follow-up, blood glucose control, and cure rate were investigated in comparison to either historical data or standard treatment cohorts or control groups [44,46]. However, in most included studies, baseline data for comparison was lacking.
Figure 1.
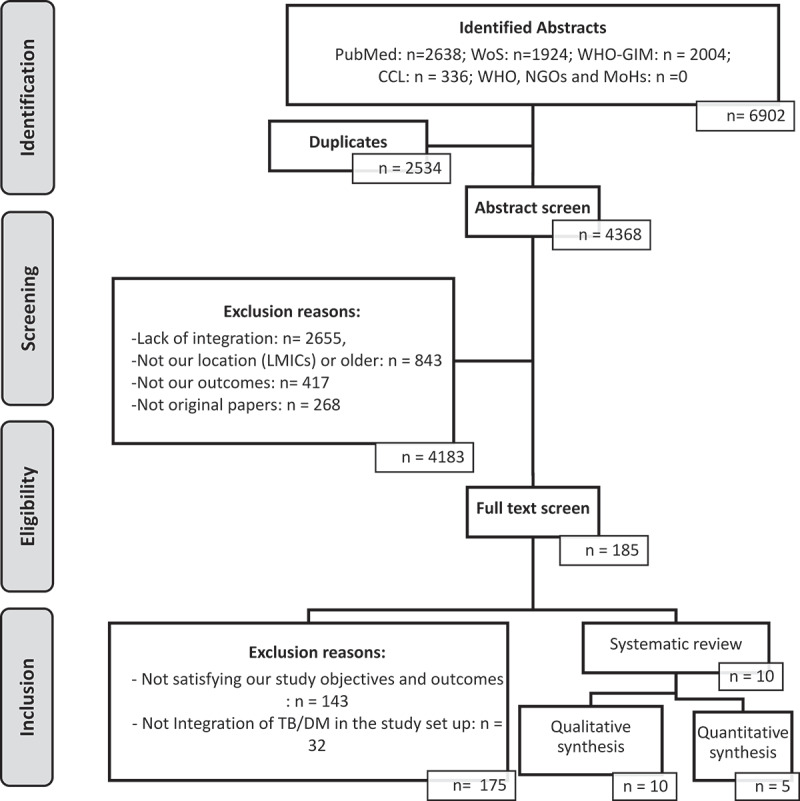
Flow diagram detailing screening of abstracts and full texts for the inclusion and exclusion of the studies into the review.
Table 3.
Description of the studies included in the systematic review.
| First Author and year | Study Title and Country | Study Design | Methodology | Outcomes Assessed | Objective, Setting and Study Duration | Description of Participants | Comparison group. | |
|---|---|---|---|---|---|---|---|---|
| 1 | Castellanos-Joya, M. et al. (2014) | Implementation of a Pilot Model for the Bidirectional Screening and Joint Management of Patients with Pulmonary Tuberculosis and Diabetes Mellitus in Mexico. | Prospective cohort. | Participants who were recruited from July 2012 to April 2013 were followed up until March 2014; and bidirectional screening was performed. | Main outcome measures were diagnoses of TB among DM, of DM among TB, and treatment loss to follow-up and Cure rate outcomes among patients with DM and TB. | The study was conducted in 15 primary care units in 5 states to investigate feasibility and effectiveness of Collaborative Framework for the Care and Control of TB and DM from July 2012 to April 2013. | TB and DM patients aged ≥20 years old who attended the clinics during study period. | A parallel group that was not exposed to joint management and a historical group was used in treatment outcomes. |
| 2 | Zhang, X. L. et al. (2015) | Integrating TB screening into annual health examinations for the rural elderly people in China. | Cross-sectional. . | Three counties in Shandong Province were randomly selected for TB screening among elderly individuals at high risk for TB. Individuals with X-rays suggestive of TB were referred to the county TB dispensary for further investigation. | Risks assessed: 1) those with symptoms of TB, 2) patients with type 2 diabetes mellitus (DM), and 3) close contacts of TBcases, 4) Treatment Loss to follow up. | The study was conducted in three counties in rural areas in Shandong Province to assess the feasibility and effects of identifying TB cases by integrating TB screening into routine health examinations from January to December 2013. | Elderly aged ≥60 years | Treatment loss to follow-up for participants of high TB risk among those with DM and TB Symptoms and those with only DM symptoms. |
| 3 | Ncube, RT. et al. (2019) | Feasibility and yield of screening for DM among TB patients in Harare, Zimbabwe. | Descriptive cross-sectional. | The patients were tested for DM using RBG and FBG. Data was double entered and analyzed by using EpiData. | Screening of TB patients for DM | The study was conducted from April to October 2017 to determine feasibility and yield of DM screening among TB patients in 2 infectious diseases Hospitals and 25 clinics in Harare. | TB patients aged ≥15 years form any of the clinics in the city of Harare. | Baseline where it is estimated that 23.7% of the population is aware of DM status. |
| 4 | Jali, MV. et al. (2013) | Bidirectional Screening of Tuberculosis Patients for Diabetes Mellitus and Diabetes Patients for Tuberculosis; India. | Prospective observational. | The data was collected from the TB Unit and DM Center during the study period. | Screening of TB patients for DM and DM patients for TB | To assess feasibility and effects of bidirectional screening among TB and DM patients within the routine healthcare setting between February to September 2012 in Bangalore. | Patients included in the study were aged ≥15 years old. | Not Specified |
| 5a | Kumar, A. et al. (2013)a | Screening of patients with tuberculosis for diabetes mellitus in India | Prospective observational study. | Screening for active TB in DM patients was followed as per the guidelines of the Revised National TB Control Programme (RNTCP). The screening for DM in TB patients followed the guidelines stipulated by the National Programme for prevention and control of Cancer, Diabetes, Cardiovascular Diseases and Stoke (NPCDCS) in India. | Screening of DM patients for TB | To assess feasibility and effects of screening patients with TB for DM within the routine healthcare setting in 8 tertiary care hospitals and more than 60 peripheral units from January 2012 to September 2012. | Patients of ages ≥15 | Not Specified. |
| 5b | Kumar, A . et al. (2013)b | Screening of patients with diabetes mellitus for tuberculosis in India |
Prospective observational study. | For project implementation, six health facilities for screening patients with DM for TB were purposively selected based on broad geographical coverage, co-located DM and TB diagnostic facilities and willingness of the staff of these clinics to participate in the project without any further financial resources. |
Screening of TB patients for DM | To assess the feasibility, results, and challenges of screening patients with diabetes mellitus (DM) for tuberculosis (TB) within the healthcare setting of six DM clinics in tertiary hospitals across India. | Patients of ages ≥15 | Not specified |
| 6 | Li, L. et al. (2012) | Screening of patients with tuberculosis for diabetes mellitus in China | Prospective observational. | The sites were selected basing on geographical locations, prevalence of TB and Urban-rural mix and TB Patients were included. Data was being collected by trained health workers and was reported quarterly. | Screening of TB patients for DM | To assess feasibility and effects of screening patients with TB for DM in 6 health facilities within routine health services from 1 September 2011 up to 31 March 2012. | TB Patients aged ≥15 years | Not Specified. |
| 7 | Segafredo, G. et al. (2019) | Integrating TB and non-communicable diseases services: Pilot experience of screening for diabetes and hypertension in patients with tuberculosis in Luanda, Angola | Descriptive cross-sectional study. | Newly diagnosed pulmonary TB (PTB) patients accessing directly observed treatment (DOT) centers were screened for DM and hypertension. Study personnel were administering a questionnaire to patients after obtaining a consent. | Screening of TB patients for DM | The study was conducted between January 2015 and December 2016 to assess the effects of integrating screening of DM and Hypertension in TB program in 6 TB centers in the city of Luanda in Angola. | TB Patients aged ≥15 years | Not Specified |
| 8 | Nsonga, J. et al.(2019) | Screening TB patients for DM in routine program setting in Kampala, Uganda | Descriptive cross-sectional study. | TB patients were screened for DM by trained health workers. FGB of ≥7.0 mmol/l was indicating DM. SPSS was used to analyze data. | Screening of TB patients for DM | A study was conducted in 10 health centers from April 2016 to December 2017 in Kampala to screen TB patients for DM in a routine setting with limited resources. | TB patients aged ≥15 years | Not specified |
| 9 | Ma, Y. et al. (2018) | Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up, in China | Retrospective Cohort analysis | Retrospective review of the medical records of all culture-positive retreatment pulmonary TB patients with type 2 DM between July 2009 and July 2013 | TB Treatment outcomes: cured, completed, failure, died, and defaulted treatment | This study used hospital records from July 2009 to July 2013 at Beijing Chest Hospital, Henan Center for Disease Control and Prevention, Shenyang Chest Hospital, the 309th Hospital of Chinese People’s Liberation Army, Beijing Research Institute for tuberculosis control to investigate whether metformin (MET) exhibited more efficacy in combination with anti-TB regimens for diabetic TB patients compared with those without MET. | Adults, and the median age of MET and non-MET group was 56.5 and 49.9 years. | Metformin treatment and non-metformin treatment |
| 10 | Ruslami, R. et al. (2021) | The effect of a structured clinical algorithm on glycemic control in patients with combined tuberculosis and diabetes in Indonesia: A randomized trial, in Indonesia | Open label randomized clinical trial | Randomization was carried out online using Research Electronic Data Capture, with permuted-block randomization (block sizes of 4, 6, and 8) generated using Stata v12. | Periodic change in Blood glucose levels | Patients were randomized between 28 April 2014 to 21 February 2017. To assess whether a package of structured counseling, clinical monitoring, and formal algorithm-based DM treatment adjustments offers better glycemic control than routine care in TB-DM patients. | Adults, mean age 53.0 years, and standard deviation of 9.4 years. | Patients assigned to the control arm only received information about TB at time of randomization and sent back to usual care. |
Abbreviations: NCDs: Non-communicable Diseases, DM: Diabetes mellitus, TB: Tuberculosis, FGB: Fasting Blood Glucose, PTB: Pulmonary TB, DOT: Directly Observed Therapy
Description of studies assessing bidirectional screening
As shown in Table 4, eight studies assessed bidirectional screening. Increase of screening for TB in DM patients ranged from 10.1% to 50.3%, and for DM in TB patients of 23.7% to 99.1%. However, seven of the eight studies had no baseline data for comparison; only a study in Zimbabwe had baseline data for comparison [46]. The information extracted from this study showed that screening coverage in TB patients for DM increased from 24% to 81%; 95% CI: 54.1, 59.8, compared to a non-integrated healthcare settings [46].
Table 4.
Evidence of effects of TB and DM integrated healthcare services on screening coverage, treatment loss to follow-up and cure rate in TB patients and PWDs in LMICs.
| Study ID | First Author and Publication year | (n/N)% of TB patients screened for DM in Non- Integrated care | (n/N)% of TB patients screened for DM in Integrated care | RD(%) (95% CI) | (n/N)% of DM patients screened for TB in Non- Integrated care | (n/N)% of DM screened for TB in Integrated care | RD(%) (95% CI) | (n/N)% TB Loss to Follow-up in TB/DM Non- Integrated Care | (n/N)% TB Loss to Follow-up in TB/DM Integrated Care | RD(%) (95% CI) | (n/N)% Cured in TB/DM Non- Integrated Care | (n/N0% Cured in TB/DM Integrated Care | RD(%) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observational Studies included in the review | |||||||||||||
| 1a | Castellanos-Joya, M. et al. (2014)a | NB | 361/883 (40.8%) | - | NB | - | 17/228 (7.5%) | 2/95 (2.1%) | −5.3% (−9.8,-0.9) | 128/228 (56.1%) | 81/95 (85.3%) | 29.1% (19.5,38.7) | |
| 1b | Castellanos-Joya, M. et al. (2014)b | NB | - | NB | 783/7763 (10.1%) | - | - | - | - | - | - | - | |
| 2 | Zhang, X. L. et al. (2015) | NB | 93094/141,931 (65.6%) | - | NB | - | - | 153/377 (40.6%) | 79/252 (31.3%) | −9.2% (−16.7,-1.7) | - | - | - |
| 3 | Ncube et al. (2019) | 383 (23.7%) | 1305 (80,7%) | 57.0% (54.1, 59.8) | NB | - | - | - | - | - | - | - | |
| 4a | Jali, MV et al. (2013)a | NB | 247/307 (80.5.0%) | - | NB | - | - | - | - | - | - | - | |
| 4b | Jali, MV et al. (2013)b | NB | - | - | NB | 2072/4118 (50.3%) | - | - | - | - | - | - | |
| 5a | Kumar et al. (2013)a | NB | - | - | NB | 13,961 (45.1%) | - | - | - | ||||
| 5b | Kumar et al. (2013)b | NB | 7467 (98.4%) | - | - | - | - | - | - | ||||
| 6 | Li, L. et al. (2012) | NB | 7947/8023 (99.1%) | - | - | - | - | - | - | ||||
| 7 | Segafredo et al. 2019) | NB | 5890/7144 (82.4%) | - | - | - | - | - | - | ||||
| 8 | Nsonga, J et al.(2019) | NB | 4016/4564 (88.0%) | - | - | - | - | - | - | ||||
| 9 | Ma, Y. (2018) | . | . | - | - | - | - | 4/42 (9.5%) | 0/16 (0.0% | −9.5% (−18.4, −0.65) | 25/42 (59.5%) | 13/16 (81.3%) | 21% (−2.5, 45.9) |
| Randomized Control Trial Reporting on Blood Glucose Control (TB-DM Patients with HbA1c of less than 8% at month 6) | |||||||||||||
| 10 | Ruslami, R.* et al. (2021) | - | - | . | 25/66 (37.9%) | 45/68 (66.2%) | 28.3% (12.1, 44.5) | ||||||
Abbreviations and symbols: NB: No baseline data for comparison, RD: Risk Difference, NCDs: Non-communicable Diseases, DM: Diabetes mellitus, TB: Tuberculosis, Blank (-): No data available or variable was not included in the assessment, Asterisk(*) is RCT study that assessed reduction in HbA1C records.
Bidirectional screening coverages and treatment loss to follow-up and cure rate among TB and DM patients were assessed in the Mexican study [44]. In this study, no baseline data was reported to calculate an effect on bidirectional screening, while the effect of joint management was analyzed by comparing with a parallel group that was not exposed to joint management as well as a historical group. In this review, we included the results from the parallel group and not the historical comparator, since parallel groups are less biased as compared to a historical comparison [51]. The study was conducted in 15 primary care units in five states in Mexico, where the researchers evaluated the feasibility and effectiveness of collaborative framework for the care and control of TB and DM. The study recruited both TB and DM patients aged ≥20 years of age who attended the clinics during the study period. Data were collected from July 2012 to April 2013. Data from this study showed that 361/885 (40.8%) TB patients were screened for DM and 783/7763 (10.1%) PWDs were screened for TB [44].
Another prospective observational implementation study in China [19] assessed feasibility and effects of screening patients with TB for DM in six health facilities within routine health services. Data from this study showed that 7947/8023 (99.1%) of TB patients were screened for DM by either random blood glucose (RBG) or fasting blood glucose (FBG), and like in the study from Mexico, no baseline data for comparison on screening coverage in this study was reported. Furthermore, sites in this study were purposely selected based on geographical locations, prevalence of TB, and urban-rural mix. All persons aged ≥15 years who were diagnosed with TB from 1 September 2011 up to 31 March 2012 were included, and trained health workers collected the data that were reported quarterly [19].
We found two studies that were conducted in India. The first study [52] was a prospective observational study; using a pre- and post-design; however, no baseline data for comparison was reported. The study aimed at assessing feasibility and effects of bidirectional screening among TB and DM patients within the routine healthcare setting between February and September 2012 in TB and DM centers in Bangalore in India. In this study, patients aged ≥15 years were recruited, and 247/307 (80.1%) TB patients were screened for DM, while 2072/4118 (50.3%) PWDs were screened for TB during the study period, respectively. Another prospective observational implementation study from India with two versions as noted by different contents, digital object identifiers (DOI), and published pages [47,53] describes the effects of integration on screening coverage for both TB patients and PWDs. The study was conducted to assess feasibility and effects of screening patients with TB for DM within the routine healthcare setting in eight tertiary care hospitals and more than 60 peripheral units across India. The study was conducted from January 2012 to September 2012. In this study, patients aged ≥15 years were included, and 8269 TB patients and 31,146 PWDs were recruited, of which 682 (8.3%) TB patients and 180 (0.6%) PWDs were known cases. During the study period, screening for DM was performed in 7467 (98.4%) of the TB patients, and 13,961 (45.1%) of PWDs were screened for TB [53].
Three studies were conducted in Africa. One study from Zimbabwe was conducted from April to October in 2017 to determine feasibility and yield of DM screening among TB patients. This study reported screening coverage of TB patients for DM by using both RBG and FBG screening tests. This study included 1617 TB patients aged ≥15 years from clinics within the city of Harare, where 24% of the study population were expected to be unaware of DM status as stated in the study [46]. Considering 24% as baseline data, the study showed that screening coverage in TB patients for DM increased from 24% to 81, RD: 57.0; 95% CI: 54.1, 59.8, compared to non-integrated healthcare setting. Further details showed that screening coverage using RBG was increasing gradually from 36% in the first quarter (April–June 2016) to 89% in fifth quarter (April–June 2017) [46]. Another study with no baseline data was conducted between January 2015 and December 2016 in Angola to assess the effects of integrating screening of DM and Hypertension in TB program in 6 TB centers in the city of Luanda. Newly diagnosed TB patients aged ≥15 years who attended the selected centers were screened for DM using FBG. In this study, 5890/7144 (82.4%) TB patients were screened for DM. A third study in Africa was conducted in Uganda from April 2016 to December 2017 to perform DM screening services among TB patients in a routine setting with limited resources. TB patients aged ≥15 years were recruited and screened for DM by trained health workers. During the implementation period, 4016/4564 (88.0%) of TB patients were screened for DM [18]. Like the Angolan study, there was no baseline data for comparison to establish the effect of integrated care.
Studies reporting on both screening coverage and treatment loss to follow-up
Three studies, one from Mexico and two from China, reported on effects of integrated care on both screening coverage and treatment loss to follow-up among TB patients [44,45,49]. In one study that was conducted in China from January to December 2013, three counties in Shandong province were examined to assess feasibility and effects of identifying TB cases by integrating TB screening into routine health examinations for the elderly people (aged ≥60 years) in rural areas, and 65.6% (93,094/141,931) elderly people were screened [45]. Although, the study lacked baseline data for comparison to quantify the effect of integrated community services on screening coverage, the analysis on the final diagnosis included loss to follow-up between different categories of the participants. It was reported that patients with DM and TB symptoms were less likely to miss a final diagnosis in comparison to participants with only an abnormal chest X-ray (31.3% (79/252 patients) versus 40.6% (153/377 patients); RD: 9.2%; 95% CI: 1.7%, 6.7%). In another study from China by Ma Y et al. [49], the authors reported 4/42 (9.5%) loss to follow-up in TB/DM patients who were not on joint metformin treatment versus none in patients who were on joint metformin treatment. Furthermore, this study reported 21% difference in cured TB/DM patients in metformin joint treatment group as compared to non-metformin group.
In a study performed in Mexico, treatment loss to follow-up was reduced from 7.5% (17/228) in the non-integrating parallel group to 2.1% (2/95) in the integrated healthcare arm (RD: −5.3%, 95% CI: –9.8, –0.9). When compared with a historical non-joint management group where treatment loss to follow-up was 16.7% (22/132), the effect of integration was more pronounced (RD: −14.6%, 95% CI: –21.5, –7.6). In the same study, we extended to extract data on cure rate. The results showed that cure rate among TB patients was 56.1% (128/228) in the non-joint management group and 85.3% (81/95) in the joint management arm (RD: 29.1%, 95% CI: 19.5%, 38.7%) [44]. Finally, a randomized controlled trials that were performed in Indonesia reported 28.3% difference in cured TB/DM patients in an integrated care than patients in traditional usual care setting [50]. Adult patients with mean age 53.0 years were included in this RCT.
Risk of bias assessment
By applying a Cochrane Risk of Bias Assessment Tool for non-randomized studies of intervention (ROBINS-I) [54,55], confounding, selection, performance related to classification of interventions, deviation from intended interventions, missing data, measurement of outcomes, and reporting biases were assessed in the nine selected observational studies [18–20,44–48]. The assessments focused on Pre-intervention, Intervention, and Post-Intervention phases in each included study. While for the assessment of risk of bias for the RCTs, we focused on randomization, blinding, deviations from intended intervention, missing data, measurement of the outcome, and selection of the reported domains. After completing the ROBINS-I and RoB tables in Microsoft Excel, the data were imported into the Risk of Bias Visualization tool [54] to display the assessment results, as shown both in Figures 2 and 3.
Figure 2.
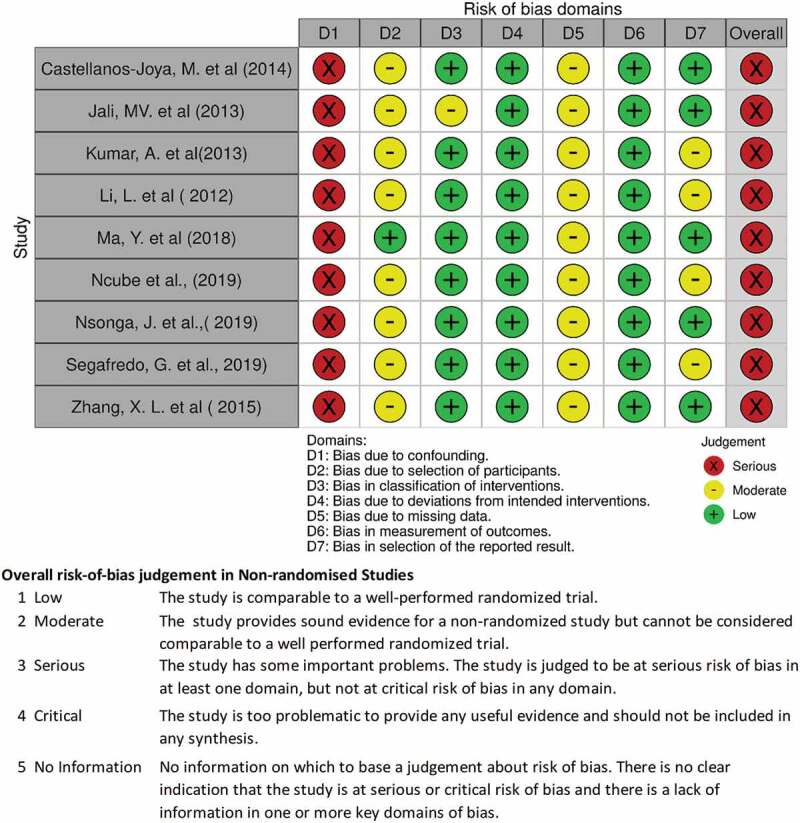
Risk of bias assessment for observational studies included in the review.
Figure 3.
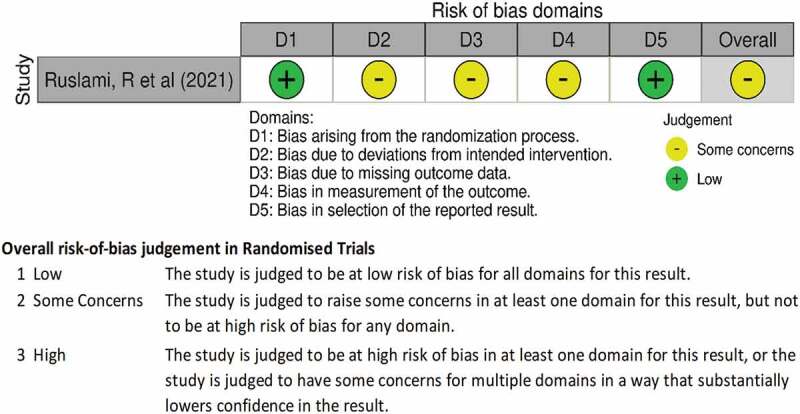
Risk of assessment of the randomized control trial included.
On the assessments of the outcome and screening of cases and controls or exposed and non-exposed, or both, we suspected misclassification biases at the inclusion of the participants since patients were included or excluded through screenings using rapid tests especially in DM screening. For instance, in most of the studies, random blood samples were collected on visit, and followed by fasting blood samples, the following day [18–20,44–48] and decisions were based on a range such as ≥6.1 mmol/l, which could be biased or be confounded by what participants had eaten before the diagnosis. Therefore, we suspected that inconsistencies in the assessments of screening results when recruiting participants could have been occurring.
The included studies did not seem to deviate from the intended interventions; however, most of them had inadequate information on the baseline data for comparison and few reported on the feasibility assessments [44,46], even though the objectives included feasibility assessment. Comparators for most studies were lacking such that, in most papers, national data or available known cases of either TB or DM patients did constitute baseline data and therefore could not be used for comparison. Since the studies used national data or available known cases, or both, as background data for these studies, this situation might have contributed to high risk of missing data in the studies. Furthermore, most studies claimed that feasibility was assessed, but it was not clear how the feasibility assessments were performed, suggesting high risk of bias on selection and on reported outcomes. Nonetheless, we anticipated presentation of robustness of results including further analyses like regression analysis of the observed effects of integration within the population and between clusters, but most studies only reported point estimates and or prevalence ratios stratified by sex and other patients’ background characteristics.
Discussion
Of the 6902 abstracts screened, ten studies, including nine observational studies and one RCT, were included. The studies were highly heterogenous and were assessed at serious risk of biases. The included studies were from Zimbabwe, Angola, Mexico, India, Uganda, Indonesia, and China. Eight studies reported on screening coverage or treatment outcomes of TB or PWDs either as a before and after comparison, or both; just 1/8 (12.5%) assessed the effects on screening coverage compared to a standard program setting in parallel which is a better comparison in implementation studies [51].
Among the included studies, there were varying settings and characteristics of participants, also methodologies and cutoff levels in screening for DM were not uniform. As a result of no baseline data for comparison, clinical as well as methodological diversities in terms of designs, risks of bias and methods, and limited number of studies included, statistical heterogeneity could not be assessed [43]. In this review, therefore, we did not perform meta-analyses for change in screening coverage and treatment outcomes because included studies were highly heterogenous. As such, we used descriptive statistics to evaluate each study that was included. However, the significant but rather weak evidence that we found in our review agrees with the results of the study on assessment of operationalization of bidirectional screening in Pakistan and that of the bidirectional screening evaluative study among TB and DM patients that was performed in India [56,57], where only 4.7% of the pre-diabetes and diabetes cases were screened for TB in Pakistan, and in India, 24% of TB patients were screened for DM. Furthermore, a study by Jerene et al. (2017) suggests that integrated screening facilitated the increase in yield of new TB and DM patients in either groups [58].
As reported in other studies, the observed low screening coverages, among others, were attributed to shortage of human resources, lack of testing materials (especially those for screening of DM among TB patients in the TB clinic); and these health systems challenges affected recording and reporting of cases especially with the sites seeing large numbers of patients [44,53]. Nevertheless, the study in China was successfully implemented within the routine system without a special budget for the implementation of this activity [19], suggesting that local considerations are important when deciding when to integrate and what package to actually include in TB and DM integrated healthcare services.
From our findings, the evidence suggests that treatment loss to follow-up may be reduced by the integration of TB and DM healthcare services as shown by the two studies from China and one study from Mexico in Table 4. The effects of integrated care on treatment loss to follow-up was moderate but significant and ranged from 5.3% to 9.5% [44,45,49].
Additional findings suggest that cure rates may increase in integrated settings and glucose control can be improved in joint treatments [44,45]. We observed a significant variability in effects across the included studies, which may, in part, be attributed to the heterogeneity between the studies and design issues. For instance, a study that was conducted in Mexico recruited hospital based patients of ages ≥15 years [44], whereas one study in China recruited community-based elderly people with risk factors for DM and TB aged ≥60 to over 80 years [45].
The age variations among participants and background residual confounding variables related to social-economic status between countries might have contributed to variability in the results, since socio-economic conditions are very important factors in determining participants adherence and compliance to treatment in healthcare. For instance, in another study that was conducted in Kyrgyzstan, the researchers found that TB and DM comorbid patients were exposed to more additional payments for healthcare services than patients within only TB disease [59], and such findings could also explain effects of social-economic factors related to access to care and utilization of services in LMICs. In addition to challenges faced in implementing integrated healthcare, studies have also shown that TB/DM bidirectional screening results in yielding more comorbid patients than in general populations in LMICs [60–62].
In addition to weak evidence on treatment loss to follow-up and screening coverage, our findings also show weak evidence to conclude that integrated TB and DM healthcare services delivery has a significant positive effect on cure rate among TB patients and blood glucose control among PWDs. However, research has shown that good glycemic control and cure rate may improve when TB and DM comorbid patients receive simultaneous treatment [50,63,64], but regardless of the few and unrobust studies that have been found in LMICs; WHO and IUATLD still encourage and recommend integrated healthcare of PWDs in TB patients to reduce the risk of recurrent TB and improve blood glucose control, respectively [65,66].
Study limitations
In this review, the searches were only limited to online and freely accessible databases; PubMed, Web of Science (WoS), WHO Global Index Medicus (WHO-GIM) and Cochrane Central Library (CCL). Due to limited time and lack of funding, it was not feasible to perform searches in non-free databases such as EMBASE and Global Health, and also collection of unpublished documents from international non-governmental organisations (NGOs) and governments in LMICs was not possible. Additionally, restricting to only English publications was a limitation. Furthermore, we believe that other important studies might have been missed since we only used the three work terms; “integrated,” ”joined,” and “combined” when searching for the relevant studies. It can happen that, in other countries, researchers might have used other related but not same terms in the studies conducted in TB and DM integrated healthcare services. With the limitations outlined herein, we believe that more studies may have been found in high income settings, other non-English speaking LMICs, and potentially identified in other databases or gray literature.
Conclusion
With few, heterogeneous and more observational studies mostly with a before-after comparison on the effect of implementing DM/TB integrated healthcare, and serious risks of bias assessments in the included studies, the synthesized evidence is weak to support the effect of TB and DM integrated care on screening coverage and treatment loss to follow-up. Nevertheless, the findings still suggested that TB/DM integrated care could have an impact on bidirectional screening and treatment loss to follow-up in both TB patients and people with DM. Therefore, decisions on local programs to integrate TB and DM healthcare services should be weighing the currently rather weak evidence, the barriers faced in the local context as well as existing healthcare guidelines for both diseases. Finally, we would recommend that further controlled implementation studies on utilization of TB and DM healthcare services, bidirectional screening coverage and treatment loss to follow-up after implementation of integration of DM and TB healthcare be performed in LMICs.
Acknowledgments
We thank the management in Department of Internal Medicine II; University of Freiburg for offering a working space and access to the computers throughout the review.
Funding Statement
There was no special funding for this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Declarations
Ethics approval and consent to participate
The study did not involve any human experiments, but only review of the existing literature. Nevertheless, the study design and data collection procedures followed the ethical principles as outlined in the World Medical Association (WMA) declaration (66), and ethical approval (reference number 157/17) was granted by University of Freiburg, Research and Ethics Committee.
Authors’ contributions
In this study project, JN, AB, and BL contributed with development of the study protocol and data extraction tools, data analysis and writing the manuscript, while DW supervised the review process, evaluation of the protocol and data extraction tools. Finally, all the authors reviewed the manuscript.
References
- [1].WHO . Noncommunicable diseases: progress monitor 2020 [Internet]. Geneva PP - Geneva: World Health Organization; 2020. Accessed 19 October 2021. Available from: https://apps.who.int/iris/handle/10665/330805 [Google Scholar]
- [2].Noubiap JJ, Nansseu JR, Nyaga UF, et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2· 3 million patients with tuberculosis. Lancet Glob Heal. 2019;7(4):e448–60. [DOI] [PubMed] [Google Scholar]
- [3].Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. 2019;157:107843. [DOI] [PubMed] [Google Scholar]
- [4].WHO . Global report on diabetes [Internet]. 978, 2020. Accessed 03 May 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes [Google Scholar]
- [5].McMurry HS, Mendenhall E, Rajendrakumar A, et al. Coprevalence of type 2 diabetes mellitus and tuberculosis in low-income and middle-income countries: a systematic review. Diabetes Metab Res Rev. 2019. Jan;35(1):e3066. [DOI] [PubMed] [Google Scholar]
- [6].Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract [Internet]. 2018;138:271–281. Available from http://www.sciencedirect.com/science/article/pii/S0168822718302031 [DOI] [PubMed] [Google Scholar]
- [7].Harries AD, Satyanarayana S, Kumar AM, et al. Epidemiology and interaction of diabetes mellitus and tuberculosis and challenges for care: a review. Public Health Action. 2013. Nov;3(Suppl 1):S3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ruslami R, Aarnoutse RE, Alisjahbana B, et al. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Heal. 2010;15(11):1289–1299. [DOI] [PubMed] [Google Scholar]
- [10].Harries A, Lin Y, Kumar AMV, et al. What can national TB control programmes in low- and middle-income countries do to end tuberculosis by 2030? F1000Research [Internet]. 2018. Jul 5;7:F1000 Faculty Rev-1011. Accessed 20 May 2019. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30026917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lönnroth K, Roglic G, Harries AD.. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol. 2014;2(9):730–739. [DOI] [PubMed] [Google Scholar]
- [12].Harries AD, Kumar AM, Satyanarayana V, et al. Diabetes mellitus and tuberculosis: programmatic management issues. Int J Tuberc Lung Dis [Internet]. 2015. Aug [cited 2017 Feb 7];19(8):879–886. Available from. http://www.ncbi.nlm.nih.gov/pubmed/26162352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harries A, Am V K, Satyanarayana S, et al. Addressing diabetes mellitus as part of the strategy for ending TB. Trans R Soc Trop Med Hyg. 2016;110(3):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alebel A, Wondemagegn AT, Tesema C, et al. Prevalence of diabetes mellitus among tuberculosis patients in Sub-Saharan Africa: a systematic review and meta-analysis of observational studies. BMC Infect Dis [Internet]. 2019;19(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jeon CY, Harries AD, Baker MA, et al. Bi-directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Heal [Internet]. 2010. Nov 1;15(11):1300–1314. [DOI] [PubMed] [Google Scholar]
- [16].Wagnew F, Eshetie S, Alebel A, et al., Meta-analysis of the prevalence of tuberculosis in diabetic patients and its association with cigarette smoking in African and Asian countries. BMC Res Notes [Internet]. 2018. May 15;11(1):298. Available from [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kumar A, Gupta D. Screening of patients with diabetes mellitus for tuberculosis in India. Trop Med Int Heal [Internet]. 2013. May cited 2017 Jun 22;18(5):646–654. Available from h tt p://d oi.wi ley.c om/10.1 111/tmi.1 2084 [DOI] [PubMed] [Google Scholar]
- [18].Nsonga J, Dongo JP, Mugabe F, et al. Screening tuberculosis patients for diabetes mellitus in a routine program setting in Kampala, Uganda: a cross-sectional study. F1000Res. 2019. Jun 17;8:872. [Internet]. Accessed 21 March 2020. https://pubmed.ncbi.nlm.nih.gov/31681473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li L, Lin Y, Mi F, et al. Screening of patients with tuberculosis for diabetes mellitus in China. Trop Med Int Heal. 2012;17(10):1294–1301. [DOI] [PubMed] [Google Scholar]
- [20].Jali M-V, Mahishale V-K, Hiremathi M-B. Bidirectional screening of tuberculosis patients for diabetes mellitus and diabetes patients for tuberculosis. Diabetes Metab J [Internet]. 2013;37(4):291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu H, Muhunthan J, Hayek A, et al. Examining the use of process evaluations of randomised controlled trials of complex interventions addressing chronic disease in primary health care—a systematic review protocol. Syst Rev [Internet]. 2016. Dec 15 [cited 2017 Feb 9];5(1):138. Available from http://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-016-0314-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tegegne BS, Mengesha MM, Teferra AA, et al. Association between diabetes mellitus and multi-drug-resistant tuberculosis: evidence from a systematic review and meta-analysis. Syst Rev. 2018;7(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Worswick J, Wayne SC, Bennett R, et al. Improving quality of care for persons with diabetes: an overview of systematic reviews-what does the evidence tell us? Syst Rev. 2013;2(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Watkins DA, Tulloch NL, Anderson ME, et al. Delivery of health care for cardiovascular and metabolic diseases among people living with HIV/AIDS in African countries: a systematic review protocol. Syst Rev. [Internet]. 2016. Dec 16 cited 2017 Feb 9;5(1):63. Available from http://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-016-0241-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Graham-Rowe E, Lorencatto F, Lawrenson JG, et al. Barriers and enablers to diabetic retinopathy screening attendance: protocol for a systematic review. Syst Rev. 2016;5(1):134. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med [Internet]. 2011. Dec 1 cited 2017 Feb 9;9(1):81. Available from http://www.ncbi.nlm.nih.gov/pubmed/21722362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deng C, Wang X, Liao Y. Current recommendations on managing tuberculosis patients with diabetes & its epidemiology. Microb Pathog. [Internet]. 2016. Mar cited 2017 Feb 9;92:43–45. Available from http://linkinghub.elsevier.com/retrieve/pii/S0882401015301911 [DOI] [PubMed] [Google Scholar]
- [28].Damery S, Flanagan S, Combes G. The effectiveness of interventions to achieve co-ordinated multidisciplinary care and reduce hospital use for people with chronic diseases: study protocol for a systematic review of reviews. Syst Rev. 2015;4(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marais BJ, Lönnroth K, Lawn SD, et al. Tuberculosis comorbidity with communicable and non-communicable diseases: integrating health services and control efforts. Lancet Infect Dis [Internet]. 2013. May cited 2017 Jun 23;13(5):436–448. Available from http://linkinghub.elsevier.com/retrieve/pii/S147330991370015X [DOI] [PubMed] [Google Scholar]
- [30].van Crevel R, van de Vijver S, Moore DAJ. The global diabetes epidemic: what does it mean for infectious diseases in tropical countries? Lancet Diabetes Endocrinol. 2017. Mar;5(6):457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jeon CY, Harries AD, Baker MA, et al. Bi-directional screening for tuberculosis and diabetes: a systematic review. Trop Med Int Heal [Internet]. 2010. Nov cited 2017 Feb 9;15(11):1300–1314. Available from h tt p://d oi.wi ley.c om/1 0.1 11 1/j.13 65-3156.2010.026 32.x [DOI] [PubMed] [Google Scholar]
- [32].Mills A. Health care systems in low- and middle-income countries. N Engl J Med. 2014;370(6):552–557. [DOI] [PubMed] [Google Scholar]
- [33].Oelke ND, Suter E, da Silva Lima MAD, et al. Indicators and measurement tools for health system integration: a knowledge synthesis protocol. Syst Rev. 2015;4(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Campbell JR, Sasitharan T, Marra F. A systematic review of studies evaluating the cost utility of screening high-risk populations for latent tuberculosis infection. Appl Health Econ Health Policy. 2015;13(4):325–340. [DOI] [PubMed] [Google Scholar]
- [35].Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- [36].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. [Internet]. 2009;62(10):e1–34. Available from http://www.sciencedirect.com/science/article/pii/S0895435609001802 [DOI] [PubMed] [Google Scholar]
- [37].Briggs CJ, Garner P. Strategies for integrating primary health services in middle‐ and low‐income countries at the point of delivery. Cochrane Database Syst Rev [Internet]. 2006. 2;Available from h ttp s://d oi.org//1 0.1002/14 651858.CD00331 8.pub 2 [DOI] [PubMed] [Google Scholar]
- [38].Lou LM, Kennedy CE, Bain‐Brickley D, et al. Integration of HIV/AIDS services with maternal, neonatal and child health, nutrition, and family planning services. Cochrane Database Syst Rev. 2012;9:1–54. [DOI] [PubMed] [Google Scholar]
- [39].World Bank . Countries and economies [Internet]. The world bank group. 2021. Accessed 06 September 2021. Available from: https://data.worldbank.org/country
- [40].Tegegne BS, Habtewold TD, Mengesha MM, et al. Association between diabetes mellitus and multi-drug-resistant tuberculosis: a protocol for a systematic review and meta-analysis. Syst Rev. 2017. Jan;6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Stovold E, Beecher D, Foxlee R, et al. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Syst Rev. 2014;3(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cochrane Airways . Data collection form– rCTs and NRS [Internet]. 2021. Accessed 04 September 2021. https://airways.cochrane.org/data-collection
- [43].Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. UK: John Wiley & Sons; 2019. [Google Scholar]
- [44].Castellanos-Joya M, Delgado-Sánchez G, Ferreyra-Reyes L, et al. Results of the implementation of a pilot model for the bidirectional screening and joint management of patients with pulmonary tuberculosis and diabetes mellitus in Mexico. PLoS One. 2014. Sep;9(9):e106961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang X-L, S-g L, H-t L, et al. Integrating tuberculosis screening into annual health examinations for the rural elderly improves case detection. Int J Tuberc Lung Dis. 2015;19(7):787–791. [DOI] [PubMed] [Google Scholar]
- [46].Ncube RT, Dube SA, Machekera SM, et al. Feasibility and yield of screening for diabetes mellitus among tuberculosis patients in Harare, Zimbabwe. Public Health Action. [Internet]. 2019. Jun 21;9(2):72–77. Available from.https://pubmed.ncbi.nlm.nih.gov/31417857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kumar A, Gupta D. Screening of patients with tuberculosis for diabetes mellitus in India. Trop Med Int Health. 2013. May;18(5):636–645. [DOI] [PubMed] [Google Scholar]
- [48].Segafredo G, Kapur A, Robbiati C, et al. Integrating TB and non-communicable diseases services: pilot experience of screening for diabetes and hypertension in patients with Tuberculosis in Luanda, Angola. PLoS One. [Internet]. 2019. Jul 5;14(7):e0218052–e0218052. Available from https://pubmed.ncbi.nlm.nih.gov/31276500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ma Y, Pang Y, Shu W, et al. Metformin reduces the relapse rate of tuberculosis patients with diabetes mellitus: experiences from 3-year follow-up. Eur J Clin Microbiol Infect Dis. 2018;37(7):1259–1263. [DOI] [PubMed] [Google Scholar]
- [50].Ruslami R, Koesoemadinata RC, Soetedjo NNM, et al. The effect of a structured clinical algorithm on glycemic control in patients with combined tuberculosis and diabetes in Indonesia: a randomized trial. Diabetes Res Clin Pract. 2021;173:108701. [DOI] [PubMed] [Google Scholar]
- [51].Robertson D, Williams GH. Clinical and translational science: principles of human research. London (UK): Academic Press; 2009. [Google Scholar]
- [52].Jali MV, Mahishale VK HM. Bidirectional screening of tuberculosis patients for diabetes mellitus and diabetes patients for tuberculosis. Chest. 2013;144(4):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kumar A, Gupta D, Nagaraja SB, et al. Screening of patients with diabetes mellitus for tuberculosis in India. Trop Med Int Heal. 2013;18(5):646–654. [DOI] [PubMed] [Google Scholar]
- [54].Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. [Internet]. 2016. Oct 12;355:i4919. Available from http://www.bmj.com/content/355/bmj.i4919.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].on behalf of the development group for ACROBAT-NRSI, Sterne JAC, Higgins JPT, Reeves BC. A cochrane risk of bias assessment tool: for Non-Randomized Studies of Interventions (ACROBAT-NRSI). Version. 2014;1 [Google Scholar]
- [56].Basir MS, Habib SS, Zaidi SMA, et al. Operationalization of bi-directional screening for tuberculosis and diabetes in private sector healthcare clinics in Karachi, Pakistan. BMC Health Serv Res [Internet]. 2019;19(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Majumdar A, Wilkinson E, Rinu PK, et al. Tuberculosis-diabetes screening: how well are we doing? A mixed-methods study from North India. Public Health Action. 2019;9(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jerene D, Hiruy N, Jemal I, et al. The yield and feasibility of integrated screening for TB, diabetes and HIV in four public hospitals in Ethiopia. Int Health. 2017;9(2):100–104. [DOI] [PubMed] [Google Scholar]
- [59].Skordis-Worrall J, Round J, Arnold M, et al. Addressing the double-burden of diabetes and tuberculosis: lessons from Kyrgyzstan. Global Health. 2017;13(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Munseri PJ, Kimambo H, Pallangyo K. Diabetes mellitus among patients attending TB clinics in Dar Es Salaam: a descriptive cross-sectional study. BMC Infect Dis [Internet]. 2019. Oct 29;19(1):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tenaye L, Mengiste B, Baraki N, et al. Diabetes mellitus among adult tuberculosis patients attending tuberculosis clinics in Eastern Ethiopia. Babu S, editor. Biomed Res Int [Internet]. 2019; 2019:7640836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ekeke N, Aniwada E, Chukwu J, et al. Screening diabetes mellitus patients for tuberculosis in Southern Nigeria: a pilot study. Adv Respir Med. 2020;88(1):6–12. [DOI] [PubMed] [Google Scholar]
- [63].Riza AL, Pearson F, Ugarte-Gil C, et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol [Internet]. 2014. Sep cited 2017 Jun 5;2(9):740–753. Available from http://linkinghub.elsevier.com/retrieve/pii/S221385871470110X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Marupuru S, Senapati P, Pathadka S, et al. Protective effect of metformin against tuberculosis infections in diabetic patients: an observational study of south Indian tertiary healthcare facility. Brazilian J Infect Dis. 2017;21(3):312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jorgensen ME, Faurholt-Jepsen D. Is there an effect of glucose lowering treatment on incidence and prognosis of tuberculosis? A systematic review. Curr Diab Rep. 2014;14(7). DOI: 10.1007/s11892-014-0505-1 [DOI] [PubMed] [Google Scholar]
- [66].Adopted by the 18th WMA general assembly Helsinki Finland . WMA declaration of Helsinki -ethical principles for medical research involving human subjects. WMA Gen Assem. 2013.18.


