ABSTRACT
Introduction
Current evidence supports the inclusion of directional preference exercises for a subgroup of patients with low back (LBP) and leg pain. Recent pain neuroscience strategies have suggested that cortical restructuring associated with movement activating the body map representation in the brain might account for the observed improvement with the directional preference approach.
Objectives
To explore whether or not a motor imagery directional preference approach would result in any changes in patients with LBP and leg pain.
Methods
A consecutive convenience sample of patients with LBP and leg pain were recruited at two outpatient physical therapy clinics. Measurements of LBP, leg pain, fear-avoidance beliefs (FABQ), pain catastrophizing (PCS), active lumbar flexion, and straight leg raise (SLR) were compared before and immediately after a virtual (motor imagery) directional preference exercise.
Results
Statistically significant differences for LBP, FABQ, PCS, active lumbar flexion, and SLR were observed, but only SLR changes met or exceeded the minimally clinically important difference (MCID).
Conclusions
A brief virtual motor imagery extension treatment yielded some immediate positive shifts in patients presenting to physical therapy with LBP and leg pain. Our results indicate that randomized comparison trials are needed to determine the effect of this intervention on the short- and longer-term outcomes in patients with LBP and leg pain.
KEYWORDS: Graded motor imagery, directional preference, low back pain, case series
Introduction
Low back pain (LBP) is the most common musculoskeletal condition treated in physical therapy, accounting for an estimated 25–40% of outpatient physical therapy visits [1,2]. One strategy commonly used by physical therapists for treating LBP with referral into the leg is directional preference [3–5]. Directional preference is the process of examining the response of a patient with LBP and associated leg pain to a repeated movement direction, i.e. extension or flexion, and if it coincides with improvement, the test becomes part of the treatment [3,6–8]. Current evidence supports the inclusion of directional preference in the treatment of LBP in physical therapy [2,9–12]. Specifically for LBP with leg pain, directional preference usually involves either an extension-bias or flexion-bias, with various studies indicating an extension protocol being the most common (estimated >80% of patients) [3,6,13]. With extension exercises, a favorable therapeutic effect results in centralization of symptoms (leg pain migrates proximally), improved range of motion, decreased pain, and decreased fear of movement [2,3,6,9–13].
In recent years, there has been an increased interest in various pain neuroscience strategies to help people in pain, including LBP [14,15]. It is well established that the physical body of a person is represented in the brain by a network of neurons, often referred to as a representation of that particular body part in the brain or body schema [16–20]. This representation refers to the pattern of activity that is evoked when a particular body part is stimulated. The most famous area of the brain associated with representation is the primary somatosensory cortex (S1) [16–19]. These neuronal representations of body parts are dynamically maintained [21–26], and it has been shown that patients with pain, including chronic LBP, display different S1 representations than people with no pain [21–26]. An interesting phenomenon associated with cortical restructuring is the fact that the body maps expand or contract, in essence increasing or decreasing the body map representation in the brain. Furthermore, these changes in shape and size of body maps seem to correlate to increased pain and disability [21,27]. Various studies have shown that physical movement is associated with restoring the cortical maps, which in turn may be associated with a decreased pain experience [6,16,21].
In patients with high levels of pain, sensitization of the nervous system, and fear of movement, physical movement itself may increase a pain experience [28,29]. An alternative approach to help restore these cortical maps is motor imagery or visualization of movement [30,31]. Various studies have shown that motor imagery activates the same areas of the brain as when actually physically moving, thus restoring the altered maps ‘without moving’ [31,32]. This approach may have some value in patients with increased fear-avoidance, especially for those with a high fear of physical activity – including movement-based treatments such as directional preference. To this point, recent directional preference research has expanded into exploring directional treatment in the face of psychosocial variables such as fear-avoidance and pain catastrophization [8]. In lieu of the emerging motor imagery research, the question arises whether the physical movement of directional preference is needed, or if visualized, motor imagery of direction preference could have some clinical value in patients presenting with LBP and leg pain, especially chronic LBP with higher levels of fear-avoidance. The aim of this case series was to explore if a motor imagery directional preference approach would yield any positive changes in patients presenting with LBP and leg pain. This was planned as a prospective pilot exploratory case series to examine effect sizes to help determine sample size for future randomized controlled trials (RCTs).
Methods
Patients
Two outpatient physical therapy clinics agreed to participate in the study by collecting data and measurements. Institutional review board approval was obtained from Southwest Baptist University, and the case series was registered with clinicaltrials.gov (NCT04394494). A consecutive convenience sample of patients attending outpatient physical therapy with LBP and leg pain, meeting the inclusion criteria and none of the exclusion criteria were asked to participate in the study. Patients were eligible if they: a) presented to physical therapy with LBP that also referred pain into either lower extremity; b) were aged 18–65; c) able to read and understand the English language; d) presented with no red flags for attending physical therapy during review of systems and evaluation; e) no prior spinal surgery; and f) demonstrated a positive response to directional preference for extension during the evaluation. A positive response to directional preference for extension was defined as a decrease in presenting symptoms and/or proximal migration of leg symptoms. We chose to limit participation to patients that demonstrated a positive response to directional preference for extension during the evaluation because we needed to determine the effect, if any, of imagined extension exercises as opposed to actual extension movements.
Participation was entirely voluntary and eligible patients agreeing to participate signed a written informed consent. Upon consent, patients completed a demographics questionnaire, which included items regarding their age, gender, duration, and location of their LBP and leg pain.
Intervention
Patients underwent a standard interview process and review of systems by the first physical therapist [33]. Patients with LBP and leg pain were tested, as part of their physical examination to determine if they responded favorably to extension-based movement as used by the McKenzie approach [3,4]. Eligible patients were instructed how cortical map alterations play a role in LBP, based on a previous study using such a neuroscience educational model [34]. This education was no longer than 5 minutes and was not a lengthy pain neuroscience education session. Following education, a series of measures were taken prior to the intervention to determine a baseline. These measurements were taken by a second physical therapist who was to remain blinded to the treatment intervention. Following measurements, the first physical therapist who was to remain blinded to the measurements placed the patients in a prone position, with their hands under their shoulders in a ‘pre-press-up’ position. However, instead of doing an active press-up, patients were asked to close their eyes and coached via visual imagery through a typical extension experience (Table 1). Each virtual extension was repeated ten times, for an estimated 5-minute total virtual extension treatment protocol.
Table 1.
Extension protocol.
|
Immediately following the experimental intervention, post-measures were completed by the second physical therapist who had been blinded to the treatment intervention. Upon completion of post-intervention measures, the formal study was completed, and the treating physical therapists would then continue their individual plan of care for each patient.
Measurements
To determine a baseline disability score, each patient completed an Oswestry Disability Index (ODI). The ODI is a 10-item questionnaire used to assess different aspects of physical function. Each item is scored from 0 to 5, with higher values representing greater disability. The total score is multiplied by 2 and expressed as a percentage. The ODI has been shown to be a valid and reliable measure of disability related to LBP [35–37].
Prior to and immediately following the experimental intervention, a series of self-reported measures and physical tests were performed:
Self-reported pain (Low back and leg) (Numeric Pain Rating Scale – NPRS): Low back and leg pain were measured with the NPRS, as has been used in various studies [38–41]. The minimal clinically important difference (MCID) for the NPRS for acute/subacute LBP is reported to be 2.0 [42] and for chronic pain 1.7 [43].
Fear avoidance beliefs (Fear Avoidance Beliefs Questionnaire – FABQ): The FABQ is a 16-item questionnaire that was designed to quantify fear and avoidance beliefs in individuals with LBP. The FABQ has two subscales: 1) a 4-item scale to measure fear avoidance beliefs about physical activity (PA) and 2) a 7-item scale to measure fear-avoidance beliefs about work (W). Each item is scored from 0 to 6 with possible scores ranging between 0 and 24 and 0 and 42 for the physical activity and work subscales, respectively. Higher scores represent an increase in fear-avoidance beliefs. The FABQ has demonstrated acceptable levels of reliability and validity in previous studies [44–46]. The presence of avoidance behavior is associated with an increased risk of prolonged disability and work loss. It is proposed that FABQ-PA >14 and FABQ-W scores >34 and are associated with a higher likelihood of not returning to work [47,48]. The MCID for the FABQ has been reported as 13.0 [49].
Pain catastrophizing: Pain catastrophizing was measured using the pain catastrophizing scale (PCS). The PCS is a self-report questionnaire that assesses inappropriate coping strategies and catastrophic thinking about pain and injury. The PCS has been used in previous studies [50,51] and demonstrated strong construct validity, reliability, and stability [52]. The PCS utilizes a 13-item, 5-point Likert scale with higher scores indicating elevated levels of catastrophization. Previous studies utilizing the PCS have shown a median score of 18 in healthy individuals and a score over 30 reported as a high level of pain catastrophizing [52]. In patients with musculoskeletal pain, the minimal detectable change (MDC) for the PCS is reported to be 9.1 [53], but the MCID has not been established.
The physical tests were performed by a single physical therapist educator with more than 30 years of clinical experience. Active lumbar flexion range of motion and passive straight leg raise have been found to have high intra-rater reliability with intraclass correlation coefficients (ICCs) reported to be between 0.81 and 0.97 [54–56]
Active lumbar flexion: Active trunk forward flexion, measured from the longest finger on the dominant hand to the floor in centimeters (cm) [57–59]. The MDC for active trunk forward flexion has been reported to be 4.5 cm [60].
Straight Leg Raise (SLR): SLR was used as a neurodynamic measurement rather than a test of hamstring length. SLR was measured with an inclinometer placed on the tibial crest 5 cm distal to the inferior border of the patella on the most affected leg [57–59]. SLR for this study kept the ankle in neutral (90 degrees) with no added dorsiflexion or plantar flexion, per previous studies [57–59]. MDC for SLR has been reported as a 5.7 degree difference [60].
Data analysis
Frequency counts for demographic data were examined for normality of distribution and the means of pre- and post-intervention measures were compared within the group using the paired-samples Student's t-test. Statistically significant findings were further evaluated by repeating the analysis using the more conservative non-parametric Wilcoxon Signed rank tests. Data analysis was performed using PASW Statistics 21 (© 2012, SPSS Inc., IBM, Chicago, IL, 60606).
Results
Ten patients met the inclusion criteria for the case series (Table 2).
Table 2.
Demographics.
| Characteristics | Patients (n = 10) |
|---|---|
| Mean age (years) (Standard deviation – SD) | 41.5 (15.3) |
| Female (%) | 4 (40%) |
|
10 (100%) |
| Mean duration of pain in days (range) (SD) | 72.3 (67.2) |
| Mean disability (Oswestry Disability Index) (SD) | 11.4 (7.1) |
| Mean LBP (NPRS) (SD) | 4.5 (1.7) |
| Mean leg pain (NPRS) (SD) | 3.7 (1.8) |
| Mean FABQ-PA (SD) | 11.7 (5.6) |
| Mean FABQ-W (SD) | 15.6 (9.3) |
| Mean PCS (SD) | 21 (7.3) |
Self-reported pain
Immediately after the virtual directional preference exercises, there was a statistically significant difference for mean LBP, which decreased from 4.5 to 3.6 (p = 0.029) (Table 3). Overall, LBP failed to improve beyond MCID for chronic LBP, but three patients met/exceeded the MCID for LBP improvements. There was no statistically significant difference for mean leg pain, from 3.7 to 2.8 (p = 0.067). Mean leg pain differences for the case series failed to meet MCID for leg pain, but six individual patients met or exceeded MCID for leg pain improvement.
Table 3.
Before and immediately after intervention measures.
| Measurement | Before motor imagery | After motor imagery | Difference |
|---|---|---|---|
| LBP | 4.5 | 3.6 | p = 0.029* |
| Leg Pain | 3.7 | 2.8 | p = 0.067 |
| FABQ-PA | 11.7 | 9.8 | p = 0.06 |
| FABQ-W | 15.6 | 13.5 | p = 0.003* |
| PCS | 21 | 19.2 | p = 0.001* |
| Active trunk flexion (cm) | 38.45 | 36.2 | p < 0.001* |
| SLR (degrees) | 59 | 66.5** | p < 0.001* |
*Significant improvement
**Exceed MCID
Psychosocial
Immediately after the virtual directional preference treatment, there was a statistically significant improvement in all psychosocial measures – FABQ-PA (p = 0.06), FABQ-W (p = 0.003), and PCS (p = 0.0014). However, none of these measures met or exceeded MCID.
Physical movements
Immediately after the virtual directional preference, there was a statistically significant improvement in active spinal flexion of 2.25 cm (p < 0.001), but it did not meet or exceed MCID for active trunk flexion. Immediately after the virtual extension exercises, there was a statistically significant improvement in mean SLR of 7.5 degrees (p < 0.001), which did exceed the MCID (Figure 1).
Figure 1.
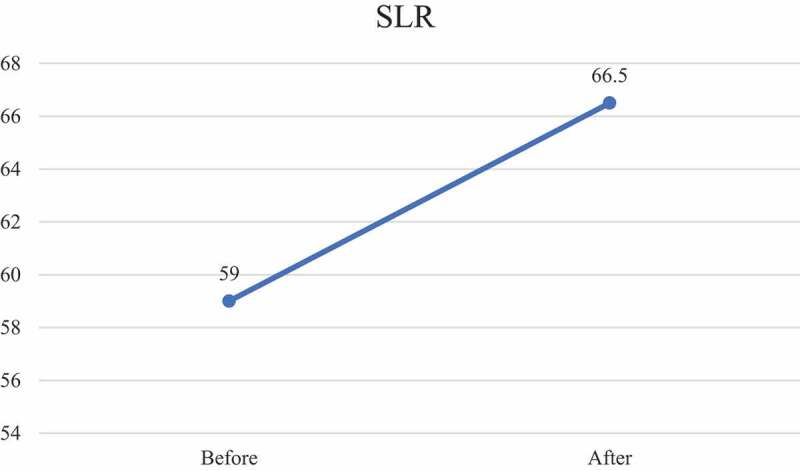
Straight leg raise before and immediately following virtual directional preference treatment.
Discussion
To date, this is the first study to explore any potential benefit of a virtual, motor imagery directional preference treatment approach. Immediately after a virtual, motor imagery extension protocol, patients with LBP and leg pain experienced positive changes in LBP, fear-avoidance, pain catastrophizing, active trunk flexion, and SLR, with only SLR meeting/exceeding MCID.
Various motor imagery studies have shown positive changes in pain ratings and movement [31,61,62]. The result of this study concurs with those findings, albeit aimed at a specific commonly proposed treatment approach of directional preference. Even though most post-intervention measures improved, only SLR exceeded MCID. This result of significantly increased SLR concurs with previous studies aiming to restore cortical body maps [34,63]. Even though this study did not measure the sensitivity of the nervous system via pressure pain thresholds (PPT), previous studies have shown a correlation between improved SLR and increased PPT in techniques aimed at body maps in the brain [63–65]. It is proposed from those studies, and perhaps supported by the results of this study, that motor imagery may in fact lead to a calming of the central and peripheral nervous system, which in turn decreases its mechanosensitivity allowing for increased movement. Considering that one of the main objectives of the directional preference model is decreased leg pain (centralization toward the low back), the improvement in SLR may be important as it fits within the McKenzie model of improvement of the extremity [66].
It is important to recognize that the total treatment was approximately only 5 minutes, yet yielded various immediate positive changes, including powerful psychosocial variables often associated with protracted recovery, i.e. high FABQ and PCS scores [49,52]. These results concur with a recent systematic review that showed that directional preference is associated with improvements in various psychometric measures, including fear-avoidance [8]. Even though the case series was not designed as such, it can be argued that patients with extremely high fear-avoidance of physical activity may indeed be appropriate candidates for such a motor imagery approach allowing for ‘movement without movement’ [63]. Furthermore, in line with a true motor imagery approach, imagined extension exercises could be seen as a precursor for actual extension movements, especially for those with higher levels of fear of movement. Future studies will need to explore whether adding motor imagery extension prior to actual extension might yield superior results compared to only active extension.
This study also adds to the body of evidence pointing to sub-grouping of patients. In this small case series, some patient’s pain measurements exceeded MCID, while others did not. These results imply that there may be a subgroup of patients presenting with LBP and leg pain, responsive to extension directional preference that may indeed greatly benefit from this approach. Future studies should further explore potential subgroups more favorable to a virtual motor imagery extension approach.
The case series contains numerous limitations. First, by its design, there were no control subjects to compare these results to. Second, only immediate outcomes were obtained, and no long-term results are reported, thus limiting the interpretation to immediate post-treatment effects. This does not address whether the findings translate or carry-over to medium- or long-term outcomes. Additionally, in lieu of the aforementioned discussions, it can be argued that a cohort of patients specifically with high-fear avoidance may have been a better sample to test this approach and assess its effect on fear-avoidance. Similarly, based on the McKenzie model, a cohort of patients with acute leg pain may have been more representative of this model. The strength of this pilot study was that the physical therapists collecting the data were adequately blinded. One PT who took all the measurements did not know what the intervention would be, and the other PT who provided the education and virtual extension protocol was blinded to measurements.
Conclusion
A brief virtual motor imagery extension treatment yielded some immediate positive shifts in patients presenting to physical therapy with LBP and leg pain. However, only SLR showed immediate clinically relevant improvement. The results indicate that randomized comparison trials are needed to determine the effect of this intervention on the short- and longer-term outcomes in patients with LBP and leg pain.
Funding Statement
The author(s) reported that there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Zheng P, et al. Stagnant physical therapy referral rates alongside rising opioid prescription rates in patients with low back pain in the United States 1997-2010. Spine (Phila Pa 1976). 2017;42(9):670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].George SZ, et al. Interventions for the management of acute and chronic low back pain: revision 2021. J Orthop Sports Phys Ther. 2021;51(11):CPG1–CPG60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hefford C. McKenzie classification of mechanical spinal pain: profile of syndromes and directions of preference. Man Ther. 2008;13(1):75–81. [DOI] [PubMed] [Google Scholar]
- [4].Halliday MH, et al. A randomized clinical trial comparing the McKenzie method and motor control exercises in people with chronic low back pain and a directional preference: 1-year follow-up. Physiotherapy. 2019;105(4):442–445. [DOI] [PubMed] [Google Scholar]
- [5].May S, Rosedale R. An international survey of the comprehensiveness of the McKenzie classification system and the proportions of classifications and directional preferences in patients with spinal pain. Musculoskelet Sci Pract. 2019;39. 10–15. [DOI] [PubMed] [Google Scholar]
- [6].Long A, Donelson R, Fung T. Does it matter which exercise? A randomized control trial of exercise for low back pain. Spine (Phila Pa 1976). 2004;29(23):2593–2602. [DOI] [PubMed] [Google Scholar]
- [7].Nechvatal P, et al. Comparison of the effect of the McKenzie method and spiral stabilization in patients with low back pain: a prospective, randomized clinical trial. J Back Musculoskelet Rehabil. 2022;35(3):641-647.doi: 10.3233/BMR-210055. [DOI] [PubMed] [Google Scholar]
- [8].Kuhnow A, et al. The McKenzie method and its association with psychosocial outcomes in low back pain: a systematic review. Physiother Theory Pract. 2021;37(12):1283–1297. [DOI] [PubMed] [Google Scholar]
- [9].May S, Aina A. Centralization and directional preference: a systematic review. Manual ther. 2012;17(6):497–506. [DOI] [PubMed] [Google Scholar]
- [10].Surkitt LD, et al. Efficacy of directional preference management for low back pain: a systematic review. Phys Ther. 2012;92(5):652–665. [DOI] [PubMed] [Google Scholar]
- [11].Karlsson M, et al. Effects of exercise therapy in patients with acute low back pain: a systematic review of systematic reviews. Syst Rev. 2020;9(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Namnaqani FI, et al. The effectiveness of McKenzie method compared to manual therapy for treating chronic low back pain: a systematic review. J Musculoskelet Neuronal Interact. 2019;19(4):492–499. [PMC free article] [PubMed] [Google Scholar]
- [13].Donelson R, et al. A prospective study of centralization of lumbar and referred pain: a predictor of symptomatic discs and anular competence. Spine. 1997;22. 1115–1122. [DOI] [PubMed] [Google Scholar]
- [14].Nijs J, et al. Thinking beyond muscles and joints: therapists’ and patients’ attitudes and beliefs regarding chronic musculoskeletal pain are key to applying effective treatment. Man Ther. 2013;18(2):96–102. [DOI] [PubMed] [Google Scholar]
- [15].Moseley GL. Reconceptualising pain according to modern pain sciences. Phys Ther Rev. 2007;12:169–178. [Google Scholar]
- [16].Wand BM, et al. Cortical changes in chronic low back pain: current state of the art and implications for clinical practice. Manual ther. 2011;16(1):15–20. [DOI] [PubMed] [Google Scholar]
- [17].Penfield W, Boldrey E. Somatic, motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60. 389–448. [Google Scholar]
- [18].Flor H. The functional organization of the brain in chronic pain. Prog Brain Res. 2000;129:313-22. doi: 10.1016/S0079-6123(00)29023 [DOI] [PubMed] [Google Scholar]
- [19].Stavrinou ML, et al. Temporal dynamics of plastic changes in human primary somatosensory cortex after finger webbing. Cereb Cortex. 2007;17(9):2134–2142. [DOI] [PubMed] [Google Scholar]
- [20].Holmes NP, Spence C. The body schema and the multisensory representation(s) of peripersonal space. Cogn Process. 2004;5(2):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Flor H, et al. Extensive reorganisation of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 1997;244. 5–8. [DOI] [PubMed] [Google Scholar]
- [22].Maihofner C, et al. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61(12):1707–1715. [DOI] [PubMed] [Google Scholar]
- [23].Moseley GL. I can’t find it! Distorted body image and tactile dysfunction in patients with chronic back pain. Pain. 2008;140(1):239–243. [DOI] [PubMed] [Google Scholar]
- [24].Lotze M, Moseley GL. Role of distorted body image in pain. Curr Rheumatol Rep. 2007;9(6):488–496. [DOI] [PubMed] [Google Scholar]
- [25].Moseley GL. Distorted body image in complex regional pain syndrome. Neurology. 2005;65(5):773. [DOI] [PubMed] [Google Scholar]
- [26].Flor H, et al. Cortical reorganisation and phantom phenomena in congenital and traumatic upper-extremity amputees. Vol. 119. Experimental Brain Research; 1998. p. 205–212. [DOI] [PubMed] [Google Scholar]
- [27].Lloyd D, et al. Differences in low back pain behavior are reflected in the cerebral response to tactile stimulation of the lower back. Spine (Phila Pa 1976). 2008;33(12):1372–1377. [DOI] [PubMed] [Google Scholar]
- [28].Vlaeyen JW, et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62(3):363–372. [DOI] [PubMed] [Google Scholar]
- [29].Vlaeyen JWS, Crombez G. Fear of movement/(re)injury, avoidance and pain disability in chronic low back pain patients. Manual Therapy. 1999;4. 187–195. [DOI] [PubMed] [Google Scholar]
- [30].Moseley GL. Graded motor imagery is effective for long standing complex regional pain syndrome. Pain. 2004;108. 192–198. [DOI] [PubMed] [Google Scholar]
- [31].Lotze M, Halsband U. Motor imagery. J Physiol (Paris). 2006;99:386–396. [DOI] [PubMed] [Google Scholar]
- [32].Gallese V, et al. Action recognition in the premotor cortex. Brain. 1996;119. 593–609. [DOI] [PubMed] [Google Scholar]
- [33].Louw A, et al. Evaluation is treatment for low back pain. J Man Manip Ther 2020. 1–10 Doi: 10.1080/10669817.2020.1703315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Louw A, et al. The effect of manual therapy and neuroplasticity education on chronic low back pain: a randomized clinical trial. J Man Manip Ther. 2017;25(5):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Deyo RA, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine (Phila Pa 1976). 1998;23(18):2003–2013. [DOI] [PubMed] [Google Scholar]
- [36].Fritz JM, Irrgang JJ. A comparison of a modified Oswestry low back pain disability questionnaire and the Quebec back pain disability scale. Phys Ther. 2001;81(2):776–788. [DOI] [PubMed] [Google Scholar]
- [37].Hakkinen A, et al. Changes in the total Oswestry index and its ten items in females and males pre- and post-surgery for lumbar disc herniation: a 1-year follow-up. Eur Spine J. 2007;16(3):347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moseley GL. Joining forces - combining cognition-targeted motor control training with group or individual pain physiology education: a successful treatment for chronic low back pain. J Man Manip Therap. 2003;11(2):88–94. [Google Scholar]
- [39].Moseley L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust J Physiother. 2002;48(4):297–302. [DOI] [PubMed] [Google Scholar]
- [40].Moseley GL. Widespread brain activity during an abdominal task markedly reduced after pain physiology education: fMRI evaluation of a single patient with chronic low back pain. Aust J Physiother. 2005;51(1):49–52. [DOI] [PubMed] [Google Scholar]
- [41].Cleland JA, Childs JD, Whitman JM. Psychometric properties of the neck disability index and numeric pain rating scale in patients with mechanical neck pain. Arch Phys Med Rehabil. 2008;89(1):69–74. [DOI] [PubMed] [Google Scholar]
- [42].Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976). 2005;30(11):1331–1334. [DOI] [PubMed] [Google Scholar]
- [43].Farrar JT, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- [44].Cleland JA, Fritz JM, Childs JD. Psychometric properties of the fear-avoidance beliefs questionnaire and Tampa scale of kinesiophobia in patients with neck pain. Am J Phys Med Rehabil. 2008;87(2):109–117. [DOI] [PubMed] [Google Scholar]
- [45].Grotle M, Vollestad NK, Brox JI. Clinical course and impact of fear-avoidance beliefs in low back pain: prospective cohort study of acute and chronic low back pain: II. Spine (Phila Pa 1976). 2006;31(9):1038–1046. [DOI] [PubMed] [Google Scholar]
- [46].Poiraudeau S, et al. Fear-avoidance beliefs about back pain in patients with subacute low back pain. Pain. 2006;124(3):305–311. [DOI] [PubMed] [Google Scholar]
- [47].Fritz JM, George SZ. Identifying psychosocial variables in patients with acute work-related low back pain: the importance of fear-avoidance beliefs. Phys Ther. 2002;82(10):973–983. [PubMed] [Google Scholar]
- [48].Burton AK, et al. Information and advice to patients with back pain can have a positive effect. A randomized controlled trial of a novel educational booklet in primary care. Spine (Phila Pa 1976). 1999;24(23):2484–2491. [DOI] [PubMed] [Google Scholar]
- [49].George SZ, Fritz JM, McNeil DW. Fear-avoidance beliefs as measured by the fear-avoidance beliefs questionnaire: change in fear-avoidance beliefs questionnaire is predictive of change in self-report of disability and pain intensity for patients with acute low back pain. Clin J Pain. 2006;22(2):197–203. [DOI] [PubMed] [Google Scholar]
- [50].Moseley GL, Nicholas MK, Hodges PW. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain. 2004;20(5):324–330. [DOI] [PubMed] [Google Scholar]
- [51].Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur J Pain. 2004;8(1):39–45. [DOI] [PubMed] [Google Scholar]
- [52].Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. [Google Scholar]
- [53].George SZ, Valencia C, Beneciuk JM. A psychometric investigation of fear-avoidance model measures in patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40(4):197–205. [DOI] [PubMed] [Google Scholar]
- [54].Chow R, Adams R, Herbert R. Straight leg raise test high reliability is not a motor memory artefact. Aust J Physiother. 1994;40(2):107–111. [DOI] [PubMed] [Google Scholar]
- [55].Neto T, et al. Reliability of the active-knee-extension and straight-leg-raise tests in subjects with flexibility deficits. J Sport Rehabil. 2015;24(4). DOI: 10.1123/jsr.2014-0220. [DOI] [PubMed] [Google Scholar]
- [56].Hunt DG, et al. Reliability of the lumbar flexion, lumbar extension, and passive straight leg raise test in normal populations embedded within a complete physical examination. Spine (Phila Pa 1976). 2001;26(24):2714–2718. [DOI] [PubMed] [Google Scholar]
- [57].Zimney K, Louw A, Puentedura EJ. Use of therapeutic neuroscience education to address psychosocial factors associated with acute low back pain: a case report. Physiother theory and Pract. 2014;30(3). 202–209. [DOI] [PubMed] [Google Scholar]
- [58].Moseley GL, Hodges PW, Nicholas MK. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain. 2004;20:324–330. [DOI] [PubMed] [Google Scholar]
- [59].Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur J Pain. 2004;8:39–45. [DOI] [PubMed] [Google Scholar]
- [60].Ekedahl H, Jonsson B, Frobell RB. Fingertip-to-floor test and straight leg raising test: validity, responsiveness, and predictive value in patients with acute/subacute low back pain. Arch Phys Med Rehabil. 2012;93(12):2210–2215. [DOI] [PubMed] [Google Scholar]
- [61].Wallwork SB, et al. Are people who do yoga any better at a motor imagery task than those who do not? Br J Sports Med. 2015;49(2):123–127. [DOI] [PubMed] [Google Scholar]
- [62].Bowering KJ, et al. Motor imagery in people with a history of back pain, current back pain, both, or neither. Clin J Pain. 2014;30(12):1070–1075. [DOI] [PubMed] [Google Scholar]
- [63].Louw A, et al. Moving without moving: immediate management following lumbar spine surgery using a graded motor imagery approach: a case report. Physiother Theory Pract. 2015;31(7):509–517. [DOI] [PubMed] [Google Scholar]
- [64].Sawyer EE, McDevitt AW, Louw A, et al. Use of pain neuroscience education, tactile discrimination, and graded motor imagery in an individual with frozen shoulder. J Orthop Sports Phys Ther. 2018;48(3):174–184. [DOI] [PubMed] [Google Scholar]
- [65].Daly AE, Bialocerkowski AE. Does evidence support physiotherapy management of adult complex regional pain syndrome type one? A systematic review. Eur J Pain. 2009;13(4):339–353. [DOI] [PubMed] [Google Scholar]
- [66].Skytte L, May S, Petersen P. Centralization: its prognostic value in patients with referred symptoms and sciatica. Spine (Phila Pa 1976). 2005;30(11):E293–9. [DOI] [PubMed] [Google Scholar]


