Abstract
Background
Capsid virus-like particles (cVLP) have proven safe and immunogenic and can be a versatile platform to counter pandemics. We aimed to clinically test a modular cVLP COVID-19 vaccine in individuals who were naive to SARS-CoV-2.
Methods
In this phase 1, single-centre, dose-escalation, adjuvant-selection, open-label clinical trial, we recruited participants at the Radboud University Medical Center in Nijmegen, Netherlands, and sequentially assigned them to seven groups. Eligible participants were healthy, aged 18–55 years, and tested negative for SARS-CoV-2 and anti-SARS-CoV-2 antibodies. Participants were vaccinated intramuscularly on days 0 and 28 with 6 μg, 12 μg, 25 μg, 50 μg, or 70 μg of the cVLP-based COVID-19 vaccine (ABNCoV2). A subgroup received MF59-adjuvanted ABNCoV2. Follow-up was for 24 weeks after second vaccination. The primary objectives were to assess the safety and tolerability of ABNCoV2 and to identify a dose that optimises the tolerability–immunogenicity ratio 14 days after the first vaccination. The primary safety endpoint was the number of related grade 3 adverse events and serious adverse events in the intention-to-treat population. The primary immunogenicity endpoint was the concentration of ABNCoV2-specific antibodies. The trial is registered with ClinicalTrials.gov, NCT04839146.
Findings
45 participants (six to nine per group) were enrolled between March 15 and July 15, 2021. Participants had a total of 249 at least possibly related solicited adverse events (185 grade 1, 63 grade 2, and one grade 3) within a week after vaccination. Two serious adverse events occurred; one was classified as a possible adverse reaction. Antibody titres were dose-dependent with levels plateauing at a vaccination dose of 25–70 μg ABNCoV2. After second vaccination, live virus neutralisation activity against major SARS-CoV-2 variants was high but was lower with an omicron (BA.1) variant. Vaccine-specific IFNγ+ CD4+ T cells were induced.
Interpretation
Immunisation with ABNCoV2 was well tolerated, safe, and resulted in a functional immune response. The data support the need for additional clinical development of ABNCoV2 as a second-generation SARS-CoV-2 vaccine. The modular cVLP platform will accelerate vaccine development, beyond SARS-CoV-2.
Funding
EU, Carlsberg Foundation, and the Novo Nordisk Foundation.
Introduction
As of July 9, 2022, there have been over 550 million cases of COVID-19 worldwide, with more than 6 million deaths.1 Vaccines against SARS-CoV-2, the causative virus, have been instrumental in controlling the pandemic. Vaccination has been implemented globally at unprecedented pace to protect susceptible populations, reduce spread, safeguard health-care systems, and diminish the global social and economic impact of non-pharmaceutical interventions to reduce COVID-19 transmission.2 However, reduced vaccine effectiveness against new SARS-CoV-2 variants, ongoing trans_mission, and the absence of universal access are major challenges. Heterologous vaccination with different existing COVID-19 vaccines is an approach to broaden protection; however, so far, it has provided little benefit over homologous boosters.3 Second-generation COVID-19 vaccines should ideally induce durable cross-protective and transmission-blocking immune responses, while being compatible with globally equitable use.
We developed a novel modular vaccine platform based on capsid virus-like particles (cVLP) that are used as scaffolds for antigen display.4 This cVLP platform uses a split-protein Tag–Catcher conjugation system (similar to the SpyTag–SpyCatcher technology)5 to allow for directional, high-density, covalent attachment of protein antigens on the cVLP surface. This cVLP platform was used to develop a COVID-19 vaccine, ABNCoV2, by attaching the receptor binding domain (RBD) of the SARS-CoV-2 Spike glycoprotein.4 The increased avidity and particle size can promote uptake by antigen presenting cells, lymph node trafficking, and B-cell activation.6 In preclinical studies in mice, ABNCoV2 was immunogenic and induced high titres of neutralising antibodies.4 cVLP-based vaccines have been successfully marketed and have been shown to be highly effective over long periods of time (eg, against human papillomavirus).7 cVLP-based vaccines can be safely used in people who are immunocompromised and in older people, two populations at high risk of severe COVID-19.8, 9
Research in context.
Evidence before this study
In the COVID-19 pandemic, mRNA vaccines, and then vectored SARS-CoV-2 vaccines, spearheaded market entry, whereas protein-based candidates failed in early clinical development due to low immunogenicity. Virus-like particle (VLP) vaccines are highly immunogenic, safe, and can be effective over long periods of time (eg, against human papillomavirus); however, development of VLP-based vaccines is often precluded by complex manufacturing procedures and the limited propensity of antigens to spontaneously form particles. We developed a simple modular capsid VLP platform that allows rapid development of VLP-based vaccines and we aimed for proof-of-concept with the SARS-CoV-2 vaccine ABNCoV2. ABNCoV2 generated robust vaccine dose-dependent neutralising antibody responses in preclinical studies and protected SARS-CoV-2-challenged Rhesus macaques. We report results of the first-in-human trial of ABNCoV2. We searched PubMed on Aug 4, 2022, for clinical trials testing SARS-CoV-2 virus-like particle vaccines with search terms “SARS-CoV-2 AND vaccine AND (VLP OR virus-like) AND (clinical trial [Filter])”, with no restrictions on publication date or language. We found one publication that reported the interim safety and immunogenicity data of a phase 1 trial with a plant-derived virus-like particle vaccine for COVID-19.
Added value of this study
Next-generation vaccines with improved tolerability, broad and durable protection, global accessibility, and transmission-blocking activity will be required for control of the SARS-CoV-2 pandemic. Our data show that ABNCoV2 is well tolerated and elicits high antibody titres, high titres of cross-neutralisation antibodies, and robust cellular responses with the preferred T-helper-1 cell pattern indicative of a protective immune status. Beyond SARS-CoV-2, the study provides successful proof-of-concept of a modular capsid VLP platform for the development of improved vaccines for globally relevant infectious diseases and pathogens of concern.
Implications of all the available evidence
ABNCoV2 is a promising complementary SARS-CoV-2 vaccine candidate and has proceeded into phase 3 clinical development. A two-dose schedule of ABNCoV2 was well tolerated and induced rapid and durable immunity. Distribution and storage of ABNCoV2 are less demanding compared with current SARS-CoV-2 vaccines, which will ease its global supply once available on the market. Modular capsid VLPs are a platform for the development of next-generation vaccines against SARS-CoV-2 and other infectious diseases.
Here, we report the results of the first-in-human clinical trial COUGH-1, designed to assess the safety, tolerability, and immunogenicity of ABNCoV2 in participants who were naive to SARS-CoV-2.
Methods
Study design and participants
COUGH-1 was a phase 1, single centre, sequential dose-escalation, adjuvant-selection, open-label trial, conducted at the Radboud University Medical Center in Nijmegen, Netherlands. Healthy participants aged 18–55 years, with no history of SARS-CoV-2 infection or vaccination were recruited. Following written informed consent, all participants underwent physical examination, haematological and biochemical screening, and were tested for current or past infection with SARS-CoV-2, HIV, and hepatitis B and hepatitis C viruses. Full details of the inclusion and exclusion criteria are provided in the protocol (appendix pp 22–23).
Ethical approval was granted by the Central Committee on Research Involving Human Subjects (NL76192.000.20). The trial is registered with ClinicalTrials.gov (NCT04839146) and the Netherlands Trial Register (NL9334).
Procedures
ABNCoV2 was administered by two intramuscular injections of 0·5 mL, 28 days apart, in the deltoid muscle of the non-dominant arm and, subsequently, the other arm. At each dose escalation, one participant was inoculated, followed by the rest of the group one week later, together with the first participant of the next group. Follow-up visits were done on days 1, 2, 7, and 14 after each vaccination and on days 42, 91, and 168 after the second vaccination. Adverse events were captured during on-site visits, through structured diaries, and by daily monitoring of body temperature for 1 week after each vaccination. Local and systemic adverse events were solicited until 7 days after ABNCoV2 administration. Unsolicited adverse events and serious adverse events (SAEs) were recorded until the end of study.
Allocation to dosage and combination with MF59-adjuvant was by sequence of enrolment. The predefined escalation schedule started with 6 μg (groups 1A and 1B), followed by 12 μg (groups 2A and 2B), 25 μg (groups 3A, 3B, and 6), 50 μg (groups 4 and 7), and 70 μg (group 5) ABNCoV2. Dose escalation occurred in groups of six participants, starting with split groups for the first three lowest doses, in which half (n=3) of the participants received the non-adjuvanted vaccine (groups 1A, 2A, and 3A) and half received the MF59-adjuvanted vaccine (groups 1B, 2B, and 3B). Additional dose escalation and the decision of whether to use adjuvant in group 4 and above depended on a review of the accumulated data up to 14 days after first vaccination in group 3B by an independent Safety Monitoring Committee (SMC). At completion of the dose escalation, the two doses nearest the optimal tolerability–immunogenicity ratio continued enrolment (into groups 6 and 7) until 12 participants received these doses of ABNCoV2.
Participants were allowed to enrol into the Dutch SARS-CoV-2 vaccination programme on the condition that it was later than 4 weeks after the final scheduled ABNCoV2 vaccination. In those enrolled in the national programme, additional follow-up visits before and two weeks after the additional vaccination were done.
ABNCoV2 consists of the Acinetobacter Phage 205 (AP205) cVLP produced in Escherichia coli and the Wuhan SARS-CoV-2 Spike RBD antigen aa319–591 (RBD; QIA0044.1), expressed in S2 cells (appendix p 9). Upon mixing the two components, a covalent isopeptide bond forms between the split-protein Tag and Catcher, which are genetically fused to the cVLP and antigen sequence, respectively.4 The final purified RBD-cVLP contains roughly 72 RBD antigens per particle. ABNCoV2 was stored frozen at –20°C (±5°C) and reconstituted in phosphate buffered saline with and without MF59. Formulated vaccines were stored at 2–8°C and used within 24 h. MF59 is an oil-in-water emulsion containing squalene, polysorbate 80, and sorbitan trioleate and is marketed as part of the influenza vaccine, Fluad Tetra (Seqirus, Holly Springs, NC, USA). MF59 mainly acts through enhanced recruitment of immune cells to the injection site and has immune-stimulatory effects in T-helper cell deficient conditions.10 The MF59 adjuvant was manufactured and provided by Seqirus.
Outcomes
The primary safety endpoint of this trial was the number of at least possibly related grade 3 adverse events and SAEs from time of first ABNCoV2 administration to the end of the follow-up period. The secondary safety endpoint was the number and severity of solicited adverse events within 1 week following administration of ABNCoV2. Solicited local adverse events were defined as pain, tenderness, erythema, and induration at the injection site. Solicited systemic adverse events were defined as headache, fatigue, fever, drowsiness, and chills. Causality to the study interventions was graded by the investigators (MJS, MBPAA, MBBM, and BGM) as unrelated, unlikely related, possibly, probably, or definitely related. Severity of adverse events was graded as mild (grade 1), moderate (grade 2), or severe (grade 3). Verbatim-recorded adverse events were coded using the Medical Dictionary for Regulatory Activities (version 24.1). For solicited and laboratory adverse events, the US Food and Drug Administration toxicity grading scale was used (appendix pp 11–12).
The primary immunogenicity endpoint was the concentration of vaccine-specific IgG antibodies 14 days after first vaccination. Exploratory immunogenicity endpoints included the concentration of vaccine-specific antibodies at baseline, during immunisation, and at follow-up. RBD-specific total IgG titres were measured by ELISA, as previously described (appendix p 4).11 RBD-specific CD4+ and CD8+ T cells were measured by flow cytometry following peptide stimulation (appendix pp 7–8).
Another exploratory endpoint was virus neutralisation of the ancestral isolate FR-4286 (B.1) and variants of concern: alpha (B.1.1.7), beta (B.1.351), and delta (B.1.617.2). We assessed serum from baseline and during immunisation and follow-up in a 50% plaque reduction neutralisation test (PRNT50). The incidence of infection with omicron variants increased sharply after the completion of the trial. Therefore, measurement of omicron (BA.1) virus neutralisation was amended to the assay list and compared with an ancestral (D614G) variant and delta variants. These measurements were done independently of the originally planned virus neutralisation panel. Virus neutralisation assays were done as previously described (appendix pp 5–6).4, 12
Statistical analysis
This study was an exploratory phase 1 clinical trial. The sample size was chosen to allow detection of large differences in adverse events and RBD-specific antibodies (appendix pp 24–26). The study was powered to detect at least one common (≥5%) adverse reaction with 90% power and, until the first SMC review, a ten-fold difference in antibody titre between non-adjuvanted and adjuvanted ABNCoV2 with 80% power. The immunogenicity assumptions were derived from preceding non-human primate studies.13
All analyses were programmed using R (version 4.1.2), and data wrangling and figures were produced with the package tidyverse (version 1.3.1).
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
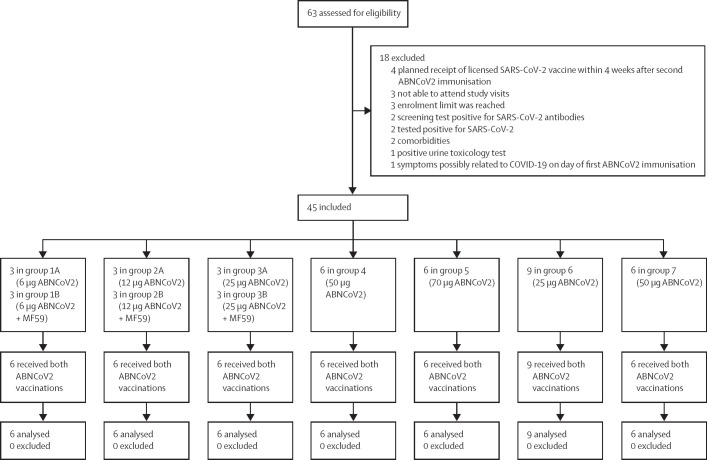
Screening took place between March 11, 2021, and June 14, 2021. In total, 45 eligible individuals who were naive to SARS-CoV-2 were enrolled and allocated to one of seven groups (six to nine per group; figure 1 ). Baseline demographics of the participants were similar among groups (table ). Vaccinations were given between March 15, 2021, and July 15, 2021. The second ABNCoV2 vaccination was given outside the prespecified time window (27–29 days) for two (4%) participants (day 30 and 35) for logistical reasons. 44 (98%) of 45 participants completed all the follow-ups. One (2%) participant in group 3A did not attend the final follow-up visit in person due to emigration but was included in the analysis. An additional unscheduled visit was conducted after the occurrence of a suspected unexpected serious adverse reaction (SUSAR). The final study visit was on Feb 25, 2022.
Figure 1.
Trial profile
Table.
Baseline demographics of the study participants
| Median age, years (range) | Median bodyweight, kg (range) | BMI, kg/m2 (range) | Sex, female:male ratio (%) | |
|---|---|---|---|---|
| Group 1A (n=3) | 31·0 (21·0–35·0) | 79·0 (61·0–96·0) | 23·0 (22·1–31·0) | 2:1 (67%) |
| Group 1B (n=3) | 27·0 (22·0–52·0) | 66·0 (59·0–69·6) | 22·4 (18·4–24·4) | 3:0 (100%) |
| Group 2A (n=3) | 25·0 (23·0–34·0) | 78·0 (76·0–89·4) | 26·0 (24·9–32·1) | 2:1 (67%) |
| Group 2B (n=3) | 37·0 (22·0–37·0) | 88·0 (62·0–94·0) | 24·9 (22·8–28·4) | 1:2 (33%) |
| Group 3A (n=3) | 27·0 (22·0–28·0) | 70·1 (61·2–79·0) | 23·6 (20·5–25·4) | 2:1 (67%) |
| Group 3B (n=3) | 48·0 (33·0–54·0) | 81·0 (72·8–91·0) | 26·4 (24·9–26·9) | 2:1 (67%) |
| Group 4 (n=6) | 25·5 (20·0–44·0) | 75·0 (60·0–97·0) | 25·0 (17·5–27·3) | 4:2 (67%) |
| Group 5 (n=6) | 20·5 (20·0–46·0) | 77·5 (66·0–90·0) | 23·7 (20·5–26·6) | 3:3 (50%) |
| Group 6 (n=9) | 24·0 (21·0–45·0) | 76·6 (61·0–91·0) | 23·3 (20·4–29·9) | 4:5 (44%) |
| Group 7 (n=6) | 24·0 (18·0–29·0) | 69·5 (60·0–88·0) | 24·5 (20·5–27·1) | 3:3 (50%) |
| Overall (n=45) | 26·0 (18·0–54·0) | 76·0 (59·0–97·0) | 24·2 (17·5–32·1) | 26:19 (58%) |
Doses of study groups were 6 μg ABNCoV2 in group 1A, 6 μg ABNCoV2 + MF59 in group 1B, 12 μg ABNCoV2 in group 2A, 12 μg ABNCoV2 + MF59 in group 2B, 25 μg ABNCoV2 in group 3A, 25 μg ABNCoV2 + MF59 in group 3B, 50 μg ABNCoV2 in group 4, 70 μg ABNCoV2 in group 5, 25 μg ABNCoV2 in group 6, and 50 μg ABNCoV2 in group 7.
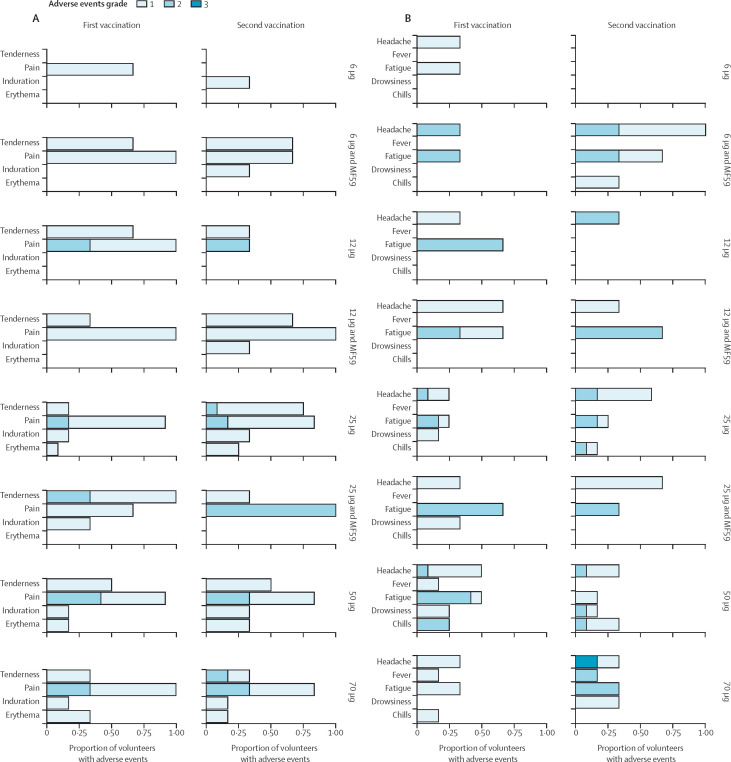
Every participant had at least one adverse event. In total, 651 adverse event episodes occurred (465 grade 1, 175 grade 2, nine grade 3, two SAE). Of those adverse events, 249 (38%) were solicited (185 grade 1, 63 grade 2, and one grade 3). Overall, ABNCoV2 was well tolerated (figure 2A and figure 2B). One unrelated SAE occurred 8 days after the second vaccination in a participant from group 7 (traumatic ligament rupture requiring hospitalisation). A second SAE was a superficial basal cell carcinoma on each upper arm in a participant from group 1B. The superficial basal cell carcinoma was diagnosed approximately 16 weeks after the second vaccination. The participant had Fitzpatrick skin type 114 and a medical history of basal cell carcinoma and melanoma. Based on the temporal and anatomical relationship between basal cell carcinoma and vaccination, the adverse event was classified as a SUSAR. It was successfully treated by radical excision. No further lesions were detected in any of the other participants during the study, including in an extra visit after the last planned follow-up.
Figure 2.
Local and systemic reactions following ABNCoV2 vaccination
(A) Related solicited local adverse events. (B) Related solicited systemic adverse events. Data represent the proportion of participants who had an adverse event of the indicated severity. The highest severity grade is shown in case there were multiple episodes of a given adverse event per participant. ABNCoV2 dose and vaccine formulation (with or without MF59) are indicated on the right side.
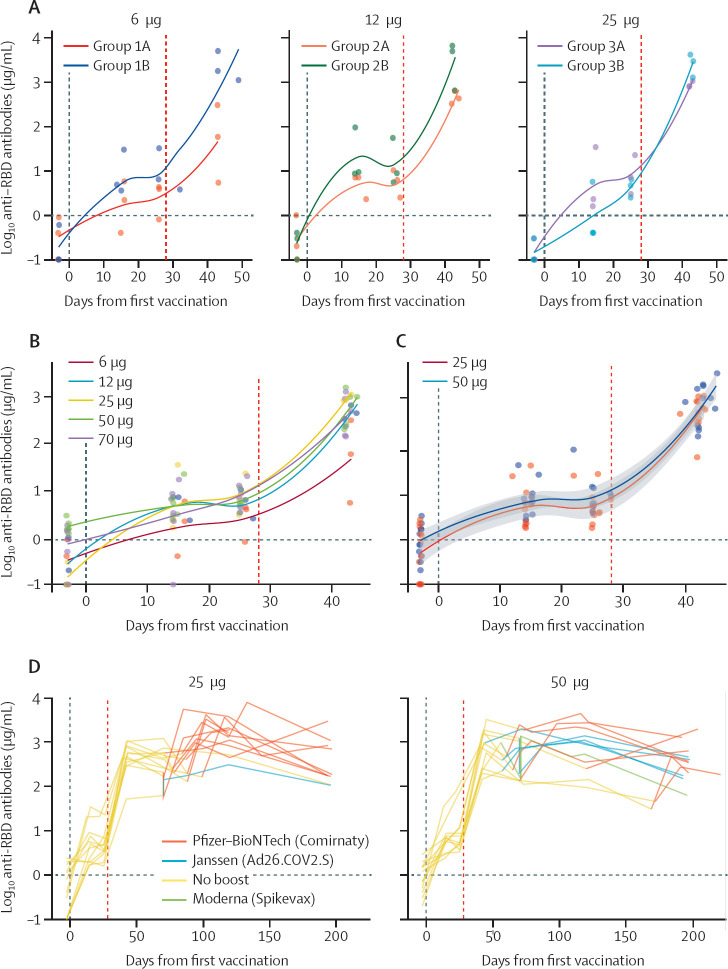
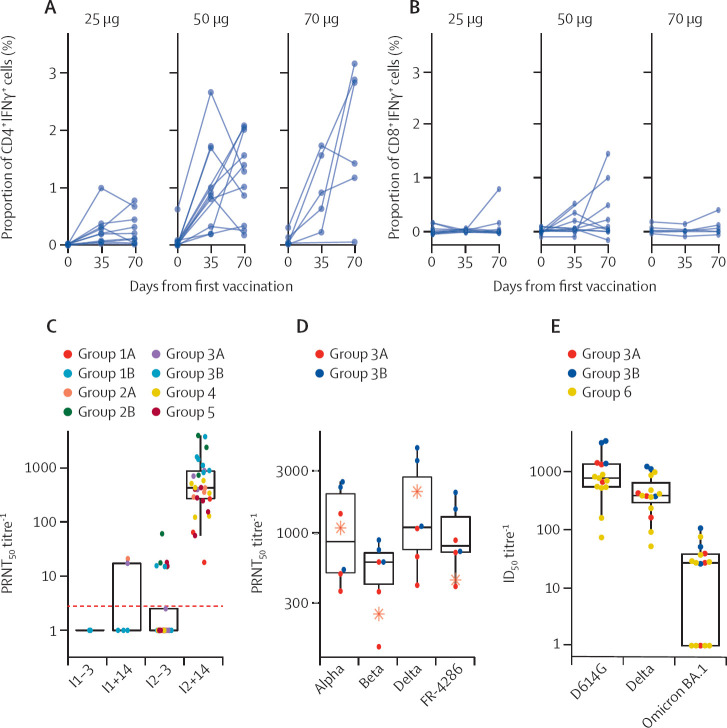
Two laboratory abnormalities occurred. One participant had grade 1 eosinophilia on day 7 after the second administration of 25 μg ABNCoV2. Another participant had dacrocytes (teardrop-shaped erythrocytes) in the whole blood cell count on day 7 after second administration of 50 μg ABNCoV2. Other haematological parameters were normal in these two patients. Both laboratory abnormalities were not associated with symptoms and normalised at the next follow-up visit (7 days later). ABNCoV2 induced seroconversion after the second vaccination in all participants irrespective of dose amount and adjuvant. The decision as to whether to proceed with or without adjuvant in groups 4 to 7 was based on the concentration of RBD-specific antibodies of group 1 to group 3 (6 μg, 12 μg, and 25 μg) up to day 14 after the second vaccination (figure 3A ). RBD-specific antibody IgG titres 14 days after the second vaccination were dose-dependent with a plateauing response pattern at around 25 μg (figure 3B). On the basis of these results, the decision was made to retest 25 μg and 50 μg as the optimal doses in group 6 and group 7 to increase the power to detect adverse events. In total, 12 participants received the optimal doses of 25 μg and 50 μg ABNCoV2. The concentration of RBD-specific antibodies of the groups receiving the optimal doses up to day 42 after the first vaccination is shown by locally estimated scatterplot smoothing fit local regression (figure 3C). The concentration of RBD-specific antibodies decayed gradually during the follow-up and could be boosted with a licensed SARS-CoV-2 vaccine in participants receiving the optimal doses (figure 3D, appendix p 10). RBD-specific CD4+ T cells were induced following immunisation with two doses of ABNCoV2 (figure 4A ). The phenotype of responding CD4+ T cells was mainly IFNγ positive, with most of the cells coexpressing TNF and CD137 (appendix p 9). Some cells also expressed the degranulation marker CD107a. The 50 μg vaccine dose induced a higher CD4+ T-cell response than 25 μg ABNCoV2, but this did not extend to the 70 μg dosage. SARS-CoV-2 RBD-specific CD8+ T cells were only marginally increased (figure 4B). Robust in-vitro activity was observed in live virus neutralisation assays 14 days after the second vaccination. PRNT50 titres were induced by all the different ABNCoV2 doses tested with and without adjuvant MF59 against an early B.1 isolate, FR-4286, representing ancestral variants. The WHO 20/136 standard (appendix p 13) lies in the same range as the neutralising antibody titres after vaccination with adjuvanted ABNCoV2 and at doses higher than 25 μg (figure 4C). Post-hoc analysis showed six-fold (95% CI 3–11) higher levels of in-vitro neutralisation activity in adjuvanted than in non-adjuvanted vaccinees. Furthermore, strong cross neutralisation was seen using serum samples from the 25 μg ABNCoV2 dose groups with and without MF59 adjuvant for the early B.1 isolate (FR-4286) and for variants of concern B.1.1.7 (alpha), B.1.351 (beta), and B.1.617.2 (delta; figure 4D). There was no reduction in neutralisation capacity against alpha or delta. A 2·2-fold reduction was seen against the beta variant virus. An independent neutralisation assay showed a 66-fold decrease in activity when comparing the omicron BA.1 with an ancestral variant (D614G; figure 4E). The same trend towards higher neutralisation titres in vaccinees receiving adjuvanted ABNCoV2 was present.
Figure 3.
SARS-CoV-2 RBD-specific antibodies
Vertical lines indicate the first and second ABNCoV2 vaccination (28 days after first vaccination). (A) Concentration of RBD-specific antibodies of groups 1 to 3 (6 μg, 12 μg, and 25 μg ABNCoV2; non-adjuvanted [groups labelled A] and MF59-adjuvanted [groups labelled B]) up to day 42 after the first vaccination (14 days after the second vaccination). (B) Concentration of RBD-specific antibodies of groups 1A, 2A, 3A, 4, and 5 (6 μg, 12 μg, 25 μg, 50 μg, and 70 μg) 14 days after second vaccination. (C) Concentration of RBD-specific antibodies of groups receiving the optimal doses 25 μg and 50 μg ABNCoV2 until day 42 after the first vaccination. (D) Concentration of RBD-specific antibodies of groups receiving the optimal doses 25 μg and 50 μg ABNCoV2 until day 196 after the first vaccination (end-of-study visit). Different colours indicate types of licensed SARS-CoV-2 vaccines that participants received during the follow-up period. RBD=receptor binding domain.
Figure 4.
Cellular and functional immune response against SARS-CoV-2
IFNγ+ cells after stimulation with SARS-CoV-2 Spike RBD class 1 and class 2 restricted pooled peptides on CD4+ T cells (A) and CD8+ T cells (B). Values are corrected for activation (no peptide). (C) SARS-CoV-2 neutralisation responses. The red line indicates the WHO 20/136 standard. (D) Virus neutralisation of alpha (B.1.1.7), beta (B.1.351), delta (B.1.617.2), and FR-4286 (B.1). Red stars indicate PRNT50 values of the WHO standard 20/136. (E) Neutralisation titres (ID50) from vaccination groups 3A (n=3), 3B (n=3), and 6 (n=9) against the ancestral D614G, delta, and omicron SARS-CoV-2 variants. I1–3=3 days before the first vaccination. I1+14=14 days after first vaccination. I2–3=3 days before the second vaccination. I2+14=14 days after second vaccination. RBD=receptor binding domain. ID50=50% inhibitory dilution. PRNT50=50% plaque reduction neutralisation test.
Discussion
In this first-in-human clinical trial of ABNCoV2, we tested a modular cVLP platform that combines flexibility in the selection of antigens with improved immunogenicity as well as good tolerability and safety in preclinical models. We found that ABNCoV2 was well tolerated and induced high IgG antibody responses against the SARS-CoV-2 RBD, peaking at 2 weeks after the second vaccination. Serum samples showed functional activity against major SARS-CoV-2 variants, antibody concentrations remained high over several months, and cellular responses were robust, with a T-helper-1 cell pattern indicative of a protective immune status.15 The immune response to the vaccine antigen was dose-dependent and MF59 showed a dose-sparing effect. At a dosage of 25 μg and higher the serological response became saturated, albeit with a tendency towards higher vaccine-specific T-cell numbers at a dose of 50 μg. Virus neutralisation activity was present after the second vaccination and from a vaccine dose of 25 μg upwards at levels similar to the WHO standard 20/136. Neutralisation activity was broad with about two-fold reduced activity against the beta variant virus. Of note, a more than ten-fold reduction was reported for BNT162b2.16 MF59 had a positive effect on virus neutralisation. Concentrations of neutralising antibodies against the omicron clade were significantly lower but similar to approved vaccines before updating to omicron BA.1 and BA.5.17 Whether ABNCoV2 will also need to be updated is currently being investigated in late-stage clinical development of ABNCoV2 (NCT05329220 and NCT05077267). A main advantage of the modular Tag–Catcher-AP205 capsid-like particle vaccine design of ABNCoV2 is the possibility to replace the current vaccine antigen relatively quickly in the event that the SARS-CoV-2 virus should acquire mutations in the RBD domain reducing the efficacy of the ABNCoV2 vaccine. ABNCoV2's tolerability was independent of dose, adjuvant, and time (first vs second vaccination). This pattern is similar to other VLP vaccines (eg, against human papillomavirus18), whereas mRNA and vectored SARS-CoV-2 vaccines are more reactogenic.19, 20 During the follow-up, two cases of basal cell carcinoma occurred in one participant and were reported as a SUSAR. Basal cell carcinoma is common and its associated mortality is very low.21 Ultraviolet radiation is the main risk factor for basal cell carcinoma, but it can develop, although rarely, on scar tissue, including vaccination scars. Basal cell carcinoma has been reported after vaccination for smallpox,22 Bacillus Calmette-Guérin,23 influenza,24 typhoid, and hepatitis A.25 The SUSAR occurred in a participant with a predisposition to skin malignancies and no additional skin anomalies were found, even when actively screened for. Nevertheless, and despite the low probability of an ABNCoV2-specific causal link, monitoring of late local reactions shall be included as clinical development progresses.
The size and design of the trial, as well as the inclusion of participants in the ongoing national vaccination campaign, precluded measuring the protective efficacy of ABNCoV2. Efficacy in preclinical models has translated reasonably well into humans for other SARS-CoV-2 vaccine candidates.26 Analogously, promising results from preclinical studies of ABNCoV2, including challenge experiments,13 as well as antibody levels and consistent virus neutralisation (as a proxy of protection27) in the current trial, advocate for ongoing clinical development of this vaccine candidate. Antibody responses induced by ABNCoV2 were in the same range as those induced after two doses of BNT162b2 and responses were boosted to peak levels in individuals receiving a heterologous vaccine (shown in figure 3C–D).28
Two participants who received 25 μg ABNCoV2 tested positive for SARS-CoV-2, 16 and 20 weeks after the second vaccination. Both participants had received one dose of BNT162b2 before the SARS-CoV-2 infection, with one participant receiving the dose 9 weeks before and the other 12 weeks before vaccination. These participants had moderate COVID-19-related symptoms, and one of them developed a grade 3 (39·0°C) fever. Whole-genome sequencing revealed delta variant (B.1.617) sub-lineage AY.122 for one and no result for the other participant, in whom viral load was very low. Of note, ten participants remained SARS-CoV-2 negative despite high-risk exposures; five who only received ABNCoV2 and five with at least one other vaccination.
The predefined immunogenicity criterion of our escalation design was based on primary data from previous trials with soluble protein vaccine candidates,29, 30 showing that antibody response following first vaccination had discriminatory power for dose selection. Additionally, the target product profile of WHO included immunogenicity following one vaccination.31 This approach turned out not to be optimal, as, after one vaccination, the response was low, variable, and did not predict response following second vaccination well. Hence, two immunisations are, at minimum, required for ABNCoV2 in vaccinees who are naive to SARS-CoV-2. cVLPs structurally resemble native viruses and can be highly immunogenic, in particular due to their size, which enables them to be drained directly to the lymph nodes, and their repetitive surface epitope display.6 cVLPs overcome risks of highly effective live attenuated vaccines (eg, vaccine-induced disease or reversion) but their immunogenicity is comparable. With ABNCoV2, we observed a dose-sparing effect that saturated at 25 μg when MF59 was added, which might be beneficial for large-scale use. Implementation will also be facilitated after development of formulations of ABNCoV2 with less stringent storage requirements from freezer to room temperature, particularly for its use in remote areas or regions with ineffective health infrastructures.
This trial had several limitations. The durability of the immune responses could not be measured in most participants as nearly all (43 of 45) received a licensed SARS-CoV-2 vaccine during the follow-up of the trial; the study population of mostly young healthy adults was not representative of the population most in need of second-generation SARS-CoV-2 vaccines; the trial was not powered to measure efficacy against infection or disease; only a relatively small set of regimens was tested; and there was no control group with a licensed vaccine, as there were no licensed vaccines available at the start of the trial.
In conclusion, the results of this trial show that ABNCoV2 was well tolerated and induced strong virus neutralising antibody responses after the second vaccination in healthy adults who are naive to SARS-CoV-2. The protein-based ABNCoV2 vaccine is not expected to require ultra-cold storage conditions (–20 and –70°C), as opposed to currently approved mRNA-based COVID-19 vaccines, easing global distribution. These findings support additional clinical development of ABNCoV2 as a second-generation vaccine and show the potential of the modular cVLP platform.
Data sharing
Deidentified participant data that underlie the results reported in this Article will be made available on request. Proposals should be directed to the corresponding author, benjamin.mordmueller@radboudumc.nl. Proposals will be reviewed and approved by the sponsor on the basis of compliance with the informed consent and scientific merit. After approval of a proposal, data requesters will need to sign a data access agreement. Data can be requested indefinitely.
Declaration of interests
MAN, AFS, AS, TGT, and CJ are listed as coinventors on a patent application covering the AP205 CLP vaccine platform technology (WO2016112921 A1) licensed to AdaptVac. CF, AFS, and WAdJ are employees at AdaptVac, a company commercialising virus-like particle display technology and vaccines, including several patents.
Acknowledgments
Acknowledgments
We thank the members of the safety monitoring committee (LW Preston Church [Sanaria, Rockville, MD, USA], Martin P Grobusch [Amsterdam UMC, Amsterdam, Netherlands], and Jürgen May [Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany]), the study monitor, Daphne Smit and Maurice van der Burgh (Radboudumc, Nijmegen, Netherlands), and the team at Radboud University Medical Center in Nijmegen, University of Copenhagen, and the Institute of Tropical Medicine of the University of Tübingen for their generous support and help. We thank the study participants for their participation in this trial. The work was funded by the EU, Advancing knowledge for the clinical and public health response to the 2019-nCoV epidemic [H2020-SC1-PHE-CORONAVIRUS-2020] and a Semper Ardens grant from Carlsberg Foundation.
COUGH-1 study group
Robert Dagil PhD, Louise Goksøyr MSc, Thomas M Hulen MSc, Christoph Janitzek PhD, Paul K Khalifé MSc, and Elena Vidal-Calvo MSc (Centre for Medical Parasitology, Department for Immunology and Microbiology, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark); Daniel S Jensen MSc and Telma Lança PhD, Sune Justesen PhD, and Olivia Lie-Andersen PhD (Immunitrack ApS, Copenhagen, Denmark); Andrea Kreidenweiss PhD (Institute of Tropical Medicine, University Hospital Tübingen, Tübingen, Germany); Karina Teelen (Department of Medical Microbiology, Radboud Institute for Molecular Life Sciences, Radboud University Medical Center, Nijmegen, Netherlands)
Contributors
MJS, MBBM, MAN, ME, and BGM conceived the study and developed the study protocol. WAdJ, MAN, AFS, and BGM acquired funding. MBBM and BGM provided oversight and supervision. HFW was the sponsor's representative of the trial. CF, CH, and RF did the antibody assays. MI, SRP, APU, AB, SR, and JB did the virus neutralisation assays. DSJ, SJ, TL, and OL-A (COUGH-1 trial study group) did the T-cell analysis. BGM did the statistical analysis. MJS and KT (COUGH-1 trial study group), MBPAA, MBBM, and BGM coordinated the trial and oversaw data collection and management. MJS, AFS, MBPAA, CF, CH, RF, WAdJ, MBBM, MAN, and BGM accessed and verified the data. MJS, AFS, MBPAA, CF, CH, RF, MI, WAdJ, MBBM, MAN, and BGM analysed the data. MJS, AFS, MBPAA, CF, MAN, and BGM wrote the original draft of the manuscript. CH, RF, ME, PGK, RtH, HFW, MI, SRP, APU, AB, SR, JB, MS, SME, TG, SC, TGT, AS, MH, and WAdJ, and RD, LG, TMH, CJ, DSJ, SJ, PKK, AK, TL, OL-A, KT, and EV-C (COUGH-1 trial study group) reviewed the manuscript. All authors had access to the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
COUGH-1 trial study group:
Merel J Smit, Adam F Sander, Maud B P A Ariaans, Cyrielle Fougeroux, Constanze Heinzel, Rolf Fendel, Meral Esen, Peter G Kremsner, Rob ter Heine, Heiman F Wertheim, Manja Idorn, Søren Riis Paludan, Alexander P Underwood, Alekxander Binderup, Santseharay Ramirez, Jens Bukh, Max Soegaard, Sayit M Erdogan, Tobias Gustavsson, Stine Clemmensen, Thor G Theander, Ali Salanti, Mette Hamborg, Willem A de Jongh, Matthew B B McCall, Morten A Nielsen, Benjamin G Mordmüller, Robert Dagil, Louise Goksøyr, Thomas M Hulen, Christoph Janitzek, Daniel S Jensen, Sune Justesen, Paul K Khalifé, Andrea Kreidenweiss, Telma Lança, Olivia Lie-Andersen, Karina Teelen, and Elena Vidal-Calvo
Supplementary Material
References
- 1.WHO WHO COVID-19 dashboard. https://covid19.who.int/
- 2.Kasis A, Timotheou S, Monshizadeh N, Polycarpou M. Optimal intervention strategies to mitigate the COVID-19 pandemic effects. Sci Rep. 2022;12 doi: 10.1038/s41598-022-09857-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jara A, Undurraga EA, Zubizarreta JR, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health. 2022;10:e798–e806. doi: 10.1016/S2214-109X(22)00112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fougeroux C, Goksøyr L, Idorn M, et al. Capsid-like particles decorated with the SARS-CoV-2 receptor-binding domain elicit strong virus neutralization activity. Nat Commun. 2021;12:324. doi: 10.1038/s41467-020-20251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakeri B, Fierer JO, Celik E, et al. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci USA. 2012;109:E690–E697. doi: 10.1073/pnas.1115485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 7.De Vincenzo R, Conte C, Ricci C, Scambia G, Capelli G. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999–1010. doi: 10.2147/IJWH.S50365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:e93–e95. doi: 10.1016/j.jinf.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonanad C, García-Blas S, Tarazona-Santabalbina F, et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc. 2020;21:915–918. doi: 10.1016/j.jamda.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko E-J, Kang S-M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum Vaccin Immunother. 2018;14:3041–3045. doi: 10.1080/21645515.2018.1495301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinzel C, Pinilla YT, Elsner K, et al. Non-invasive antibody assessment in saliva to determine SARS-CoV-2 exposure in young children. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.753435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underwood AP, Sølund C, Fernandez-Antunez C, et al. Neutralisation titres against SARS-CoV-2 are sustained 6 months after onset of symptoms in individuals with mild COVID-19. EBioMedicine. 2021;71 doi: 10.1016/j.ebiom.2021.103519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkmann A, Koopman G, Mooij P, et al. A capsid virus-like particle-based SARS-CoV-2 vaccine induces high levels of antibodies and protects rhesus macaques. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.857440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 15.Sattler A, Angermair S, Stockmann H, et al. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest. 2020;130:6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 17.Tan C-W, Lim B-L, Young BE, et al. Comparative neutralisation profile of SARS-CoV-2 omicron subvariants BA.2.75 and BA.5. Lancet Microbe. 2022;3:e898. doi: 10.1016/S2666-5247(22)00220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiller JT, Castellsagué X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. 2021;325:2201–2202. doi: 10.1001/jama.2021.5374. [DOI] [PubMed] [Google Scholar]
- 21.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 22.Rich JD, Shesol BF, Horne DW., 3rd Basal cell carcinoma arising in a smallpox vaccination site. J Clin Pathol. 1980;33:134–135. doi: 10.1136/jcp.33.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Hur N, Avni J, Neuman Z. Basal cell carcinoma following BCG vaccination. Report of two cases. Dis Chest. 1963;44:653–655. doi: 10.1378/chest.44.6.653. [DOI] [PubMed] [Google Scholar]
- 24.Pace A, Degaetano J. Basal cell carcinoma developing in an influenza vaccine scar. Australas J Dermatol. 2004;45:75–76. doi: 10.1111/j.1440-0960.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith VH, Soon C, Dharma B, Eltigani EA, Bedlow AJ, Carr RC. Basal cell carcinomas arising in travel vaccination scars. Clin Exp Dermatol. 2008;33:515–516. doi: 10.1111/j.1365-2230.2008.02750.x. [DOI] [PubMed] [Google Scholar]
- 26.Moore JP, Klasse PJ. COVID-19 vaccines: “warp speed” needs mind melds, not warped minds. J Virol. 2020;94:e01083–e01120. doi: 10.1128/JVI.01083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 28.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esen M, Kremsner PG, Schleucher R, et al. Safety and immunogenicity of GMZ2—a MSP3-GLURP fusion protein malaria vaccine candidate. Vaccine. 2009;27:6862–6868. doi: 10.1016/j.vaccine.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Mordmüller B, Sulyok M, Egger-Adam D, et al. First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate to prevent pregnancy-associated malaria. Clin Infect Dis. 2019;69:1509–1516. doi: 10.1093/cid/ciy1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO WHO target product profiles for covid-19 vaccines, version 3. April 29, 2020. https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data that underlie the results reported in this Article will be made available on request. Proposals should be directed to the corresponding author, benjamin.mordmueller@radboudumc.nl. Proposals will be reviewed and approved by the sponsor on the basis of compliance with the informed consent and scientific merit. After approval of a proposal, data requesters will need to sign a data access agreement. Data can be requested indefinitely.