Abstract
The superfamily Yponomeutoidea, one of the early-derived groups in the order Lepidoptera, consists of 11 families. However, mitochondrial genome (mitogenome) sequences, popularly used for phylogeny and evolutionary tracing, are available for only seven species across six genera and five families. Thus, a larger variety of mitogenome sequences in Yponomeutoidea are required to improve our understanding of lepidopteran phylogeny and genomic evolution. In this study, we present the complete mitogenome of Attevaaurea (Fitch, 1856), the first species in the family Attevidae (superfamily Yponomeutoidea, order Lepidoptera) to be sequenced. The complete mitogenome comprises 16,329 bp and contains a typical set of genes and one non-coding region. Within Yponomeutoidea, the mitogenome of A.aurea has a unique trnI-trnM-trnQ arrangement at the A + T-rich region and ND2 junction and trnA-ND3 arrangement at the trnG and trnR junction. Twelve of the 13 protein-coding genes (PCGs) of A.aurea have a typical ATN starting codon, whereas COI has the atypical CGA codon, which is frequently found in the starting region of lepidopteran COI. Phylogenetic analyses, based on the concatenated sequences of 13 PCGs and two rRNA genes, using the Maximum Likelihood method, revealed a sister relationship between Attevidae and Praydidae with moderately low nodal support (bootstrap support = 64%).
Keywords: mitochondrial genome, Attevaaurea , phylogeny, Attevidae
Introduction
The superfamily Yponomeutoidea is one of the earliest groups to develop external feeding mechanisms in the order Lepidoptera and comprises ~ 1,800 species across 11 families (Sohn et al. 2013). However, only seven species in six genera across five families have available mitochondrial genome (mitogenome) sequences. Thus, the characterisation of the mitogenomes of more families will significantly contribute to the study of genomic evolution and subsequent phylogenetic analysis within this superfamily, as well as other early-derived lepidopteran clades.
The ailanthus webworm (Attevaaurea Fitch, 1856) is a small, colourful moth predominantly found north of Costa Rica, across the USA and in southern Quebec and Ontario, Canada (Wilson et al. 2010). Populations distributed south of Costa Rica in Uruguay and Argentina are known as A.pustulella (Fabricius, 1787), the former classification of A.aurea in North America (Wilson et al. 2010).
In this study, we present the complete mitogenome of A.aurea, the first species in the family Attevidae (superfamily Yponomeutoidea, order Lepidoptera) to be sequenced. The sequence was analysed in terms of its mitogenome characteristics and phylogenetic position within the superfamily Yponomeutoidea. Additionally, the DNA barcoding region of A.aurea was compared to that of previously-registered A.aurea and A.pustulella, which have been used for extensive phylogenetic analysis (Wilson et al. 2010), to further confirm sequence divergence between the two species.
Materials and methods
Sample collection, DNA extraction, PCR and sequencing
In 2011, a brood of A.aurea was collected from the Paint Branch Trail at the University of Maryland (College Park, MA, USA; 38°59’39’’N, 76°56’5’’W). In this study, DNA was extracted from the whole body of one adult male using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). Using Lepidoptera-specific primers (Suppl. material 1, Kim et al. 2012), three overlapping long fragments (LFs; COI to ND4, ND5 to lrRNA and lrRNA to COI) were amplified. These LFs were then used as templates for the amplification of 26 short fragments (SFs) using the same Lepidoptera-specific primers (Suppl. material 1, Kim et al. 2012). All products were sequenced in both forward and reverse transcriptional directions by Sanger's methods. The whole body of the specimen was consumed in the process. Thus, other individuals of the brood were moved as voucher specimens to the Gongju National University of Education (Gongju, South Korea) and labelled with accession nos. GNUE-I-0001–GNUE-I-0003.
Boundary delimitation and annotation
Individual SF sequences were manually assembled into complete mitogenomes using SeqMan (DNASTAR, Madison, WI, USA). The identification and boundary delimitation of each gene and secondary structure folding of tRNAs were performed using the MITOS Web Server (http://mitos.bioinf.uni-leipzig.de/index.py) and using the default search mode, Mito/Chloroplast as the searching source and the genetic code of invertebrate mitogenomes for tRNA isotype prediction (Lowe and Chan 2016). Where necessary, mitogenome sequences of species in the superfamily Yponomeutoidea registered in GenBank were downloaded and aligned for improved annotation by following the protocols presented by Cameron 2014.
Phylogenetic analysis
Phylogenetic analysis was conducted using 25 available mitogenomes in 23 species (including A.aurea) in the superfamilies Gracillarioidea, Yponomeutoidea and Tineoidea. We selected Gracillarioidea and Tineoidea, along with Yponomeutoidea, because of the previously established sister-group relationship between Yponomeutoidea and Gracillarioidea and of the branching of Tineoidea as a lineage basal to these two superfamilies (Timmermans et al. 2014, Breinholt et al. 2018, Bao et al. 2019, Kawahara et al. 2019). Two species within the superfamily Nepticuloidea (Stigmellaroborella and Astrotischeria sp.) were used as outgroups. Thirteen protein-coding genes (PCGs) and two rRNA genes (including those of two outgroup species) were aligned using RevTrans ver. 2.0 (Wernersson and Pedersen 2003) and concatenated using SequenceMatrix ver. 1.8 (Vaidya et al. 2011). The Maximum Likelihood method was applied using CIPRES Portal ver. 3.1 (Miller et al. 2010) for phylogenetic analyses, based on the GTR + Gamma + I model, which was selected using jModelTest (Posada 2008).
Data resources
Genome sequence data used in this study are openly available from the GenBank database of the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov) under the accession no. ON480203. All datasets used in this study were published at Zenodo on 03 July 2022 (Zenodo. https://doi.org/10.5281/zenodo.6791899).
Results and discussion
Genome summary
The complete 16,392-bp mitogenome of A.aurea is composed of typical gene sets (two rRNAs, 22 tRNAs and 13 PCGs) and a major non-coding A + T-rich region (Table 1; GenBank accession no. ON480203). Twelve of the 13 PCGs have the typical ATN start codon, whereas COI has the atypical CGA codon, which is found in the majority of other available mitogenome sequences of Tineoidea, Gracillarioidea and Yponomeutoidea species (data not shown), as well as Lepidoptera species (Kim et al. 2010, Park et al. 2016, Kim et al. 2018, Jeong et al. 2021). Ten PCGs end with typical stop codon TAA, whereas COII and ND4 have incomplete, single-thymine stop codons. The A/T content is 79.9% in PCGs, 82.4% in tRNAs, 82.5% in the whole genome, 86.5% in lrRNA, 87.6% in srRNA and 98.0% in the A + T-rich region (data not shown).
Table 1.
Summary of Attevaaurea mitochondrial genome.
| Gene | Nucleotide number | Size | Anticodon | Start codon | Stop codon | O/S |
| trnI | 1-64 | 64 | GAT 29-31 | |||
| trnM | 65-132 | 68 | CAT 96-98 | -8 | ||
| trnQ | 141-209 | 69 | TTG 177-179 | -41 | ||
| ND2 | 251-1276 | 1026 | ATT | TAA | +2 | |
| trnW | 1275-1340 | 64 | TCA 1305-1307 | +8 | ||
| trnC | 1333-1401 | 69 | GCA 1369-1371 | -7 | ||
| trnY | 1409-1474 | 66 | GTA 1441-1443 | -5 | ||
| COI | 1480-3015 | 1536 | CGA | TAA | -54 | |
| trnL2 | 3070-3136 | 67 | TAA 3100-3102 | |||
| COII | 3137-3818 | 682 | ATG | T-tRNA | ||
| trnK | 3819-3889 | 71 | CTT 3849-3851 | +1 | ||
| trnD | 3889-3954 | 66 | GTC 3919-3921 | |||
| ATP8 | 3955-4116 | 162 | ATC | TAA | +7 | |
| ATP6 | 4110-4781 | 672 | ATG | TAA | +1 | |
| COIII | 4781-5569 | 789 | ATG | TAA | -2 | |
| trnG | 5572-5640 | 69 | TCC 5602-5604 | -332 | ||
| trnA | 5973-6039 | 67 | TGC 6002-6004 | -103 | ||
| ND3 | 6143-6517 | 375 | ATA | TAA | -28 | |
| trnR | 6546-6607 | 62 | TCG 6573-6575 | -4 | ||
| trnN | 6612-6677 | 66 | GTT 6642-6644 | +1 | ||
| trnS1 | 6677-6737 | 61 | GCT 6698-6700 | -23 | ||
| trnE | 6761-6826 | 66 | TTC 6791-6793 | +2 | ||
| trnF | 6825-6892 | 68 | GAA 6856-6858 | -3 | ||
| ND5 | 6896-8629 | 1716 | ATT | TAA | ||
| trnH | 8630-8694 | 65 | GTG 8659-8661 | |||
| ND4 | 8695-10036 | 1342 | ATG | T-tRNA | +1 | |
| ND4L | 10036-10323 | 288 | ATG | TAA | -2 | |
| trnT | 10326-10390 | 65 | TGT 10357-10359 | |||
| trnP | 10391-10455 | 65 | TGG 10424-10426 | -1 | ||
| ND6 | 10457-10981 | 525 | ATT | TAA | -3 | |
| CytB | 10985-12136 | 1152 | ATG | TAA | -10 | |
| trnS2 | 12147-12215 | 69 | TGA 12179-12181 | +2 | ||
| ND1 | 12214-13170 | 957 | ATG | TAA | -1 | |
| trnL1 | 13172-13238 | 67 | TAG 13207-13209 | |||
| lrRNA | 13239-14559 | 1321 | ||||
| trnV | 14560-14625 | 66 | TAC 14594-14596 | |||
| srRNA | 14626-15393 | 768 | ||||
| A + T–rich region | 15394-16392 | 999 | ||||
| Non-underlined and underlined genes indicate forward and reverse transcriptional directions, respectively. tRNAs are denoted as one-letter symbols in accordance with the IUPAC-IUB single-letter amino acid codes, except those encoding leucine and serine, which are labelled L1 for the CTN, L2 for the TTR, S1 for the AGN and S2 for the TCN codon families. O/S denotes the number of the overlapping(+)/intergenic space sequence(-). | ||||||
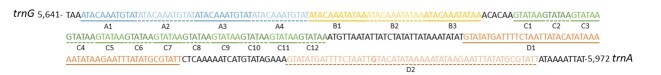
The genes of A.aurea are interleaved with a total 627 bp, spread over 17 regions ranging in size between 1 and 332 bp (Table 1). Most intergenic spacer sequences (ISSs) are short (1–20 bp), but four locations have longer ISSs (41–332 bp). Examination with the naked eye revealed that three of those four ISSs (trnQ-ND2, COI- trnL2 and trnA-ND3) have no notable features, except high A/T content (90.24–96.30%; data not shown). However, the longest of these ISSs (332 bp), located between trnG and trnA, has four tandem repeat units with varying copy numbers (Fig. 1; A1–A4, B1–B3, C1–C12 and D1–D2). All copies of each repeat unit have identical sequences, except for one nucleotide substitution (A for G) in repeat unit D.
Figure 1.
Four tandem repeat units found between trnG and trnA with various copy numbers (A1–A4, B1–B3, C1–C12 and D1–D2). The nucleotide position is indicated at each end of the sequence in relation to the mitochondrial genome of Attevaaurea.
Gene rearrangement
Compared with that of other Lepidoptera species, the A.aurea mitogenome has a very rare trnI-trnM-trnQ arrangement (underlining indicates gene inversion) at the A + T-rich region and ND2 junction (Fig. 2). Monopislongella (Walker, 1863) (family Tineidae, superfamily Tineoidea) is the only species previously known to exhibit the trnI-trnM-trnQ arrangement in the Ditrysia clade, including the superfamilies Gracillarioidea, Yponomeutoidea, and Tineoidea (Jeong et al. 2021). Conversely, the majority of ditrysian Lepidoptera species have the gene order trnM-trnI-trnQ at the same junction (Fig. 2, Kim et al. 2014). This differs from the ancestral trnI-trnQ-trnM order found in most insects (Fig. 2, Boore 1999), including ancient, non-ditrysian lepidopteran groups, such as Hepialoidea and Nepticuloidea (Cao et al. 2012, Timmermans et al. 2014). Moreover, the A.aurea mitogenome has the trnA-ND3 arrangement at the trnG and trnR junctions instead of the ND3-trnA arrangement found in almost all Lepidoptera species, including all those in Gracillarioidea, Yponomeutoidea, and Tineoidea (Fig. 2, Kim et al. 2014, Jeong et al. 2021). Thus far, only seven species in six genera across fiv families in Yponomeutoidea have had their mitogenomes sequenced. Thus, further analysis of this superfamily is required to make any conclusive remarks about the evolution of this rearrangement. Nevertheless, current analysis indicates that the arrangement of the family Attevidae is an autapomorphic characteristic of the superfamily Yponomeutoidea (data not shown).
Figure 2.

Linear arrangement of the mitochondrial genome of A.aurea. Gene sizes are not drawn to scale. Non-underlined and underlined gene names indicate forward and reverse transcriptional directions, respectively. Translocated genes are indicated by lines with arrows.
Comparison of DNA barcoding sequence
The comparison between the DNA barcoding sequences of current A.aurea and those of A.aurea previously registered on GenBank, including those registered by Wilson et al. (2010), showed a 0.00–1.67% divergence. Compared with A.pustulella DNA barcoding sequences, there was a divergence of at least 3.95% (data not shown). This reflects the findings of a previous study that A.aurea, distributed between Costa Rica and southern Quebec and Ontario, is indeed A.aurea and that phylogenetic results demonstrate a clear separation of A.aurea from other Attevidae species, including A.pustulella (Wilson et al. 2010).
Phylogenetic analysis
Phylogenetic analysis revealed overall lower nodal supports for familial relationships within Yponomeutoidea. A sister relationship between the families Attevidae and Praydidae, each of which is represented by a single species, was supported, but the nodal support for this relationship was not high (bootstrap support (BS) = 64%; Fig. 3). Within the Ditrysia clade, Gracillarioidea and Yponomeutoidea exhibit a sister relationship with the highest nodal support, placing Tineoidea sister to the two superfamilies with the highest support (Fig. 3). Previously, Sohn et al. (2013), using 8.0–18.9 kb of 8–27 genes from 11 families in Yponomeutoidea, also revealed a sister relationship between Attevidae and Praydidae and this relationship was supported with relatively high nodal support. In terms of relationships between superfamilies, our findings are consistent with previous studies based on mitogenomic, molecular, morphological, genomic and transcriptome data that proposed a sister-group relationship between Yponomeutoidea and Gracillarioidea, with Tineoidea diverging earlier within ditrysian Lepidoptera (Heikkilä et al. 2014, Timmermans et al. 2014, Breinholt et al. 2018, Bao et al. 2019, Kawahara et al. 2019). Additional phylogenetic relationships within the early-derived groups of Lepidoptera could be determined, based on further taxonomic research with a wider scope.
Figure 3.
Phylogenetic tree of the three ditrysian superfamilies included in this study: Tineoidea, Gracillarioidea and Yponomeutoidea. The number at each node indicates the bootstrap value. The scale bar indicates the number of substitutions per site. The GenBank accession number of each species is shown in brackets after its scientific name.
Conclusions
This mitogenome of A.aurea has a unique trnI-trnM-trnQ arrangement at the A + T-rich region and ND2 junction and trnA-ND3 arrangement at the trnG and trnR junction, which is unprecedented in Yponomeutoidea. Thus, additional mitogenome sequences are required from other genera, subfamilies and families to understand the taxonomic extent of this arrangement in Yponomeutoidea. Phylogenetic analysis revealed a sister relationship between Attevidae and Praydidae, consistent with the results of a previous large-scale molecular phylogenetic study, but nodal support was not high in this study. The result of our DNA barcoding sequence comparison supports the finding of a previous study that A.aurea, occurring north of Costa Rica in the USA and southern Quebec and Ontario, is genetically distinct from A.pustulella distributed from Costa Rica south to Uruguay and Argentina. Including that of A.aurea, only nine mitogenome sequences, representing seven genera across six families, are currently available for the superfamily Yponomeutoidea. Thus, more mitogenome sequences from the early-derived groups of Lepidoptera, including Yponomeutoidea, are essential for a greater understanding of mitogenome evolution and phylogenetic relationships in this order.
Supplementary Material
List of primers used to amplify and sequence the Attevaaurea mitochondrial genome
Jeong et al.
Data type
Primer list
File: oo_710151.docx
Acknowledgements
This study was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry through the Agriculture, Food and Rural Affairs Convergence Technologies Program and Educating Creative Global Leader Program, funded by the Ministry of Agriculture, Food and Rural Affairs (grant no. 321001-03).
Funding program
This study was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry through the Agriculture, Food and Rural Affairs Convergence Technologies Program and Educating Creative Global Leader Program, funded by the Ministry of Agriculture, Food and Rural Affairs (grant no. 321001-03).
Hosting institution
Chonnam National University, Gwangju, South Korea
Ethics and security
We declare that there are no violations of the guidelines of the authors’ respective institutions and local, national and international regulations.
Conflicts of interest
We declare no potential conflicts of interest.
Funding Statement
The Ministry of Agriculture, Food and Rural Affairs
Author contributions
Conceptualisation, J.-C.S., J.S.J. and I.K.; fieldwork, J.-C.S.; data analysis and interpretation, J.S.J., J.S.P., H.K.O. and M.J.K.; writing—original draft, J.S.J., J.-C.S., M.J.K. and I.K.; writing—review and editing, J.-C.S., J.S.P., H.K.O. and I.K.; supervision, I.K.; project administration, J.S.P. and I.K.; funding acquisition, I.K.
Conflicts of interest
We declare no potential conflicts of interest.
References
- Bao Lu, Zhang Yonghen, Gu Xing, Gao Yuefang, Yu Youben. The complete mitochondrial genome of Eterusiaaedea (Lepidoptera, Zygaenidae) and comparison with other zygaenid moths. Genomics. 2019;111(5):1043–1052. doi: 10.1016/j.ygeno.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Boore J. L. Animal mitochondrial genomes. Nucleic Acids Research. 1999;27(8):1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breinholt Jesse W., Earl Chandra, Lemmon Alan R., Lemmon Emily Moriarty, Xiao Lei, Kawahara Akito Y. Resolving relationships among the megadiverse butterflies and moths with a novel pipeline for anchored phylogenomics. Systematic Biology. 2018;67(1):78–93. doi: 10.1093/sysbio/syx048. [DOI] [PubMed] [Google Scholar]
- Cameron Stephen L. How to sequence and annotate insect mitochondrial genomes for systematic and comparative genomics research. Systematic Entomology. 2014;39(3):400–411. doi: 10.1111/syen.12071. [DOI] [Google Scholar]
- Cao Yong-Qiang, Ma Chuan, Chen Ji-Yue, Yang Da-Rong. The complete mitochondrial genomes of two ghost moths, Thitarodesrenzhiensis and Thitarodesyunnanensis: the ancestral gene arrangement in Lepidoptera. BMC Genomics. 2012;13(1) doi: 10.1186/1471-2164-13-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä Maria, Mutanen Marko, Kekkonen Mari, Kaila Lauri. Morphology reinforces proposed molecular phylogenetic affinities: a revised classification for Gelechioidea (Lepidoptera) Cladistics. 2014;30(6):563–589. doi: 10.1111/cla.12064. [DOI] [PubMed] [Google Scholar]
- Jeong Su Yeon, Park Jeong Sun, Kim Min Jee, Kim Sung- Soo, Kim Iksoo. The complete mitochondrial genome of Monopislongella Walker, 1863 (Lepidoptera: Tineidae) Mitochondrial DNA Part B. 2021;6(8):2159–2161. doi: 10.1080/23802359.2021.1944389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Akito Y, Plotkin David, Espeland Marianne, Meusemann Karen, Toussaint Emmanuel F A, Donath Alexander, Gimnich France, Frandsen Paul B, Zwick Andreas, Dos Reis Mario, Barber Jesse R, Peters Ralph S, Liu Shanlin, Zhou Xin, Mayer Christoph, Podsiadlowski Lars, Storer Caroline, Yack Jayne E, Misof Bernhard, Breinholt Jesse W, et al. Phylogenomics reveals the evolutionary timing and pattern of butterflies and moths. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(45):22657–22663. doi: 10.1073/pnas.1907847116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Jong Sun, Park Jeong Sun, Kim Min Jee, Kang Pil Don, Kim Seon Gon, Jin Byung Rae, Han Yeon Soo, Kim Iksoo. Complete nucleotide sequence and organization of the mitochondrial genome of eri-silkworm, Samiacynthiaricini (Lepidoptera: Saturniidae) Journal of Asia-Pacific Entomology. 2012;15(1):162–173. doi: 10.1016/j.aspen.2011.10.002. [DOI] [Google Scholar]
- Kim Jong Seok, Kim Min Jee, Jeong Jun Seong, Kim Iksoo. Complete mitochondrial genome of Saturniajonasii (Lepidoptera: Saturniidae): Genomic comparisons and phylogenetic inference among Bombycoidea. Genomics. 2018;110(5):274–282. doi: 10.1016/j.ygeno.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Kim Min Jee, Wan Xinlong, Kim Ki Gyoung, Hwang Jae Sam, Kim Iksoo. Complete nucleotide sequence and organization of the mitogenome of endangered Eumenisautonoe (Lepidoptera: Nymphalidae) African Journal of Biotechnology. 2010;9(5):735–754. doi: 10.5897/ajb09.1486. [DOI] [Google Scholar]
- Kim Min Jee, Wang Ah Rha, Park Jeong Sun, Kim Iksoo. Complete mitochondrial genomes of five skippers (Lepidoptera: Hesperiidae) and phylogenetic reconstruction of Lepidoptera. Gene. 2014;549(1):97–112. doi: 10.1016/j.gene.2014.07.052. [DOI] [PubMed] [Google Scholar]
- Lowe Todd M., Chan Patricia P. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Research. 2016;44 doi: 10.1093/nar/gkw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Mark A., Pfeiffer Wayne, Schwartz Terri. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. 2010 Gateway Computing Environments Workshop (GCE) 2010 doi: 10.1109/gce.2010.5676129. [DOI]
- Park Jeong Sun, Kim Min Jee, Jeong Su Yeon, Kim Sung Soo, Kim Iksoo. Complete mitochondrial genomes of two gelechioids, Mesophlepsalbilinella and Dichomerisustalella (Lepidoptera: Gelechiidae), with a description of gene rearrangement in Lepidoptera. Current Genetics. 2016;62(4):809–826. doi: 10.1007/s00294-016-0585-3. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution. 2008;25(7):1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Sohn Jae-Cheon, Regier Jerome C., Mitter Charles, Davis Donald, Landry Jean-François, Zwick Andreas, Cummings Michael P. A Molecular Phylogeny for Yponomeutoidea (Insecta, Lepidoptera, Ditrysia) and its implications for classification, biogeography and the evolution of host plant use. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0055066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans Martijn J. T.N., Lees David C., Simonsen Thomas J. Towards a mitogenomic phylogeny of Lepidoptera. Molecular Phylogenetics and Evolution. 2014;79:169–178. doi: 10.1016/j.ympev.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Vaidya Gaurav, Lohman David J., Meier Rudolf. SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics. 2011;27(2):171–180. doi: 10.1111/j.1096-0031.2010.00329.x. [DOI] [PubMed] [Google Scholar]
- Wernersson R., Pedersen AG. RevTrans: multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Research. 2003;31(13):3537–3539. doi: 10.1093/nar/gkg609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson John, Landry Jean-François, Janzen Daniel, Hallwachs Winnie, Nazari Vazrick, Hajibabaei Mehrdad, Hebert Paul. Identity of the ailanthus webworm moth (Lepidoptera, Yponomeutidae), a complex of two species: evidence from DNA barcoding, morphology and ecology. ZooKeys. 2010;46:41–60. doi: 10.3897/zookeys.46.406. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of primers used to amplify and sequence the Attevaaurea mitochondrial genome
Jeong et al.
Data type
Primer list
File: oo_710151.docx