Abstract
Background
The North Caucasus is an extensive region with a multitude of landscapes and high biological diversity. Amongst various ecosystems, the xerophytic sub-Mediterranean forests of the Abrau Peninsula (Utrish State Nature Reserve) and its vicinity are unique but have been poorly studied. The diversity of diatoms in North Caucasian ecosystems have been studied partially and only little information is available about their presence and distribution on the Abrau Peninsula. Here, we present a comprehensive check-list of diatoms sampled during a July 2021 field campaign. Samples were collected in 67 sites, including 39 permanent streams, 21 temporal (puddles) and seven permanent waterbodies. Results of the current study contribute to improving the knowledge about diatoms in the north-western Caucasus and its sub-Mediterranean ecosystems in particular.
New information
Here, we provide a detailed dataset that contains 215 freshwater and brackish diatom occurrences collected during a field campaign in July 2021. A total of 88 diatom (Bacillariophyta) taxa which belong to 12 orders, 25 families and 39 genera were collected. The genera with the highest number of occurrences per site were Gomphonema (26), Nitzschia (22), Navicula (20), Cocconeis (14), Amphora (14), Achnanthidium (14) and Planothidium (11). The genera with the highest number of infrageneric taxa were Nitzschia (8), Navicula (7), Gomphonema (6) and Mastogloia (5). Naviculablazencicae, known as the endemic of the Lake Prespa (Levkov 2007) is found from two sites in our study. Three specimens of the genus Mastogloia could not be assigned to a known species and may represent new diatom species. Distribution and ecology data are provided for each taxa. Occurrence data are given. Statistical analysis of diatom communities showed a significant dependence on habitat type and their ecological conditions.
Keywords: Bacillariophyta, freshwater ecosystems, coastal ecosystems, new records, species check-list, Utrish State Nature Reserve
Introduction
Diatoms are a widely distributed group of algae whose representatives populate both aquatic (marine and freshwater) and terrestrial ecosystems, such as soils, mosses, wet walls and rocks (Round et al. 1990, Smol and Stoermer 2010) and play a key role in the nutrient cycle and energy flux (Benoiston et al. 2017). In seas and oceans, organic carbon produced by diatoms is consumed rapidly and serves as a base for marine food webs. In coastal waters, diatoms support most productive fisheries. In the open ocean, a relatively large proportion of diatom organic matter sinks rapidly from the surface, becoming food for deep-water organisms (Armbrust 2009). Soils and other terrestrial ecosystems have more severe effects for diatoms and differ from aquatic ecosystems in diatom species composition, although diatoms can be the dominant algal group at periods of the year with high soil moisture (Foets et al. 2020).
Diatoms are regularly used as biological indicators for the water quality environmental assessment (Patrick 1973, Reid et al. 1995, Kelly et al. 1998, Battarbee et al. 2002). The analysis of diatom communities and their biodiversity is a useful tool to secure an ecological and sustainable use of the water resources and the correct elaboration of guidelines for their preservation, in particular, in specially protected natural areas. Some recent studies have shown that natural springs in protected areas may act as biodiversity hotspots (Falasco and Bona 2011, Falasco et al. 2012).
Different ecological groups of Black Sea diatoms have been actively studied, especially from the perspective of water quality assessment (Petrov and Nevrova 2007, Ryabushko et al. 2017, Ryabushko et al. 2019, Ryabushko et al. 2021). Additionally, there is ongoing research on diatom diversity of specially protected natural sea areas of the Black Sea (Nevrova 2015, Ryabushko et al. 2018, Polyakova and Davidovich 2019, Ryabushko et al. 2021, Davidovich and Polyakova 2022). In a recent study of diatom communities in the water area surrounding Bolshoi Utrish (Anapa District, Russia), it was found that 77% of the biomass and 25% of the total phytoplankton abundance was composed of Bacillariophyta species (Yasakova and Kolesnikov 2021). Inland research of diatom communities have mainly been focused on Abrau Lake, the largest freshwater lake in the Abrau Peninsula (Kovaleva 2005, Kovaleva 2018). There are, however, a number of freshwater waterbodies and streams in the Abrau Peninsula and the nearby Black Sea coastal zone that are still understudied in terms of diatom diversity and distribution. We assume that some sampling locations (freshwater streams) on the territory of Utrish State Nature Reserve, especially without anthropogenic disturbance, potentially might be hidden hotspots of diatom biodiversity.
This study presents a taxonomical characterisation and occurrence dataset of the diatoms found in Mediterranean ecosystems of the Abrau Peninsula, north-western Caucasus, particularly in protected areas of Utrish State Nature Reserve. We aim to contribute to the current knowledge of diatom diversity and distribution in the freshwater and brackish inland water in the north-western Caucasus and its sub-Mediterranean ecosystems in particular.
Project description
Title
Diatom diversity, distribution and ecology in Mediterranean ecosystems of Abrau Peninsula, north-western Caucasus.
Personnel
Samples were collected on 12-20 July 2021 on the Abrau Peninsula by Alisa Neplyukhina and Angelina Paskhina. Identifications were made by Alisa Neplyukhina. Statistical analyses were performed by Daniil Korobushkin and Ruslan Saifutdinov. The text was written by Alisa Neplyukhina, Daniil Korobushkin and Ruslan Saifutdinov.
Study area description
The Abrau Peninsula is located between the city of Anapa and Abrau-Durso settlement in Novorossiysk District, Krasnodar Krai, Russia. Most of the Abrau Peninsula is under the protection of the Utrish State Nature Reserve (hereinafter referred to as "Utrish") and is not affected or disturbed by human activity, with the exception of the coastal zone and suburbs. The Abrau Peninsula has a humid subtropical (Cfa) and Mediterranean climate (Csa) according to the Köppen climate classification with cool rainy winters without stable snow cover and with hot dry summers (Chen and Chen 2013). The mean annual precipitation ranges from 480 mm (Anapa) to 788 mm (Novorossiysk), the mean July and February temperatures for both localities are 21℃ and 2℃, respectively (weatherbase, CantyMedia 2022).
The study area belongs to the Mediterranean ecoregion (Olson et al. 2001, Ogureeva et al. 2018) and is the only place in Russia covered by Mediterranean forests. The vegetation here forms three major belts (Bocharnikov et al. 2020, Seregin and Suslova 2007): 1. coastal slopes with sub-Mediterranean xerophytic forests and shrublands with pistachio (Pistaciamutica), juniper (Juniperusexcelsa, J.oxycedrus, J.foetidissima), oak (Quercuspubescens) and oriental hornbeam (Carpinusorientalis); 2. piedmont and low-mountain area with a combination of mesophilic and xerophilic forests and a predominance of two oak species (Q.pubescens, Q.petrea), oriental hornbeam and junipers; 3. low mountains with mesophilic deciduous forests with a domination of oak (Q.petrea), hornbeam (Carpinuscaucasica), lime (Tiliabegoniifolia), maple (Acerlaetum), ash (Fraxinusexcelsior) and beech (Fagusorientalis). A distinctive feature of the Utrish flora is unique pronounced Mediterranean core tertiary relict elements. It is inhabited by numerous rare, endemic species of flora and fauna.
Freshwater habitats are represented by permanent and temporary streams flowing to the Black Sea, as well as temporary small waterbodies (hereinafter referred as "puddles") scattered across the Abrau Peninsula. Brackish habitats are represented by small permanent lagoons located along the coastline.
Sampling methods
Study extent
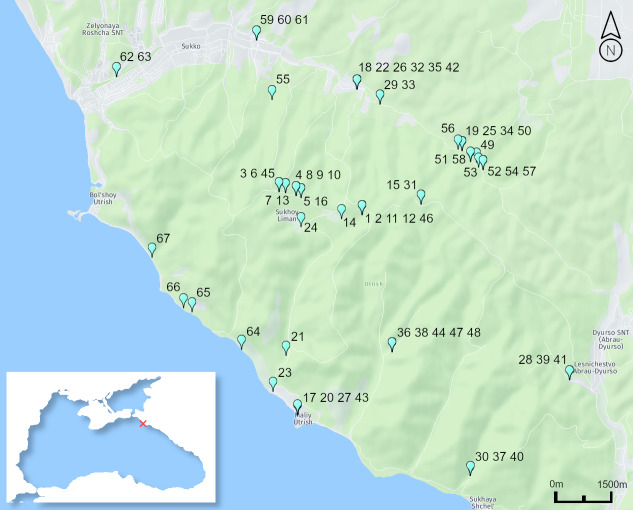
Diatoms were collected from 67 sampling sites, including 39 permanent streams, 21 temporal waterbodies (puddles) and seven permanent waterbodies (lakes and lagoons) collected on the Abrau Peninsula, north-western Caucasus, Russia (Fig. 1, Table 1).
Figure 1.
Study area and sampling sites location (Map source credits: https://wego.here.com).
Table 1.
Samples code, location of the sampling sites, site type and sample type on the Abrau Peninsula.
| Sampling code | Laboratory codename | Latitude (ºN) | Longitude (ºW) | Site type | Salinity | Sample type | Commentary |
| 1 | UT-2021-1 | 44.7582 | 37.4783 | Stream | Freshwater | Rock scrap | No diatoms found |
| 2 | UT-2021-2 | 44.7582 | 37.4783 | Stream | Freshwater | Rock scrap | |
| 3 | UT-2021-3 | 44.7637 | 37.4498 | Temporary (Puddle) | Freshwater | Rock scrap | |
| 4 | UT-2021-4 | 44.7627 | 37.4556 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 5 | UT-2021-5 | 44.7623 | 37.4573 | Temporary (Puddle) | Freshwater | Sediment | |
| 6 | UT-2021-6 | 44.7637 | 37.4497 | Temporary (Puddle) | Freshwater | Soil | |
| 7 | UT-2021-7 | 44.7635 | 37.4520 | Temporary (Puddle) | Freshwater | Soil | |
| 8 | UT-2021-8 | 44.7627 | 37.4556 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 9 | UT-2021-9 | 44.7627 | 37.4556 | Temporary (Puddle) | Freshwater | Sediment | |
| 10 | UT-2021-10 | 44.7627 | 37.4556 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 11 | UT-2021-11 | 44.7582 | 37.4782 | Stream | Freshwater | Rock scrap | No diatoms found |
| 12 | UT-2021-12 | 44.7582 | 37.4782 | Stream | Freshwater | Rock scrap | No diatoms found |
| 13 | UT-2021-13 | 44.7635 | 37.4520 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 14 | UT-2021-14 | 44.7572 | 37.4713 | Temporary (Puddle) | Freshwater | Rock scrap | No diatoms found |
| 15 | UT-2021-15 | 44.7606 | 37.4986 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 16 | UT-2021-16 | 44.7623 | 37.4573 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 17 | UT-2021-17 | 44.7097 | 37.4561 | Permanent | Freshwater | Rock scrap | |
| 18 | UT-2021-18 | 44.7887 | 37.4765 | Stream | Freshwater | Sediment | |
| 19 | UT-2021-19 | 44.7737 | 37.5125 | Stream | Freshwater | Sediment | |
| 20 | UT-2021-20 | 44.7094 | 37.4562 | Permanent | Freshwater | Sediment | |
| 21 | UT-2021-21 | 44.7239 | 37.4522 | Temporary (Puddle) | Freshwater | Soil | No diatoms found |
| 22 | UT-2021-22 | 44.7887 | 37.4765 | Stream | Freshwater | Rock scrap | No diatoms found |
| 23 | UT-2021-23 | 44.7151 | 37.4476 | Permanent | Freshwater | Soil | |
| 24 | UT-2021-24 | 44.7552 | 37.4574 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 25 | UT-2021-25 | 44.7737 | 37.5125 | Stream | Freshwater | Rock scrap | |
| 26 | UT-2021-26 | 44.7887 | 37.4764 | Stream | Freshwater | Sediment | |
| 27 | UT-2021-27 | 44.7093 | 37.4561 | Permanent | Freshwater | Sediment | |
| 28 | UT-2021-28 | 44.7178 | 37.5495 | Stream | Freshwater | Sediment | |
| 29 | UT-2021-29 | 44.7851 | 37.4844 | Stream | Freshwater | Sediment | |
| 30 | UT-2021-30 | 44.6946 | 37.5154 | Stream | Freshwater | Rock scrap | |
| 31 | UT-2021-31 | 44.7606 | 37.4986 | Temporary (Puddle) | Freshwater | Soil | |
| 32 | UT-2021-32 | 44.7888 | 37.4765 | Stream | Freshwater | Rock scrap | |
| 33 | UT-2021-33 | 44.7851 | 37.4844 | Stream | Freshwater | Rock scrap | |
| 34 | UT-2021-34 | 44.7737 | 37.5125 | Stream | Freshwater | Rock scrap | |
| 35 | UT-2021-35 | 44.7888 | 37.4765 | Stream | Freshwater | Rock scrap | No diatoms found |
| 36 | UT-2021-36 | 44.7247 | 37.4885 | Stream | Freshwater | Rock scrap | No diatoms found |
| 37 | UT-2021-37 | 44.6941 | 37.5152 | Stream | Freshwater | Rock scrap | |
| 38 | UT-2021-38 | 44.7247 | 37.4885 | Stream | Freshwater | Rock scrap | No diatoms found |
| 39 | UT-2021-39 | 44.7178 | 37.5495 | Stream | Freshwater | Rock scrap | |
| 40 | UT-2021-40 | 44.6945 | 37.5154 | Stream | Freshwater | Rock scrap | No diatoms found |
| 41 | UT-2021-41 | 44.7178 | 37.5495 | Stream | Freshwater | Rock scrap | No diatoms found |
| 42 | UT-2021-42 | 44.7888 | 37.4765 | Stream | Freshwater | Rock scrap | No diatoms found |
| 43 | UT-2021-43 | 44.7093 | 37.4561 | Permanent | Freshwater | Sediment | |
| 44 | UT-2021-44 | 44.7247 | 37.4885 | Stream | Freshwater | Sediment | |
| 45 | UT-2021-45 | 44.7638 | 37.4498 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 46 | UT-2021-46 | 44.7582 | 37.4782 | Stream | Freshwater | Sediment | No diatoms found |
| 47 | UT-2021-47 | 44.7247 | 37.4885 | Stream | Freshwater | Rock scrap | No diatoms found |
| 48 | UT-2021-48 | 44.7247 | 37.4885 | Stream | Freshwater | Rock scrap | No diatoms found |
| 49 | UT-2021-49 | 44.7709 | 37.5175 | Temporary (Puddle) | Freshwater | Rock scrap | No diatoms found |
| 50 | UT-2021-50 | 44.7737 | 37.5125 | Stream | Freshwater | Rock scrap | No diatoms found |
| 51 | UT-2021-51 | 44.7710 | 37.5156 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 52 | UT-2021-52 | 44.7690 | 37.5197 | Stream | Freshwater | Rock scrap | No diatoms found |
| 53 | UT-2021-53 | 44.7697 | 37.5183 | Temporary (Puddle) | Freshwater | Soil | No diatoms found |
| 54 | UT-2021-54 | 44.7690 | 37.5197 | Stream | Freshwater | Rock scrap | |
| 55 | UT-2021-55 | 44.7862 | 37.4474 | Temporary, Stream | Freshwater | Sediment | |
| 56 | UT-2021-56 | 44.7741 | 37.5111 | Stream | Freshwater | Sediment | |
| 57 | UT-2021-57 | 44.7691 | 37.5199 | Stream | Freshwater | Rock scrap | |
| 58 | UT-2021-58 | 44.7711 | 37.5155 | Temporary (Puddle) | Freshwater | Sediment | No diatoms found |
| 59 | UT-2021-59 | 44.8007 | 37.4420 | Stream | Freshwater | Rock scrap | |
| 60 | UT-2021-60 | 44.8007 | 37.4421 | Stream | Freshwater | Rock scrap | No diatoms found |
| 61 | UT-2021-61 | 44.8007 | 37.4420 | Stream | Freshwater | Rock scrap, Sediment | |
| 62 | UT-2021-62 | 44.7918 | 37.3940 | Stream | Freshwater | Rock scrap | |
| 63 | UT-2021-63 | 44.7918 | 37.3940 | Stream | Freshwater | Rock scrap, Sediment | |
| 64 | UT-2021-64 | 44.7254 | 37.4368 | Stream | Freshwater | Rock scrap | |
| 65 | UT-2021-65 | 44.7345 | 37.4199 | Permanent | Brackish | Rock scrap | |
| 66 | UT-2021-66 | 44.7354 | 37.4170 | Permanent | Brackish | Rock scrap | |
| 67 | UT-2021-67 | 44.74781 | 37.4061 | Stream | Brackish | Moss squeeze, Rock scrap |
Sampling description
Material for this research was collected in July 2021. Sampling was carried out after the annual peak of summer precipitation in June (CantyMedia 2022) and performed after a week of strong rains (CantyMedia 2022). This made it possible to collect material from both permanent and temporary waterbodies. Diatom samples were collected from 67 sites on the Abrau Peninsula (Table 1). The sampling sites differed in salinity from brackish to freshwater. Sample types include 36 rock scrap samples, 24 sediments samples, six soil samples and one moss squeeze sample. Diatom samples were collected in 50 ml plastic containers and immediately fixed with Lugol's solution (2 ml to 50 ml of sample) in order to keep other algae groups in their best condition for futher research (Sadchikov 2003). Material was cleaned from the organics in accordance with the hot peroxide method following Kelly et al. (2001). Light microscopical investigations were performed in bright-field optics using a Leica DM 750 microscope, equipped with a Leica ICC50 HD digital camera. Permanent slides were prepared with Naphrax®. For the scanning electron microscopy investigation, drops of cleaned material were air-dried on pieces of aluminium foil, mounted on brass stubs with double-sided carbon tape and coated with Au in a S150A Sputter Coater (Edwards, UK) ion coater. Scanning electron microscopic investigations were conducted using TESCAN MIRA 3 LMH (TESCAN, Czech Republic) in the Joint Usage Center «Instrumental methods in ecology» at the IEE RAS. All prepared LM slides and SEM stubs are stored in the collection of the Laboratory for Ecology of Aquatic Communities and Invasions, IEE RAS.
Quality control
For diatom identification, a number of manuals were used (Lange-Bertalot 2001, Kulikovskiy et al. 2016, Cantonati et al. 2017). Valid diatom taxon names were verified according to Guiry and Guiry (2022). Data on diatom ecology are given according to Kulikovskiy et al. (2016), Cantonati et al. (2017) and Guiry and Guiry 2022 .
Step description
The data have been published as a Darwin Core Archive (DwC-A), which is a standardised format for sharing biodiversity data as a set of one or more data tables. The core data table contains 215 occurrences (Neplyukhina et al. 2022).
Statistical analysis: Similarity between diatom communities of Abrau Peninsula was evaluated using hierarchical cluster analysis. Before analysis, the data were prepared via the dplyr 1.0.8. package (Wickham et al. 2018) into species x communities matrix with presence/absence data. Data on diatoms were pooled into communities according to their presence in the habitat type (stream, waterbody or puddle) and according to the sampling method (scrap, sediment, moss and soil). A detailed description of habitat type selection and sampling methods is given in Table 1. Distances between communities were calculated using a binary method and the Ward.D2 method was selected for the hierarchical clustering procedure. Additionally for each cluster, bootstrap probability value (BP) and approximately unbiased (AU) probability values (p-values) were calculated via multi-scale bootstrap on 10000 resamplings using the package pvclust 2.2-0 (Suzuki and Shimodaira 2006). To define our clusters, we used a significance level of p < 0.05, i.e. the AU value equal or higher than 95. The obtained dendrogram was customised with the dendextend 1.15.2 package (Galili 2015). The above analyses were performed in R 4.1.2 (R Core Team 2021) with R Studio interface (R studio Inc.). To analyse the correlation between the species richness of diatoms belonging to a particular ecological group and their presence in various habitats of the Abrau Peninsula, the principal component analysis (PCA) was applied. Sampled habitats (freshwater puddles, freshwater streams, freshwater waterbodies and brackish waterbodies in accordance with Table 1) and separability preferences (eutrophic, mesotrophic, oligotrophic, polluted water) were selected as active variables, while environment preferences (freshwater, brackish, marine) were chosen as additional (passive) ones. Prior to the analysis, data were Z-transformed to homogenise the variance. PCA analysis were performed using Statistica 13.0 software (TIBCO Software Inc., USA).
Geographic coverage
Description
Utrish State Nature Reserve, Abrau Peninsula, north-western Caucasus, Russia
Coordinates
44.694123 N and 44.800702 N Latitude; 37.394033 E and 37.5495 E Longitude.
Taxonomic coverage
Description
All diatoms were identified to genus or species/intraspecific level. In total, 88 infrageneric taxa were identified belonging to two classes, 12 orders, 25 families and 39 genera distributed in the subphylum Bacillariophytina, 11 of them being identified only to genus level. The taxonomic coverage of the diatoms found in studied material is given in Table 2. The diatom species list with their ecological preferences, distribution and occurrence is given in Table 3.
Table 2.
Taxonomic coverage of diatoms from studied samples.
| Orders | Families | Genera | Total taxa | Total species |
| Achnanthales | 3 | 4 | 10 | 8 |
| Bacillariales | 1 | 3 | 13 | 13 |
| Bacillariophyta ordo incertae sedis | 1 | 1 | 1 | 1 |
| Cymbellales | 2 | 7 | 14 | 12 |
| Fragilariales | 3 | 3 | 3 | 3 |
| Licmophorales | 1 | 1 | 1 | |
| Mastogloiales | 1 | 1 | 5 | 2 |
| Naviculales | 10 | 13 | 28 | 22 |
| Rhabdonematales | 1 | 2 | 2 | 2 |
| Rhopalodiales | 1 | 1 | 1 | 1 |
| Surirellales | 1 | 1 | 3 | 2 |
| Thalassiophysales | 1 | 2 | 7 | 7 |
Table 3.
List of diatom species found in samples with notes on their ecology, distribution and occurrence (number of samples). Data on ecology and distribution are given according to Kulikovskiy et al. 2016, Cantonati et al. (2017), and Guiry and Guiry 2022 .
| Taxa | Abbreviation for taxa | Habitat | Distribution | Saprobility | Water chemistry | Accuracy |
| Achnanthesbrevipesvar.brevipes C.Agardh | ACHBRE | Brackish, Marine | Widely distributed | 1 | ||
| Achnanthes sp. | ACHSP | 1 | ||||
| Achnanthidiumminutissimum (Kützing) Czarnecki | ACHNMIN | Freshwater | Cosmopolitan | 8 | ||
| Achnanthidium sp. | ACHNSP | 1 | ||||
| Achnanthidiumstraubianum (Lange-Bertalot) Lange-Bertalot | ACHNSTR | Freshwater | Arctic-alpine | Mesotrophic, Eutrophic | Calcium-bicarbonate rich | 5 |
| Amphorainariensis Krammer | AMINA | Freshwater | Widely distributed | Oligotrophic, Mesotrophic | 4 | |
| Amphoraindistincta Levkov | AMINDI | Freshwater | Widely distributed | Oligotrophic | 6 | |
| Amphoraovalis (Kützing) Kützing s.l. | AMOV | Freshwater | Cosmopolitan | Oligotrophic, Mesotrophic, Eutrophic | 1 | |
| Amphorapediculus (Kützing) Grunow in A.W.F.Schmidt | AMPED | Freshwater, Brackish | Widely distributed | Oligotrophic | 3 | |
| Brachysiraaponina Kützing | BRACH | Marine, Brackish | Widely distributed | 3 | ||
| Caloneiscf.vasileyevae Lange-Bertalot, Genkal & Vekhov | CALVAS | Freshwater | Holarctic | 4 | ||
| Cocconeiseuglypta Ehrenberg | COCCEU | Freshwater, Brackish | Cosmopolitan | Mesotrophic, Eutrophic | Alkaline | 3 |
| Cocconeislineata Ehrenberg | COCCLIN | Freshwater, Brackish | Cosmopolitan | Mesotrophic, Eutrophic | Alkaline | 9 |
| Cocconeispediculus Ehrenberg | COCCPED | Freshwater | Cosmopolitan | Mesotrophic, Eutrophic | Alkaline | 1 |
| Cocconeisplacentula Ehrenberg s.l. | COCCPLAT | Freshwater, Brackish | Cosmopolitan | Oligotrophic, Mesotrophic, Eutrophic | 1 | |
| Craticulaaccomoda (Hustedt) D.G.Mann in Round, R.M.Crawford & D.G.Mann | CRATACC | Freshwater | Cosmopolitan | Eutrophic, Polluted water | 1 | |
| Craticulacf.buderi (Grunov ex Van Heurck) D.G.Mann. | CRATBUD | Freshwater, Brackish | Widely distributed | 1 | ||
| Craticuladissociata (E.Reichardt) E.Reichardt | CRATDISS | Freshwater | Holarctic | Eutrophic | 1 | |
| Craticulamolestiformis (Hustedt) Mayama | CRATMOL | Freshwater | Cosmopolitan | Eutrophic, Polluted water | 1 | |
| Ctenophora sp. | CTENSP | 1 | ||||
| Cymbellaaffinis Kützing | CYMAFF | Freshwater | Widely distributed, Alpine | Oligotrophic, Mesotrophic | Сalcium-bicarbonate rich | 3 |
| Cymbellahantzschiana Krammer | CYMHANTZ | Freshwater | Widely distributed | Oligotrophic, Mesotrophic | 3 | |
| Cymbopleura sp. | CYMSP | 1 | ||||
| Diatomatenuis C.Agardh | DIATTEN | Freshwater, Brackish | Cosmopolitan | 2 | ||
| Diploneiscf.carloswetzelii Lange-Bertalot & Fuhrmann | DIPCAR | Freshwater | 1 | |||
| Diploneiskrammeri Lange-Bertalot & E.Reichardt | DIPKRAM | Freshwater | Arctic-Alpine | Oligotrophic, Mesotrophic | Alkaline, Сalcium-bicarbonate rich | 2 |
| Diploneisoculata (Brébisson) Cleve | DIPOCU | Freshwater, Brackish | Cosmopolitan | Oligotrophic, Mesotrophic | Сalcium-bicarbonate rich | 1 |
| Encyonopsismicrocephala (Grunow) Krammer | ENCYMIC | Freshwater | Cosmopolitan | Oligotrophic, Mesotrophic | Сalcium-bicarbonate rich | 4 |
| Encyonopsissubminuta Krammer & E.Reichardt in Krammer | ENCYSUBM | Freshwater | Holarctic | Oligotrophic, Mesotrophic | Сalcium-bicarbonate rich | 1 |
| Fallaciacf.subhamulata (Grunow) D.G.Mann in Round, R.M.Crawford & D.G.Mann | ENCYSUBH | Freshwater | Holarctic | Oligotrophic, Mesotrophic | Alkaline | 2 |
| Fragilariformabicapitata (A.Mayer) D.M.Williams & Round | FRAGBIC | Freshwater | Holarctic | Oligotrophic, Mesotrophic, Eutrophic | Acidic, Siliceous | 2 |
| Frustuliavulgaris (Thwaites) De Toni | FRUSTV | Freshwater | Cosmopolitan | Mesotrophic, Eutrophic | 2 | |
| Geissleria sp. | GEISSP | Freshwater | 1 | |||
| Gomphonemaangustum C.Agardh | GOMANG | Freshwater | Cosmopolitan | Calcium-bicarbonate rich | 1 | |
| Gomphonemapumilumvar.rigidum E.Reichardt & Lange-Bertalot | GOMPUM | Freshwater | Cosmopolitan | Oligotrophic, Mesotrophic | Calcium-bicarbonate rich | 9 |
| Gomphonemapygmaeum J.Kociolek & E.Stoermer | GOMPYG | Freshwater | Holarctic | 4 | ||
| Gomphonemamicropus Kützing | GOMMIC | Freshwater, Brackish | Cosmopolitan | Oligotrophic, Mesotrophic | Alkaline | 4 |
| Gomphonemaparvulum (Kützing) Kützing s.l. | GOMPAR | Freshwater | Cosmopolitan | Mesotrophic, Eutrophic | Alkaline | 6 |
| Gomphonemasubclavatum (Grunow) Grunow | GOMSUB | Freshwater | Oligotrophic | 2 | ||
| Halamphorabicapitata (M.H.Hohn & J.Hellerman) J.G.Stepanek & Kociolek | HALABI | Holarctic | 4 | |||
| Halamphoracoffeiformis (C.Agardh) Mereschkowsky | HALACOFFE | Brackish | Cosmopolitan | 1 | ||
| Halamphoramontana (Krasske) Levkov | HALAMON | Freshwater | Cosmopolitan | Oligotrophic, Mesotrophic | Alkaline | 4 |
| Hantzschiaamphioxys (Ehrenberg) Grunow in Cleve & Grunow | HANTZAM | Freshwater | Cosmopolitan | Mesotrophic, Eutrophic | 3 | |
| Hantzschiaabundans Lange-Bertalot | HANTZAB | Freshwater | Cosmopolitan | Mesotrophic, Eutrophic | 1 | |
| Humidophilacontenta (Grunow) R.L.Lowe & al. | HUMCON | Freshwater, Aerophilic | Cosmopolitan | 3 | ||
| Luticolaacidoclinata Lange-Bertalot in Lange-Bertalot & Metzeltin | LUTAC | Freshwater, Aerophilic | Holarctic | Oligotrophic | Weakly acidic | 1 |
| Luticolacf.ventricosa (Kützing) D.G.Mann in Round, R.M.Crawford & D.G.Mann | LUTVEN | Freshwater, Aerophilic | Cosmopolitan | 1 | ||
| Luticolamutica (Kützing) D.G.Mann in Round, R.M.Crawford & D.G.Mann | LUTMUT | Freshwater, Brackish, Aerophilic | Cosmopolitan | 2 | ||
| Luticolanivalis (Ehrenberg) D.G.Mann in Round, R.M.Crawford & D.G.Mann | LUTNIV | Freshwater, Aerophilic | Holarctic | Oligotrophic | 1 | |
| Mastogloialanceolata Thwaites ex W. Smith | MASTL | Brackish,Marine | 2 | |||
| Mastogloiapusillavar.pusilla Grunow | MASTP | Brackish, Marine | 1 | |||
| Mastogloia sp.1 | MAST1 | Brackish, Marine | 1 | |||
| Mastogloia sp.2 | MAST2 | Brackish, Marine | 1 | |||
| Mastogloia sp.3 | MAST3 | Brackish, Marine | 2 | |||
| Meridioncircularevar.constrictum (Ralfs) Van Heurck | MERCIR | Freshwater | Holarctic | Oligotrophic, Mesotrophic | 2 | |
| Naviculaantonii Lange-Bertalot | NAVANT | Freshwater | 7 | |||
| Naviculablazencicae Z.Levkov & S.Krstic | NAVBLA | Freshwater | Alpine | 2 | ||
| Naviculacincta (Ehrenb.) Ralfs in A.Pritch. | NAVCINC | 1 | ||||
| Naviculacryptotenella | NAVCRY | 2 | ||||
| Navicula sp. | NAVSP | 4 | ||||
| Naviculatripunctata (O.F.Müller) Bory in Bory de Saint-Vincent | NAVTRI | Freshwater | Cosmopolitan | Eutrophic | 3 | |
| Naviculavulpina Kützing | NAVVUL | Freshwater | Cosmopolitan | Oligotrophic, Mesotrophic | Сalcium-bicarbonate rich | 1 |
| Navicymbulapussila (Grunow) Krammer | NAVYPUS | Brackish | Cosmopolitan | Сalcium-bicarbonate rich | 1 | |
| Neidiomorphabinodiformis (Krammer) M.Cantonati, Lange-Bertalot & N.Angeli | NEIDBI | Freshwater | Holarctic | Oligotrophic | 1 | |
| Nitzschiaclausii Hantzsch | NITZCLAUS | Freshwater, Brackish | Cosmopolitan | Mesotrophic | 1 | |
| Nitzschiadenticula Grunow | NITZDEN | Freshwater | Widely distributed | Oligotrophic, Mesotrophic | Сalcium-bicarbonate rich | 4 |
| Nitzschialinearis W.Smith | NITZLIN | Freshwater | Holarctic | Eutrophic | Alkaline | 4 |
| Nitzschiaschwabei Krasske ex Lange-Bertalot | NITZSCH | Brackish | Holarctic | 4 | ||
| Nitzschiatenuis W.Smith | NITZTE | Freshwater | Holarctic | Eutrophic | 1 | |
| Nitzschiathermaloides Hustedt | NITZTHE | Marine, Brackish | Holarctic | 2 | ||
| Nitzschiatubicola Grunow in Cleve & Grunow | NITZTU | Marine, Brackish | Cosmopolitan | 5 | ||
| Nitzschiavaldestriata Aleem & Hustedt | NITZVA | Freshwater, Brackish | Widely distributed | 1 | ||
| Pinnulariabertrandiivar.angustefasciata Krammer | PINNBET | Freshwater | Holarctic | 1 | ||
| Pinnulariaborealisvar.scalaris (Ehrenberg) Rabenhorst | PINNBOR | Freshwater | Widely distributed | Siliceous | 1 | |
| Planothidiumfrequentissimum (Lange-Bertalot) Lange-Bertalot | PLANFRE | Freshwater | Cosmopolitan | Oligotrophic, Mesotrophic | Alkaline | 11 |
| Playaensiscitrus (Krasske) E.Reichardt | PLAYCI | Freshwater | Widely distributed | 1 | ||
| Pleurosigmaelongatum W.Smith | PLEU | 1 | ||||
| Pseudostaurosirabrevistriata (Grunow) D.M.Williams & Round | PSEUSBRE | Freshwater, Brackish | Cosmopolitan | Oligotrophic, Mesotrophic, Eutrophic | Calcium-bicarbonate rich | 1 |
| Reimeriauniseriata S.E.Sala, J.M.Guerrero & M.E.Ferrario | REIMUN | Freshwater | Widely distributed | 4 | ||
| Rhopalodiagibba (Ehrenberg) O.Müller | RHOGI | Freshwater | Cosmopolitan | Oligotrophic, Mesotrophic, Eutrophic | Alkaline | 1 |
| Sellaphora sp. | SELLSP | 1 | ||||
| Stauroformaexiguiformis (Lange-Bertalot) R.J.Flower, V.J.Jones & Round | STAUREXI | Freshwater | Cosmopolitan | Eutrophic | Acidic | 1 |
| Surirellaangusta Kützing | SURAN | Freshwater | Widely distributed | Mesotrophic, Eutrophic | 1 | |
| Surirellaovalis Brébisson | SUROV | Brackish, Marine | Cosmopolitan | 1 | ||
| Surirella sp. | SURSP | Brackish, Marine | 1 | |||
| Tryblionellaangustata W.Smith | TRYAN | Freshwater, Brackish, Marine | Cosmopolitan | 2 | ||
| Tryblionellaapiculata W.Gregory | TRYAP | Freshwater, Brackish, Marine | Cosmopolitan | Oligotrophic, Mesotrophic | 2 | |
| Tryblionellahungarica (Grunow) Frenguelli | TRYHUN | Brackish | Cosmopolitan | Mesotrophic | 5 |
Temporal coverage
Notes
July 12-20, 2022
Usage licence
Usage licence
Creative Commons Public Domain Waiver (CC-Zero)
Data resources
Data package title
Diatoms of Utrish State Nature Reserve, Abrau Peninsula (Russia)
Resource link
https://www.gbif.org/occurrence/download?dataset_key=021f55ef-ec0c-427e-9ba5-bbfa0778bd64
Alternative identifiers
Number of data sets
1
Data set 1.
Data set name
Diatoms of Utrish State Nature Reserve, Abrau Peninsula (Russia)
Data format
Darwin Core Archive
Data format version
1.1 published on 2022-06-20
Description
This dataset presents the first data on the distribution of freshwater and brackish diatoms on Abrau Peninsula and especially in the territory of the Utrish State Nature Reserve. The data in this occurrence resource have been published as a Darwin Core Archive (DwC-A), which is a standardised format for sharing biodiversity data as a set of one or more data tables. The core data table contains 215 occurrences. This IPT archives the data and, thus, serves as the data repository.
Data set 1.
| Column label | Column description |
|---|---|
| id | The ID of the record. |
| type | The nature of the resource. |
| basisOfRecord | The specific nature of the data record. |
| occurrenceID | Identifier of the record, coded as a global unique identifier. |
| eventID | Identifier of the event, unique for the dataset. |
| eventDate | Time interval when the event occurred. |
| country | Country of the sampling site. |
| countryCode | Code of the country where the event occurred. |
| LocationID | Identifier of sampling location for this dataset. |
| samplingProtocol | Description of sample collection method. |
| locationRemarks | Notes about the features of sampling site. |
| decimalLatitude | The geographic latitude of the sampling site. |
| decimalLongitude | The geographic longitude of the sampling site. |
| geodeticDatum | The spatial reference system upon which the geographic coordinates are based. |
| coordinateUncertaintyInMetres | The indicator for the accuracy of the coordinate location in metres, described as the radius of a circle around the stated point location. |
| recordedBy | A list (concatenated and separated) of names of people responsible for collecting material and recording the original Occurrence. |
| identifiedBy | A list (concatenated and separated) of names of people who assigned the Taxon to the subject. |
| taxonID | The identifier for the set of taxon information (data associated with the Taxon class). Specific identifier to the dataset. |
| scientificName | The name with authorship applied on the first identification of the specimen. |
| acceptedNameUsage | The specimen accepted name, with authorship. |
| kingdom | Kingdom name. |
| phylum | Phylum name. |
| class | Class name. |
| order | Order name. |
| family | Family name |
| genus | Genus name. |
| specificEpithet | The name of the first or species epithet of the scientificName. |
| infraspecificEpithet | The name of the lowest or terminal infraspecific epithet of the scientificName, excluding any rank designation. |
| taxonRank | The taxonomic rank of the most specific name in the scientificName. |
| scientificNameAuthorship | The authorship information for the scientificName. |
| identificationQualifier | Contains commentaries about taxon identification (marks sp., sensu lato etc.) |
Additional information
Diatom diversity and occurrence
This study presents 215 diatom (Bacillariophyta) occurrences in 67 sites on the Abrau Peninsula, belonging to 88 different infrageneric taxa from 39 genera, 25 families, 12 orders and one class (Table 2). Eleven of the 88 taxa have been identified only to genus level. No diatoms were found in 30 out of 67 samples. The families with the highest number of occurrences (> 10%) were Bacillariaceae (35; 16.3%), Gomphonemataceae (30; 14%), Naviculaceae (24; 11.2%), Achnanthidiaceae (24; 11.2%) and Catenulaceae (23; 10.7%). These families also were with the highest number of taxa: Bacillariaceae (13), Naviculaceae (8), Catenulaceae (7) and Gomphonemataceae (7), except for Achnanthidiaceae with three taxa. Additionally, the family Cymbellaceae was represented with a high number of taxa (7) despite the low occurrence rate (only 6.5%). The families with lower occurrences (< 3) were Bacillariophyceae insertae sedis, Neidiaceae, Pleurosigmataceae, Rhopalodiaceae, Staurosiraceae and Ulnariaceae (1; 0.5%) and Amphipleuraceae, Brachysiraceae, Fragilarialceae and Pinnulariaceae (2; 1%). All these families are families with the smallest number of diatom taxa: one in all, except for Pinnulariaceae with two taxa. The genera with the highest number of occurrences were Gomphonema (26), Nitzschia (22), Navicula (20), Amphora (14), Cocconeis (14), Planothidium (11) and Achnanthes (10). Thirty-six genera had less than five occurrences.
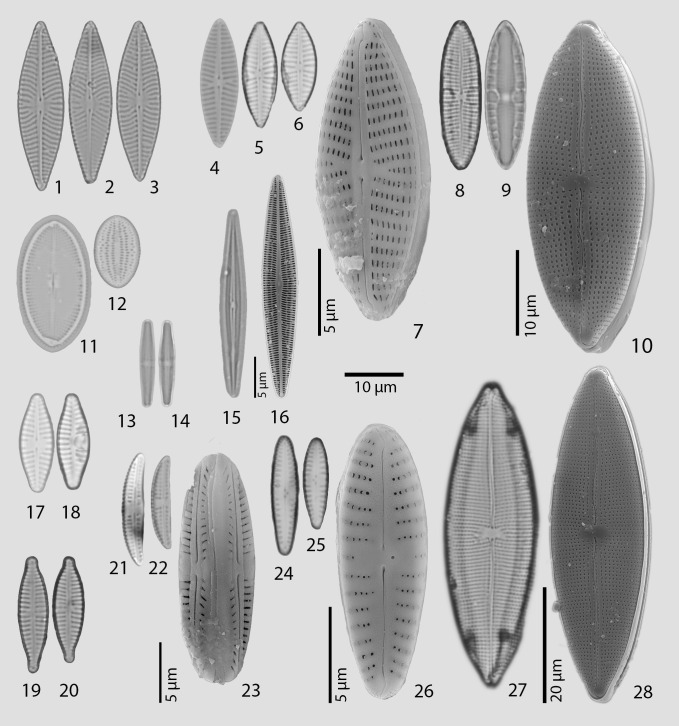
The most common species were Planothidiumfrequentissimum (11 samples), Cocconeisplacentula (9 samples), Gomphonemapumilumvar.rigidum (9 samples), Achnanthidiumminutissimum (8 samples), Naviculaantonii (7 samples), Amphorainariensis (6 samples) and Gomphonemaparvulum (6 samples) (Fig. 2).
Figure 2.
LM and SEM images of the most common and some other diatoms findings on Abrau Peninsula: 1-3 Naviculablazencicae; 4-7 – Naviculaantonii; 8-10 – Mastogloia sp.2; 11, 12 – Cocconeisplacentula s.l.; 13, 14 – Achnanthidiumminutissimum; 15, 16 – Brachysiraaponina; 17, 18 – Planothidiumfrequentissimum; 19, 20 – Gomphonemaparvulum s.l.; 21-23 – Amphorainariensis; 24-26 – Gomphonemapumilumvar.rigidum; 27, 28 – Mastogloialanceolata. Scale bar = 10 µm and applies for all images, except SEM pictures 7, 10, 16, 23, 26, 28. LM – light microscopy, SEM – scanning electron microscopy.
The richiest sites in number of taxa were UT-2021-67 (20 taxa), UT-2021-20 (freshwater puddle, 16 taxa), UT-2021-28 (freshwater waterbody sediment, 14 taxa), UT-2021-25 (soil sample of puddle, 11 taxa), UT-2021-54 (freshwater waterbody with antropogenic impact, 11 taxa) and UT-2021-66 (coastline brackish lagoon, 11 taxa).
The UT-2021-67 site is a quite unique sampling site, where freshwater from the Zhemchuzhnyj Waterfall stream mixes with seawater and rocks with water from the stream being covered with moss. From this site, we sampled both rock scrap and moss squeeze and found the highest diversity of diatom taxa (Fig. 3).
Figure 3.

The view on Zhemchuzhnyj Waterfall, the hotspot of diatom diversity in Utrish Nature State Reserve. Rocks with algal film (left), stream water falls on stones covered with moss (right).
Light microscope (LM) and scanning electron microscope (SEM) images of the most frequently occurring species and some others are represented in Fig. 2.
Naviculablazencicae Levkov (Fig. 2, 1-3) was originally described by Levkov and colleagues (Levkov et al. 2007) from North Macedonia and, until now, has been known as the endemic of Lake Ohrid. In the study, it was found in two sampling locations represented by two freshwater temporal waterbodies (UT-2021-05 and UT-2021-09).
One of Mastogloia species, referred to as Mastogloia sp.2 (Fig. 2, 8-10), held a unique combination of morphometric characteristics (paratecta and raphe structural features) which we were unable to identify as a known species. Probably the same species was also found by A. Kaleli (Kaleli 2019) in similar habitats of coastline brackish waterbodies. According to the published illustration (see Mastogloia sp.1 in Kaleli 2019), the valves collected by A. Kaleli are quite similar to Mastogloia sp.2 in the current study and supposedly belonged to the same species, although it has also not been identified and needs additional verification. Beside that, two other Mastogloia species (Mastogloia sp.1 and Mastogloia sp.3) which were found in the current research are also likely to be new species and require further study.
Data analysis
The cluster analysis revealed a considerable modulation effect of habitat type on the floristic composition of diatom communities of the Abrau Peninsula (Fig. 4). Diatom communities collected from streams, regardless of the sampling method, were significantly dissimilar to the communities collected from waterbodies and communities collected from scraps and soils of puddles (p < 0.05). In turn, communities collected from waterbodies were combined with communities sampled from scraps and soils of puddles and formed significant clusters (p < 0.01).
Figure 4.
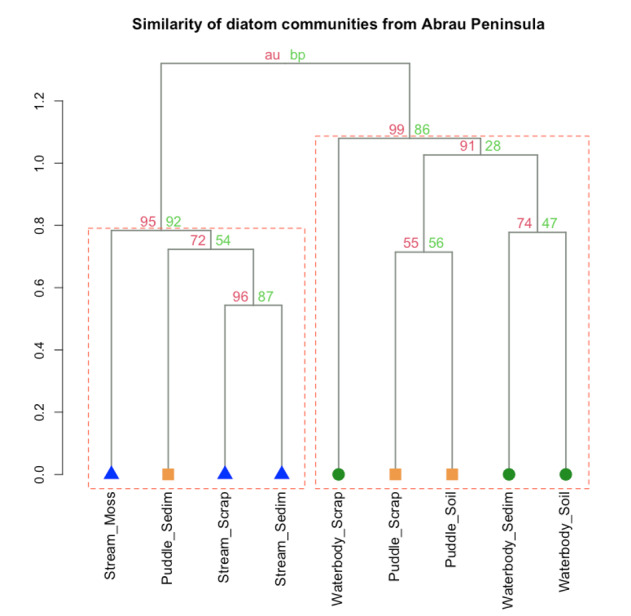
Hierarchical cluster analysis using the presence/absence matrix of diatom communities collected by different sampling methods from various biotopes of Abrau Peninsula (binary method, Ward.D2 clustering). Different symbols at the nodes of clusters illustrates biotope types: triangles – streams, squares – puddles (temporary waterbodies) and circles – permanent waterbodies. Right part of labels illustrates the type of sampling method: Moss – moss squeeze, Sedim – sediment from the bottom of waterbody or stream, Scrap – scrapping from the stones and Soil – soil in the littoral zone of waterbodies. Values at branches are approximately unbiased p-values (red colour) and bootstrap probabilities (green colour) in percentage. Clusters that are framed by red dashed line are supported by a p-value < 0.05.
The results of cluster analysis suggest that the floristic composition of diatom communities from streams is quite different from that in small ephemeral water objects (puddles) and stagnant water bodies (such as ponds, lakes and lagoons). Although some of the species living in streams might sometimes be present in puddles (see Fig. 4), the floristic composition of streams is most likely conservative and does not mix with other types of water objects.
The ecological conditions of marine and brackish waterbodies were obviously antagonistic to freshwater, thus the PCA by factor 1 clearly and predictably separated the frequency of freshwater and marine and brackish species (Fig. 5). The frequency of occurrence of oligotrophic and mesotrophic species strongly and positively correlated with freshwater streams of Abrau Peninsula. Only here were collected freshwater species such as Amphorainariensis, A.pediculus, Cymbellaaffinis, C. hantzschiana, Encyonopsismicrocephala, Gomphonemapygmaeum, Naviculatripunctata and Reimeriauniseriata. The majority of collected eutraphentic species tended to be from freshwater puddles (e.g. Craticuladissociata, C.molestiformis, Gomphonemaparvulum) and, to a lesser extent, freshwater waterbodies of the study area. The latter were positively correlated with the occurrence of species that prefer polluted water and, conversely, were antagonistic to the habitats of oligotrophic species and stream habitats. This may be due to the location of this type of waterbodies mainly near recreational areas and settlements. Aerophilic species did not show any strong correlation with the studied habitat types.
Figure 5.
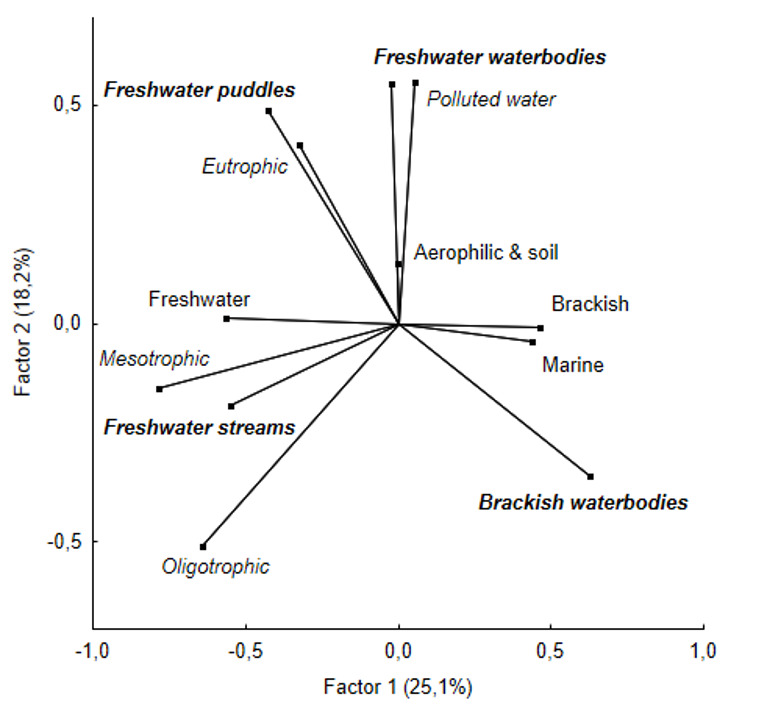
Relationship between frequency of species occurrences with different separability (italic, active variables) and environment preferences (normal, supplementary variables) and in various investigated habitats (bold and italic, active variables) determined using the principal component analysis (PCA).
Acknowledgements
This work was supported by the Russian Science Foundation, project #19-74-10104. The identification of the diatoms was supported by RFBR, project #20-34-90011. The study was conducted using the Joint Usage Center «Instrumental methods in ecology» at the IEE RAS. The authors are grateful to O.N. Bykhalova, Deputy Director of Research at Utrish State Nature Reserve for the opportunity to work in the Reserve and for her help with fieldwork and samples collection. We also want to thank our friend, Ekaterina Petyukova, for help during the field compaign and collection of some samples. We would like to thank Dmitry A. Chudaev (Lomonosov Moscow State University, Moscow, Russia) for valuable comments on diatom identification. We thank all reviewers and redactors for their comments and suggestions that helped us significantly improve manuscript.
Author contributions
AN and DK worked out the concept of the study. AN and AP carried out sampling collection in July 2021 on the Abrau Peninsula and Utrish Nature State Reserve. AN prepared samples and permanent microscopic slides and identified diatoms. RS and DK performed statistical analyses of obtained data. AN, RS, AP and DK worked on preparation of the Darwin Core archive dataset and text of the manuscript. All authors agree with the final version of the paper.
References
- Armbrust E. Virginia. The life of diatoms in the world's oceans. Nature. 2009;459(7244):185–192. doi: 10.1038/nature08057. [DOI] [PubMed] [Google Scholar]
- Battarbee R. W., Jones V. J., Flower R. J., Cameron N. G., Bennion H., Carvalho L., Juggins S. In: Tracking environmental change using lake sediments. Terrestrial, Algal, and Siliceous Indicators. 2nd ed. Smol J. P., Birks H. J. B., Last W. M., Bradley R. S., Alverson K., editors. Vol. 3. Springer; Netherland: 2002. Diatoms.371. [DOI] [Google Scholar]
- Benoiston Anne-Sophie, Ibarbalz Federico M., Bittner Lucie, Guidi Lionel, Jahn Oliver, Dutkiewicz Stephanie, Bowler Chris. The evolution of diatoms and their biogeochemical functions. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1728) doi: 10.1098/rstb.2016.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocharnikov M. V., Petrushina M. N., Suslova E. G. Spatial Organization of the Vegetation and Landscapes of the Sub-Mediterranean Forest and Woodland Belt on the Abrau Peninsula (Northwestern Caucasus) Arid Ecosystems. 2020;9(4):237–247. doi: 10.1134/s2079096119040024. [DOI] [Google Scholar]
- Cantonati M, Kelly M. G., Lange-Bertalot H., editors. Freshwater Benthic Diatoms of Central Europe. Over 800 Common Species Used in Ecological Assessment. Koeltz Botanical Books; 2017. 942 [Google Scholar]
- CantyMedia weatherbase. http://www.weatherbase.com. [2022-03-21T00:00:00+02:00]. http://www.weatherbase.com
- Chen Deliang, Chen Hans Weiteng. Using the Köppen classification to quantify climate variation and change: An example for 1901–2010. Environmental Development. 2013;6:69–79. doi: 10.1016/j.envdev.2013.03.007. [DOI] [Google Scholar]
- Davidovich N. A., Polyakova S. L. Assessment of the species diversity of the genus Pseudo-nitzschia H. Peragallo, 1900 (Bacillariophyta) in plankton near Karadag using multidimensional statistical analysis. Russian Journal of Marine Biology. 2022;47(6):515–518. doi: 10.1134/s1063074021060031. [DOI] [Google Scholar]
- Falasco Elisa, Bona Francesca. Diatom community biodiversity in an Alpine protected area: a study in the Maritime Alps Natural Park. Journal of Limnology. 2011;70(2) doi: 10.4081/jlimnol.2011.157. [DOI] [Google Scholar]
- Falasco Elisa, Ector Luc, Ciaccio Elisabetta, Hoffmann Lucien, Bona Francesca. Alpine freshwater ecosystems in a protected area: a source of diatom diversity. Hydrobiologia. 2012;695(1):233–251. doi: 10.1007/s10750-012-1114-0. [DOI] [Google Scholar]
- Foets Jasper, Wetzel Carlos E., Teuling Adriaan J., Pfister Laurent. Temporal and spatial variability of terrestrial diatoms at the catchment scale: controls on productivity and comparison with other soil algae. PeerJ. 2020;8 doi: 10.7717/peerj.9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili Tal. dendextend: an R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics. 2015;31(22):3718–3720. doi: 10.1093/bioinformatics/btv428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiry M. D., Guiry G. AlgaeBase: World-Wide Electronic Publication. http://www.algaebase.org. [2022-03-15T00:00:00+02:00]. http://www.algaebase.org
- Kaleli Aydın. Benthic diatom composition of Iztuzu Coastal Lake, Dalyan (Aegean Sea, Turkey) Aquatic Sciences and Engineering. 2019;34(4):122–130. doi: 10.26650/ase2019575987. [DOI] [Google Scholar]
- Kelly M. G., Cazaubon A., Coring E., Dell'Uomo A., Ector L., Goldsmith B., Guasch H., Hürlimann J., Jarlman A., Kawecka B., Kwandrans J., Laugaste R., Lindstrøm E. -A., Leitao M., Marvan P., Padisák J., Pipp E., Prygiel J., Rott E., Sabater S., van Dam H., Vizinet J. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. Journal of Applied Phycology. 1998;10(2):215–224. doi: 10.1023/a:1008033201227. [DOI] [Google Scholar]
- Kelly M. G., Adams C., Graves A. C., Jamieson J., Krokowski J., Lycett E. B., Murray-Bligh J., Prichard S., Wilkins C. The Trophic Diatom Index: A User's Manual. Revised version. Environment Agency; Bristol: 2001. 135. [Google Scholar]
- Kovaleva G. V. Mikrovodorosli ozera Abrau (Krasnodarskij Kraj) [Microalgae Of The Abrau Lake (Krasnodar Region)] Botanicheskij zhurnal. 2005;90(5):681–695. Russian. [Google Scholar]
- Kovaleva G. V. K flore diatomovyh vodoroslej Ust'-Manychskogo vodohranilishcha (Zapadenskij i Shahaevskij limany)[Diatoms flora of he Ust-Manych water storage reservoir (Shakhayevsky and Zapadensky Limans)] Trudy Yuzhnogo Nauchnogo Centra Rossijskoj Akademii Nauk. 2018;7:69–103. Russian. [Google Scholar]
- Kulikovskiy M. S., Glushchenko A. M., Genkal S. I., Kuznetsova I. V. Opredelitel' diatomovych vodoroslei Rossii. Filigran; Yaroslavl: 2016. 803. Russian. [Google Scholar]
- Lange-Bertalot H. Navicula sensu stricto. 10 genera separated from Navicula sensu lato. Frustulia. Diatoms of Europe, 2. Gantner Verlag; 2001. 526. [Google Scholar]
- Levkov Z., Krstic S., Metzeltin D., Nakov T. Diatoms of lakes Prespa and Ohrid. About 500 taxa from ancient lake system. Iconographia Diatomologica, 16. Ruggell; 2007. 607. [Google Scholar]
- Neplyukhina A, Saifutdinov R, Paskhina A, Korobushkin D. GBIF.org; 2022. [2022-04-22T00:00:00+03:00]. Diatoms of Utrish State Nature Reserve, Abrau Peninsula (Russia). A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences. Occurrence dataset. 01. [DOI] [Google Scholar]
- Nevrova E. L. Ocenka raznoobraziya diatomovyh bentosa (Bacillariophyta) u poberezh'ya Karadaga (Chyornoe more, Krym) [Evaluation of benthic diatoms diversity (Bacillariophyta) near Karadag shore] In: Gaevskaya A. V., Morozova A. L., editors. 100 Let Karadagskoj Nauchnoj Stancii Im. T.I. Vyazemskogo. Н.Орiанда; Simferopol: 2015. Russian. [Google Scholar]
- Ogureeva G. N., Leonova N. B., Emelyanova L. G., Buldakova E. V., Kadetov N. G., Arkhipova M. V., Miklyaeva I. M., Bocharnikov M. V., Dudov S. V., Ignatova I. A., Ignatova I. A., Muchnik E. E., Urbanavichus G. P., Danilenko A. K., Rumyantsev V. Y., Leontieva O. A., Romanov A. A., Konstantinov P. A. WWF Russia, Moscow; 2018. Map “The Biomes of Russia” (scale 1: 7 500 000) [Google Scholar]
- Olson David M., Dinerstein Eric, Wikramanayake Eric D., Burgess Neil D., Powell George V. N., Underwood Emma C., D'amico Jennifer A., Itoua Illanga, Strand Holly E., Morrison John C., Loucks Colby J., Allnutt Thomas F., Ricketts Taylor H., Kura Yumiko, Lamoreux John F., Wettengel Wesley W., Hedao Prashant, Kassem Kenneth R. Terrestrial Ecoregions of the World: A New Map of Life on Earth. BioScience. 2001;51(11) doi: 10.1641/0006-3568(2001)051[0933:teotwa]2.0.co;2. [DOI] [Google Scholar]
- Patrick R. Use of algae, especially diatoms, in the assessment of water quality. ASTM Special Technical Publications. 1973;528:76–95. [Google Scholar]
- Petrov A., Nevrova E. Database on Black Sea benthic diatoms (Bacillariophyta): its use for a comparative study of diversity pecularities under technogenic pollution impacts; Proceedings Ocean Biodiversity Informatics; International Conference on Marine Biodiversity Data Management; Hamburg, Germany. 29 November – 1 December, 2004; Paris: VLIZ Special Publication; 2007. 153-165 [Google Scholar]
- Polyakova Svetlana L., Davidovich Nickolai A. Abundance dynamics and size distribution of cells of the toxicogenic species from the genus Pseudo-nitzschia near the coast of Karadag (next to the biological station) Issues of modern algology (Voprosi sovremennoi algologii) 2019:64–68. doi: 10.33624/2311-0147-2019-2(20)-64-68. [DOI]
- Team R Core. Foundation for Statistical Computing, Vienna, Austria; 2021. R: A language and environment for statistical computing. [Google Scholar]
- Reid M. A., Tibby J. C., Penny D., Gell P. A. The use of diatoms to assess past and present water quality. Australian Journal of Ecology. 1995;20(1):57–64. doi: 10.1111/j.1442-9993.1995.tb00522.x. [DOI] [Google Scholar]
- Round F. E., Crawford R. M., Mann D. G. Diatoms: biology and morphology of the genera. Cambridge university press; Cambridge: 1990. 747. [Google Scholar]
- Ryabushko Larisa I., Balycheva Daria S., Ryabushko Vitaly I. Microphytobenthos diatoms of the Black Sea: Biodiversity and ecology. Ecologica Montenegrina. 2017;14:48–59. doi: 10.37828/em.2017.14.6. [DOI] [Google Scholar]
- Ryabushko Larisa I., Lishaev Denis N., Kovrigina Nelya P. Species diversity of epilithon diatoms and the quality of the waters of the Donuzlav Gulf ecosystem (Crimea, the Black Sea) Diversity. 2019;11(7) doi: 10.3390/d11070114. [DOI] [Google Scholar]
- Ryabushko Larisa, Miroshnichenko Ekaterina, Blaginina Anastasia, Shiroyan Armine, Lishaev Denis. Diatom and cyanobacteria communities on artificial polymer substrates in the Crimean coastal waters of the Black Sea. Marine Pollution Bulletin. 2021;169 doi: 10.1016/j.marpolbul.2021.112521. [DOI] [PubMed] [Google Scholar]
- Ryabushko L. I., Balycheva D. S., Pospelova N. V., Begun A. A. Diatomovie vodorosli (Bacillariophyta) microfitobentosa i fitoplanktona osobo ohranyaemih prirodnih akvatorii v pribrejie Chernogo i Yaponskogo morei [Diatoms (Bacillariophyta) of microphytobenthos and phytoplankton of specially protected natural water areas in Black and Japanese Seas] Biota i Sreda. 2018;4:5–24. Russian. [Google Scholar]
- Ryabushko L. I., Shiroyan A. G., Lishaev D. N. Diatomovye vodorosli epifitona makrofitov krymskogo pribrezh'ya Chyornogo morya [Diatoms of macrophyte epiphyton of the Crimean coast of the Black Sea] Trudy Karadagskoj nauchnoj stancii im. T.I. Vyazemskogo - prirodnogo zapovednika RAN. 2021:3–11. doi: 10.21072/eco.2021.15.01. Russian. [DOI]
- Sadchikov A. P. Metody izuchenia presnovodnogo fitoplanktona. Universitet i shkola; Moscow: 2003. 157. Russian. [Google Scholar]
- Seregin Alexey P., Suslova Elena G. Contribution to the vascular plant flora of the Utrish area, a relic sub-Mediterranean ecosystem of the Russian Black Sea Coast. Willdenowia. 2007;37(2) doi: 10.3372/wi.37.37207. [DOI] [Google Scholar]
- Smol J. P., Stoermer E. F., editors. The diatoms: applications for the environmental and earth sciences. Second edition. Cambridge University Press; 2010. 667. [DOI] [Google Scholar]
- Suzuki R., Shimodaira H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22(12):1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- Wickham H., François R., Henry L., Müller K. dplyr: A Grammar of Data Manipulation. R package. https://CRAN.R-project.org/package=dplyr. 2018 1.0.8.
- Yasakova O. N., Kolesnikov M. V. Sostoyanie fitoplanktonnogo soobshchestva akvatorii v rajone mysa Bol'shoj Utrish v oktyabre 2018 goda [The State Of The Phytoplankton Community In The Water Area In The Area Of Cape Bolshoi Utrish In October 2018] Nazemnye I Morskie Ekosistemy Poluostrova Abrau: Istoriya, Sostoyanie, Ohrana Nauchie trudi. 2021;5 Russian. [Google Scholar]