PURPOSE
Despite therapeutic advances in the treatment of ovarian cancer (OC), 5-year survival remains low, and patients eventually die from recurrent, chemotherapy-resistant disease. The National Cancer Gynecologic Cancer Steering Committee identified the integration of scientifically defined subgroups as a top strategic priority in clinical trial planning.
METHODS
A group of experts was convened to review the scientific literature in OC to identify validated predictive biomarkers that could inform patient selection and treatment stratification. Here, we report on these findings and their potential for use in future clinical trial design on the basis of hierarchal evidence grading.
RESULTS
The biomarkers were classified on the basis of mechanistic targeting, including DNA repair and replication stress, immunotherapy and tumor microenvironment, oncogenic signaling, and angiogenesis. Currently, BRCA mutations and homologous recombination deficiency to predict poly (ADP-ribose) polymerase inhibitor response are supported in OC by the highest level of evidence. Additional biomarkers of response to agents targeting the pathways above have been identified but require prospective validation.
CONCLUSION
Although a number of biomarkers of response to various agents in OC have been described in the literature, high-level evidence for the majority is lacking. This report highlights the unmet need for identification and validation of predictive biomarkers to guide therapy and future trial design in OC.
INTRODUCTION
Ovarian cancer (OC) is the eighth most commonly occurring cancer in women and the 18th most commonly occurring cancer worldwide.1 In the United States, there were an estimated 21,410 new cases diagnosed and 13,770 deaths from OC in 2021. Initial treatment requires expert multidisciplinary care, which typically involves primary debulking surgery or neoadjuvant chemotherapy (NACT).2 Despite therapeutic advances, the 5-year relative survival in the United States remains low (49.1%), and patients eventually die from recurrent, chemotherapy-resistant disease.3
CONTEXT
Key Objective
To evaluate the predictive value of existing and emerging biomarkers in ovarian cancer (OC) for use in future clinical trial design on the basis of hierarchical evidence grading.
Knowledge Generated
Multiple potential biomarkers have been identified, but few have been found to be predictive on the basis of high-level evidence showing treatment/outcome interaction. Currently, BRCA mutations and homologous recombination deficiency to predict poly (ADP-ribose) polymerase inhibitor response are supported in OC by the highest level of evidence. Mismatch repair deficiency and high tumor mutation burden are predictive of response to pembrolizumab irrespective of cancer type and may be relevant to rare OCs exhibiting these alterations. Additional biomarkers of response to agents targeting DNA repair and replication stress, immune response, oncogenic signaling, and angiogenesis have been identified but require prospective validation.
Relevance
This report highlights the unmet need for identification and validation of predictive biomarkers to guide therapy and future trial design in OC.
The US National Cancer Institute (NCI) Gynecologic Cancer Steering Committee (GCSC) identified targeting scientifically defined OC subgroups to advance precision therapy as a top strategic priority in clinical trial planning. On the basis of this input, the NCI convened an OC Clinical Trials Planning Meeting (OCCTPM) in February 2021. The objectives of the OCCTPM were to review the molecular and immunologic landscape in OC, to develop trials on the basis of validated biomarkers, and to design novel combination therapies to overcome drug resistance. Before the meeting, a group of experts were assembled to cull the scientific literature for data on validated discriminants to inform treatment-focused groups and to identify validated markers for patient selection and treatment stratification. Reports were generated on several key areas including antiangiogenic therapy, DNA damage repair, hormone-related pathways, immunotherapy, gene signatures, epigenetics, and oncogenic signaling. We summarize the key findings from these individual reports below and identify potential candidate approaches for clinical trial planning and design on the basis of hierarchal evidence grading.
METHODS
Predictive biomarkers differentially segregate expected benefit from a defined therapy and measure the likelihood of better or worse outcomes in response to a specific biomarker-targeted intervention. Predictive biomarkers are differentiated from prognostic biomarkers by the requirement for a statistically significant treatment outcome by biomarker interaction.4,5 This difference can be shown using ERBB2/HER2 amplification as an example of both a prognostic and predictive biomarker. HER2-amplified cancers have a worse overall prognosis compared with nonamplified cancers.6 Additionally, HER2-amplified cancers show increased responsiveness to HER2-targeted agents.7 Validated tests allowing the selection of patients with predictive biomarkers are known as companion diagnostics. These are defined by the US Food and Drug Administration (FDA) as medical devices that provide essential information for the safe and effective use of a corresponding drug or biological product.8
Biomarkers are further categorized depending on their use in clinical trial design and hypothesis testing. Integral biomarkers are inherent to study design and are used to determine trial eligibility or stratification or can be used as primary end points. Integrated biomarkers are incorporated prospectively into trials for hypothesis testing, often for prospective validation of their treatment interactive effect to allow promotion to use as integral biomarkers. Finally, exploratory biomarker testing can be planned for hypothesis generation leading eventually to implementation as integrated elements and then for integral use. Considerations for appropriate use of biomarkers in clinical trial design include a number of variables including assay performance characteristics, ease of implementation, costs, and strength of available evidence to support their use.
Several standardized guidelines for reporting the strength of evidence supporting the use of a given biomarker have been proposed. ASCO Tumor Markers Guidelines Committee recommended five Levels of Evidence (LOEs) to determine the clinical utility of a tumor marker.9 Initially published in 1996, this LOE scale has been widely used and includes domains involving patients, specimens, assays, and statistical analyses. More recently, the LOE scale was revised by Simon and al. to provide more precise definitions for the types of studies that might be used to analyze the clinical utility of a given prognostic or predictive biomarker.10 Level I evidence consists of a prospective trial designed to address the tumor marker in question or a prospective trial not designed to address the tumor marker but accompanied by one or more validation studies with consistent results. The consistent results from these validation studies must be equally compelling and performed using the same assay or similar assays that clearly identified the same marker.7 Use of the estrogen receptor (ER) to predict endocrine therapy benefit in breast cancer is an example of a biomarker with supported by level 1 evidence, including both prospectively designed trials and multiple validation studies.11 Level II evidence includes prospective trials not designed to address the tumor marker without confirmatory validation studies or with supportive evidence from two or more prospective observational studies. Level III/IV evidence includes singular prospective observational and retrospective studies correlating the biomarker with an outcome. It is important to note that the LOE for a particular biomarker is specific to the tumor type, drug, and clinical setting in which it has been validated. These criteria, qualified by correlation to treatment outcome, have been used for the grading of LOE in this summary report.
RESULTS
Data supporting biomarkers with level I and II evidence (Table 1) and select biomarkers of unclear predictive value (level III/IV; Table 2) and those not found to be predictive of clinical benefit (Table 3) are discussed below. The biomarkers have been categorized on the basis of cancer-associated pathways targeted by specific drugs.
TABLE 1.
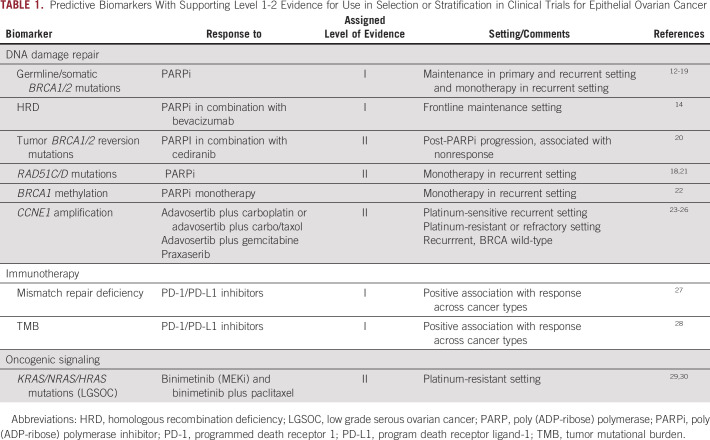
Predictive Biomarkers With Supporting Level 1-2 Evidence for Use in Selection or Stratification in Clinical Trials for Epithelial Ovarian Cancer
TABLE 2.
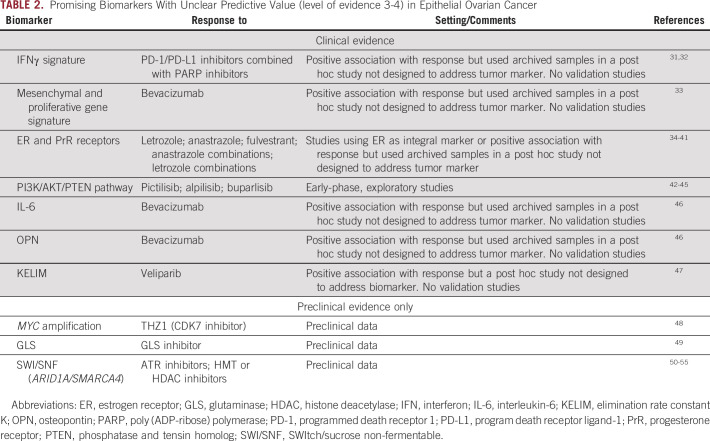
Promising Biomarkers With Unclear Predictive Value (level of evidence 3-4) in Epithelial Ovarian Cancer
TABLE 3.
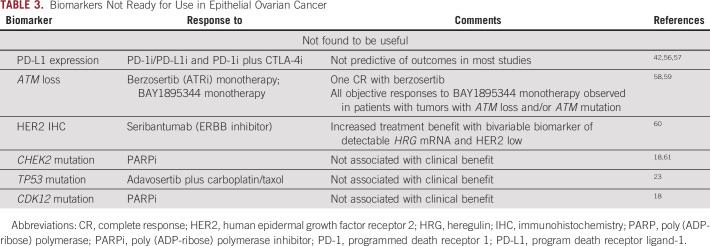
Biomarkers Not Ready for Use in Epithelial Ovarian Cancer
DNA REPAIR AND REPLICATION STRESS
Homologous recombination repair (HRR) is a pathway responsible for repair of double-stranded DNA breaks. High-grade serous OC (HGSOC) is characterized by chromosomal instability due to impaired HRR pathways, known as HR deficiency (HRD). This results in the inability of a cell to perform HRR, thus requiring the use of alternative, less reliable repair pathways.62 These generate patterns of chromosomal alterations referred to as genomic scars, which are permanent even in the event of HRR restoration.63-65 HRD in cancer cells has many underlying causes, of which the most prevalent in OC are somatic and germline mutations in BRCA1 or BRCA2 (BRCAm).66
BRCA Mutations
The presence of germline or somatic BRCAm has consistently correlated with benefit from poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi) in multiple studies, including maintenance after platinum response in both the primary12-14 and recurrent15-17 settings, and for treatment of recurrent disease.18,19 Many randomized clinical trials used BRCAm as criteria for eligibility or prospective stratification, including SOLO1 and SOLO2 (olaparib maintenance for frontline [ClinicalTrials.gov identifier: NCT01844986] and platinum-senstive [ClinicalTrials.gov identifier: NCT01874353] BRCAm OC), PRIMA (frontline niraparib maintenance; ClinicalTrials.gov identifier: NCT02655016), PAOLA-1 (frontline olaparib maintenance alone or with bevacizumab; ClinicalTrials.gov identifier: NCT02477644) NOVA (platinum-sensitive recurrent niraparib maintenance; ClinicalTrials.gov identifier: NCT01847274), and ARIEL4 (recurrent, platinum-resistant or partially platinum-sensitive; ClinicalTrials.gov identifier: NCT02855944). Trials including non-BRCAm cancers prospectively stratified on the basis of BRCAm status and preplanned a hierarchical statistical design to evaluate benefit across stratifications although they did not examine a treatment outcome by biomarker interaction. All demonstrated the greatest magnitude of benefit in patients with BRCAm cancers.
HRD Clinical Assays
Current HRD assays measure mutational profiles or genomic scars, which are a historical mark of previous HRD and do not provide dynamic measurements of real-time HRR function. Use of HRD as a predictive biomarker is complicated by this caveat. Multiple prospective randomized clinical trials have demonstrated statistically significant differential benefit of PARPi in patients with HRD cancers compared with those without HRD, athough none have formally tested the biomarker interaction.12,15,16 However, there is heterogeneity in the assays used to define HRD across clinical trials.
The Myriad Genetics myChoice CDx incorporates BRCA mutation sequencing with a genomic instability score that is a composite of three different measures of genomic instability: loss of heterozygosity (LOH), telomeric allelic imbalance and large-scale state transitions which have been shown to be associated with HRD and response to platinum agents.63,65,67,68 HRD is defined by this assay as BRCAm or BRCA wildtype with genomic instability score cutoff ≥ 42. The PRIMA and NOVA studies used the Myriad Genetics myChoice Cdx HRD for stratification of patients. Both BRCAm and the larger HRD population, which included BRCAm, had greater benefit than biomarker-negative cases.12,15 The Myriad Genetics myChoice HRD Plus assay was used in the PAOLA-1 trial to stratify between HRD and non-HRD or HR-proficient cancers (≥ 42 = HRD).14 Analysis of the PAOLA-1 results again showed greater benefit to olaparib for patients with BRCAm and HRD biomarker-positive cancers compared with biomarker-negative cancers when given in combination with bevacizumab compared with bevacizumab alone. Greater magnitude of benefit from treatment with niraparib in platinum-sensitive recurrent HRD OC was also shown in the Quadra study using the MyChoice assay.19 On the basis of these studies, Myriad Genetics myChoice CDx assay has been approved by the FDA as a companion diagnostic for therapy with olaparib in combination with bevacizumab in frontline maintenance and for single-agent niraparib therapy in the recurrent settting.
The Foundation Medicine NGS LOH assay (≥ 16% = LOH-high = HRD) was used for prospective patient stratification in ARIEL-3 (ClinicalTrials.gov identifier: NCT01968213). This assay measures the percentage of genomic LOH. Patients who had LOH high tumors had a greater magnitude of clinical benefit from rucaparib than those who did not.16 In ARIEL-2 (ClinicalTrials.gov identifier: NCT01891344), a higher magnitude of benefit was observed in patients with HRD tumors (≥ 14% = LOH-high) compared with the LOH low subgroup (0.62, P = .011).18
BRCA Reversion Mutations
Secondary somatic reversion mutations in BRCA1/2 restore an open reading frame and create a functional protein and, by restoring HRR, generate resistance to PARPi and platinum.69-73 The impact of reversion mutations on rucaparib response/resistance was examined in ARIEL2, a prospective phase II trial of rucaparib in recurrent HGSOC.18,74 In a retrospective analysis, cell-free DNA was sequenced from serial plasma samples collected from patients with BRCAm carcinomas receiving rucaparib in ARIEL2. BRCA reversion mutations were identified in 18% (2 of 11) of platinum-refractory and 13% (5 of 38) of platinum-resistant patients in pretreatment blood collected, compared with 2% (1 of 48) of patients with platinum-sensitive cancers (P = .049). Patients without BRCA reversion mutations detected in pretreatment cell-free DNA had significantly longer rucaparib progression-free survival (PFS) than those with reversion mutations (median, 9.0 v 1.8 months; hazard ratio, 0.12; P < .0001).
RAD51C and RAD51D Mutations and RAD51C Promoter Methylation
Additional genes and proteins are involved in the HRR pathway, including the RAD51 family. The impact of RAD51C and RAD51D mutations on response to rucaparib was also assessed in ARIEL2.18,75 Rucaparib was shown to be active in ovarian carcinoma with RAD51C or RAD51D mutations, with five partial responses (PsR) among seven evaluable patients with RAD51C/D mutations treated with rucaparib. The results from multivariable analysis also identified RAD51C/D mutation as a significant prognosticator of objective response rate (odds ratio [OR], 20.658; 95% CI, 1.865 to 228.889; P = .0136).75 However, because this was a single-arm trial, prognostic versus predictive characteristics of biomarkers cannot be separated. The role of RAD51C/D in PARPi sensitivity/resistance is also supported by the finding of RAD51C/D secondary somatic mutations that restore the open reading frame (ie, reversion mutations) in some OCs with acquired PARPi resistance.21 In a study of 12 patients with RAD51C promoter-methylated HGSOC supplemented with patient-derived xenograft models, methylation of RAD51C promoter has been also demonstrated to be associated with sensitivity to platinum and PARPi while loss of methylation even in a single gene copy was sufficient to confer PARP inhibitor resistance.76 Given the lack of prospective testing and validation studies, evidence for RAD51C mutation and promoter methylation status as biomarkers is considered level 3, but the data nevertheless highlight a strong rationale for inclusion of these biomarkers into prospective studies.
Homozygous BRCA1 Methylation
Methylation of the BRCA1 promoter can lead to functional BRCA1 loss if present in both alleles or there is methylation of one allele combined with a LOH event resulting in loss of the other allele. A correlation of loss of BRCA1 function due to homozygous BRCA1 promoter methylation with response to rucaparib was demonstrated in ARIEL2.18,22,75 Testing was retrospectively performed on the pretreatment biopsy and was adjusted for BRCA1 copy number and LOH to determine zygosity. The homozygous BRCA1 methylation cancer subgroup had a median PFS of 14.5 months (95% CI, 4.8 to 18.3, n = 6) which was comparable with the BRCAm subgroup (12.8 months; 95% CI, 9.0 to 14.7, n = 40) and longer than BRCAwt, non–BRCA1-methylated cases (5.5 months, 95% CI, 5.0 to 6.2; P = .062, log-rank test, n = 143). Objective response rate was significantly better in the methylated subgroup compared with BRCAwt patients with non–BRCA1-methylated tumors (P = .0014, Fisher exact test), with five of six patients with homozygous BRCA1 methylation achieving a partial response. A sixth patient had a 33% reduction in target lesions not confirmed by subsequent imaging. In paired samples and in patient derived xenograft models, methylation was frequently lost in pretreatment biopsies compared with earlier samples and appears to be another mechanism of acquired PARPi resistance.22
CCNE1 Amplification
CCNE1 amplification is identified as a dynamic event enriched in patients with platinum-resistant OC.77 Tumors with CCNE1 amplification were found to have a prolonged response to the WEE1 inhibitor adavosertib in combination with chemotherapy (carboplatin alone or with paclitaxel) in platinum-sensitive TP53-mutant OC.23 CCNE1 amplification in platinum-resistant or -refractory OC was correlated with better response rate in the combination arm in a study of gemcitabine with adavosertib or placebo (Fisher exact test P = .013); this did not translate to improved PFS or overall survival (OS; P > .10).24 Twelve of 19 (63%) patients with recurrent BRCAwt disease and high CCNE1 copy number and/or mRNA expression measured on pretreatment core biopsy samples had PFS ≥ 6 months when treated with single-agent CHK1/2 inhibitor prexasertib.25 Additionally, when Cyclin E1 and E2 are bound, this activates CDK2 (cyclin-dependent kinase 2) and drives G1/S progression of the cell cycle. Overexpression of CDK2 is associated with abnormal regulation of the cell cycle. Tumors with CCNE1 amplification have been associated with preclinical response to CDK2 inhibitors which are currently under development.78,79 In summary, CCNE1 amplification is emerging as a potential biomarker of response to adavosertib, prexasertib, and CDK2 inhibitors, and prospective vadlidation trials are needed to further support these findings.
IMMUNOTHERAPY AND TUMOR MICROENVIRONMENT
The majority of clinical trials using immune checkpoint blockade in OC have been disappointing to date. Mismatch repair (MMR) deficiency and/or high tumor mutational burden (TMB) are the only level 1 evidence biomarkers with a strong correlation to response to anti–programmed death receptor 1 (PD-1) inhibition in solid tumors.27,28 However, MMR is a rare event in OC, occurring in < 5% overall and most common in low-grade endometrioid or clear cell type OC.80TMB over 10 mutations/megabase DNA is considered TMB high,81 and this is a rare event in OC.31,32,82 The median TMB in high-grade serous OC is 3.6 mutations/Mb, and the mean TMB is 5.3 mutations/Mb.
Expression of program death receptor ligand-1 (PD-L1) has been demonstrated to enrich for responders to immune checkpoint blockade across a number of cancer types. PD-L1 expression in OC at any cutoff has not shown response to anti–PD-1/PD-L1 inhibition; the lack of activity of this intervention makes measure of value of PD-L1 as a biomarker fraught.42,56,57 Similar to PD-L1 expression, studies of genomic biomarkers to date have failed to identify predictors of response to immunotherapy in OC.
A study by Färkkilä et al identified potential predictors of response to niraparib and pembrolizumab that may be worthy of further prospective exploration. In this study, which retrospectively analyzed specimens from 62 women enrolled in the TOPACIO trial for platinum-resistant OC (ClinicalTrials.gov identifier: NCT02657889), mutational signature 3, reflecting defective homologous recombination DNA repair, and positive interferon gamma gene expression signature, as a surrogate of exhausted CD8+ T cells in the tumor microenvironment, were found to be associated with an improved outcome31 (Table 2). However, this was not a randomized trial, so these findings could be due to a lack of a comparator arm.
ONCOGENIC SIGNALING
Several oncogenic pathways have been explored for therapeutic targeting across a number of OC histologic subtypes. Post hoc analysis of the negative trial MILO/ENGOT-ov11 (ClinicalTrials.gov identifier: NCT01849874) tested binimetinib versus physician's choice chemotherapy (PCC) in recurrent or persistent low-grade serous carcinoma. This trial found that patients with low-grade serous OCs bearing KRAS mutations had better response to binimetinib, a small-molecule inhibitor of MEK1/2.29 This study included 303 patients of whom 215 had tumor testing data available. The frequency of KRAS mutation was evenly distributed between the two groups, 32%-34%, and was significantly associated with objective response to treatment with binimetinib (OR, 3.4; 95% CI, 1.53 to 7.66; unadjusted P = .003) but not with PCC (OR, 2.13; 95% CI, 0.67 to 6.81; P = .2). The positive GOG-0281 trial of trametinib versus PCC (which included hormonal therapy options) had molecular data for 134 of 260 randomly assigned patients for a preplanned analysis.83 A treatment by KRAS/NRAS/BRAF mutation interaction analysis adjusted for multiple comparisons trended to predictive significance for response rate (P = .11) and was negative for PFS.
There are additional biomarkers that are promising but unclear in terms of predicting response to treatment of specific agents. Mutations in phosphatidylinositol-3-kinase (PI3K) pathway have been evaluated in the setting of PI3K inhibitor therapy and have not been identified as critical factors for predicting response. Mutations in PIK3CA and phosphatase and tensin homolog (PTEN) were found to be associated with response in patients with advanced stage solid tumors in a phase I trial of pictilisib/GDC-0941(ClinicalTrials.gov identifier: NCT00876122). Three of 60 participants had OC, one of whom had stable disease for 4 months.43 Combination studies of olaparib with PI3K inhibitors such as alpelisib or buparlisib have also been explored. There was no relationship between presence of alteration and response to treatment combinations although PI3K alterations were detected in 33%-44% of tumors.43-45,84 Additional oncogenic signaling pathways may be of future clinical significance for treatment response, but currently, these are only based on preclinical models. For example, in preclinical studies, c-MYC amplification has shown to be a potential predictor of response to bromodomain and extraterminal inhibitors85 or to olaparib and palbociclib.86 Emerging evidence also indicates that mutations in members of SWI/SNF chromatin remodeling complex such as ARID1A and SMARCA4 may sensitize the tumors to epigenetic therapies such as histone deacetylase inhibitors and EZH2 inhibitors.50-55 A trial of EZH2 inhibitor tazemetostat in ARID1A-mutated ovarian clear cell carcinoma (NRG-GY014) has recently completed accrual (ClinicalTrials.gov identifier: NCT03348631).
ER and progesterone receptors (PrRs) have been studied in the context of response to aromatase inhibitors and ER antagonists such as letrozole, anastrozole, and fulvestrant.34-41 Several studies in heavily pretreated women with elevated ER-alpha tumor expression demonstrated evidence of disease stabilization compared with patients with tumors with lower ER-alpha expression. Most patients progressed within 6 months. There is limited evidence to date that ER and PrR are functionally active on different OC types, which may explain in part the limited benefit observed for these agents.
ANGIOGENESIS
Several analytes have been examined for relationship to outcome with antiangiogenic agents. To date, none of these have had locked down values validated to show a treatment-by-biomarker effect. Examples include the mesenchymal and proliferative gene expression signature, and plasma interleukin-6 (IL-6), and osteopontin. The mesenchymal and proliferative gene signatures in high-grade serous OC have been shown to be associated with inferior survival. Evaluation of these signatures within the context of phase III ICON7 clinical trial (ClinicalTrials.gov identifier: NCT00483782) evaluating a combination of bevacizumab and carboplatin/paclitaxel chemotherapy in newly diagnosed OCs demonstrated improved outcomes with bevacizumab in the mesenchymal and proliferative signature subgroup.33 However, these data were derived from a post hoc analysis of ICON7 data and have not been prospectively validated in other studies. Circulating IL-6 concentration is an additional promising biomarker, identified as part of the angiome analysis of seven putative biomarkers (IL-6, Ang-2, osteopontin, stromal cell-derived factor-1, VEGF-D, IL-6 receptor [IL-6R], and GP130). This cassette was analyzed retrospectively in plasma of patients enrolled in GOG 218, double-blind, placebo-controlled, phase III trial in newly diagnosed stage III or stage IV epithelial OC comparing chemotherapy with/without bevacizumab incorporated with/without bevacizumab maintenance.46,87 The data were dichotomized at the median. Patients with high IL-6 levels had a longer PFS and OS when treated with bevacizumab compared with placebo. Osteopontin was found to be a negative prognostic marker for both PFS and OS in this angiome analysis. Validation requires, ideally, a prospective analysis using a locked down cutoff for IL-6.
CANCER ANTIGEN-125 ELIMINATION RATE CONSTANT K
The modeled cancer antigen-125 (CA-125) Elimination rate constant K (KELIM), determined on the basis of CA-125 clearance during the first 100 days of chemotherapy initiation, is a validated early marker of tumor chemosensitivity and prognosis.88,89 Evaluation of KELIM as a predictor of response to maintenance PARP inhibitor response was also performed in an exploratory analysis of the phase III VELIA/GOG-3005 study, which evaluated veliparib vs. placebo administered concurrently with chemotherapy followed by veliparib vs. placebo maintenance (ClinicalTrials.gov identifier: NCT02470585).47 Overall, high KELIM values appeared to enrich for patients with improved benefit from veliparib. However, it remains difficult to separate the predictive from prognostic value of KELIM, particularly since PARPi are only approved in the maintenance setting. Evaluation of KELIM as a predictive factor of future benefit from PARPi maintenance in other completed trials could be of value.
DISCUSSION
Although multiple biomarkers have been evaluated in the OC therapeutic landscape, few have been shown to be predictive on the basis of high-level evidence showing treatment/outcome interaction. Currently, use of BRCA mutations and HRD to predict for response to PARPi is supported by the highest LOE in OC. MMR deficiency and TMB have been shown to be predictors of response to pembrolizumab across several tumor types thus demonstrating Level 1 evidence. These biomarkers have limited applicability to OC, as very few OCs exhibit MMR deficiency or high TMB. Additional biomarkers involved in DNA damage repair (BRCA1/2 reversion mutations, RAD51C/D mutations, biallelic BRCA 1 methylation, CCNE1 amplification) and oncogenic signaling (KRAS, NRAS, and HRAS mutations) are supported by Level 2 evidence and require validation testing. Although additional biomarkers such as interferon gamma signature and mesenchymal or proliferative gene signatures, PI3K/AKT/PTEN pathway mutations, and IL-6 are promising as predictors of response to some agents, further supporting evidence is needed to establish their predictive ability in OC.
Given the increasing recognition that incorporation of integrated and exploratory biomarkers into trials is essential to understand the predictors and mechanisms of response, the majority of NRG-sponsored trials now include collection of archival tissue and pretreatment and on-treatment blood. In addition, incorporation of novel agents into the neoadjuvant setting provides an opportunity to sample both pretreatment and on-treatment tissue which will enable better understanding on the impact of novel agents on cancer cells and the tumor microenvironment. A recently completed trial, NRG-GY007 (ClinicalTrials.gov identifier: NCT02713386), collected tumors and blood at baseline and post-NACT with paclitaxel and carboplatin with/without ruxolitinib. These tumors and blood specimens are currently undergoing analysis. A recently activated trial, NRG-GY027 (ClinicalTrials.gov identifier: NCT05276973), incorporates ipatasertib (AKT-inhibitor) with NACT. In an effort to identify biomarkers for response, tumors collected at baseline/pre-NACT will undergo whole-exome sequencing to evaluate for PTEN, PIK3CA, PIK3R1, AKT1, TP53, KRAS, NF1, TSC1/TSC2, and tumors collected post-NACT will be evaluated for changes in downstream pathway expression for pGSK3β, p-PRAS40, p4EB1, pERK, and p-AKT. Additional exploratory biomarkers such as immune cell population differences in blood using mass cytometry between responders and nonresponders have also been proposed.
The LOE scale used here also has limitations. Mainly, it is unclear how to grade LOE for biomarkers without supporting evidence in OC but with established and consistent predictive value or validation studies performed in multiple tumor types. One such example is TMB, which consistently predicts response to PD-1 inhibitors; the poor outcome to PD-1 and PD-L1 inhibitors is as anticipated given the low TMB of OC. Available LOE scales do not address whether biomarker data are reliable if dependent solely on evidence from other disease types to inform use as an integral biomarker in OC. It is also questionable whether validation studies performed in other tumor types can be considered as a LOE for OC. For the purpose of this review, we have considered these where multiple, consistent studies are available to supplement evidence in OC.
This review highlights the need for correlative science as a key component of every clinical trial in OC. Furthermore, biomarker discovery and validation are imperative not only in OC but within specific histologic subtypes. Future trial design should allow for biomarker discovery through correlative studies and validation through prospective use of integrated biomarkers.
ACKNOWLEDGMENT
The authors would like to thank Ramy Serour and Jean Lynn for their assistance throughout all aspects of the CTPM and help in preparing this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Josee-Lyne Ethier
Honoraria: AstraZeneca, Merck, GlaxoSmithKline
Consulting or Advisory Role: Merck, AstraZeneca, GlaxoSmithKline
Katherine C. Fuh
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Aravive
Consulting or Advisory Role: Aravive
Research Funding: Merck, Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: AVB-S6-500
Rebecca Arend
Employment: Signatera
Consulting or Advisory Role: VBL Therapeutics, GlaxoSmithKline, Merck, Seagan, Sutro Biopharma, KIYATEC, Caris Life Sciences, Leap Therapeutics
Research Funding: Champions Oncology, Exelixis, GlaxoSmithKline, Immunogen, Merck
Travel, Accommodations, Expenses: Caris Life Sciences, GlaxoSmithKline, VBL Therapeutics
Gottfried E. Konecny
Speakers' Bureau: Clovis Oncology, AstraZeneca, Merck, GlaxoSmithKline
Research Funding: Merck (Inst), Lilly (Inst)
Panagiotis A. Konstantinopoulos
Consulting or Advisory Role: Merck, Vertex, AstraZeneca, Pfizer/EMD Serono, Tesaro, Bayer, Alkermes, Repare Therapeutics, Kadmon, Mersana, Novartis
Research Funding: Pfizer (Inst), Lilly (Inst), Tesaro (Inst), Merck Serono (Inst), AstraZeneca (Inst), Merck (Inst), Bayer (Inst), Bristol-Myers Squibb/Sanofi (Inst), Novartis (Inst)
Kunle Odunsi
Leadership: Society for Immunotherapy of Cancer, AACR, CRI Associate Director
Stock and Other Ownership Interests: Tactiva Therapeutics
Honoraria: KIYATEC
Consulting or Advisory Role: Regeneron, Celsion, GlaxoSmithKline, Dailchi-Sanyo
Research Funding: AstraZeneca, Tesaro
Patents, Royalties, Other Intellectual Property: Roswell
Travel, Accommodations, Expenses: Immunovaccine
Elizabeth M. Swisher
Leadership: IDEAYA Biosciences
Dmitriy Zamarin
Stock and Other Ownership Interests: ImmunOs Therapeutics, Calidi Biotherapeutics, Accurius
Consulting or Advisory Role: Synlogic, Agenus, Genentech/Roche, Memgen, Celldex, AstraZeneca, Crown Bioscience, Synthekine, GlaxoSmithKline, Hookipa Biotech, Tessa Therapeutics, Xencor, Targovax
Research Funding: AstraZeneca/MedImmune (Inst), Genentech (Inst), Plexxikon (Inst), Bristol Myers Squibb (Inst), Synthekine (Inst)
Patents, Royalties, Other Intellectual Property: I hold a patent regarding the use of recombinant Newcastle Disease Virus (NDV) for cancer therapy (Inst)
No other potential conflicts of interest were reported.
SUPPORT
D.Z. is supported by the National Cancer Institute Cancer Center Core Grant No. P30-CA008748 and GOG Foundation Scholar Investigator Award. K.C.F. reports grant funding 5R01CA234553-03 (KCF), Research Scholar Grant-19-080-01-TBG, from the American Cancer Society (KCF), and GOG Foundation Scholar Investigator Award. P.A.K. acknowledges funding P50CA240243 and R01CA258553 from NIH/NCI and W81XWH2110604/OC200122 from Department of Defense.
J.-L.E. and K.C.F. contributed equally to this work. E.C.K. and D.M. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Provision of study materials or patients: Elizabeth M. Swisher
Collection and assembly of data: All authors
Data analysis and interpretation: Josee-Lyne Ethier, Katherine C. Fuh, Gottfried E. Konecny, Panagiotis A. Konstantinopoulos, Elizabeth M. Swisher, Elise C. Kohn, Dmitriy Zamarin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Josee-Lyne Ethier
Honoraria: AstraZeneca, Merck, GlaxoSmithKline
Consulting or Advisory Role: Merck, AstraZeneca, GlaxoSmithKline
Katherine C. Fuh
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Aravive
Consulting or Advisory Role: Aravive
Research Funding: Merck, Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: AVB-S6-500
Rebecca Arend
Employment: Signatera
Consulting or Advisory Role: VBL Therapeutics, GlaxoSmithKline, Merck, Seagan, Sutro Biopharma, KIYATEC, Caris Life Sciences, Leap Therapeutics
Research Funding: Champions Oncology, Exelixis, GlaxoSmithKline, Immunogen, Merck
Travel, Accommodations, Expenses: Caris Life Sciences, GlaxoSmithKline, VBL Therapeutics
Gottfried E. Konecny
Speakers' Bureau: Clovis Oncology, AstraZeneca, Merck, GlaxoSmithKline
Research Funding: Merck (Inst), Lilly (Inst)
Panagiotis A. Konstantinopoulos
Consulting or Advisory Role: Merck, Vertex, AstraZeneca, Pfizer/EMD Serono, Tesaro, Bayer, Alkermes, Repare Therapeutics, Kadmon, Mersana, Novartis
Research Funding: Pfizer (Inst), Lilly (Inst), Tesaro (Inst), Merck Serono (Inst), AstraZeneca (Inst), Merck (Inst), Bayer (Inst), Bristol-Myers Squibb/Sanofi (Inst), Novartis (Inst)
Kunle Odunsi
Leadership: Society for Immunotherapy of Cancer, AACR, CRI Associate Director
Stock and Other Ownership Interests: Tactiva Therapeutics
Honoraria: KIYATEC
Consulting or Advisory Role: Regeneron, Celsion, GlaxoSmithKline, Dailchi-Sanyo
Research Funding: AstraZeneca, Tesaro
Patents, Royalties, Other Intellectual Property: Roswell
Travel, Accommodations, Expenses: Immunovaccine
Elizabeth M. Swisher
Leadership: IDEAYA Biosciences
Dmitriy Zamarin
Stock and Other Ownership Interests: ImmunOs Therapeutics, Calidi Biotherapeutics, Accurius
Consulting or Advisory Role: Synlogic, Agenus, Genentech/Roche, Memgen, Celldex, AstraZeneca, Crown Bioscience, Synthekine, GlaxoSmithKline, Hookipa Biotech, Tessa Therapeutics, Xencor, Targovax
Research Funding: AstraZeneca/MedImmune (Inst), Genentech (Inst), Plexxikon (Inst), Bristol Myers Squibb (Inst), Synthekine (Inst)
Patents, Royalties, Other Intellectual Property: I hold a patent regarding the use of recombinant Newcastle Disease Virus (NDV) for cancer therapy (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.World Cancer Research Fund : Cancer survival statistics. https://www.wcrf.org/dietandcancer/cancer-survival-statistics/
- 2.Lheureux S, Gourley C, Vergote I, et al. : Epithelial ovarian cancer. Lancet 393:1240-1253, 2019 [DOI] [PubMed] [Google Scholar]
- 3.SEER Cancer Stat Facts: Ovarian Cancer. National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/statfacts/html/ovary.html [Google Scholar]
- 4.Cummings J, Raynaud F, Jones L, et al. : Fit-for-purpose biomarker method validation for application in clinical trials of anticancer drugs. Br J Cancer 103:1313-1317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohn EC, Ivy SP: Confronting the care delivery challenges arising from precision medicine. Front Oncol 6:106, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson BK, Najjar O, Damast S, et al. : Human epidermal growth factor 2 (HER2) in early stage uterine serous carcinoma: A multi-institutional cohort study. Gynecol Oncol 159:17-22, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fader AN, Roque DM, Siegel E, et al. : Randomized phase II trial of carboplatin-paclitaxel versus carboplatin-paclitaxel-trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol 36:2044-2051, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Mansfield EA: FDA perspective on companion diagnostics: An evolving paradigm. Clin Cancer Res 20:1453-1457, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Hayes DF, Bast RC, Desch CE, et al. : Tumor marker utility grading system: A framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst 88:1456-1466, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Simon RM, Paik S, Hayes DF: Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 101:1446-1452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) : Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 378:771-784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Martín A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Ray-Coquard I, Pautier P, Pignata S, et al. : Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019 [DOI] [PubMed] [Google Scholar]
- 15.Mirza MR, Monk BJ, Herrstedt J, et al. : Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154-2164, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Coleman RL, Oza AM, Lorusso D, et al. : Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390:1949-1961, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pujade-Lauraine E, Ledermann JA, Selle F, et al. : Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 18:1274-1284, 2017. [Erratum: Lancet Oncol 18:e510, 2017] [DOI] [PubMed] [Google Scholar]
- 18.Swisher EM, Lin KK, Oza AM, et al. : Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol 18:75-87, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Moore KN, Secord AA, Geller MA, et al. : Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 20:636-648, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Lheureux S, Oaknin A, Garg S, et al. : EVOLVE: A multicenter open-label single-arm clinical and translational phase II trial of cediranib plus olaparib for ovarian cancer after PARP inhibition progression. Clin Cancer Res 26:4206, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Kondrashova O, Nguyen M, Shield-Artin K, et al. : Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov 7:984-998, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondrashova O, Topp M, Nesic K, et al. : Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun 9:3970, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oza AM, Estevez-Diz M, Grischke E-M, et al. : A biomarker-enriched, randomized phase II trial of adavosertib (AZD1775) plus paclitaxel and carboplatin for women with platinum-sensitive TP53-mutant ovarian cancer. Clin Cancer Res 26:4767, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Lheureux S, Cristea MC, Bruce JP, et al. : Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397:281-292, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J-M, Nair J, Zimmer A, et al. : Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: A first-in-class proof-of-concept phase 2 study. Lancet Oncol 19:207-215, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leijen S, Van Geel R, Sonke GS, et al. : Phase II study of WEE1 inhibitor AZD1775 plus carboplatin in patientswith tp53-mutated ovarian cancer refractory or resistant to first-line therapy within 3 months. J Clin Oncol 34:4354-4361, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Marabelle A, Le DT, Ascierto PA, et al. : Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol 38:1-10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marabelle A, Fakih M, Lopez J, et al. : Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21:1353-1365, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Monk BJ, Grisham RN, Banerjee S, et al. : MILO/ENGOT-ov11: Binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum. J Clin Oncol 38:3753-3762, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grisham RN, Moore KN, Gordon MS, et al. : Phase Ib study of binimetinib with paclitaxel in patients with platinum-resistant ovarian cancer: Final results, potential biomarkers, and extreme responders. Clin Cancer Res 24:5525, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Färkkilä A, Gulhan DC, Casado J, et al. : Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun 11:1459, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lampert EJ, Zimmer A, Padget M, et al. : Combination of PARP inhibitor olaparib, and PD-L1 inhibitor durvalumab, in recurrent ovarian cancer: A proof-of-concept phase II study. Clin Cancer Res 26:4268, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kommoss S, Winterhoff B, Oberg AL, et al. : Bevacizumab may differentially improve ovarian cancer outcome in patients with proliferative and mesenchymal molecular subtypes. Clin Cancer Res 23:3794-3801, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman A, Gabra H, Langdon SP, et al. : CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: Identification of an endocrine-sensitive subgroup. Clin Cancer Res 8:2233-2239, 2002 [PubMed] [Google Scholar]
- 35.Smyth JF, Gourley C, Walker G, et al. : Antiestrogen therapy is active in selected ovarian cancer cases: The use of letrozole in estrogen receptor-positive patients. Clin Cancer Res 13:3617-3622, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Ramirez PT, Schmeler KM, Milam MR, et al. : Efficacy of letrozole in the treatment of recurrent platinum- and taxane-resistant high-grade cancer of the ovary or peritoneum. Gynecol Oncol 110:56-59, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Kok PS, Beale P, O'Connell RL, et al. : PARAGON (ANZGOG-0903): A phase 2 study of anastrozole in asymptomatic patients with estrogen and progesterone receptor-positive recurrent ovarian cancer and CA125 progression. J Gynecol Oncol 30:e86, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argenta PA, Thomas SG, Judson PL, et al. : A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol Oncol 113:205-209, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Colon-Otero G, Zanfagnin V, Hou X, et al. : Phase II trial of ribociclib and letrozole in patients with relapsed oestrogen receptor-positive ovarian or endometrial cancers. ESMO Open 5:e000926, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colon-Otero G, Weroha SJ, Foster NR, et al. : Phase 2 trial of everolimus and letrozole in relapsed estrogen receptor-positive high-grade ovarian cancers. Gynecol Oncol 146:64-68, 2017 [DOI] [PubMed] [Google Scholar]
- 41.Gershenson DM, Sun CC, Iyer RB, et al. : Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol 125:661-666, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matulonis UA, Shapira-Frommer R, Santin AD, et al. : Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann Oncol 30:1080-1087, 2019 [DOI] [PubMed] [Google Scholar]
- 43.Sarker D, Ang JE, Baird R, et al. : First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) Inhibitor, in patients with advanced solid tumors. Clin Cancer Res 21:77-86, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konstantinopoulos PA, Barry WT, Birrer M, et al. : Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: A dose-escalation and dose-expansion phase 1b trial. Lancet Oncol 20:570-580, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Mak VCY, Zhou Y, et al. : Deregulated Gab2 phosphorylation mediates aberrant AKT and STAT3 signaling upon PIK3R1 loss in ovarian cancer. Nat Commun 10:716, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez Secord A, Bell Burdett K, Owzar K, et al. : Predictive blood-based biomarkers in patients with epithelial ovarian cancer treated with carboplatin and paclitaxel with or without bevacizumab: Results from GOG-0218. Clin Cancer Res 26:1288-1296, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.You B, Sehgal V, Hosmane B, et al. : CA-125 KELIM as a potential complementary tool for predicting veliparib benefit: An exploratory analysis from the VELIA/GOG-3005 study. J Clin Oncol 10.1200/JCO.22.00430 [epub ahead of print on July 22, 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng M, Kwiatkowski NP, Zhang T, et al. : Targeting MYC dependency in ovarian cancer through inhibition of CDK7 and CDK12/13. eLife 7:e39030, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen Y-A, Hong J, Asaka R, et al. : Inhibition of the MYC-regulated glutaminase metabolic Axis is an effective synthetic lethal approach for treating chemoresistant ovarian cancers. Cancer Res 80:4514, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dykhuizen EC, Hargreaves DC, Miller EL, et al. : BAF complexes facilitate decatenation of DNA by topoisomerase IIα. Nature 497:624-627, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williamson CT, Miller R, Pemberton HN, et al. : ATR inhibitors as a synthetic lethal therapy for tumours deficient in ARID1A. Nat Commun 7:13837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bitler BG, Aird KM, Garipov A, et al. : Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med 21:231-238, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bitler BG, Wu S, Park PH, et al. : ARID1A-mutated ovarian cancers depend on HDAC6 activity. Nat Cell Biol 19:962-973, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurashima K, Kashiwagi H, Shimomura I, et al. : SMARCA4 deficiency-associated heterochromatin induces intrinsic DNA replication stress and susceptibility to ATR inhibition in lung adenocarcinoma. NAR Cancer 2:zcaa005, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta M, Concepcion CP, Fahey CG, et al. : BRG1 loss predisposes lung cancers to replicative stress and ATR dependency. Cancer Res 80:3841-3854, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zamarin D, Burger RA, Sill MW, et al. : Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: An NRG Oncology study. J Clin Oncol 38:1814-1823, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Disis ML, Taylor MH, Kelly K, et al. : Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol 5:393-401, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yap TA, O'Carrigan B, Penney MS, et al. : Phase I trial of first-in-class ATR inhibitor M6620 (VX-970) as monotherapy or in combination with carboplatin in patients with advanced solid tumors. J Clin Oncol 38:3195-3204, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yap TA, Tan DA, Terbuch A, et al. : First-in-human trial of the oral ataxia telangiectasia and RAD3-related (ATR) inhibitor BAY 1895344 in patients with advanced solid tumors. Cancer Discov 11:80-91, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu JF, Ray-Coquard I, Selle F, et al. : Randomized phase II trial of seribantumab in combination with paclitaxel in patients with advanced platinum-resistant or -refractory ovarian cancer. J Clin Oncol 34:4345-4353, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abida W, Campbell D, Patnaik A, et al. : Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: Analysis from the phase II TRITON2 study. Clin Cancer Res 26:2487-2496, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lord CJ, Ashworth A: The DNA damage response and cancer therapy. Nature 481:287-294, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Abkevich V, Timms KM, Hennessy BT, et al. : Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer 107:1776, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wagle N, Berger MF, Davis MJ, et al. : High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov 2:82-93, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birkbak NJ, Wang ZC, Kim J-Y, et al. : Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov 2:366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stover EH, Konstantinopoulos PA, Matulonis UA, et al. : Biomarkers of response and resistance to DNA repair targeted therapies. Clin Cancer Res 22:5651-5660, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Maxwell KN, Wubbenhorst B, Wenz BM, et al. : BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun 8:319, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Popova T, Manié E, Rieunier G, et al. : Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res 72:5454, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Noordermeer SM, van Attikum H: PARP inhibitor resistance: A tug-of-war in BRCA-mutated cells. Trends Cell Biol 29:820-834, 2019 [DOI] [PubMed] [Google Scholar]
- 70.Sakai W, Swisher EM, Karlan BY, et al. : Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451:1116-1120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swisher EM, Sakai W, Karlan BY, et al. : Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res 68:2581-2586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards SL, Brough R, Lord CJ, et al. : Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451:1111-1115, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Norquist B, Wurz KA, Pennil CC, et al. : Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol 29:3008-3015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin KK, Harrell MI, Oza AM, et al. : Reversion mutations in circulating tumor DNA predict primary and acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov 9:210, 2019 [DOI] [PubMed] [Google Scholar]
- 75.Swisher EM, Kwan TT, Oza AM, et al. : Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (parts 1 and 2). Nat Commun 12:2487, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nesic K, Kondrashova O, Hurley RM, et al. : Acquired RAD51C promoter methylation loss causes PARP inhibitor resistance in high-grade serous ovarian carcinoma. Cancer Res 81:4709-4722, 2021 [DOI] [PubMed] [Google Scholar]
- 77.Patch A-M, Christie EL, Etemadmoghadam D, et al. : Whole–genome characterization of chemoresistant ovarian cancer. Nature 521:489-494, 2015 [DOI] [PubMed] [Google Scholar]
- 78.Etemadmoghadam D, Au-Yeung G, Wall M, et al. : Resistance to CDK2 inhibitors is associated with selection of polyploid cells in CCNE1-amplified ovarian cancer. Clin Cancer Res 19:5960-5971, 2013 [DOI] [PubMed] [Google Scholar]
- 79.McDonald ER III, de Weck A, Schlabach MR, et al. : Project DRIVE: A compendium of cancer dependencies and synthetic lethal relationships uncovered by large-scale, deep RNAi screening. Cell 170:577-592.e10, 2017 [DOI] [PubMed] [Google Scholar]
- 80.Leskela S, Romero I, Cristobal E, et al. : Mismatch repair deficiency in ovarian carcinoma: Frequency, causes, and consequences. Am J Surg Pathol 44:649-656, 2020 [DOI] [PubMed] [Google Scholar]
- 81.Choucair K, Morand S, Stanbery L, et al. : TMB: A promising immune-response biomarker, and potential spearhead in advancing targeted therapy trials. Cancer Gene Ther 27:841-853, 2020 [DOI] [PubMed] [Google Scholar]
- 82.Liu YL, Selenica P, Zhou Q, et al. : BRCA mutations, homologous DNA repair deficiency, tumor mutational burden, and response to immune checkpoint inhibition in recurrent ovarian cancer. JCO Precis Oncol:665-679, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gershenson DM, Miller A, Brady WE, et al. : Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): An international, randomised, open-label, multicentre, phase 2/3 trial. The Lancet 399:541-553, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matulonis UA, Wulf GM, Barry WT, et al. : Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann Oncol 28:512-518, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li C, Bonazzoli E, Bellone S, et al. : Mutational landscape of primary, metastatic, and recurrent ovarian cancer reveals c-MYC gains as potential target for BET inhibitors. Proc Natl Acad Sci USA 116:619-624, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yi J, Liu C, Tao Z, et al. : MYC status as a determinant of synergistic response to Olaparib and Palbociclib in ovarian cancer. EBioMedicine 43:225-237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burger RA, Brady MF, Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Colomban O, Tod M, Leary A, et al. : Early modeled longitudinal CA-125 kinetics and survival of ovarian cancer patients: A GINECO AGO MRC CTU study. Clin Cancer Res 25:5342-5350, 2019 [DOI] [PubMed] [Google Scholar]
- 89.You B, Colomban O, Heywood M, et al. : The strong prognostic value of KELIM, a model-based parameter from CA 125 kinetics in ovarian cancer: Data from CALYPSO trial (a GINECO-GCIG study). Gynecol Oncol 130:289-294, 2013 [DOI] [PubMed] [Google Scholar]