PURPOSE
Cardiovascular disease is a significant cause of late morbidity and mortality in survivors of childhood cancer. Clinical informatics tools could enhance provider adherence to echocardiogram guidelines for early detection of late-onset cardiomyopathy.
METHODS
Cancer registry data were linked to electronic health record data. Structured query language facilitated the construction of anthracycline-exposed cohorts at a single institution. Primary outcomes included the data quality from automatic anthracycline extraction, sensitivity of International Classification of Disease coding for heart failure, and adherence to echocardiogram guideline recommendations.
RESULTS
The final analytic cohort included 385 pediatric oncology patients diagnosed between July 1, 2013, and December 31, 2018, among whom 194 were classified as no anthracycline exposure, 143 had low anthracycline exposure (< 250 mg/m2), and 48 had high anthracycline exposure (≥ 250 mg/m2). Manual review of anthracycline exposure was highly concordant (95%) with the automatic extraction. Among the unexposed group, 15% had an anthracycline administered at an outside institution not captured by standard query language coding. Manual review of echocardiogram parameters and clinic notes yielded a sensitivity of 75%, specificity of 98%, and positive predictive value of 68% for International Classification of Disease coding of heart failure. For patients with anthracycline exposure, 78.5% (n = 62) were adherent to guideline recommendations for echocardiogram surveillance. There were significant association with provider adherence and race and ethnicity (P = .047), and 50% of patients with Spanish as their primary language were adherent compared with 90% of patients with English as their primary language (P = .003).
CONCLUSION
Extraction of treatment exposures from the electronic health record through clinical informatics and integration with cancer registry data represents a feasible approach to assess cardiovascular disease outcomes and adherence to guideline recommendations for survivors.
INTRODUCTION
Cardiovascular disease (CVD) is a significant cause of late morbidity and mortality in survivors of childhood cancer.1 An analysis from the Childhood Cancer Survivor Study (CCSS) showed an increased incidence of serious CVD, including cardiomyopathy, coronary artery disease, arrhythmias, and valvular disease, among survivors of childhood cancer compared with siblings.2 Indeed, survivors demonstrated seven times higher mortality rates compared with the general population, with cardiac causes of death second only to subsequent malignancy.1-3 The cumulative incidence of cardiomyopathy by age 45 years was 4.8% among all survivors with an earlier onset in adulthood compared with siblings and higher incidence rates for survivors with anthracycline exposure.2,4
CONTEXT
Key Objective
To automatically extract cumulative anthracycline exposure from the electronic health record through standard query language, construct cardiovascular risk-based cohorts of children with cancer, and assess provider adherence to guideline recommendations for echocardiograms.
Knowledge Generated
Automatic extraction of cumulative anthracycline exposure was highly consistent with manual chart review for children with cancer, which represents a feasible approach for cardiovascular risk stratification of survivors. Significant differences in provider adherence to echocardiogram guidelines were observed with regard to patient race and ethnicity and primary language.
Relevance
Anthracycline exposure is an important predictor of late cardiotoxicity, and automatic extraction of this key data element from the electronic health record, integrated with cancer registry data, can guide equitable population health management of survivors of childhood cancer. Furthermore, this serves as a model to promote interoperability at other institutions and expansion to include other critical treatment exposures.
Chemotherapy, most notably anthracyclines, and chest radiation are cardiotoxic.5 There is a strong dose-response relationship between cumulative anthracycline dose and cardiomyocyte injury, primarily with a marked increase in risk after 250 mg/m2.6 Although recent cardioprotective strategies, such as dexrazoxane,7,8 and reduction of anthracycline exposures resulted in a modest decrease in the cumulative incidence of heart failure in survivors treated in more recent decades,9 cumulative anthracycline dose persists as a major predictor of CVD. Socioeconomic factors influence overall cardiovascular health10 and survivors report lower physical activity levels,11 which could further exacerbate treatment-related effects.12 Therefore, early diagnosis of treatment-related cardiovascular sequelae represents key targets for interventions aimed at cardioprotection, such as adherence to screening guidelines, preventive care, and healthy lifestyles.
As the knowledge of late cardiac effects expands through well-designed longitudinal cohort studies, such as the CCSS, the implementation of guideline-based care is essential. On the basis of treatment exposures, the Children's Oncology Group (COG) routinely publishes guidelines for long-term follow-up care, including detection of cardiotoxicity.13 The International Guideline Harmonization Group provides another layer for evidence-based survivorship care.14 Together these inform screening guidelines for early detection of cardiovascular complications.15 Analyses have proven the cost-effectiveness of following guideline-based screening frequency of echocardiograms.16,17 Moreover, gene-wide association studies and bioinformatics advances in precision medicine promise to further refine risk stratification for anthracycline-induced cardiotoxicity among survivors.18-22
Learning health systems23,24 and the application of real-world data to improve survivorship care are synergistic with national efforts in clinical oncology such as the Cancer Moonshot to enhance data sharing.25 The Childhood Cancer Data Initiative aims to build an infrastructure to integrate data from multiple sources,26 and the Cancer Informatics for Cancer Centers organization reflects the growing interest on harness informatics, data science, and population science methods within the field of oncology.27 The Minimal Common Oncology Data Elements (mCODE) Initiative focuses on the application of data standards to promote interoperability of cancer-related information.28 The Childhood Cancer Data Initiative and mCODE need tangible, reproducible methods, such as the integration of electronic health record (EHR) and cancer registry data,29 to apply current knowledge to improve survivorship care and cultivate multi-institutional collaborations.
The primary aim of this study was to use a clinical informatics approach to automatically extract cumulative anthracycline exposure data from the EHR to construct cardiovascular risk-adapted cohorts of children with cancer to assess early CVD burden and adherence to guideline-based recommendations for echocardiogram surveillance.
METHODS
Cancer Registry and Patient Information
The Duke Health Institutional Review Board approved this study. Cancer centers accredited by the Commission on Cancer are required to report all newly diagnosed cases to the National Cancer Database.30 Cancer registry data provided the base cohort, and EHR data elements were integrated and linked by the medical record number. Cardiovascular risk-adapted cohorts were constructs based on patients with documented anthracycline exposure, ≤ 18 years of age at time of diagnosis, and reported to the cancer registry with a malignancy between July 1, 2013 (the date of EHR implementation) and December 31, 2018 (Fig 1). Only analytic cases were included, which are defined by the National Cancer Database as cases newly diagnosed and received all or part of the first course of treatment at the reporting facility. Patient age at diagnosis, race and ethnicity, and mortality data were captured from the cancer registry. Patient gender was extracted from the EHR using the Duke Enterprise Data Unification Content Explorer (DEDUCE). DEDUCE is an institutional self-service query tool that uses a data dictionary and standard query language (SQL) to automatically extract data elements from the Duke Medicine enterprise data warehouse.31 SQL was used to extract primary language data directly from the EHR database Clarity.
FIG 1.
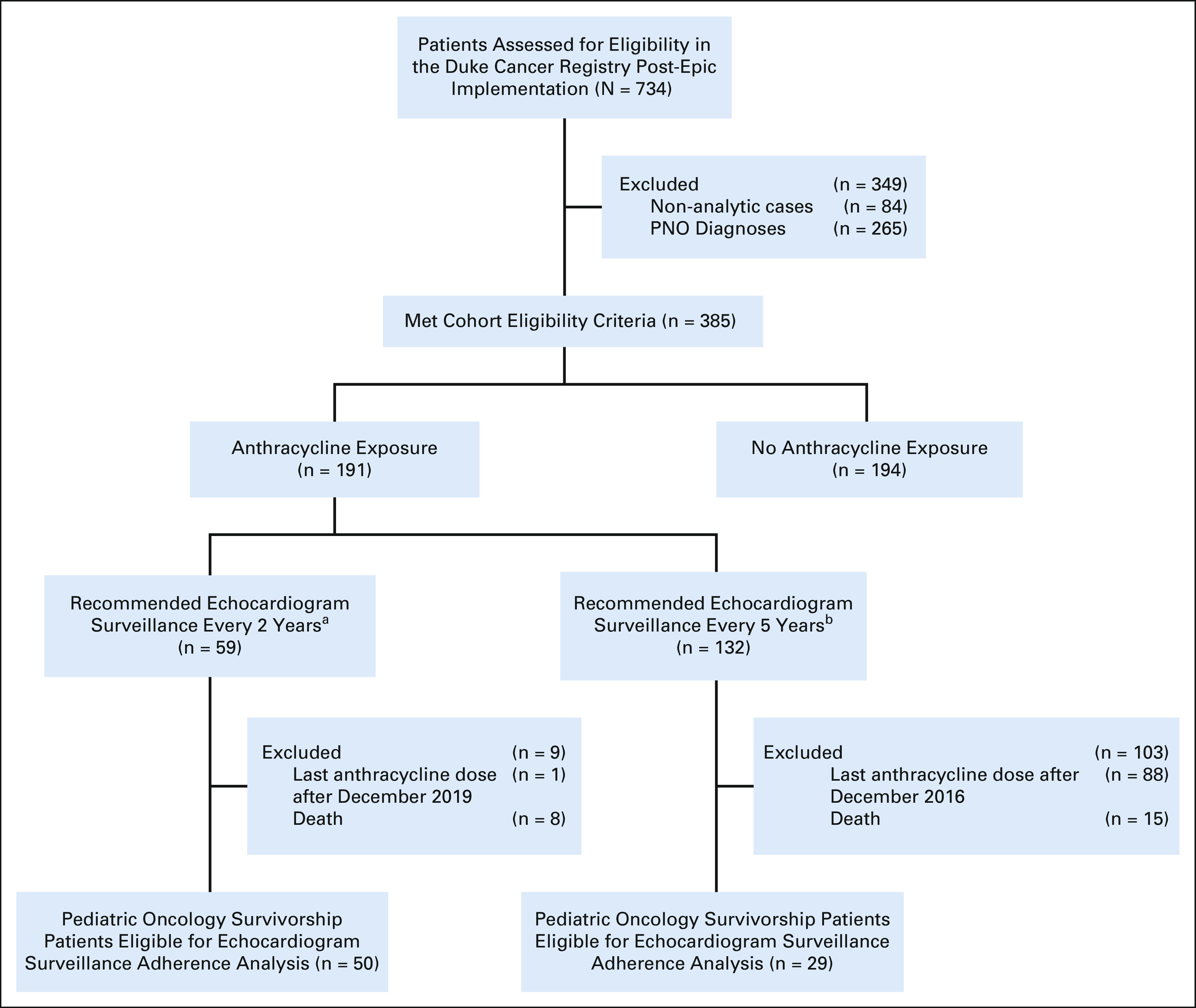
Construction of the final analytic childhood cancer cumulative anthracycline exposure cohort on the basis of inclusion and exclusion criteria from cancer registry data for patients diagnosed between July 1, 2013, and December 31, 2018. aCumulative anthracycline dose ≥ 250 mg/m2 or cumulative anthracycline dose < 250 and chest radiation ≥ 15 Gy. bCumulative anthracycline dose < 250 mg/m2 and chest radiation < 15 Gy. PNO, pediatric neuro-oncology.
This research was submitted to and approved by the Duke University Health System Institutional Review Board (Pro00104185) on December 11, 2019.
Disease Classification
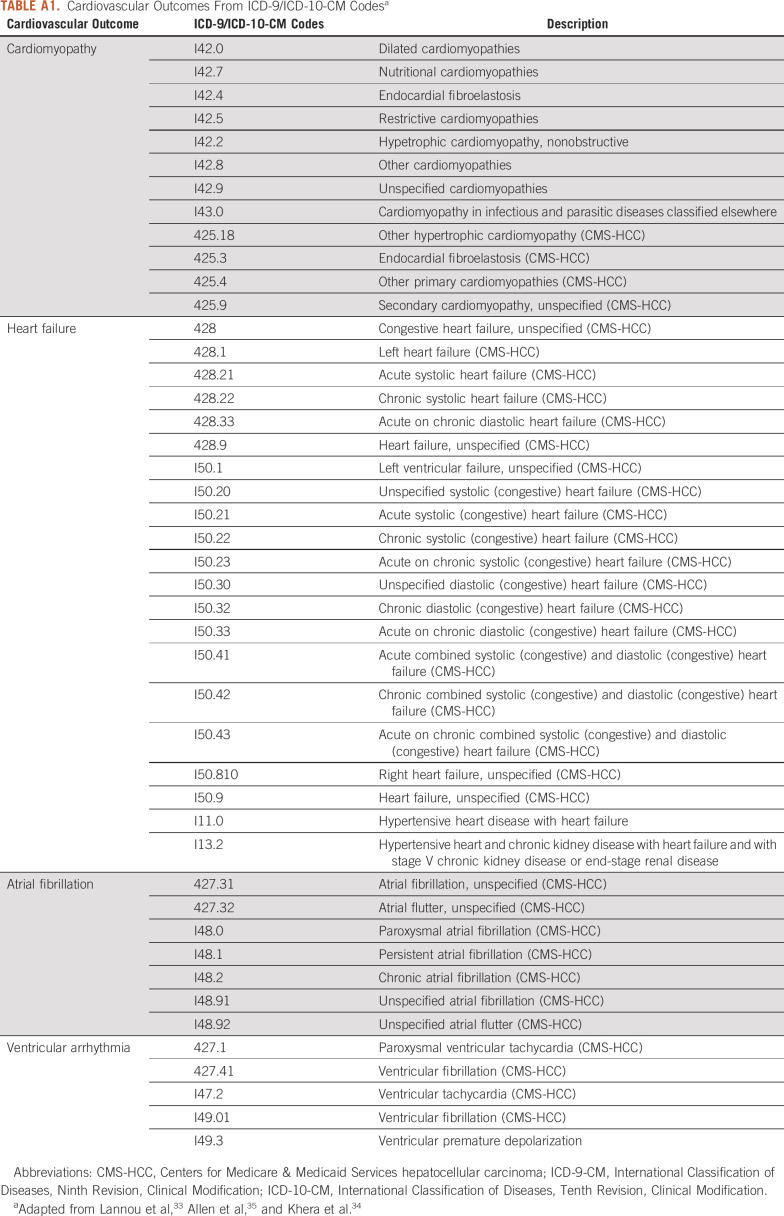
The International Classification of Diseases for Oncology, Third Revision, from the cancer registry, and the disease groupings from the International Classification of Childhood Cancer, Third Revision,32 were used to identify a pediatric oncology cohort. Patients with a primary CNS tumor were excluded (n = 262), as they are historically less likely to receive anthracyclines.5 For cardiovascular outcomes, all International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) were extracted from the EHR using DEDUCE. Established classifications for cardiomyopathy, heart failure, atrial fibrillation, and ventricular arrhythmias provided a framework to capture major cardiac outcomes during the treatment and early survivorship periods (Appendix Table A1).33-36
Anthracycline Exposure
Discrete data elements for cumulative anthracycline exposure were extracted through SQL coding from the Epic EHR reporting database Clarity. The lifetime dose-tracking table, built into Epic by the informatics team at Duke, captures the total cumulative dose for each type of anthracycline, measured in milligrams per meter squared. The body surface area was measured using the documented height and weight at the time of administration. Cumulative anthracycline exposure was calculated by the doxorubicin isotoxic equivalent dose with the total dose of doxorubicin multiplied by one, the total dose of daunorubicin multiplied by 0.5, the total dose of epirubicin multiplied by 0.67, the total dose of idarubicin multiplied by 5, and the total dose of mitoxantrone multiplied by 4.13,37 High and low anthracycline exposure cutoffs were defined as a cumulative dose of ≥ 250 mg/m2 and < 250 mg/m2, respectively (Appendix Tables A2 and A3).
Echocardiogram Guideline Adherence and Cohort Construction
DEDUCE was used to automatically extract the date of every echocardiogram from the EHR on the basis of Current Procedural Terminology (CPT) coding.38 Congruent with the COG Long-Term Follow-Up Guidelines, Version 5.0,13 adherence was defined as an echocardiogram within 27 months of the last administration of an anthracycline for the patients with cumulative exposure ≥ 250 mg/m2 or exposure < 250 mg/m2 and chest radiation ≥ 15 Gy. For patients with cumulative exposure < 250 mg/m2 and chest radiation < 15 Gy, adherence was defined as an echocardiogram within 63 months of the last administration of an anthracycline.
Manual EHR Review
Manual EHR review of patient data including cumulative anthracycline exposure, chest radiation data (which were not captured in the SQL methods), causes of death, and echocardiogram parameters for heart failure facilitated both quality assurance and a sensitivity analysis for International Classification of Disease (ICD) coding of heart failure in this cohort. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Duke University Medical Center.39,40 REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for data integration and interoperability with external sources. The REDCap project designer facilitated discrete fields for each patient to record the date of each echo, echocardiogram parameters (ejection fraction and shortening fraction), ICD cardiac codes, diagnosis of heart failure by cardiology, and documented heart failure symptoms.
Statistical Analyses
Patients reported to the cancer registry with a non-CNS diagnosis were grouped by no, low, and high anthracycline exposures. Continuous variables are presented as means (standard error [SE]), and differences were compared using the analysis of variance test across the three groups. Categorical variables are presented as counts (proportions), and the χ2 test was used to compare differences. For patients with anthracycline exposure and who met the eligibility criteria for echocardiogram guideline adherence, differences in continuous variables were compared using the t-test, and differences in categorical variables were compared using the χ2 test. All statistical analyses were conducted using Stata/SE version 16.1 (Stata Corp LLC, College Station, TX).
RESULTS
Post-EHR Implementation Anthracycline Exposure Cohort Construction
A total of 734 patients age ≤ 18 years at the time of diagnosis were captured in the institutional cancer registry between July 1, 2013, and December 31, 2018. We excluded 265 CNS patients on the basis of International Classification of Diseases for Oncology, Third Revision coding, and International Classification of Childhood Cancer, Third Revision classification.32 Of the remaining 469 patients, 84 patients coded as nonanalytic cases in the cancer registry were excluded, leaving a total of 385 patients in the final analytic cohort (Fig 1).
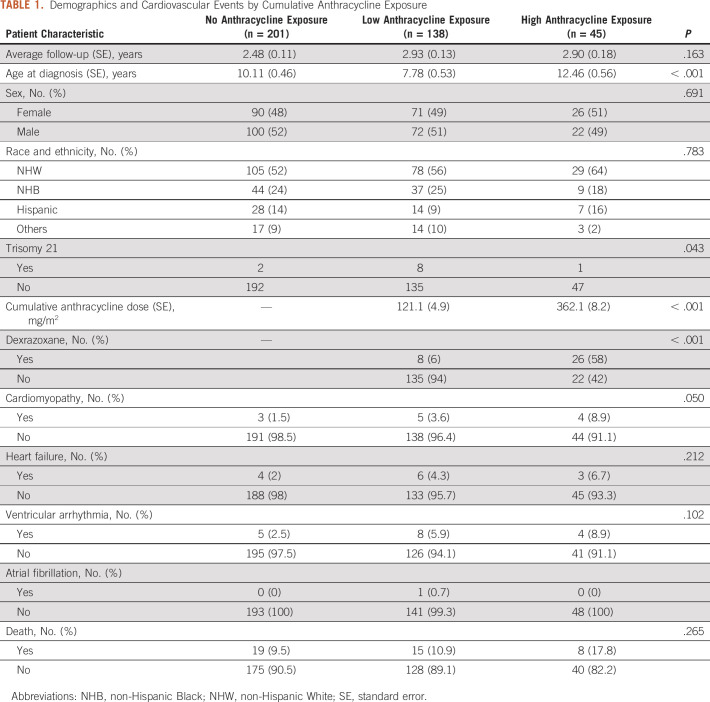
SQL was used to automatically extract cumulative anthracycline exposure from Clarity. A total of 191 patients received an anthracycline, among whom 48 received a high cumulative dose (≥ 250 mg/m2) and 143 received an intermediate or low dose (< 250 mg/m2). One hundred ninety-four patients did not receive anthracyclines. The high anthracycline exposure group received a mean cumulative anthracycline dose of 362.1 (SE 8.2) mg/m2 compared with a mean dose of 121.1 (SE 4.9) mg/m2 in the low anthracycline exposure group and were more likely to receive dexrazoxane (58% v 6%) for cardioprotection (P < .001). There were no statistical differences between the high, low, and no anthracycline-exposed groups with regard to sex (P = .691) or race and ethnicity (P = .783). There was significant heterogeneity in the age at diagnosis (P < .001) and diagnosis of Trisomy 21 (P = .043) among the different groups (Table 1).
TABLE 1.
Demographics and Cardiovascular Events by Cumulative Anthracycline Exposure
Quality Assurance of SQL Coding for Anthracycline Exposure
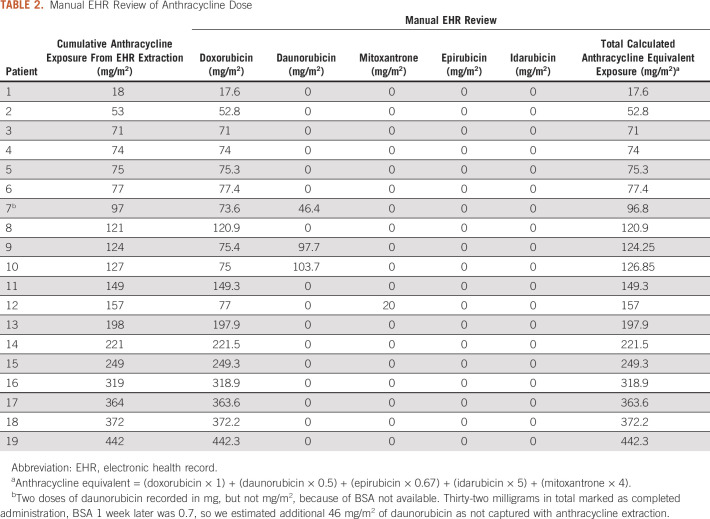
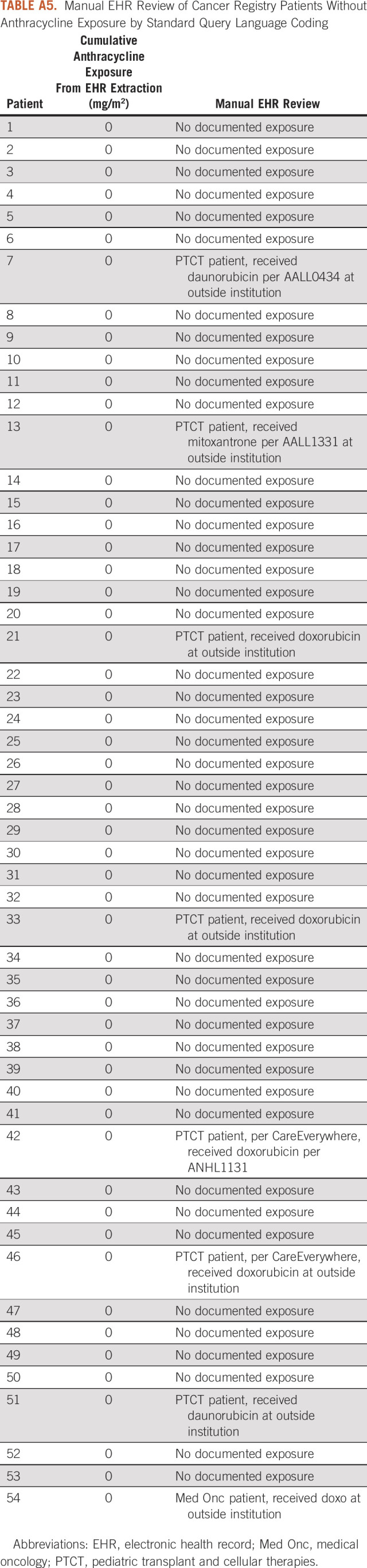
Manual EHR review of anthracycline exposures was conducted for quality assurance and was highly consistent with the automatic extraction through SQL (Table 2). Of the 10% reviewed (n = 19), one patient with confirmed daunorubicin administration did not have a documented body surface area, which resulted in an underestimate of the cumulative anthracycline exposure by 23 mg/m2. Additionally, we manually reviewed 10% of patients in the cancer registry that did not have anthracycline exposure from SQL automatic extraction (n = 53). Of these patients, 85% (n = 46) had no record of anthracycline administration, 13% (n = 7) were pediatric hematopoietic stem cell transplant (HSCT) patients with documented anthracycline exposure at an outside institution, and one was a medical oncology patient who received anthracyclines at an outside institution.
TABLE 2.
Manual EHR Review of Anthracycline Dose
Cardiac Events Among Anthracycline-Exposed Groups
DEDUCE facilitated the automatic extraction of all ICD-9-CM and ICD-10-CM codes from the EHR and the identification of diagnoses for cardiovascular outcomes of interest (Table 1). Of patients with high anthracycline exposure, 8.9% had documented cardiomyopathy, 6.7% had heart failure, and 8.9% had ventricular arrhythmia during or after therapy. There were no significant differences in cardiomyopathy (P = .050), heart failure (P = .212), ventricular arrhythmia (P = .102), or death (P = .265) among the anthracycline exposure groups (Table 1) during the follow-up period. Diagnoses of CVD before the date of initial diagnosis for malignancy were excluded.
Sensitivity Analysis for ICD-9-CM and ICD-10-CM Classification and Manual Chart Review of Echocardiogram for Anthracycline-Exposed Groups
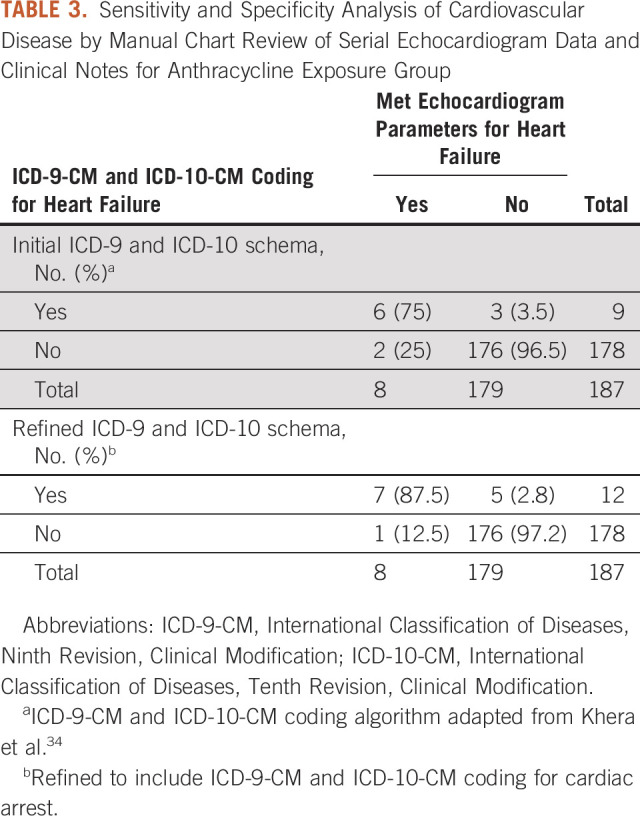
All echocardiogram reports from the anthracycline-exposed groups were manually reviewed. Excluding patients with a previous diagnosis of heart failure, 4.3% of patients met parameters for heart failure (n = 8) and 4.8% of patients had an ICD-9-CM and ICD-10-CM code for heart failure (n = 9). This yielded a sensitivity of 75% and a specificity of 98% of this ICD-9-CM and ICD-10-CM coding approach to detect heart failure parameters on echocardiogram with a positive predictive value of 67% and a negative predictive value of 99%. Refinement of the ICD-based coding schema to include cardiac arrest increased the sensitivity to 87.5% and decreased the positive predictive value to 58% (Table 3). A manual chart review of all discordant cases was performed (Appendix Tables A4 and A5).
TABLE 3.
Sensitivity and Specificity Analysis of Cardiovascular Disease by Manual Chart Review of Serial Echocardiogram Data and Clinical Notes for Anthracycline Exposure Group
Guideline Adherence to Echocardiogram Surveillance Recommendations
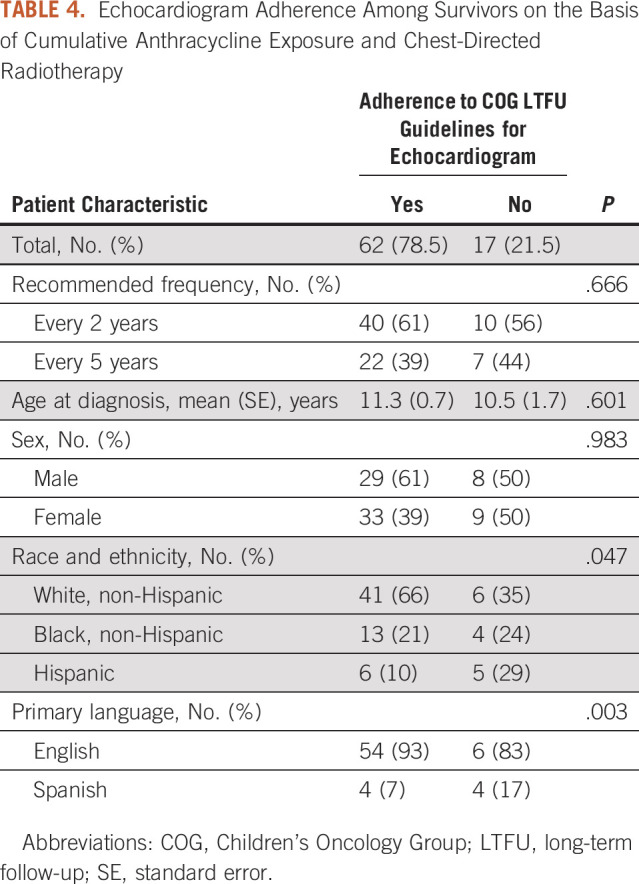
Within the anthracycline-exposed cohorts, we performed a landmark analysis to ensure equivalent follow-up time to assess adherence to guideline recommendations for surveillance echocardiograms. For the group recommended for echocardiogram surveillance every 2 years, we excluded patients with last anthracycline administration after December 2019 (n = 1) and death during the follow-up period (n = 8) to allow for a 27-month follow-up time. For the group recommended to receive echocardiogram surveillance every 5 years, we excluded patients with last anthracycline administration after December 2016 (n = 88) and death during the follow-up period (n = 15) to allow for a 63-month follow-up time. Twenty-two percent of patients were not adherent to the COG Long-Term Follow-Up Guidelines for echocardiogram surveillance (n = 17; Table 4). Of these 17 patients, three did not retrieve any CPT code for echocardiogram on automatic data extraction. With manual chart review, one met criteria for guideline adherence, one did not meet criteria for guideline adherence, and one was treated exclusively by medical oncology and did not complete the recommended treatment. There were no differences among the anthracycline exposure groups (P = .666), age at diagnosis (P = .601), and sex (P = .983).
TABLE 4.
Echocardiogram Adherence Among Survivors on the Basis of Cumulative Anthracycline Exposure and Chest-Directed Radiotherapy
Disparities in Echocardiogram Surveillance by Race and Ethnicity and Primary Language
On the basis of χ2 tests, there was a significant association between race and ethnicity (P = .047) and primary language (P = .003) and adherence to echocardiogram surveillance guidelines. Eight-seven percent of non-Hispanic White patients received echocardiograms within the recommended time period compared with 76% of non-Hispanic Black patients and 55% of Hispanic patients. With regard to primary language, 90% of English-speaking patients received guideline-based care for echocardiogram surveillance compared with 50% of Spanish-speaking patients (Table 4).
DISCUSSION
This study supports the utility of SQL to automatically extract cumulative anthracycline exposure from the EHR, using cancer registry data as a base cohort. Manual EHR review revealed excellent concordance of 95% with results from the automated SQL data extraction. One patient had two missed doses of daunorubicin because of lack of a recorded body surface area at the time of administration; however, this did not lead to misclassification for recommended echocardiogram frequency. The discrete structure of the lifetime dose tracking of anthracycline exposure in Epic, accessible through SQL, represents a model for other key chemotherapy exposures to monitor the late effects.41,42
The major limitations for these automated approaches for EHR data extraction include the potential to underestimate exposure to anthracyclines because of the receipt of some care at outside institutions. The exclusion of nonanalytic cases helps mitigate undocumented anthracycline exposures. Nevertheless, 15% of patients had free text documentation of anthracycline administration at outside institutions. Nearly all of these patients were pediatric hematopoietic stem-cell transplant patients who received a portion of their care at an outside institution. This led to misclassification of patients as having no anthracycline exposure when they likely had low anthracycline exposure. Anthracycline exposure is a critical data element, for both care during active treatment and survivorship, to inform the risk of cardiotoxicity. This underscores the need for increased interoperability of these structured data elements unique to oncology to facilitate health information exchange for treatment received at outside institutions.
The application of established ICD-9-CM and ICD-10-CM coding schema for heart failure33-35 illustrates the opportunity to capture early CVD for children with cancer. The use of dexrazoxane for cardioprotection may account for the lack of significant difference in cardiac events between the low and high anthracycline groups; however, this may also reflect insufficient power because of the small sample size. Interinstitutional collaborations for surveillance of cardiac events would increase sample size and benefit from the informatics methods presented in this study. Manual review of echocardiogram parameters yielded a modest sensitivity of 75% and a positive predictive value of 67%, which calls for further refinement to detect heart failure on a population health level. Current advances in natural language processing43-45 and machine learning46 offer next steps to improve the detection of heart failure in the general population.
Echocardiogram adherence,38 on the basis of CPT coding and enhanced by manual EHR review for quality assurance, showed that nearly a fifth of survivors with anthracycline exposure received suboptimal guideline-based care during the early follow-up period. With regard to echocardiogram adherence, this single institution does not capture echocardiograms at outside facilities, as an absence of data does not definitively equate to an absence of care. Nevertheless, the tight follow-up window after the last known anthracycline administration represents a relatively early period when patients would more likely be seen for active follow-up at the primary treatment site. The differences in guideline-based echocardiogram surveillance by race and ethnicity and primary language raise questions of health equity. Although adjustment for sociodemographic or language barriers was limited by cohort size, this observation compels further investigation to the root causes of these disparities to promote equitable survivorship-focused care.
Knowledge gaps for interventions to improve guideline-adherent care for survivors47 highlight the need for clinical informatics approaches, such as SQL and ICD or CPT coding, to facilitate population health–level solutions. The significant heterogeneity in treatment exposures and risk of late effects48-51 underscores the complexity of providing appropriate care for survivors. Real-world data from the EHR serve as scaffolding for future implementation science-based interventions across the oncology continuum, including survivorship,52 through the iterative, evidence-generating dynamic sustainability framework53 to refine clinical practice. While longitudinal cohort studies, such as CCSS,54 offer considerable insight, future advances in survivorship care would benefit from a learning health systems approach given the dynamic nature of pediatric oncology care with recent de-escalation of treatment regimens, newer targeted agents, cardioprotective strategies, and immunotherapy. For cardiomyopathy specifically, review of existing guidelines calls for ongoing evaluation of potential interventions to mitigate late effects.15 Subspecialty clinic attendance significantly improves adherence to cardiomyopathy screening guidelines for adult survivors.55
The structured data elements presented in our analysis for anthracycline exposures and CVD among children with cancer align well with national efforts to advance clinical research informatics. The mCODE initiative seeks to enhance cancer care delivery through conformance to Fast Healthcare Interoperability Resources (FHIR) standards, approved by HL7, to increase data interoperability.28 Using SMART on FHIR provides a level of EHR independence.56 Although the work presented in this study was done using Epic, it should be replicable with any EHR vendor-supporting FHIR. Applications in precision medicine in oncology for clinical decision support57 and recent advances with the SMART/HL7 bulk data access standard58 bolster future directions for population health in pediatric oncology. The integration of cancer registry and EHR data provides a useful framework, albeit the main limitations in our study being the relatively small sample size and lack of robust follow-up time to detect late effects. Longitudinal, interinstitutional collaborations that leverage structured data elements and interoperability within pediatric oncology will be increasingly important as new agents with potential cardiotoxicity change the landscape of survivorship care in the future.59,60
APPENDIX
TABLE A1.
Cardiovascular Outcomes From ICD-9/ICD-10-CM Codesa
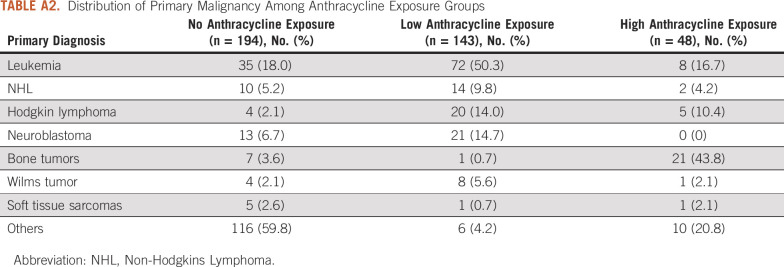
TABLE A2.
Distribution of Primary Malignancy Among Anthracycline Exposure Groups
TABLE A3.
Cause of Death for Anthracycline Exposure Groups
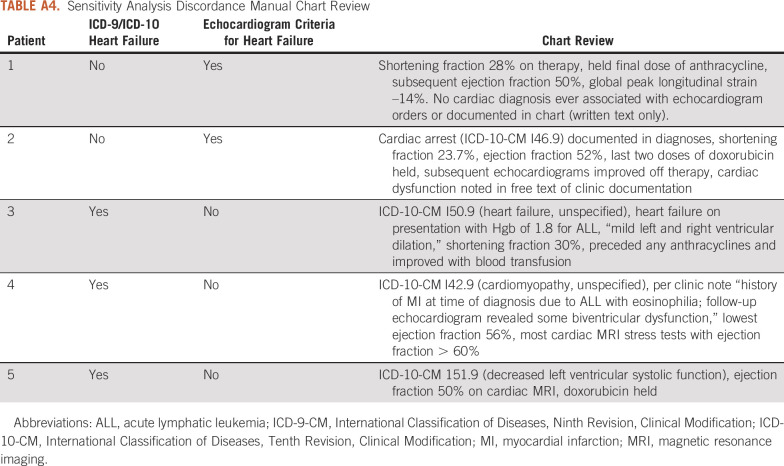
TABLE A4.
Sensitivity Analysis Discordance Manual Chart Review
TABLE A5.
Manual EHR Review of Cancer Registry Patients Without Anthracycline Exposure by Standard Query Language Coding
Steve Power
Stock and Other Ownership Interests: Merck (I)
Susan G. Kreissman
Leadership: Champions Oncology (I), Allterum Therapeutics (I)
Stock and Other Ownership Interests: Champions Oncology (I), Newave Pharmaceutical (I)
Consulting or Advisory Role: Champions Oncology (I), Newave Pharmaceutical (I), Allterum Therapeutics (I), Grid Therapeutics (I), AiCure (I)
Michel Khouri
Employment: Chimerix (I), SpringWorks Therapeutics Inc (I)
Stock and Other Ownership Interests: Chimerix (I), SpringWorks Therapeutics Inc (I)
Consulting or Advisory Role: Pfizer, Eidos Therapeutics, Alnylam Pharmaceuticals Inc
Speakers' Bureau: Alnylam Pharmaceuticals Inc
No other potential conflicts of interest were reported.
SUPPORT
D.H.N. was supported by the NIH Transfusion Medicine and Hematology (5T32 HL007057-44) training grant effective July 1, 2019, to June 30, 2021.
DATA SHARING STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. While the SQL is not allowed to be published within the manuscript due to contractual obligations to Epic, authors can request that the query be published within Epic's query library that is accessible to all Epic users (including researchers).
AUTHOR CONTRIBUTIONS
Conception and design: David H. Noyd, Susan G. Kreissman, Kevin C. Oeffinger, Warren A. Kibbe
Collection and assembly of data: David H. Noyd, Amy Berkman, Claire Howell, Steve Power
Data analysis and interpretation: David H. Noyd, Steve Power, Susan G. Kreissman, Andrew P. Landstrom, Michel Khouri, Kevin C. Oeffinger, Warren A. Kibbe
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Steve Power
Stock and Other Ownership Interests: Merck (I)
Susan G. Kreissman
Leadership: Champions Oncology (I), Allterum Therapeutics (I)
Stock and Other Ownership Interests: Champions Oncology (I), Newave Pharmaceutical (I)
Consulting or Advisory Role: Champions Oncology (I), Newave Pharmaceutical (I), Allterum Therapeutics (I), Grid Therapeutics (I), AiCure (I)
Michel Khouri
Employment: Chimerix (I), SpringWorks Therapeutics Inc (I)
Stock and Other Ownership Interests: Chimerix (I), SpringWorks Therapeutics Inc (I)
Consulting or Advisory Role: Pfizer, Eidos Therapeutics, Alnylam Pharmaceuticals Inc
Speakers' Bureau: Alnylam Pharmaceuticals Inc
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rose-Felker K, Border WL, Hong BJ, et al. : Cardio-oncology related to heart failure: Pediatric considerations for cardiac dysfunction. Heart Fail Clin 13:311-325, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GT, Oeffinger KC, Chen Y, et al. : Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 31:3673-3680, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, et al. : Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst 100:1368-1379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulrooney DA, Armstrong GT, Huang S, et al. : Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: A cross-sectional study. Ann Intern Med 164:93-101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates JE, Howell RM, Liu Q, et al. : Therapy-related cardiac risk in childhood cancer survivors: An analysis of the Childhood Cancer Survivor Study. J Clin Oncol 37:1090-1101, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco JG, Sun CL, Landier W, et al. : Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes—A report from the Children's Oncology Group. J Clin Oncol 30:1415-1421, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asselin BL, Devidas M, Chen L, et al. : Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: A report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 34:854-862, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaikh F, Dupuis LL, Alexander S, et al. : Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: A systematic review and meta-analysis. J Natl Cancer Inst 108:djv357, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Mulrooney DA, Hyun G, Ness KK, et al. : Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: Report from the Childhood Cancer Survivor Study cohort. BMJ 368:l6794, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz WM, Kelli HM, Lisko JC, et al. : Socioeconomic status and cardiovascular outcomes: Challenges and interventions. Circulation 137:2166-2178, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antwi GO, Jayawardene W, Lohrmann DK, et al. : Physical activity and fitness among pediatric cancer survivors: A meta-analysis of observational studies. Support Care Cancer 27:3183-3194, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Chow EJ, Oeffinger KC, et al. : Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst 112:256-265, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Children's Oncology Group : Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5.0. Monrovia, CA, Children's Oncology Group, 2018 [Google Scholar]
- 14.Kremer LCM, Mulder RL, Oeffinger KC, et al. : A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 60:543-549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armenian SH, Hudson MM, Mulder RL, et al. : Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 16:e123-e136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrhardt MJ, Ward ZJ, Liu Q, et al. : Cost-effectiveness of the International Late Effects of Childhood Cancer Guideline Harmonization Group screening guidelines to prevent heart failure in survivors of childhood cancer. J Clin Oncol 38:3851-3862, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong FL, Bhatia S, Landier W, et al. : Cost-effectiveness of the Children's Oncology Group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med 160:672-683, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leong SL, Chaiyakunapruk N, Lee SW: Candidate gene association studies of anthracycline-induced cardiotoxicity: A systematic review and meta-analysis. Sci Rep 7:39, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McOwan TN, Craig LA, Tripdayonis A, et al. : Evaluating anthracycline cardiotoxicity associated single nucleotide polymorphisms in a paediatric cohort with early onset cardiomyopathy. Cardiooncology 6:5, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrykey K, Andelfinger GU, Laverdière C, et al. : Genetic factors in anthracycline-induced cardiotoxicity in patients treated for pediatric cancer. Expert Opin Drug Metab Toxicol 16:865-883, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Aminkeng F, Ross CJ, Rassekh SR, et al. : Recommendations for genetic testing to reduce the incidence of anthracycline-induced cardiotoxicity. Br J Clin Pharmacol 82:683-695, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatia S: Genetics of anthracycline cardiomyopathy in cancer survivors: JACC: CardioOncology state-of-the-art review. JACC CardioOncol 2:539-552, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman CP, Wong AK, Blumenthal D: Achieving a nationwide learning health system. Sci Transl Med 2:57cm29, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Bertagnolli MM, Anderson B, Norsworthy K, et al. : Status update on data required to build a learning health system. J Clin Oncol 38:1602-1607, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffee EM, Dang CV, Agus DB, et al. : Future cancer research priorities in the USA: A Lancet Oncology Commission. Lancet Oncol 18:e653-e706, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute : Childhood cancer data initiative. https://www.cancer.gov/research/areas/childhood/childhood-cancer-data-initiative
- 27.Barnholtz-Sloan JS, Rollison DE, Basu A, et al. : Cancer Informatics for Cancer Centers (CI4CC): Building a community focused on sharing ideas and best practices to improve cancer care and patient outcomes. JCO Clin Cancer Inform 4:108-116, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osterman TJ, Terry M, Miller RS: Improving cancer data interoperability: The promise of the minimal common oncology data elements (mCODE) initiative. JCO Clin Cancer Inform 4:993-1001, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noyd DH, Neely NB, Schroeder KM, et al. : Integration of cancer registry and electronic health record data to construct a childhood cancer survivorship cohort, facilitate risk stratification for late effects, and assess appropriate follow-up care. Pediatr Blood Cancer 68:e29014, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Boffa DJ, Rosen JE, Mallin K, et al. : Using the National Cancer Database for outcomes research: A review. JAMA Oncol 3:1722-1728, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Horvath MM, Rusincovitch SA, Brinson S, et al. : Modular design, application architecture, and usage of a self-service model for enterprise data delivery: The Duke Enterprise Data Unified Content Explorer (DEDUCE). J Biomed Inform 52:231-242, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steliarova-Foucher E, Stiller C, Lacour B, et al. : International Classification of Childhood Cancer, third edition. Cancer 103:1457-1467, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Lannou S, Mansencal N, Couchoud C, et al. : The public health burden of cardiomyopathies: Insights from a nationwide inpatient study. J Clin Med 9:920, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khera R, Kondamudi N, Zhong L, et al. : Temporal trends in heart failure incidence among Medicare beneficiaries across risk factor strata, 2011 to 2016. JAMA Netw Open 3:e2022190, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen LA, Yood MU, Wagner EH, et al. : Performance of claims-based algorithms for identifying heart failure and cardiomyopathy among patients diagnosed with breast cancer. Med Care 52:e30-e38, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czaja AS, Collins K, Valuck RJ, et al. : Validity of administrative claims-based algorithms for ventricular arrhythmia and cardiac arrest in the pediatric population. Pharmacoepidemiol Drug Saf 29:1499-1503, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Feijen EAM, Leisenring WM, Stratton KL, et al. : Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol 5:864-871, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balasubramanian S, Kipps AK, Smith SN, et al. : Pediatric echocardiography by work relative value units: Is study complexity adequately captured? J Am Soc Echocardiogr 29:1084-1091, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Minor BL, et al. : The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95:103208, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377-381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clemens E, van den Heuvel-Eibrink MM, Mulder RL, et al. : Recommendations for ototoxicity surveillance for childhood, adolescent, and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCare Consortium. Lancet Oncol 20:e29-e41, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skinner R, Mulder RL, Kremer LC, et al. : Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol 18:e75-e90, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Adekkanattu P, Jiang G, Luo Y, et al. : Evaluating the portability of an NLP system for processing echocardiograms: A retrospective, multi-site observational study. AMIA Annu Symp Proc 2019:190-199, 2019 [PMC free article] [PubMed] [Google Scholar]
- 44.Nath C, Albaghdadi MS, Jonnalagadda SR: A natural language processing tool for large-scale data extraction from echocardiography reports. PLoS One 11:e0153749, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Lee S, Martin E, et al. : Enhancing ICD-code-based case definition for heart failure using electronic medical record data. J Card Fail 26:610-617, 2020 [DOI] [PubMed] [Google Scholar]
- 46.Kilic A: Artificial intelligence and machine learning in cardiovascular health care. Ann Thorac Surg 109:1323-1329, 2020 [DOI] [PubMed] [Google Scholar]
- 47.Zabih V, Kahane A, O'Neill NE, et al. : Interventions to improve adherence to surveillance guidelines in survivors of childhood cancer: A systematic review. J Cancer Surviv 13:713-729, 2019 [DOI] [PubMed] [Google Scholar]
- 48.Frobisher C, Glaser A, Levitt GA, et al. : Risk stratification of childhood cancer survivors necessary for evidence-based clinical long-term follow-up. Br J Cancer 117:1723-1731, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edgar AB, Duffin K, Borthwick S, et al. : Can intensity of long-term follow-up for survivors of childhood and teenage cancer be determined by therapy-based risk stratification? BMJ Open 3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace WH, Blacklay A, Eiser C, et al. : Developing strategies for long term follow up of survivors of childhood cancer. BMJ 323:271-274, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eiser C, Absolom K, Greenfield D, et al. : Follow-up after childhood cancer: Evaluation of a three-level model. Eur J Cancer 42:3186-3190, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Mitchell SA, Chambers DA: Leveraging implementation science to improve cancer care delivery and patient outcomes. J Oncol Pract 13:523-529, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chambers DA, Glasgow RE, Stange KC: The dynamic sustainability framework: Addressing the paradox of sustainment amid ongoing change. Implement Sci 8:117, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robison LL, Armstrong GT, Boice JD, et al. : The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol 27:2308-2318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marr KC, Agha M, Sutradhar R, et al. : Specialized survivor clinic attendance increases adherence to cardiomyopathy screening guidelines in adult survivors of childhood cancer. J Cancer Surviv 11:614-623, 2017 [DOI] [PubMed] [Google Scholar]
- 56.Mandel JC, Kreda DA, Mandl KD, et al. : SMART on FHIR: A standards-based, interoperable apps platform for electronic health records. J Am Med Inform Assoc 23:899-908, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warner JL, Rioth MJ, Mandl KD, et al. : SMART precision cancer medicine: A FHIR-based app to provide genomic information at the point of care. J Am Med Inform Assoc 23:701-710, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu D, Sahu R, Ignatov V, et al. : High performance computing on flat FHIR files created with the new SMART/HL7 bulk data access standard. AMIA Annu Symp Proc 2019:592-596, 2019 [PMC free article] [PubMed] [Google Scholar]
- 59.Moslehi JJ: Cardiovascular toxic effects of targeted cancer Therapies. N Engl J Med 375:1457-1467, 2016 [DOI] [PubMed] [Google Scholar]
- 60.Chow EJ, Leger KJ, Bhatt NS, et al. : Paediatric cardio-oncology: Epidemiology, screening, prevention, and treatment. Cardiovasc Res 115:922-934, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. While the SQL is not allowed to be published within the manuscript due to contractual obligations to Epic, authors can request that the query be published within Epic's query library that is accessible to all Epic users (including researchers).