PURPOSE
Early detection of cancer risk is essential as it is associated with a higher chance of survival, more successful treatment, and improved quality of life. Genetic testing helps at-risk patients estimate the likelihood of developing cancer in a lifetime. This study aims to indentify the factors (perceived susceptibility, severity, benefits, and self-efficacy) that impact one's decision to take the genetic test.
METHODS
We examined the impacts of different factors of the health belief model on the engagement of patients in genetic testing using data from the National Cancer Institute's 2020 cross-sectional nationally representative data published in 2021. Complete surveys were answered by 3,865 participants (weighted population size = 253,815,197). All estimates were weighted to be nationally representative of the US population using the jackknife weighting method for parameter estimation. We used multivariable logistic regression to test our hypotheses for patients who have taken the genetic test for cancer risk detection. We adjusted the multivariate model for age, education, income, race, sex, cancer history, familial cancer history, and education.
RESULTS
We tested five hypotheses using the health belief model. Respondents who had genetic testing were more likely to rely on their health care providers and genetic counselors to make their decisions. Respondents who had genetic tests also reported less reliability on other sources than doctors: for the internet and social media (odds ratio = 0.33; P < .001) and for journals and magazines (odds ratio = 0.48; P = .007).
CONCLUSION
The findings show that patients generally rely on suggestions from their health care providers and counselors in genetic testing decisions. These findings also indicate that health care providers play a critical role in helping patients decide whether to use genetic testing to detect cancer risk in the early stages.
INTRODUCTION
Genetic testing can encourage cancer preventive strategies by identifying gene mutations, particularly among individuals with a family cancer history who are typically prone to genetic ailments.1-5 Technological advancements have allowed genetic testing to be a viable option for cancer risk prediction.
CONTEXT
Key Objective
Using the health belief model to investigate the factors that affect engagement in genetic testing for cancer risk detection among patients who are at risk of developing cancer.
Knowledge Generated
We found that genetic counselors and health care providers are the most reliable source of information influencing patients’ decisions to take genetic testing for cancer risk detection.
Relevance
Our study provides information that can support genetic counselors and health care providers in their efforts to sensitize patients who are prone to developing cancer about the importance of early screening and the role of genetic testing in decreasing the probability of cancer development and facilitating personalized treatments.
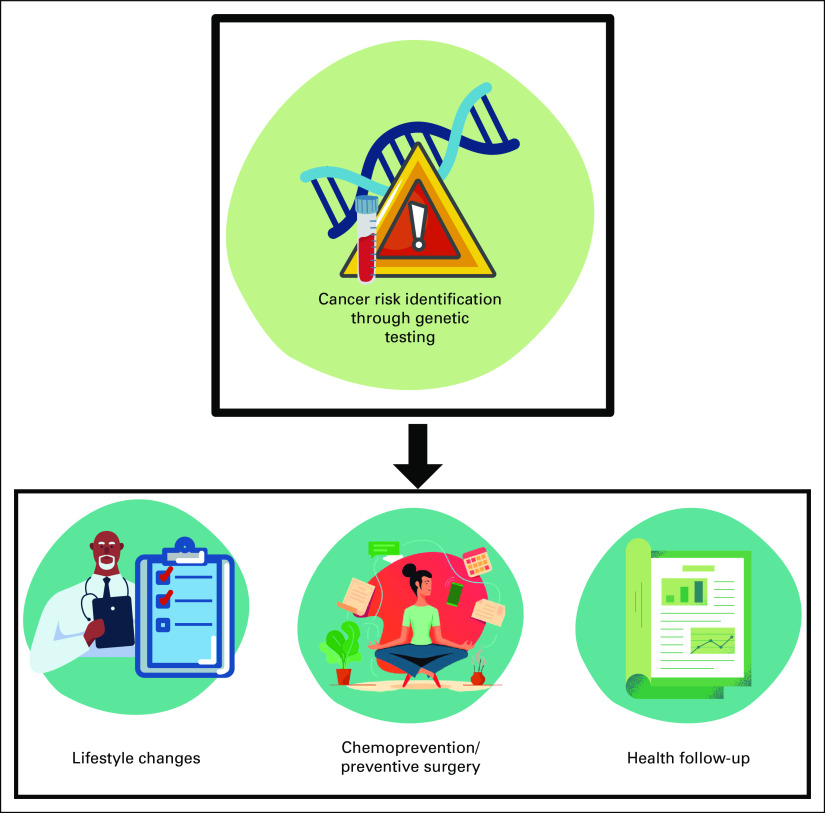
Prediagnostic genetic testing enables individuals to take surveillance and preventative measures and protocols, including enhanced screening modalities (eg, mammography, colonoscopy, etc) and prophylactic preventive treatments (eg, prophylactic surgical procedures or chemoprevention).6 In addition, genetic testing can support early disease detection of cancer before it progresses and becomes difficult to treat6 and therefore might help reduce mortality and control costs.7 Figure 1 summarizes some of the importance and potential role of genetic testing in prediagnostic scenarios.
FIG 1.
Prediagnostic genetic testing.
Despite the importance of genetic testing, not many are willing to participate in this practice.8 Moreover, not much research has been dedicated to capturing the factors that influence one's decision to take a genetic test. Mistrust of medical research, limited access to genetic counseling, and cost have been identified by the literature as factors responsible for disparities in genetic services.9 Technical barriers such as data storage8 and data-sharing issues10 also play a significant role. Many people hesitate to participate in genetic testing as they may lack an understanding of the risks and benefits of the genetic testing.11
Several other factors can influence the intention to participate in genetic testing, explained by the health belief model (HBM). HBM is an expectancy-value theory developed originally to explain adherence to preventive health care regimens and predict treatment compliance.12 The HBM specifies that three clusters of subjective beliefs determine the likelihood that health-protective behavior will be undertaken.13 Knowing an individual’s sense of perceived threat of developing a disease and the perceived benefits and barriers provides a better understanding of some of the beliefs and attitudes that determine a behavior's adoption. This knowledge also contributes to developing interventions designed to increase the behavior adoption rate among patients. In addition, individuals' adherence to generic testing may be affected by their degree of confidence in genetic testing and the patients' perceived self-efficacy.13 Therefore, this study explores four hypotheses testing the impact of perceived susceptibility, severity, benefits, and self-efficacy on patients' decisions to take a genetic test.
Hypothesis 1 (H1): The patients' perceived severity is associated with their decision to do genetic testing.
Hypothesis 2 (H2): The patients' perceived benefits or barriers are associated with their decision to do genetic testing.
Hypothesis 3 (H3): The patients' perceived susceptibility is associated with their decision to do genetic testing.
Hypothesis 4 (H4): The patients' perceived self-efficacy and the source that they get information from (cues to action) are associated with their decision to do genetic testing.
METHODS
Data Source
We used data from the Health Information National Trends Survey (HINTS) data repository. The surveys are collected by the National Cancer Institute to provide updates on health communication usage and practices across the US population. Information about the HINTS framework and methodology is available elsewhere.14
Data Analysis
First, descriptive analysis statistics were conducted using demographic attributes and the other factors that we considered following the HBM components for this study. We used weighted data for our analysis to correct for nonbias responses using a jackknife method.15 We estimated the proportions of respondents who undertook the genetic test on the basis of their sources of information for testing, health knowledge, and perception of genetic testing. The analysis also controlled for potential confounding factors such as demographics, income, cancer history, and family cancer history. The control variables were used as covariates in the regression model. We implemented chi-square tests of independence using replicate weights, which were analyzed using the R Survey package with type JKn to include the weight samples across the data set. We used a multivariable logistic regression model to test our hypotheses for patients who undertook the genetic test, adjusting for the covariates.
Measures
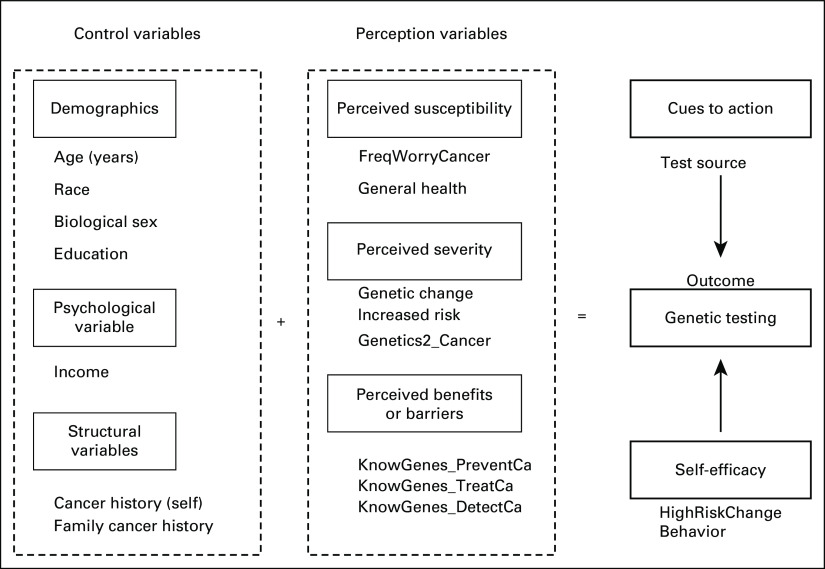
Figure 2 summarizes all the categorized variables used in this study as model measures. The outcome variable is the health-promoting behavior that an HBM model tests the person's aptitude to engage in. In our case, we consider GeneticTesting as the outcome (yes, no) to know whether a person has ever done a genetic test.
FIG 2.
The conceptual framework of the health belief model applied to patients’ decisions about genetic testing.
Control variables (modifying variable) are demographics, cancer history, family cancer history, and psychological and structural variables that indirectly affect perceptions of health-related behaviors by affecting perceived seriousness, susceptibility, benefits, and barriers.16
In this study, demographic attributes are age (18-34, 35-44, 45-64, and ≥ 65 years), sex (male and female), race (Hispanic, White, Black, and Others), and education (≤high school, ongoing college, and graduated from college or more). The psychological variable considered was income (high-income population and low-income population). For the structural variables, we considered family cancer history (FamiliarFamilyCancer2) and cancer history to check whether the respondent was ever diagnosed with cancer and the family history of cancer (yes, no).
Perceived susceptibility variables refer to a person's subjective perception of the risk of acquiring cancer. We consider the variable GeneralHealth as a general indicator of how the patients perceive their health status (good, bad) and the variable FreqWorryCancer as characterizing whether a respondent is worried about getting cancer (yes, no).
Perceived severity variables refer to a person's feelings on the seriousness of contracting cancer. The variables are (1) GeneticChangeIncreasedRisk, which characterizes the readiness of respondents to know that they have a genetic change that increases their chances of getting cancer (yes, no), and (2) Genetics2_Cancer, which investigates how much a person thinks that inherited genes can determine whether they will develop cancer (yes, no).
Perceived benefits/barriers variables are variables that approach how a person perceives the various actions taken to reduce the threat of the disease or augment it. In our study, we consider the three variables KnowGenes_PreventCa, KnowGenes_TreatCa, and KnowGenes_DetectCa, respectively, to determine how much knowing genetic information is essential for preventing and detecting cancer (yes, no).
Cues to action variables characterize the triggers that stimulate decision making regarding a recommended health action.17 We consider, in this case, the variable that we created and called TestSource. This variable combines many questions that try to identify the source of information from which the person heard, read, or learned about genetic testing (family or friend, health care provider or genetic counselor, social media or internet, and journals or magazines).
Self-efficacy variables characterize the person's perception of their ability to perform the recommended behavior in a successful way.18 The variable considered here to evaluate that is HighRiskChangeBehavior which asks this question: “Do you agree with the statement that if you found out from a genetic test that you were at high risk of cancer, you would change your behaviors such as diet, exercise, and getting routine medical tests?” (yes, no). Please see the Data Supplement for all of the used survey questions.
RESULTS
Descriptive Statistics
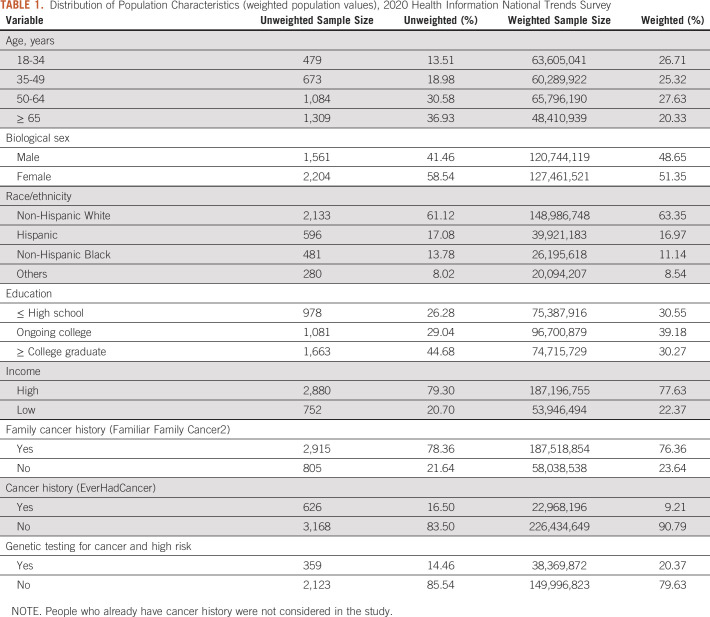
There were 3,865 participants (weighted population size = 253,815,197) who were offered genetic testing in health care settings. Table 1 shows the demographics of the respondents included in the study. A total of 2,393 (67.51%) respondents were above 50 years old, and 2,133 (61.12%) were White (non-Hispanic). One thousand six hundred sixty-three (44.68%) respondents had college graduation or more, and 79.30% belonged to the high-income population. Among these participants, 20.37% only accepted to undertake the genetic test. We focused on this population to understand the factors that encourage them to decide compared with those who did not undertake any cancer genetic test.
TABLE 1.
Distribution of Population Characteristics (weighted population values), 2020 Health Information National Trends Survey
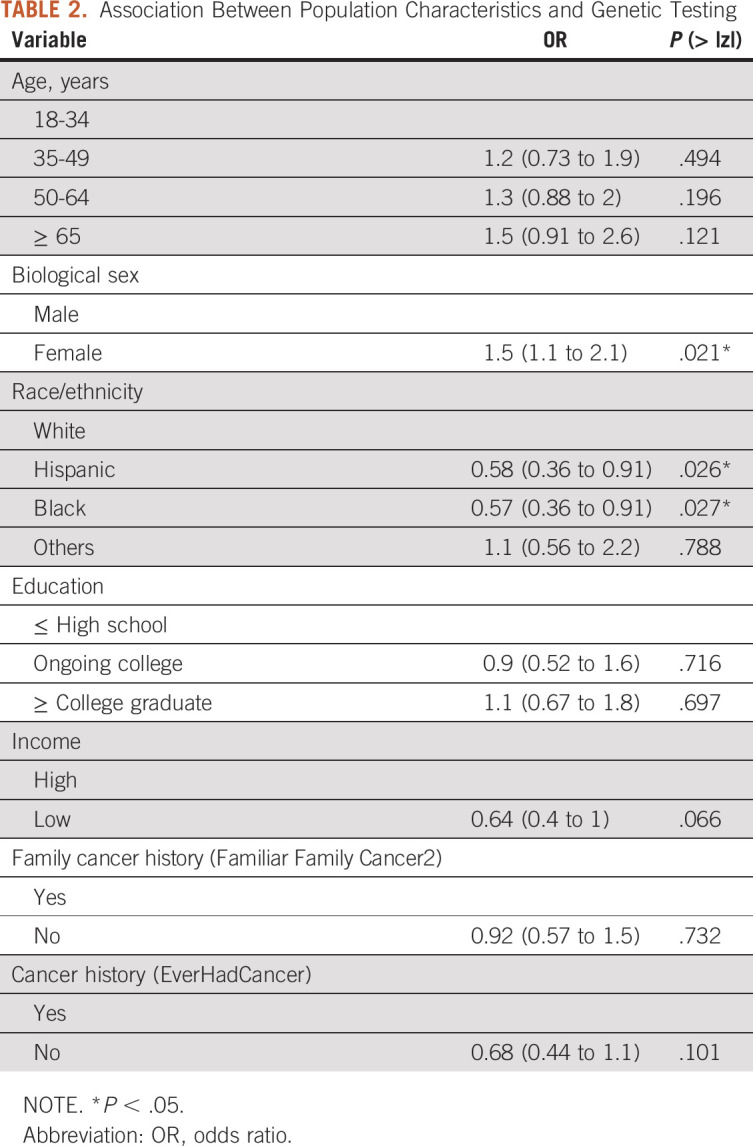
Control Variables
According to the analysis shown in Table 2, sex and race were significant predictors of patients' decisions to do genetic testing. Females were significantly more likely to take the genetic tests than males (odds ratio [OR] = 1.55; P = .021). Non-Hispanic Black and Hispanic populations are less likely to take genetic tests than non-Hispanic White patients (OR = 0.57, P = .027; OR = 0.58, P = .026, respectively). Income, family cancer history, and cancer history were not significantly associated with genetic testing decisions (P = .06, P = .73, and P = .10, respectively).
TABLE 2.
Association Between Population Characteristics and Genetic Testing
Perceived Severity
As illustrated in Table 3, we did not find a significant influence of perceived severity on taking genetic testing. So, we fail to accept H1. Two variables represent perceived severity: genetic change increased risk (P = .395) and Genetics2_Cancer (P = .253).
TABLE 3.
Association Between Population Perception, Self-Efficacy, and Cues to Action, and Genetic Testing
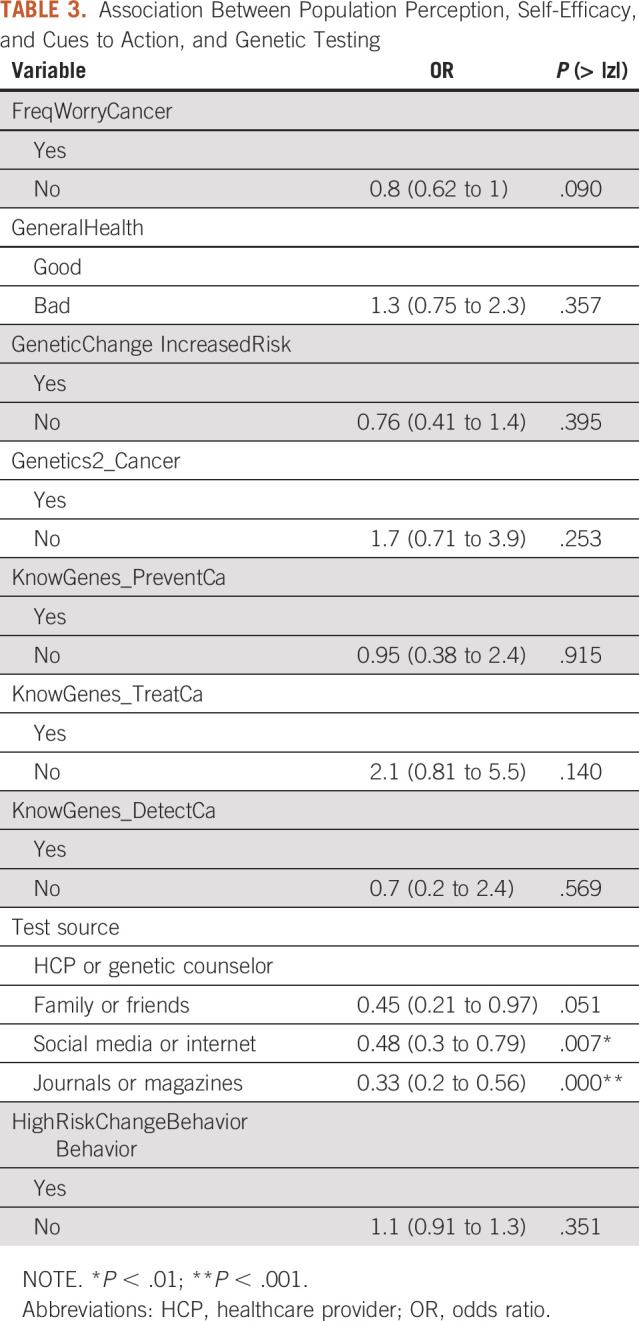
Perceived Benefits and Barriers
As shown in Table 3, knowledge about how much genetic information can help prevent and detect cancer was not correlated with the decision to take the genetic tests (P = .915, P = .140, and P = .589, respectively). We thus fail to accept H2.
Perceived Susceptibility
There was no significant correlation between perceived susceptibility and the decision to undertake the genetic test (Table 3). Worrying about having cancer did not affect patients' decisions (P = .09). The general health perception was also not correlated with the decision to take the genetic test (P = .357). Therefore, we fail to accept H3.
Cues to Action
The results also showed that providers and genetic counselors are more likely to affect the patients' decision to take genetic testing. People who take genetic tests seek out information from more reliable sources such as health care providers and genetic counselors rather than from family and friends, the internet and social media (OR = 0.33, P < .001), or journals and magazines (OR = 0.48, P = .007). Therefore, we fail to reject H4.
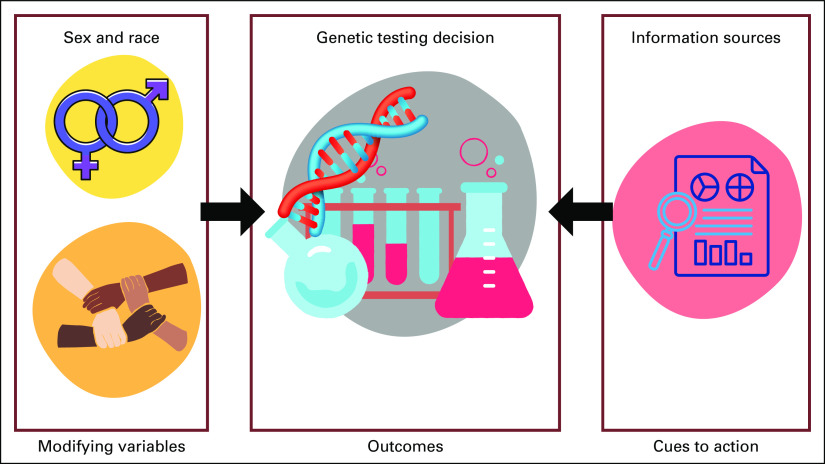
With these results, we updated the health belief model components on the basis of the accepted hypotheses. To sum up, the demographic variables and the source of treatment-related information affect the patient's decision to take the genetic tests significantly. Figure 3 illustrates the significant findings.
FIG 3.
Evaluation of the conceptual framework of the health belief model applied on the genetic testing.
DISCUSSION
Genetic testing is typically performed in presymptomatic circumstances to determine a hereditary predisposition or risk of relapse.19 Its medical value in some clinical settings is helpful, especially with cancer, which often involves long-term treatments, care involving different health professionals, expensive drugs, access to medical equipment, and putting an enormous burden on society. This study uses nationwide data to capture the significant impact of race, biological sex, and information source on patients' decision to undertake genetic testing for cancer risk screening. The novelty of this study lies in the use of well-established HBM.
According to our study, patients typically receive information about genetic testing from various sources, including health care providers, genetic counselors, friends, the internet, and magazines. However, our study showed that patients who received the genetic tests for health risk and cancer were more likely to receive guidance from genetic counselors or health care providers. In other words, our study highlights the importance of structured guidance and expert information in engaging or motivating patients to perform essential tasks. But recently, different companies offering services or products related to genetic tests started advertising them using all means of communication, including radio, magazines, television, and the internet. Therefore, the role of informing individuals about the importance of genetic testing in medicine is not limited to the professionals anymore, and secondary information sources can play an important role, particularly when access to experts is limited. It has become easier for patients looking for health-related information to obtain all they need to know to build their knowledge about this market without professional help.20 However, the lack of professional guidance may contribute to confusion and thus nonengagement in genetic testing.
The study also identifies gender disparities in genetic testing. According to our findings, females were significantly more likely to take the genetic tests than males. Similar results were reported in a study according to which, in 2015, females received three times as many genetic testing as men (73% v 27%; P < .001). The disparity persisted irrespective of cancer diagnosis.21 The gender disparity in genetic testing was also discussed in the 2021 Annual Meeting of American Society of Clinical Oncology (ASCO), where a study involving 1,320 patients (664 men and 656 women) found that only 19.3% of men were referred to genetics for cancer screening compared with 80.3% of women. Moreover, 32.8% of men who were referred did not complete the screening compared with 13.7% of women.22
Disparities were also significant among minorities. According to our study, non-Hispanic Black and Hispanic populations were less likely to have taken genetic tests than non-Hispanic White patients. Such disparities may prevail because of various factors not limited to insurance coverage, access to health care, awareness, history of family cancer, and most importantly, referrals. For example, according to a 2021 study, non-Hispanic White patients are more likely to be referred to genetic testing because of family cancer history than all other ethnicities.23 Although our study did not find a significant association between education and the decision to undertake genetic testing, previous studies have reported their significance. A study reported a strong association between race and knowledge about genetic testing.24 According to the study, non-Hispanic Black patients and Hispanic patients had significantly lower knowledge compared with non-Hispanic White patients. Other studies have identified a lack of trust in the medical community's potential use of genetic information as a factor preventing minorities from undertaking genetic testing.25
We also hypothesized that individuals worried about cancer should be more motivated to gather all possible health-related information through genetic testing to manage cancer risk. However, the decision making in cancer is a complex nonlinear process that is affected by not only personal knowledge but also emotions.26 However, emotions (anxiety, uncertainty, etc) may prevail, affecting the thinking process and, therefore, the decisions related to life-critical situations. For instance, the risk perception of genetic testing between individuals with a family history of cancer and those without previous family experience of the disease was demonstrated to have no differences in levels of concerns.27 Supporting the literature, our study reported no significant impact of family history of cancer on the decision to undertake genetic testing.
In summary, patients should be encouraged and provided resources to help them decide to undergo genetic testing that may reveal cancer and health risk. Pretest counseling should also include education on the limitations of current genetic testing technology, including the risk of false-negative results, and the uncertainties associated with genetic variants of unknown significance. Furthermore, we should strive to enhance individual empowerment and shared decision making and promote a model that is not opposed to the implementation of genetic testing but imposes informational commitments upon the companies that offer them. Our findings can guide genetic counselors, health care providers, and oncologists as they are the main impactors of at-risk patients' opinions and decisions. To improve the genetic testing rate and improve patients' awareness about the importance of genetic tests, especially in the early diagnosis and prevention of cancer, health care staff can emphasize salient factors in their strategic plans to educate and sensitize patients about the early prevention benefits and the importance of the role of genetic testing in diagnosis and treatment paths.
It is important to point out some limitations of this study. Some variables that could influence the psychobehavioral impact of genetic testing were not included. The examination of trait measures like risk propensity, health literacy, and numeracy could have provided more information on how patients make health care decisions. In addition, future studies need to consider that consumers' attitudes toward genetic testing differ by country, on the basis of cultural differences. One limitation of the HBM is that it does not account for environmental factors that may prohibit or promote the recommended action. Future research would also benefit from investigating this topic in different contexts, accounting for patients' comorbidities, different clinical history, or other diseases.
In conclusion, recent scientific breakthroughs and technological advances have improved our understanding of cancer and how we diagnose and treat it, leading to more precise, predictable, robust, and customized health care for the individual patient. It is imperative to leverage new technologies to generate new data and support the application and advancement of genetic testing. Future studies need to investigate possible factors influencing patients' reactions to predictive genetic testing on the basis of different populations' characteristics. To help empower patients at risk to make informed decisions about genetic testing, health counselors play a critical role in educating their patients about cancer risks and informing them through a reliable source of information that they trust.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Safa Elkefi, Avishek Choudhury
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/cci/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Daly MB, Pal T, Berry MP, et al. : Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:77-102, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Lancaster JM, Powell CB, Chen LM, et al. : Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 136:3-7, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Chen M, Luo D: A CRISPR path to cutting-edge materials. N Engl J Med 382:85-88, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Nassar SF, Raddassi K, Ubhi B, et al. : Precision medicine: Steps along the road to combat human cancer. Cells 9:2056, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Easton DF, Pharoah PD, Antoniou AC, et al. : Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 3723:2243-2257, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute : Genetic Testing Fact Sheet, 2017. https://www.cancer.gov/about-cancer/causes-prevention/genetics/genetic-testing-fact-sheet [Google Scholar]
- 7.Guillem JG, Wood WC, Moley JF, et al. : ASCO/SSO review of current role of risk-reducing surgery in common hereditary cancer syndromes. J Clin Oncol 248:4642-4660, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Phillips KA, Deverka PA, Hooker GW, et al. : Genetic test availability and spending: Where are we now? Where are we going? Health Aff (Millwood) 37:710-716, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salloum RG, George TJ, Silver N, et al. : Rural-urban and racial-ethnic differences in awareness of direct-to-consumer genetic testing. BMC Public Health 18:277, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landry LG, Ali N, Williams DR, et al. : Lack of diversity in genomic databases is A barrier to translating precision medicine research into practice. Health Aff (Millwood) 37:780-785, 2018 [DOI] [PubMed] [Google Scholar]
- 11.National Library of Medicine, National Institute of Health : What are some of the challenges facing precision medicine and the Precision Medicine Initiative. MedlinePlus Trusted Health Information for You, 2020. https://medlineplus.gov/genetics/understanding/precisionmedicine/challenges/ [Google Scholar]
- 12.Janz NK, Becker MH: The health belief model: A decade later. Health Educ Q 11:1-47, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui TR, Ghazal S, Bibi S, et al. : Use of the health belief model for the assessment of public knowledge and household preventive practices in Karachi, Pakistan, a dengue-endemic city. PLoS Negl Trop Dis 101:e0005129, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westat : Health Information National Trends Survey 4 (HINTS 4), Cycle 4 Methodology Report. National Cancer Institute, 2015. https://hints.cancer.gov/docs/HINTS_4_Cycle_4_Methodology_Report.pdf [Google Scholar]
- 15.Heffner JL, Mull KE: Smartphone ownership among US adult cigarette smokers: 2014 Health Information National Trends Survey (HINTS) data. J Med Internet Res 19:e305, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glanz K, Rimer BK, Viswanath K: Health Behavior and Health Education: Theory, Research, and Practice. San Francisco, CA, Jossey-Bass, 2008, pp 45-51 [Google Scholar]
- 17.Carpenter CJ: A meta-analysis of the effectiveness of health belief model variables in predicting behavior. Health Commun 25:661-669, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Chhatre S, Wittink MN, Gallo JJ, et al. : Sources of information for learning and decision-making in men with localized prostate cancer. Am J Mens Health 14:1557988320945461, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliveri S, Pravettoni G: The disclosure of direct-to-consumer genetic testing: Sounding out the psychological perspective of consumers. Biol Med 8, 2016 [Google Scholar]
- 20.Sun L, Brentnall A, Patel S, et al. : A cost-effectiveness analysis of multigene testing for all patients with breast cancer. JAMA Oncol 5:1718-1730, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Childers KK, Maggard-Gibbons M, Macinko J, et al. : National distribution of cancer genetic testing in the United States: Evidence for a gender disparity in hereditary breast and ovarian cancer. JAMA Oncol 4:876-879, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith W, Smith K, Sessions W, et al. : An evaluation of gender discrepancies in genetic referrals for BRCA testing for indicated malignancies. J Clin Oncol 39, 2021. (suppl; abstr 10584) [Google Scholar]
- 23.Chapman-Davis E, Zhou ZN, Fields JC, et al. : Racial and ethnic disparities in genetic testing at a hereditary breast and ovarian cancer center. J Gen Intern Med 36:35-42, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suther S, Kiros GE: Barriers to the use of genetic testing: A study of racial and ethnic disparities. Genet Med 11:655-662, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Saulsberry K, Terry SF: The need to build trust: A perspective on disparities in genetic testing. Genet Test Mol biomarkers 17:647-648, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzocco K, Masiero M, Carriero MC, et al. : The role of emotions in cancer patients' decision-making. Ecancermedicalscience 13:914, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ertmański S, Metcalfe K, Trempała J, et al. : Identification of patients at high risk of psychological distress after BRCA1 genetic testing. Genet Test Mol Biomarkers 13:325-330, 2009 [DOI] [PubMed] [Google Scholar]