PURPOSE
In metastatic triple-negative breast cancer (mTNBC), consistent biomarkers of immune checkpoint inhibitor (ICI) therapy benefit remain elusive. We evaluated the immune, genomic, and transcriptomic landscape of mTNBC in patients treated with ICIs.
METHODS
We identified 29 patients with mTNBC treated with pembrolizumab or atezolizumab, either alone (n = 9) or in combination with chemotherapy (n = 14) or targeted therapy (n = 6), who had tumor tissue and/or blood available before ICI therapy for whole-exome sequencing. RNA sequencing and CIBERSORTx-inferred immune population analyses were performed (n = 20). Immune cell populations and programmed death-ligand 1 expression were assessed using multiplexed immunofluorescence (n = 18). Clonal trajectories were evaluated via serial tumor/circulating tumor DNA whole-exome sequencing (n = 4). Association of biomarkers with progression-free survival and overall survival (OS) was assessed.
RESULTS
Progression-free survival and OS were longer in patients with high programmed death-ligand 1 expression and tumor mutational burden. Patients with longer survival also had a higher relative inferred fraction of CD8+ T cells, activated CD4+ memory T cells, M1 macrophages, and follicular helper T cells and enrichment of inflammatory gene expression pathways. A mutational signature of defective repair of DNA damage by homologous recombination was enriched in patients with both shorter OS and primary resistance. Exploratory analysis of clonal evolution among four patients treated with programmed cell death protein 1 blockade and a tyrosine kinase inhibitor suggested that clonal stability post-treatment was associated with short time to progression.
CONCLUSION
This study identified potential biomarkers of response to ICIs among patients with mTNBC: high tumor mutational burden; presence of CD8+, CD4 memory T cells, follicular helper T cells, and M1 macrophages; and inflammatory gene expression pathways. Pretreatment deficiencies in the homologous recombination DNA damage repair pathway and the absence of or minimal clonal evolution post-treatment may be associated with worse outcomes.
INTRODUCTION
Triple-negative breast cancer (TNBC) has an aggressive clinical course with high rates of metastatic recurrence within 2-3 years of diagnosis.1,2 Until recently, patients with metastatic TNBC (mTNBC) were treated with sequential chemotherapy regimens, which produce a median overall survival (OS) of 13-18 months.3,4
CONTEXT
Key Objective
The biology underlying immune checkpoint inhibitor (ICI) responsiveness in metastatic triple-negative breast cancer (mTNBC) remains largely unknown. We performed genomic analysis of TNBC tumors to investigate molecular determinants of benefit or resistance to ICI in mTNBC.
Knowledge Generated
Patients with longer survival outcomes more frequently had programmed death-ligand 1–positive tumors and higher median mutational burden. Tumors from durable responders had a higher relative fraction of follicular helper T cells and activated CD4+ memory T cells and a higher expression of genes involved in the inflammatory response. Reductions in cancer cell fractions of primary clones and those bearing strong immunogenic targets or driver genes were associated with ICI benefit.
Relevance
Even in the setting of deep multiomic characterization, no single biomarker performs optimally to predict ICI benefit in mTNBC. Composite biomarkers will likely be required to achieve this goal. Alternatively, an early biomarker of response, including circulating tumor DNA change, may offer a functional readout.
The addition of immune checkpoint inhibitors (ICIs) to chemotherapy in the first-line setting for patients with programmed death-ligand 1 (PD-L1)–positive mTNBC has improved progression-free survival (PFS) and OS and has been considered the standard treatment for this population.5-8 However, not all these patients benefit from this approach, and response rates are lower in patients who have received prior therapy in the metastatic setting.9,10 Furthermore, there are questions about the broad utility of PD-L1 testing, including reproducibility, and it is understood that PD-L1 positivity does not explain all the immunogenicity of breast cancer. Moreover, PD-L1 status is not predictive of benefit of immunotherapy in the neoadjuvant setting.
Recent voluntary withdrawal of atezolizumab from the market reinforces the critical importance to identify more robust biomarkers for ICI benefit to guide therapy within this population. Although the biology underlying ICI resistance in TNBC remains largely unknown and there is a paucity of genomic data from patients who received ICI, in this study, we performed genomic analysis of TNBC tumors, with the objective of investigating the molecular determinants of benefit or resistance to ICI in patients with mTNBC.
METHODS
Study Cohort and Clinical Annotation
All patients with confirmed mTNBC, as defined by American Society of Clinical Oncology/College of American Pathologists guidelines, were retrospectively included if they had tumor tissue available and were treated with programmed cell death protein 1 (PD-1)/PD-L1 inhibitors as monotherapy or combined with chemotherapy or targeted therapy at the Dana-Farber Cancer Institute (Boston, MA). This project received approval from the Dana-Farber/Harvard Cancer Center Institutional Review Board (DF/HCC Protocols #05-246 and #13-364) and was conducted in accordance with the ethical guidelines outlined by the Belmont Report.
Patient charts were reviewed to determine the temporal relationship between available biopsy samples and ICI exposure. Responses were retrospectively collected on the basis of RECIST version 1.111 prospectively assessed on each clinical trial. PFS was defined as the date of starting immunotherapy to the date of progression, death, or last follow-up. OS was defined as the date of starting immunotherapy until the date of death or last follow-up. Patients alive and without progression at last follow-up were censored for PFS, and those still alive were censored for OS.
Genomic and Transcriptomic Profiling
Whole-exome sequencing (WES) was performed on baseline tumor and blood samples from 25 patients treated with anti–PD-1, anti–PD-L1, or PD-1 blockade with either a tyrosine kinase inhibitor or chemotherapy. Four of these patients also had WES performed after treatment (two formalin-fixed, paraffin-embedded and two liquid biopsies) to evaluate tumor clonal evolution (Data Supplement). Methods for detection of somatic point mutations, indels, copy number, mutational signature, and clonal evolution; HLA/neoantigen prediction; and transcriptomic analyses are described in the Data Supplement. Tumor mutational burden (TMB; mutation per megabase [muts/Mb]) was calculated as the total number of mutations detected for a given tumor sample divided by the length of the total genomic target region captured with the exome assay. Samples with a TMB of ≥ 10 muts/Mb were classified as hypermutated.12
RNA sequencing (RNA-seq) was performed on 18 baseline tumor samples that also had WES performed. Baseline tumor and immune cell populations from 18 patients were assessed using multiplex immunofluorescence (mIF) panels that included CD4, CD8, PD-1, PD-L1, and cytokeratin on samples collected before the initiation of ICI therapy (Data Supplement).13 A full description of the mIF methodology is included in the Data Supplement. The association between potential biomarkers and clinical benefit and resistance to ICI was assessed.14
Statistical Analysis
Statistical analyses were performed using R version 4.0.3. Categorical variables were compared using the Fisher's exact test, and continuous variables were compared using the Student's t-test or Wilcoxon rank-sum test as appropriate. The Kaplan-Meier method was used to compare survival outcomes (PFS and OS) of dichotomized groups (eg, high v low PD-L1) using the survival package, with statistical significance computed using the log-rank test at a significance level of P < .05. Pretreatment gene expression information was compared across patients in four groups: OS greater than versus < 2 years, PFS greater than versus < 9 months, PD-L1 expression above versus below median as assessed by mIF, and durable responders (never progressed) versus patients with intrinsic resistance to therapy. Given that the median PFS for patients treated in the arm containing the PD-1/L1 inhibitor in the IMPassion1305 and KEYNOTE-3557 studies was 7.5 months and 9.7 months, respectively, we chose 9 months as the benchmark PFS cutoff for declaring clinical benefit in this study. For similar reasons, we chose 2 years as the benchmark OS cutoff for declaring clinical benefits in this study.
RESULTS
Patient Characteristics
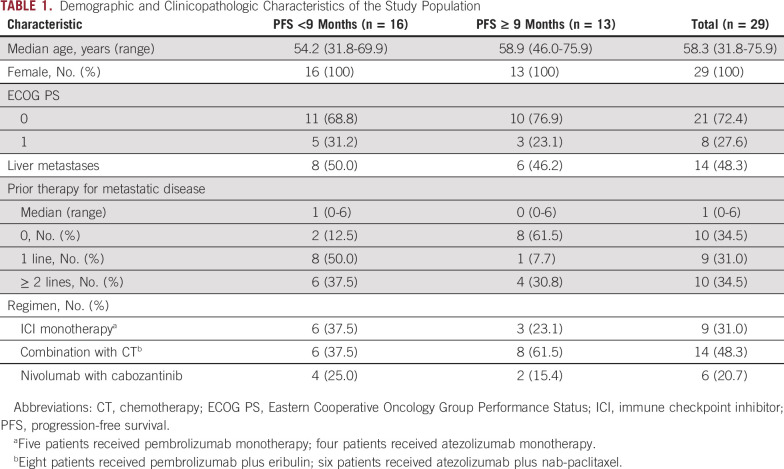
We identified 29 patients with mTNBC treated with an ICI alone (pembrolizumab, n = 5; atezolizumab, n = 4) or as part of a combination regimen with chemotherapy (eribulin plus pembrolizumab, n = 8; nab-paclitaxel plus atezolizumab, n = 6) or a targeted therapy (cabozantinib plus nivolumab, n = 6) who had tumor tissue and/or blood available for sequencing obtained before and after ICI therapy (Fig 1A).
FIG 1.
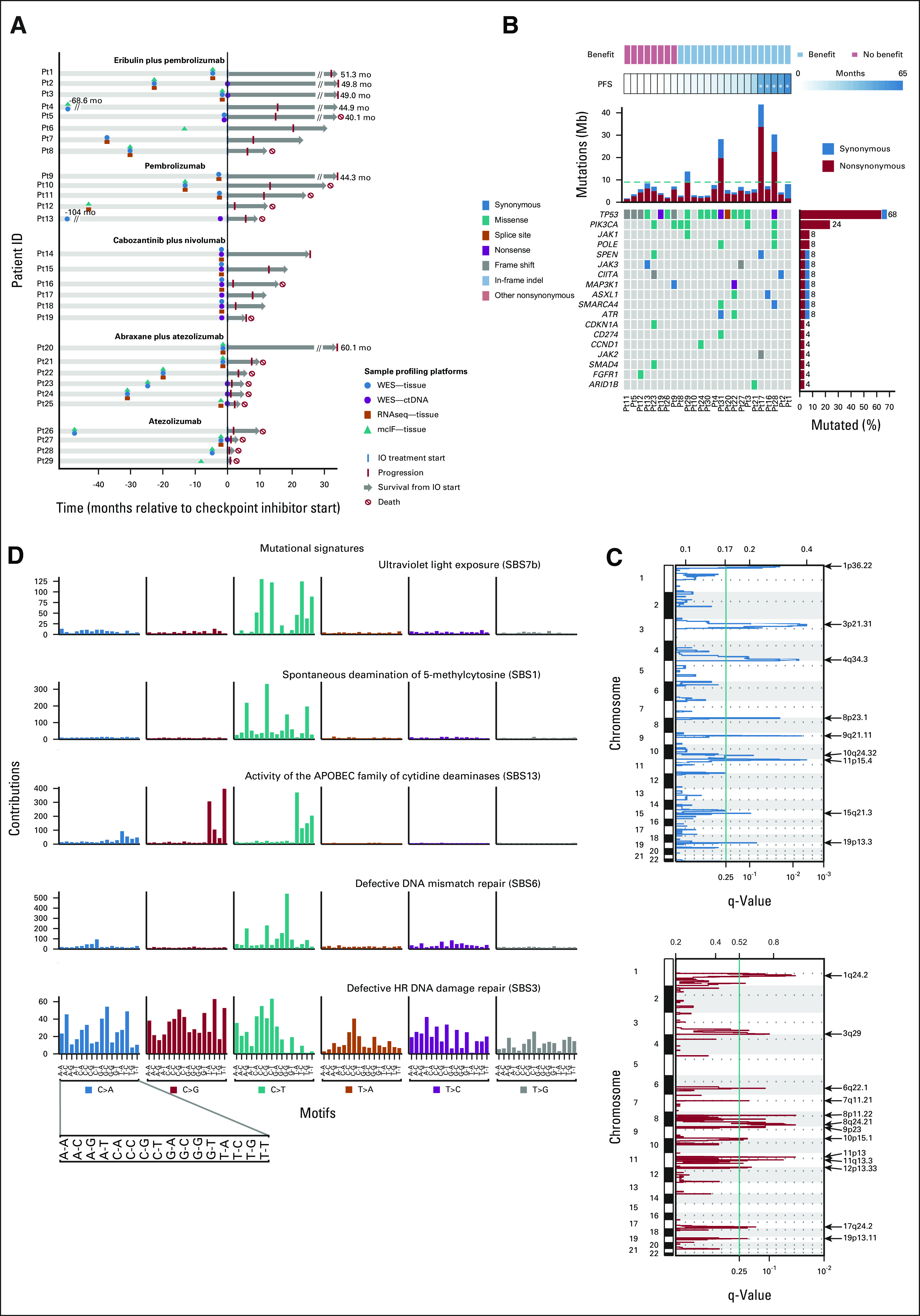
Genomic characteristics of the study population. (A) Summary of clinical history, sample collection, and molecular profiling of patients included in this study. (B) Most frequent mutations observed in 25 tumor samples collected before starting ICI-based therapy. Top tracks, benefit status, and PFS per patient. Patients are sorted by increasing PFS. Five patients with durable responses are marked with an asterisk. Top histogram, mutation rate per sample. Right histogram, frequency of somatic alterations. heatmap, and distribution of synonymous and nonsynonymous mutation events. (C) Recurrent focal deletions (top panel) and amplifications (bottom panel) identified by GISTIC2. (D) Mutational signatures prevalent in the cohort. ctDNA, circulating tumor DNA; HR, homologous recombination; ICI, immune checkpoint inhibitor; mcIF, multicolor immunofluorescence; PFS, progression-free survival; RNASeq, RNA sequencing; SBS, single base substitution; WES, whole-exome sequencing.
Patient characteristics are reported in Table 1. To investigate possible biomarkers of response and resistance to ICI-based therapy, patients were grouped into those who had a PFS < 9 months and those with a PFS ≥ 9 months. More patients with a PFS ≥ 9 months were treatment-naïve (61.5%) compared with patients with a PFS < 9 months (12.5%). In addition, patients with a PFS ≥ 9 months were more likely to have been treated with an ICI in combination with chemotherapy (61.5%) than patients with a PFS < 9 months (31.3%).
TABLE 1.
Demographic and Clinicopathologic Characteristics of the Study Population
Genomic Features of the TNBC Cohort
Next, we analyzed the prevalence of somatic mutation and copy number events in the cohort. Among the most frequently mutated genes were several well-known cancer drivers: TP53 (68%); PIK3CA (24%); and JAK1, POLE, JAK3, MAP3K1, ASXL1, SMARCA4, and ATR (8% each; Fig 1B and Data Supplement). Only TP53 and PIK3CA were identified as recurrently mutated in the cohort (P < .05), consistent with results from previous studies.15-17 In addition, we identified multiple copy number alterations, including previously described arm-level events in TNBC (deletions in 5q, 8p, and 17p; amplifications in 1q, 8q, and 10p) that were recurrent in more than half of the patients (Fig 1C and Data Supplement).15-18 An analysis of the mutational spectrum and signatures using a Signature Analyzer revealed that two of four predominant mutational processes in the cohort were defective DNA mismatch and DNA double-stranded break repair by homologous recombination, without evidence of somatic inactivation of BRCA1/2 genes in the majority of patients and somatic BRCA1 mutation in 2 of 14 patients (Pt21 and Pt26) for which defective DNA double-stranded break repair by homologous recombination was the dominant mutational signature (Fig 1D and Data Supplement).
Tumor Genomic Characteristics and Outcomes
We evaluated the association of tumor genomic features with PFS on ICI therapy and OS. Median TMB by DNA WES was higher in patients with the PFS ≥ 9 months compared with patients with the PFS < 9 months (P = .024; Fig 2A) and the OS ≥ 2 years compared with patients with the OS < 2 years (P = .033; Fig 2B). Of note, the neoantigen load was not different between the high- versus low-PFS or low-OS groups (Data Supplement).
FIG 2.
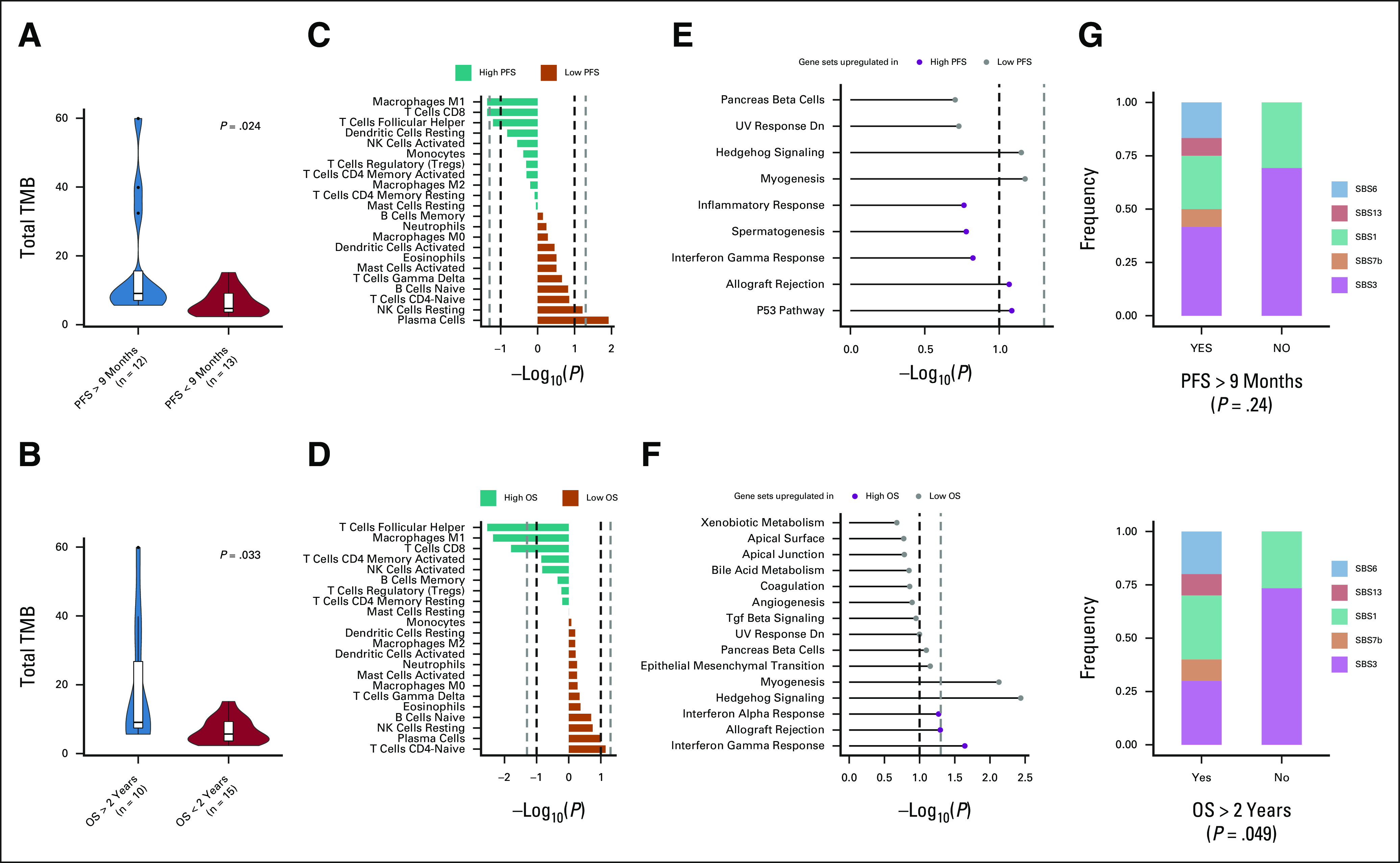
Genomic differences between patients with high and low PFS (≥ 9 months v < 9 months) and high and low OS (≥ 2 years v < 2 years). (A) Median pretreatment TMB by PFS. (B) Median pretreatment TMB by OS. (C) Immune cell populations according to CIBERSORTx by PFS. (D) Immune cell populations according to CIBERSORTx by OS. (E) Hallmark gene sets (GSEA) enriched for each group by PFS. The dark and light vertical dotted lines correspond to P value thresholds of .1 and .05, respectively. (F) Hallmark gene sets (GSEA) enriched for each group by OS. (G) Defective HR DNA damage repair (SBS3) was identified as the dominant signature in a higher proportion of patients with poorer OS (bottom panel), but not PFS (top panel). GSEA, gene set enrichment analyses; HR, homologous recombination; OS, overall survival; PFS, progression-free survival; TMB, tumor mutational burden.
In RNAseq data, CIBERSORTx analysis of 22 inferred immune subsets revealed significantly higher relative inferred fractions of CD8+ T cells and M1 macrophages among patients with the PFS ≥ 9 months versus PFS < 9 months and OS ≥ 24 months (all P < .05; Figs 2C and 2D). In addition, gene set enrichment analyses (GSEA) of RNAseq data demonstrated that hallmarks like hedgehog signaling and myogenesis were enriched among patients with lower OS (< 2 years; all P < .001), whereas allograft rejection (P = .086), interferon (IFN)-α responses (P = .054), and IFN-γ (P = .023) were positively associated with longer OS (first degree relative [FDR] ≤ 0.25; Figs 2E and Figs 2F). We also found that defective homologous recombination DNA damage repair signature (SBS3) was over-represented as the dominant signature in samples with lower OS (P = .048; Fig 2G).
PD-L1 Expression and Outcomes
Patients with a combined positive score value (defined as total PDL1+/cytokeratin+ × 100) of the median presented improved PFS (P < .005) and OS (P = .018; Figs 3A-F); higher total PD-L1 expression was also associated with improved survival outcomes (Data Supplement). The median TMB did not differ between patients with PD-L1–high versus PD-L1–low tumors (data not shown). GSEA analysis showed that PD-L1–high tumors were enriched for hallmarks such as allograft rejection (P < .001), IFN-γ response (P < .001), and inflammatory response (P = .008) compared with PD-L1–low tumors. By contrast, transforming growth-factor-β signaling (P = .031) and myogenesis (P = .037) hallmarks were enriched in PD-L1–low compared with PD-L1–high tumors (FDR ≤ 0.25; Data Supplement).
FIG 3.
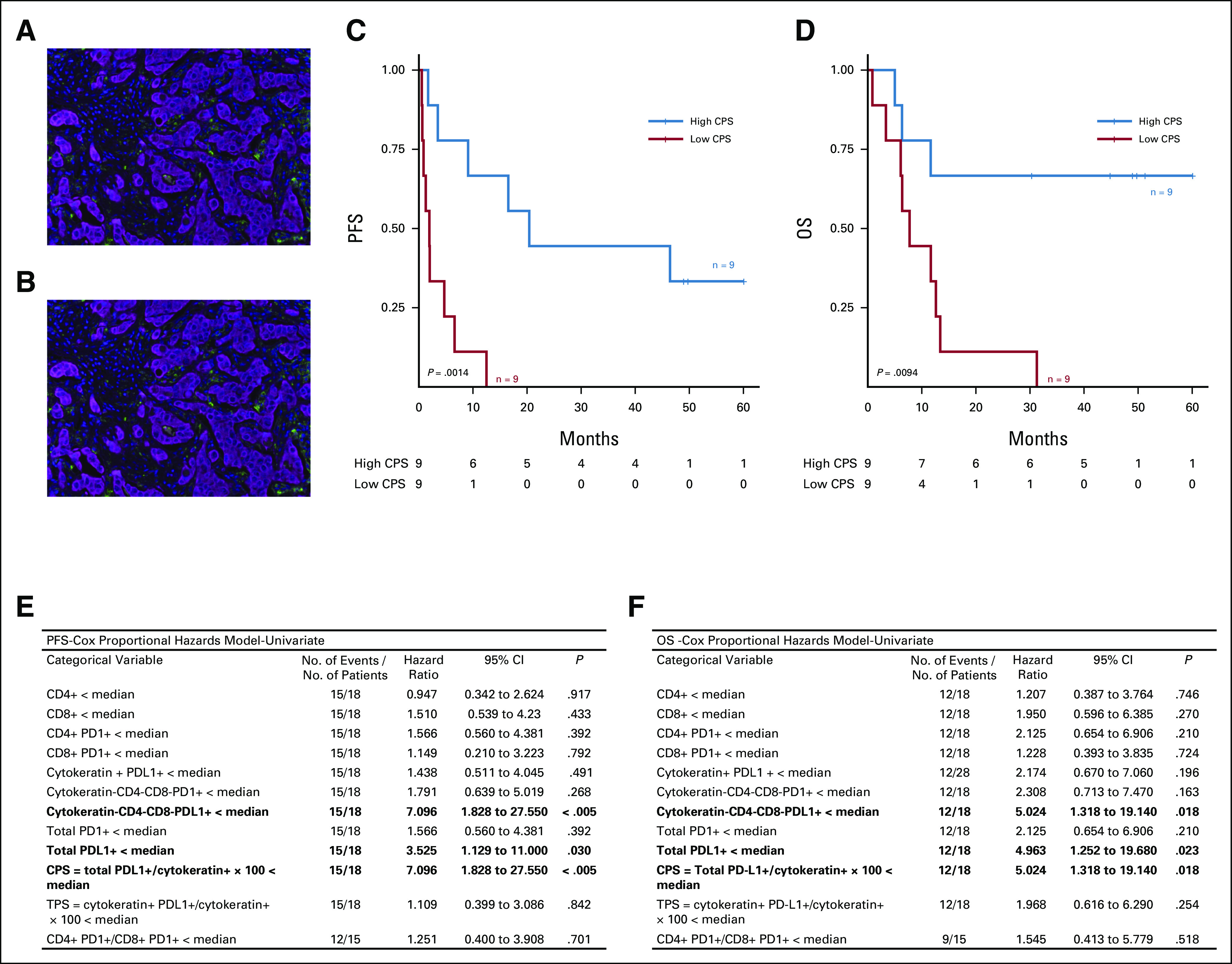
PD-L1 positivity is associated with prolonged PFS and OS in patients treated with immune checkpoint inhibitors. Image of one of the mIF panels, evaluating PD-L1 and cytokeratin in a patient with (A) low and (B) high PD-L1 expression on infiltrating cells. Kaplan-Meier curves for (C) PFS and (D) OS among patients with low and high CPS. Univariate Cox proportional hazards analysis of association between (E) PFS and (F) OS and immune-infiltrating cells assessed by mIF. CPS, combined positive score; mIF, multiplex immunofluorescence; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; TPS, tissue polypeptide specific antigen.
We also investigated association of RNAseq features with PD-L1 IHC expression. Using CIBERSORTx, we observed a higher relative fraction of M1 macrophages (P = .004), CD8+ T cells (P = .009), and follicular helper T cells (P = .013) among PD-L1–high tumors compared with PD-L1–low tumors. By contrast, the relative fraction of CD4+ T cells was higher in patients with PD-L1–low tumors (P = .029; Data Supplement).
Molecular Features of TNBC with Durable Response to Immunotherapy
In this study cohort, five patients with durable response to immunotherapy, here defined as being free of disease progression at the time of analysis (durable responders), had PFS rates ranging from 26 to 60 months. Durable responders tended to have higher TMB than patients with no benefit although the result was not statistically significant, likely because of small numbers (P = .13; Fig 4A). Three of three durable responders with mIF data had PD-L1 positivity, compared with three of six patients with intrinsic ICI resistance (P = .46; data not shown). Interestingly, we found that the tumor from only one of the five durable responders seemed to be driven by defects in the homologous repair machinery compared with the tumors of seven of eight patients (87.5%) with intrinsic resistance (P = .032), consistent with the observed association of this signature with worse OS (Fig 4B).
FIG 4.
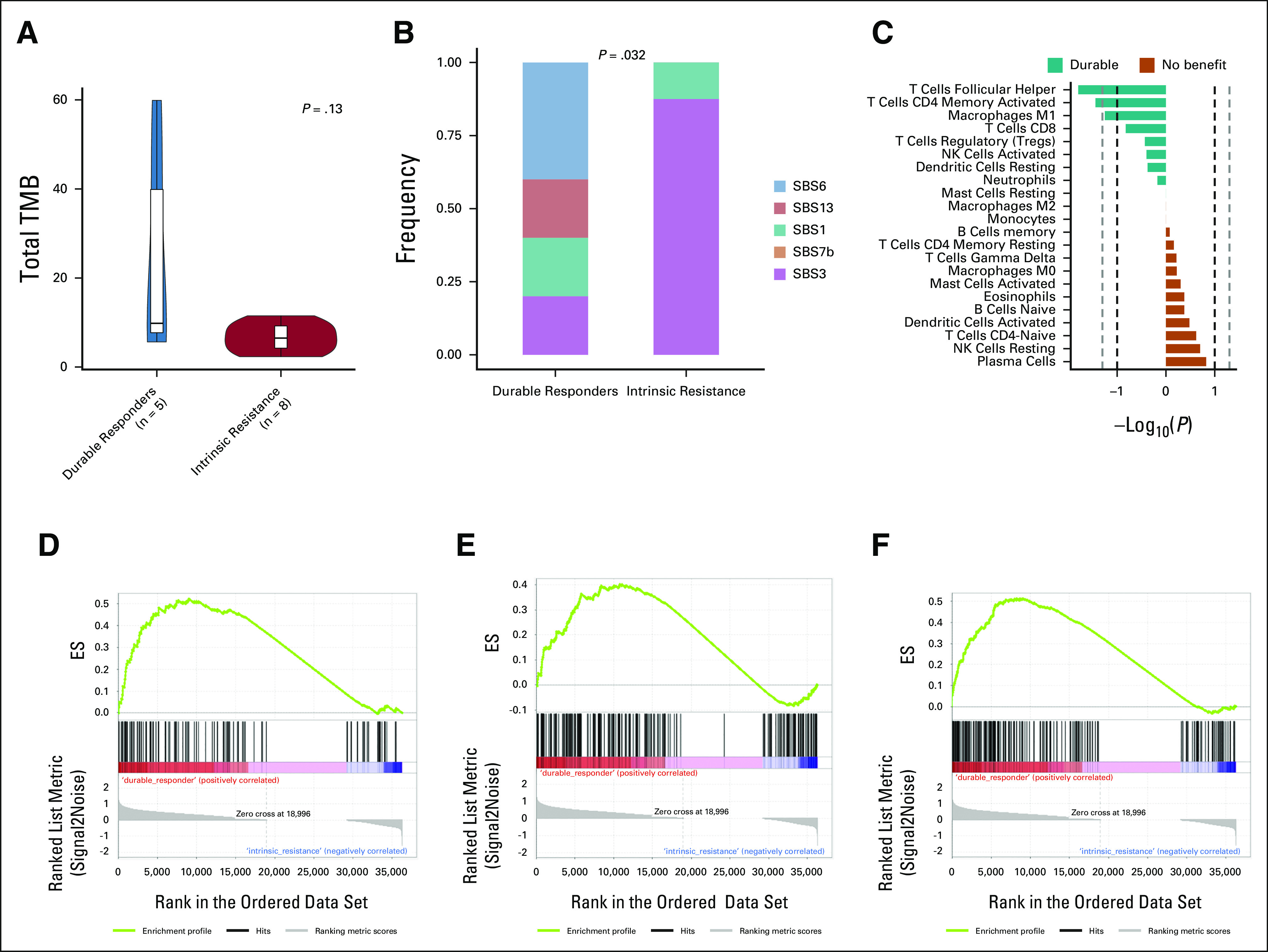
Transcriptomic and genomic differences between pretreatment samples from patients with durable benefit (never progressed to immunotherapy) and patients with intrinsically resistant tumors. (A) Median pretreatment TMB by benefit. (B) Frequency of mutational signatures according to benefit to immunotherapy. (C) Immune cell populations according to CIBERSORTx by benefit to immunotherapy. Hallmark gene sets (GSEA) of durable responders versus patients with no benefit for (D) PI3K-AKT-mTOR signaling (P = .023), (E) heme metabolism (P = .023), and (F) inflammatory response (P = .046) enriched in durable responders (enrichment plot). AKT, protein kinase B; ES, enrichment score; GSEA, gene set enrichment analyses; HR, homologous recombination; ICI, immune checkpoint inhibitor; mTOR, mammalian target of rapamycin; NK, natural killer; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; PI3K, phosphatidylinositol 3-kinase; SBS, single base substitution; TMB, tumor mutational burden.
Using CIBERSORTx, we found a significantly higher relative fraction of follicular helper T cells (P = .016) and activated CD4+ memory T cells (P = .036) among patients with durable responses compared with patients with intrinsic resistance to ICI-based regimens (Fig 4C). GSEA revealed that patients with durable benefit to immunotherapy presented tumors enriched for hallmarks such as PI3K-AKT-mTOR signaling (P = .023), heme metabolism (P = .023), and inflammatory response (P = .046) compared with patients with intrinsic resistance (FDR ≤ 0.25; Figs 4D-4F).
Tumor Evolution during Immunotherapy
We postulated that reduction in tumor burden or disease control in response to anti–PD-1 treatment may be associated with depletion in tumor clones that are sensitive to antitumor immunity. To test this hypothesis, we examined the association of clonal evolution with time to progression in four patients treated with the anti–PD-1 agent nivolumab and cabozantinib, an inhibitor of multiple tyrosine kinases including MET, AXL, and VEGFR2 (Fig 1A).
One patient (Pt14) had an almost complete depletion of the primary clone encoding neoepitopes with a number of strong predicted binders to the patient's HLA alleles, including one derived from a nonsynonymous mutation in the cancer driver gene INPPL1 (Fig 5A). This patient remained without progression of disease 26 months after treatment at last follow-up. Pt15 had decreases in cancer cell fractions of two subclones, along with increased cancer cell fractions in a third subclonal population, and had an intermediate PFS of 13 months (Fig 5B).19 By contrast, two other patients (Pt19 and Pt18) showed no change in clonal structure at progression and had the earliest times to progression at 2 and 6 months after initiation of therapy (Figs 5C and 5D). We further observed higher TMB and immune cytolytic activity in the patient with a durable response compared with other patients (Figs 5E and 5F). Moreover, Pt14 was marked by a pretreatment infiltrate composition that was relatively increased in antitumor (CD8 T cells, CD4 memory activated, follicular helper T cells, M1 macrophages, activated NK cells, and activated dendritic cells) and decreased in protumor (CD4 memory resting and M0 and M2 macrophages) immune cell fractions compared with other patients (Fig 5G).
FIG 5.
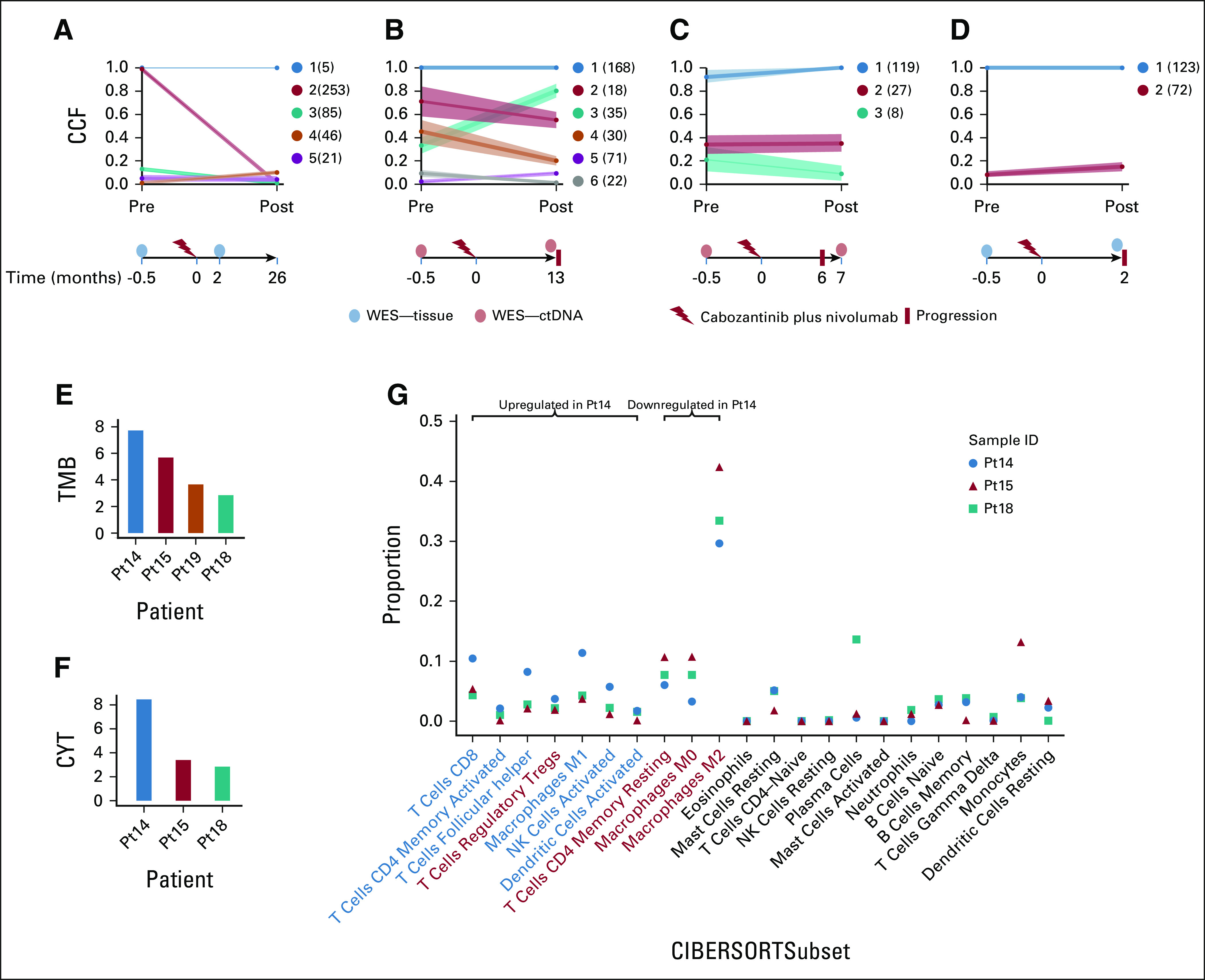
Clonal evolution is associated with response to ICI. All four patients were treated with nivolumab plus cabozantinib, and clonal evolutionary analysis was performed using PhylogicNDT. (A) Pt14 (FFPE samples) was marked by an almost complete loss of the clonal population and was disease-free at 26 months post-therapy. (B) Pt15 (ctDNA samples) showed modest decreases in some subclones and progressed after 13 months. (C) Pt19 (ctDNA) and (D) Pt18 (FFPE) had virtually no change in clonal cancer cell fractions and were associated with early relapses (6 and 2 months post-treatment, respectively). (E) TMB was highest in Pt14, who was progression-free post-treatment, and progressively decreased with time to progression in patients 15, 19, and 18. (F) CYT had a similar trend within these patients (RNAseq was unavailable for patient 19). (G) Pt14 has increased antitumor and decreased protumor (M0 and M2 macrophages) immune compartment proportions compared with other samples (CIBERSORTx). Immune cell labels: antitumor (blue)and protumor (red). CCF, cancer cell fraction; ctDNA, circulating tumor DNA; CYT, cytolytic activity; FFPE, formalin-fixed, paraffin-embedded; ICI, immune checkpoint inhibitor; NK, natural killer; Pt, patient; TMB, tumor mutational burden; TTP, time to progression; WES, whole-exome sequencing.
DISCUSSION
In this study, we analyzed a unique cohort by incorporating multidimensional profiling of immune, genomic, and transcriptomic features associated with survival outcomes in patients with mTNBC treated with ICI monotherapy or combination regimens. Moreover, this cohort included multiple ICI durable responders, with a PFS of at least 26 months and offers the opportunity to interrogate genomic factors associated with these exceptional responders. Consistent with previous studies,5,7,20-22 we found that patients with longer PFS and OS more frequently had PD-L1–positive tumors and higher median TMB. In addition, we observed that patients with durable responses after ICI had tumors with a higher relative fraction of follicular helper T cells and activated CD4+ memory T cells and a higher expression of genes involved in the inflammatory response. These data further support the hypothesis that patients with a T-cell–inflamed mTNBC phenotype are more likely to derive benefit from PD-1–containing/PD-L1–containing therapies.
It is clear that PD-L1 is a suboptimal biomarker.23 Thus, it is important to identify additional biomarkers that can refine our ability to predict which patients will benefit from ICIs. In the present study, the median TMB was significantly higher among patients who achieved PFS ≥ 9 months and OS ≥ 2 years on ICI-containing regimens, consistent with other studies in breast cancer.20-22,24,25 As this study demonstrates, even in the setting of deep characterization including multiomics, multicolor immunofluorescence, and standard tissue markers, there is not (to date) one single biomarker that performs optimally to predict ICI benefit. We hypothesize that pretreatment, a composite biomarker that builds upon the best performing features among distinct data types may be required. Alternatively, an early biomarker of response (eg, circulating tumor DNA change) may offer a functional readout, as has been seen in other cancer types.26
Previous studies in mTNBC have noted that increased tumor-infiltrating lymphocyte frequencies are correlated with improved response to ICI-containing regimens.27-29 In our study, tumors from patients with longer survival after ICI had a higher expression of genes in IFN-γ1, inflammatory response, and allograft rejection pathways and also had higher relative fractions of CD8+ T cells, follicular helper T cells, and M1 macrophages. These results are in concordance with the exploratory analysis of the IMpassion130 study, which showed that patients who experienced the greatest benefit from the addition of atezolizumab to chemotherapy had a high CD8-positive cell infiltration and immune-inflamed tumors.30 Conversely, patients in our study with PD-L1–low tumors also had an increased expression of TGF-β signaling genes. Increased TGF-β1 expression has been associated with T-cell exclusion, higher tumor grade, axillary lymph node metastasis, and shorter disease-free survival in patients with TNBC.31,32 In vitro, higher levels of TGF-β1 are associated with increased migration and invasion of TNBC cells.31
In contrast to other solid tumor types treated with ICI, durable responses lasting more than 24 months are infrequent in mTNBC.5-7 We present data from five exceptional responders without disease progression (ranging from 26 to 60 months). Compared with patients with intrinsic resistance, the pretreatment tumors from patients with durable responses had a significantly higher relative fraction of follicular helper T cells and activated CD4+ memory T cells. The importance of CD4+ T cells as regulators of immune responses has been shown in primary breast cancer.33 We also found that durable responses had significantly increased inflammatory gene response. In addition, these patients with durable benefit had tumors with higher expression of genes in the PI3K-Akt-mTOR signaling pathway, inflammatory response, and heme metabolism. Loss of PTEN has been suggested to be associated with resistance to ICI in mTNBC although the specific mechanism is unclear.20 One possibility is that tumors with increased PI3K/Akt activity present with higher levels of PD-L1 expression in mTNBC,34 which in turn yields greater sensitivity to regimens that target PD-1/PD-L1 interactions. We also found that durable responses had significantly higher expression of genes in the heme metabolism pathway. Previously, it was shown that ICI-activated CD8+ T cells promote tumor cell lipid peroxidation and sensitize tumors to ferroptosis in a IFN-γ–dependent manner.35 The combination of ferroptosis activators and ICI could be a promising approach to increase the proportion of patients who benefit from immunotherapy in breast cancer.
Finally, our study of evolutionary trajectories of tumor clones after ICI treatment in a small cohort suggests that changes in clonal architecture, particularly reductions in cancer cell fractions of primary clones and those bearing strong immunogenic targets or driver genes, could serve as an early indicator of treatment effectiveness. Of note, analysis of two of the four patients in this cohort was based on WES of circulating DNA, demonstrating the feasibility of developing an early response test on the basis of readily available liquid biopsy samples.
This study has limitations. First, we identified a small sample size of patients with mTNBC who were treated with different ICI-containing regimens in different lines of metastatic disease, from 0 to 6 lines of therapy in the metastatic setting. Larger prospective studies should be conducted to validate the association between high TMB, immune infiltrates, and expression of IFN pathway genes with the response to ICI-containing regimens in patients with mTNBC. Second, information about PD-L1 status using immunohistochemical assays with either the SP142 or 22C3 antibody was not available because this was not clinically required when the patients were consented to these clinical trials. Instead, we performed a mIF assay to evaluate the expression of PD-L1 in the tumor microenvironment and the type of PD-L1–positive cells. Immunostaining results with the PD-L1 antibody clone (405.9A11)36 used in our work have been shown to be highly correlated with the results of immunostaining with other commercially available clones (eg, 22c3, E1L3N, and SP142) and predictive of clinical response to PD-1 blockade in previous studies.37 Finally, although data suggest that distinct metastatic sites reflect variation in the immune microenvironment (eg, fewer tumor-infiltrating lymphocytes in liver metastases), in our population, just two biopsies came from the liver; thus, this small number prevents us from performing specific analyses around specific metastatic sites.
In summary, we present a comprehensive analysis of multiomic profiling of patients with mTNBC receiving ICIs to date. We confirm prior findings regarding the association of PD-L1 status and high TMB with response to ICIs in breast cancer; additionally, we found potential novel associations of response to these agents, including higher infiltration of CD8-positive cells and higher expression of genes in IFN-γ, inflammatory response and allograft rejection pathways, and clonal evolution while on ICIs.
ACKNOWLEDGMENT
T.E.K. acknowledges grant support from the National Institutes of Health (T32CA009172). K.A.C. acknowledges grant support from the National Institutes of Health (T32CA247815-01). S.A.S acknowledges support from the NCI (R50RCA211482). J.F. acknowledges support from the NIH (U24CA224331).
Romualdo Barroso-Sousa
Consulting or Advisory Role: AstraZeneca/Daiichi Sankyo, Pfizer, Roche, Libbs, MSD, Sanofi, Lilly, AstraZeneca/Merck
Speakers' Bureau: Lilly, Libbs, AstraZeneca/Daiichi Sankyo, Roche, Pfizer, MSD
Research Funding: AstraZeneca/Daiichi Sankyo, Bristol Myers Squibb
Travel, Accommodations, Expenses: AstraZeneca/Daiichi Sankyo, MSD, Lilly
Katharine Collier
Research Funding: Conquer Cancer Foundation
Edward T. Richardson III
Honoraria: MJH Healthcare Holdings, LLC
Research Funding: AstraZeneca (Inst)
Tanya Keenan
Employment: 5AM Ventures (I), Merck
Stock and Other Ownership Interests: Trillium Therapeutics (I), Alnylam (I), Aeglea BioTherapeutics (I), Crinetics Pharmaceuticals (I)
Research Funding: Merck (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Cyclacel
Ryan C. Brennick
Employment: Foundation Medicine
Patrick A. Ott
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Merck
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Celldex (Inst), ARMO BioSciences (Inst), Neon Therapeutics (Inst), CytomX Therapeutics (Inst), Genentech (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Xencor, Oncorus
Expert Testimony: Boehringer Ingelheim
F. Stephen Hodi
Employment: Dana-Farber Cancer Institute
Leadership: Bicara Therapeutics
Stock and Other Ownership Interests: Apricity Health, Torque, Pionyr, Bicara Therapeutics
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech/Roche, EMD Serono, Sanofi, Bristol Myers Squibb, Surface Oncology, Compass Therapeutics, Partners Therapeutics, Pionyr, Torque, Rheos Medicines, Boston Pharmaceuticals, Checkpoint THerapeutics, Eisai, Bioentre, Gossamer Bio, Iovance Biotherapeutics, Trillium Therapeutics, CatalYm, Amgen, Immunocore
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech/Roche (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy, patent pending royalties received on MICA-related disorders application to institution per institutional IP policy, Angiopoietin-2 Biomarkers Predictive of Anti-immune checkpoint response (Inst), Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms, Methods of Using Pembrolizumab and Trebananib (Inst)
Travel, Accommodations, Expenses: Novartis, Bristol Myers Squibb
Other Relationship: Bristol Myers Squibb, Genentech/Roche
Deborah A. Dillon
Consulting or Advisory Role: Oncology Analytics, Novartis
Research Funding: Canon Medical System (Inst)
Travel, Accommodations, Expenses: Novartis
Nancy U. Lin
Stock and Other Ownership Interests: Artera Inc
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, Denali Therapeutics, AstraZeneca, Prelude Therapeutics, Voyager Therapeutics, Affinia Therapeutics, Pfizer, Olema Pharmaceuticals, Aleta Biotherapeutics, Artera
Research Funding: Genentech (Inst), Pfizer (Inst), Seattle Genetics (Inst), Merck (Inst), Zion (Inst), Olema Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Eliezer M. Van Allen
Stock and Other Ownership Interests: Syapse, Tango Therapeutics, Genome Medical, Microsoft, Ervaxx, Monte Rosa Therapeutics, Manifold Bio
Consulting or Advisory Role: Syapse, Roche, Third Rock Ventures, Takeda, Novartis, Genome Medical, Invitae, Illumina, Tango Therapeutics, Ervaxx, Janssen, Monte Rosa Therapeutics, Manifold Bio
Speakers' Bureau: Illumina
Research Funding: Bristol Myers Squibb, Novartis, Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Patent on discovery of retained intron as source of cancer neoantigens (Inst), Patent on discovery of chromatin regulators as biomarkers of response to cancer immunotherapy (Inst), Patent on clinical interpretation algorithms using cancer molecular data (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Scott Rodig
Leadership: Immunitas
Stock and Other Ownership Interests: Immunitas
Honoraria: Perkin Elmer, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb
Research Funding: Bristol Myers Squibb, Merck, Affimed Therapeutics, Kite, a Gilead company
Patents, Royalties, Other Intellectual Property: Patent pending for use of antigalectin 1 antibodies for diagnostic use
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Genentech/Roche
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Elizabeth A. Mittendorf
Honoraria: Physicians' Education Resource
Consulting or Advisory Role: Roche/Genentech, Merck, Exact Sciences, Roche
Research Funding: GlaxoSmithKline
Uncompensated Relationships: Bristol Myers Squibb, Lilly
Open Payments Link: https://openpaymentsdata.cms.gov/physician/899522/summary
Catherine J. Wu
Stock and Other Ownership Interests: BioNTech, BioNTech (I)
Research Funding: Pharmacyclics/Janssen
Nikhil Wagle
Stock and Other Ownership Interests: Relay Therapeutics, Flare Therapeutics
Consulting or Advisory Role: Lilly, Relay Therapeutics, Flare Therapeutics
Research Funding: Puma Biotechnology, AstraZeneca
Daniel G. Stover
Consulting or Advisory Role: Novartis
Sachet A. Shukla
Stock and Other Ownership Interests: Agenus, Agios, BreakBio Corp, Bristol Myers Squibb, Lumos Pharma
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Paxman, Seattle Genetics, Odonate Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Immunomedics/Gilead, Mersana, Certara Inc, 4D Pharma, Ellipses Pharma, OncoSec, Chugai Pharma, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Infinity Pharmaceuticals, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Zetagen
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Cyclacel (Inst), Nektar (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, December 8-11, 2020 (virtual symposium).
SUPPORT
Supported by a medical oncology grant from the Dana-Farber Cancer Institute, Susan G. Komen for the Cure (CCR17480903 to D.G.S.), and a Pelotonia Young Investigator Award (D.G.S).
R.B.-S., J.F., and K.C. contributed equally to this work. D.G.S., S.A.S. and S.M.T. are cosenior authors.
DATA SHARING STATEMENT
The clinical data in this study are derived from patients from clinical trials starting enrollment prior to 2019. Since these patients come from trials sponsored by five different drug companies (Merck, Eisai, EMS, Exelixis, and Genentech), complete data are controlled access and can be provided upon request to the corresponding authors. All mutation, copy number, and expression data along with the clinical annotations are provided in the manuscript and its supplemental materials.
AUTHOR CONTRIBUTIONS
Conception and design: Romualdo Barroso-Sousa, Katrina Z. Kao, Ryan C. Brennick, Victoria Attaya, Scott Rodig, Eric P. Winer, Catherine J. Wu, Nikhil Wagle, Daniel G. Stover, Sachet A. Shukla, Sara M. Tolaney
Financial support: Nancy U. Lin, Nikhil Wagle, Sara M. Tolaney
Administrative support: Eliezer M. Van Allen, Sara M. Tolaney
Provision of study materials or patients: Romualdo Barroso-Sousa, Michael P. Manos, Nancy U. Lin, Nikhil Wagle, Sara M. Tolaney
Collection and assembly of data: Romualdo Barroso-Sousa, Katrina Z. Kao, Tanya Keenan, Ofir Cohen, Michael P. Manos, Ryan C. Brennick, Patrick A. Ott, F. Stephen Hodi, Deborah A. Dillon, Victoria Attaya, Nancy U. Lin, Eliezer M. Van Allen, Scott Rodig, Nikhil Wagle, Sachet A. Shukla, Sara M. Tolaney
Data analysis and interpretation: Romualdo Barroso-Sousa, Juliet Forman, Katharine Collier, Zachary T. Weber, Tejas R. Jammihal, Katrina Z. Kao, Patrick A. Ott, F. Stephen Hodi, Nancy U. Lin, Eliezer M. Van Allen, Scott Rodig, Elizabeth A. Mittendorf, Nikhil Wagle, Daniel G. Stover, Sachet A. Shukla, Sara M. Tolaney
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Romualdo Barroso-Sousa
Consulting or Advisory Role: AstraZeneca/Daiichi Sankyo, Pfizer, Roche, Libbs, MSD, Sanofi, Lilly, AstraZeneca/Merck
Speakers' Bureau: Lilly, Libbs, AstraZeneca/Daiichi Sankyo, Roche, Pfizer, MSD
Research Funding: AstraZeneca/Daiichi Sankyo, Bristol Myers Squibb
Travel, Accommodations, Expenses: AstraZeneca/Daiichi Sankyo, MSD, Lilly
Katharine Collier
Research Funding: Conquer Cancer Foundation
Edward T. Richardson III
Honoraria: MJH Healthcare Holdings, LLC
Research Funding: AstraZeneca (Inst)
Tanya Keenan
Employment: 5AM Ventures (I), Merck
Stock and Other Ownership Interests: Trillium Therapeutics (I), Alnylam (I), Aeglea BioTherapeutics (I), Crinetics Pharmaceuticals (I)
Research Funding: Merck (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Cyclacel
Ryan C. Brennick
Employment: Foundation Medicine
Patrick A. Ott
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Merck
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Celldex (Inst), ARMO BioSciences (Inst), Neon Therapeutics (Inst), CytomX Therapeutics (Inst), Genentech (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Xencor, Oncorus
Expert Testimony: Boehringer Ingelheim
F. Stephen Hodi
Employment: Dana-Farber Cancer Institute
Leadership: Bicara Therapeutics
Stock and Other Ownership Interests: Apricity Health, Torque, Pionyr, Bicara Therapeutics
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech/Roche, EMD Serono, Sanofi, Bristol Myers Squibb, Surface Oncology, Compass Therapeutics, Partners Therapeutics, Pionyr, Torque, Rheos Medicines, Boston Pharmaceuticals, Checkpoint THerapeutics, Eisai, Bioentre, Gossamer Bio, Iovance Biotherapeutics, Trillium Therapeutics, CatalYm, Amgen, Immunocore
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech/Roche (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy, patent pending royalties received on MICA-related disorders application to institution per institutional IP policy, Angiopoietin-2 Biomarkers Predictive of Anti-immune checkpoint response (Inst), Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms, Methods of Using Pembrolizumab and Trebananib (Inst)
Travel, Accommodations, Expenses: Novartis, Bristol Myers Squibb
Other Relationship: Bristol Myers Squibb, Genentech/Roche
Deborah A. Dillon
Consulting or Advisory Role: Oncology Analytics, Novartis
Research Funding: Canon Medical System (Inst)
Travel, Accommodations, Expenses: Novartis
Nancy U. Lin
Stock and Other Ownership Interests: Artera Inc
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, Denali Therapeutics, AstraZeneca, Prelude Therapeutics, Voyager Therapeutics, Affinia Therapeutics, Pfizer, Olema Pharmaceuticals, Aleta Biotherapeutics, Artera
Research Funding: Genentech (Inst), Pfizer (Inst), Seattle Genetics (Inst), Merck (Inst), Zion (Inst), Olema Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Eliezer M. Van Allen
Stock and Other Ownership Interests: Syapse, Tango Therapeutics, Genome Medical, Microsoft, Ervaxx, Monte Rosa Therapeutics, Manifold Bio
Consulting or Advisory Role: Syapse, Roche, Third Rock Ventures, Takeda, Novartis, Genome Medical, Invitae, Illumina, Tango Therapeutics, Ervaxx, Janssen, Monte Rosa Therapeutics, Manifold Bio
Speakers' Bureau: Illumina
Research Funding: Bristol Myers Squibb, Novartis, Sanofi (Inst)
Patents, Royalties, Other Intellectual Property: Patent on discovery of retained intron as source of cancer neoantigens (Inst), Patent on discovery of chromatin regulators as biomarkers of response to cancer immunotherapy (Inst), Patent on clinical interpretation algorithms using cancer molecular data (Inst)
Travel, Accommodations, Expenses: Roche/Genentech
Scott Rodig
Leadership: Immunitas
Stock and Other Ownership Interests: Immunitas
Honoraria: Perkin Elmer, Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb
Research Funding: Bristol Myers Squibb, Merck, Affimed Therapeutics, Kite, a Gilead company
Patents, Royalties, Other Intellectual Property: Patent pending for use of antigalectin 1 antibodies for diagnostic use
Travel, Accommodations, Expenses: Roche, Bristol Myers Squibb
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Genentech/Roche
Research Funding: Genentech (Inst)
Other Relationship: InfiniteMD
Elizabeth A. Mittendorf
Honoraria: Physicians' Education Resource
Consulting or Advisory Role: Roche/Genentech, Merck, Exact Sciences, Roche
Research Funding: GlaxoSmithKline
Uncompensated Relationships: Bristol Myers Squibb, Lilly
Open Payments Link: https://openpaymentsdata.cms.gov/physician/899522/summary
Catherine J. Wu
Stock and Other Ownership Interests: BioNTech, BioNTech (I)
Research Funding: Pharmacyclics/Janssen
Nikhil Wagle
Stock and Other Ownership Interests: Relay Therapeutics, Flare Therapeutics
Consulting or Advisory Role: Lilly, Relay Therapeutics, Flare Therapeutics
Research Funding: Puma Biotechnology, AstraZeneca
Daniel G. Stover
Consulting or Advisory Role: Novartis
Sachet A. Shukla
Stock and Other Ownership Interests: Agenus, Agios, BreakBio Corp, Bristol Myers Squibb, Lumos Pharma
Sara M. Tolaney
Consulting or Advisory Role: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech, Eisai, Sanofi, Bristol Myers Squibb, Paxman, Seattle Genetics, Odonate Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Immunomedics/Gilead, Mersana, Certara Inc, 4D Pharma, Ellipses Pharma, OncoSec, Chugai Pharma, BeyondSpring Pharmaceuticals, OncXerna Therapeutics, Infinity Pharmaceuticals, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Zetagen
Research Funding: Genentech/Roche (Inst), Merck (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Novartis (Inst), Bristol Myers Squibb (Inst), Eisai (Inst), AstraZeneca (Inst), NanoString Technologies (Inst), Cyclacel (Inst), Nektar (Inst), Immunomedics (Inst), Odonate Therapeutics (Inst), Sanofi (Inst), Seattle Genetics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Dent R, Trudeau M, Pritchard KI, et al. : Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 13:4429-4434, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Lin NU, Vanderplas A, Hughes ME, et al. : Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118:5463-5472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waks AG, Winer EP: Breast cancer treatment: A Review. JAMA 321:288-300, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Garrido-Castro AC, Lin NU, Polyak K: Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov 9:176-198, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid P, Adams S, Rugo HS, et al. : Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108-2121, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Schmid P, Rugo HS, Adams S, et al. : Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:44-59, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Cortes J, Cescon DW, Rugo HS, et al. : KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer [abstract]. J Clin Oncol 38, 2020. (suppl 15; abstr 1000) [DOI] [PubMed] [Google Scholar]
- 8.Merck : Merck Announces Phase 3 KEYNOTE-355 Trial Met Primary Endpoint of Overall Survival (OS) in Patients With Metastatic Triple-Negative Breast Cancer Whose Tumors Expressed PD-L1 (CPS ≥10). https://www.merck.com/news/merck-announces-phase-3-keynote-355-trial-met-primary-endpoint-of-overall-survival-os-in-patients-with-metastatic-triple-negative-breast-cancer-whose-tumors-expressed-pd-l1-cps-%E2%89%A510/ [Google Scholar]
- 9.Adams S, Schmid P, Rugo HS, et al. : Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 30:397-404, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Adams S, Loi S, Toppmeyer D, et al. : Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30:405-411, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Marabelle A, Fakih M, Lopez J, et al. : Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21:1353-1365, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Carey CD, Gusenleitner D, Lipschitz M, et al. : Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 130:2420-2430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wander SA, Cohen O, Gong X, et al. : The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov 10:1174-1193, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Network : Comprehensive molecular portraits of human breast tumours. Nature 490:61-70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang YZ, Ma D, Suo C, et al. : Genomic and transcriptomic landscape of triple-negative breast cancers: Subtypes and treatment strategies. Cancer Cell 35:428-440, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Staaf J, Glodzik D, Bosch A, et al. : Whole-genome sequencing of triple-negative breast cancers in a population-based clinical study. Nat Med 25:1526-1533, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stover DG, Parsons HA, Ha G, et al. : Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol 36:543-553, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachireddy P, Ennis C, Nguyen VN, et al. : Distinct evolutionary paths in chronic lymphocytic leukemia during resistance to the graft-versus-leukemia effect. Sci Transl Med 12:eabb7661, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barroso-Sousa R, Keenan TE, Pernas S, et al. : Tumor mutational burden and PTEN alterations as molecular correlates of response to PD-1/L1 blockade in metastatic triple-negative breast cancer. Clin Cancer Res 26:2565-2572, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emens LA, Molinero L, Adams S, et al. : Tumour mutational burden and clinical outcomes with first-line atezolizumab and nab-paclitaxel in triple-negative breast cancer: Exploratory analysis of the phase III IMpassion130 trial. Ann Oncol 31:S360-S361, 2020 [Google Scholar]
- 22.Winer EP, Lipatov O, Im S-A, et al. : Association of tumor mutational burden (TMB) and clinical outcomes with pembrolizumab (pembro) versus chemotherapy (chemo) in patients with metastatic triple-negative breast cancer (mTNBC) from KEYNOTE-119. J Clin Oncol 38:1013-1013, 2020. (suppl 15; abstr 1013) [Google Scholar]
- 23.Ribas A, Hu-Lieskovan S: What does PD-L1 positive or negative mean? J Exp Med 213:2835-2840, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alva AS, Mangat PK, Garrett-Mayer E, et al. : Pembrolizumab (P) in patients (pts) with metastatic breast cancer (MBC) with high tumor mutational burden (HTMB): Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J Clin Oncol 37, 2019. (suppl 15; abstr 1014) [DOI] [PubMed] [Google Scholar]
- 25.Barroso-Sousa R, Li T, Reddy S, et al. : Nimbus: A phase 2 trial of nivolumab plus ipilimumab for patients with hypermutated her2-negative metastatic breast cancer (MBC). Cancer Res 82:4S, 2022. (abstr GS2-10) [Google Scholar]
- 26.Nabet BY, Esfahani MS, Moding EJ, et al. : Noninvasive early identification of therapeutic benefit from immune checkpoint inhibition. Cell 183:363-376, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emens LA, Cruz C, Eder JP, et al. : Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: A phase 1 study. JAMA Oncol 5:74-82, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid P, Adams S, Rugo HS, et al. : IMpassion130: Updated overall survival (OS) from a global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab (atezo) + nab-paclitaxel (nP) in previously untreated locally advanced or metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 37, 2019. (suppl 15; abstr 1003) [Google Scholar]
- 29.Loi S, Adams S, Schmid P, et al. : Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): Results from KEYNOTE-086. Ann Oncol 28:v608, 2017 [Google Scholar]
- 30.Emens LA, Goldstein LD, Schmid P, et al. : The tumor microenvironment (TME) and atezolizumab + nab-paclitaxel (A+nP) activity in metastatic triple-negative breast cancer (mTNBC): IMpassion130. J Clin Oncol 39, 2021. (suppl 15; abstr 1006) [Google Scholar]
- 31.Ding MJ, Su KE, Cui GZ, et al. : Association between transforming growth factor-β1 expression and the clinical features of triple negative breast cancer. Oncol Lett 11:4040-4044, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruosso T, Gigoux M, Manem VSK, et al. : Spatially distinct tumor immune microenvironments stratify triple-negative breast cancers. J Clin Invest 129:1785-1800, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu-Trantien C, Loi S, Garaud S, et al. : CD4⁺ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest 123:2873-2892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittendorf EA, Philips AV, Meric-Bernstam F, et al. : PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res 2:361-370, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Green M, Choi JE, et al. : CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569:270-274, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahoney KM, Sun H, Liao X, et al. : PD-L1 antibodies to its cytoplasmic domain most clearly delineate cell membranes in immunohistochemical staining of tumor cells. Cancer Immunol Res 3:1308-1315, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaule P, Smithy JW, Toki M, et al. : A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol 3:256-259, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]