PURPOSE
The CNS is a recurrent site of progression in anaplastic lymphoma kinase (ALK)–rearranged (ALK+) lung cancer. Lorlatinib is a third-generation ALK inhibitor developed to penetrate the CNS and overcome ALK resistance mutations. We conducted a phase II study to evaluate the intracranial activity of lorlatinib in patients with CNS-only progression on second-generation ALK inhibitors.
METHODS
Patients with ALK+ lung cancer who had intracranial progression on ≥ 1 ALK inhibitor without measurable extracranial disease received lorlatinib 100 mg once daily. The primary end point was intracranial disease control rate at 12 weeks per modified RECIST v1.1. Secondary end points included intracranial progression-free survival, intracranial objective response rate, and safety/tolerability.
RESULTS
Twenty-three patients were enrolled between November 2016 and January 2019. Fifteen (65%) patients had irradiated CNS metastases, with a median of 20.2 months between radiation and lorlatinib. Control of intracranial disease was observed in 21 (95%) evaluable patients at 12 weeks. The intracranial objective response rate was 59% with six complete and seven partial responses. The median intracranial progression-free survival was 24.6 months (95% CI, 20.2 to not reached). With a median follow-up of 16.8 months, nine patients developed disease progression, including four patients with CNS progression. The most common treatment-related adverse events were hypercholesterolemia (96%), hypertriglyceridemia (87%), edema (65%), cognitive effects (52%), and mood effects (43%). Three patients discontinued treatment because of toxicity, including two patients with fatal respiratory events.
CONCLUSION
Lorlatinib induced durable intracranial disease control in patients with CNS-only relapse on second-generation ALK inhibitors, suggesting that tumors with CNS-limited progression on brain-penetrant ALK tyrosine kinase inhibitors remain ALK-dependent.
INTRODUCTION
Anaplastic lymphoma kinase (ALK)–rearranged (ALK+) non–small-cell lung cancer (NSCLC) demonstrates a predisposition toward CNS metastasis, with approximately 30%-40% of patients presenting with brain involvement.1,2 Because of this CNS tropism and high rates of CNS progression on the first-generation ALK tyrosine kinase inhibitor (TKI) crizotinib,1 current management strategies for ALK+ NSCLC prioritize ALK TKIs with proven blood-brain barrier penetration as first-line treatment.3,4 Compared with crizotinib, second-generation ALK TKIs (eg, alectinib and brigatinib) induce more robust intracranial responses, decrease the cumulative incidence of brain metastases, and yield more durable systemic and intracranial responses.3-5 However, resistance to treatment is largely inevitable, and the CNS remains a recurrent site of progression, even on second-generation ALK TKIs.3,5
CONTEXT
Key Objective
A subset of patients with ALK-rearranged lung cancer will experience CNS-only progression on second-generation anaplastic lymphoma kinase (ALK) inhibitors. The efficacy of the third-generation ALK inhibitor lorlatinib in this context is not well-defined.
Knowledge Generated
We conducted a phase II clinical trial to evaluate the intracranial activity of lorlatinib in patients with CNS-only progression on second-generation ALK inhibitors. In this trial, which included 23 patients, intracranial disease control was observed in 95% of patients with a corresponding intracranial objective response rate of 59%. Responses were durable with a median intracranial progression-free survival of 24.6 months.
Relevance
This prospective clinical trial suggests that lorlatinib has robust intracranial activity in patients with CNS-only progression on second-generation ALK inhibitors. Given the challenges of characterizing molecular mechanisms underlying CNS-specific progression events, this study supports empiric use of lorlatinib for patients experiencing this unique pattern of progression.
Lorlatinib is a potent, third-generation ALK TKI designed to penetrate the CNS and developed to overcome ALK mutations that confer resistance to second-generation ALK TKIs.6 In the phase I/II study that led to its initial approval, treatment with lorlatinib resulted in objective intracranial responses in 56% of patients relapsing on a second-generation ALK TKI with a median duration of intracranial response of 12.4 months.7,8 In the study, the cumulative incidence of extracranial progression on lorlatinib exceeded the cumulative incidence of intracranial progression, consistent with a CNS protective effect.8,9 Among patients receiving lorlatinib as their initial therapy in the phase III CROWN study, 97% were free from intracranial progression at 18 months, strongly supporting the drug's ability to delay CNS relapses.10
For a subset of patients, the CNS is the only site of progression on first- and second-generation ALK TKIs.5 Among patients progressing on crizotinib, isolated CNS progression is primarily attributable to limited blood-brain barrier penetration.11 As several next-generation ALK TKIs have significant CNS penetration, it is possible that true resistance may account for some CNS-specific relapses. However, the molecular underpinnings of CNS-specific progression on second-generation ALK TKIs have not been robustly characterized, largely because of inaccessibility of brain metastases for sampling. In a handful of studies that analyzed CSF from patients with CNS progression on ALK therapies, ALK kinase domain mutations and key alterations in other genes have been detected, suggesting that ALK-dependent and ALK-independent mechanisms may contribute to CNS-specific resistance.12,13
Here, we conducted a phase II study (ClinicalTrials.gov identifier: NCT02927340) to evaluate the intracranial activity of lorlatinib in patients with ALK+ NSCLC and CNS-only progression on ALK TKIs without measurable, active extracranial disease. When our study launched, this subset of patients was not eligible to enroll in the ongoing phase I/II lorlatinib study. Because of its selectivity, lorlatinib is optimized to overcome resistance in tumors with ALK-dependent resistance mechanisms.14 Thus, this study was designed to provide insight into whether CNS metastases that are resistant to second-generation ALK TKIs retain ALK dependence.
METHODS
Study Design
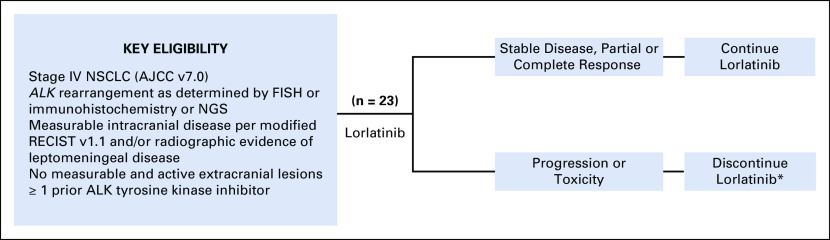
ClinicalTrials.gov identifier: NCT02927340 is an open-label, investigator-initiated, single-arm phase II trial of lorlatinib in patients with ALK-rearranged NSCLC with CNS metastasis and no sites of active, measurable extracranial disease (Appendix Fig A1). The study was conducted at two institutions (Massachusetts General Hospital and Dana-Farber Cancer Institute) and has completed full enrollment of 23 patients. The Protocol was approved by the Institutional Review Board. Written informed consent was obtained from all patients before screening. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonization.
Patients age ≥ 18 years with a histologically or cytologically confirmed diagnosis of stage IV (American Joint Committee on Cancer v7.0) NSCLC harboring an ALK rearrangement as determined by the US Food and Drug Administration–approved Vysis fluorescence in situ hybridization or Ventana immunohistochemistry assays or local and commercial next-generation sequencing platforms were enrolled. Patients were required to have at least one measurable (≥ 5 mm) intracranial lesion according to modified RECIST version 1.1 or evidence of leptomeningeal disease on imaging.15 CSF evaluation was not mandated to confirm leptomeningeal disease. Untreated and treated CNS metastases were permitted. To be eligible to enroll after local therapy, patients had to have new CNS metastases or irradiated CNS metastases that demonstrated unequivocal progression (defined as a > 20% increase in longest diameter). Patients with symptomatic CNS lesions were eligible if the Eastern Cooperative Oncology Group performance status was ≤ 2. Steroid use was permitted to address neurologic symptoms, provided that the steroid dose was stable or decreasing for at least 1 week before enrollment. Patients with measurable extracranial lesions were excluded, except for those with extracranial lesions that were not felt to represent active sites of disease on the basis of a prolonged period of stability. There was no limit on prior systemic therapies, including ALK TKIs.
Lorlatinib was administered at a starting dose of 100 mg once daily in continuous 21-day cycles. Adverse events (AEs) were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Dose reductions and interruptions were allowed. All patients underwent baseline tumor assessments, including brain imaging by contrast-enhanced magnetic resonance imaging (MRI) and computed tomography (CT) scans of the chest, abdomen, and pelvis. The study mandated a MRI slice thickness of 1 mm for brain metastases between 5 and 10 mm in size.
CT and MRI scans were performed every 6 weeks for the initial eight cycles and then every 9 weeks. Apart from patients with known pelvic metastases, on-treatment CT scans were limited to chest and abdominal imaging. Response assessment was performed centrally using RECIST v1.1 for extracranial evaluation and modified RECIST v1.1 for intracranial evaluation.15 Patients with ongoing clinical benefit were allowed to continue treatment beyond progression at the treating investigator's discretion. Participants were given the option to undergo CSF sampling before treatment and at relapse to characterize tumor-related alterations in cell-free DNA.
Statistical Design
The primary end point was intracranial disease control (defined as complete response, partial response, or stable disease) at 12 weeks according to modified RECIST v1.1.15 Key secondary end points included median intracranial progression-free survival (PFS), median intracranial duration of response, intracranial objective response rate, median extracranial PFS, and safety and tolerability of lorlatinib. The target rate of effectiveness was an intracranial disease control rate (DCR) at 12 weeks of 85%. An intracranial DCR of 60% or less was considered ineffective. The study design had a 90% power to detect this difference, with a one-side α level of .07. All patients who underwent intracranial restaging at 12 weeks were included in the efficacy analysis. Patients were not censored at extracranial progression, provided that they demonstrated ongoing CNS disease control and continued on lorlatinib. The data cutoff for this analysis was July 31, 2020. Analyses were performed using SAS version 9.4.
RESULTS
Patient Characteristics
Between November 2016 and January 2019, 23 patients were enrolled. One patient was inadvertently enrolled with nonmeasurable intracranial disease and was excluded from the efficacy analysis but included in the safety analysis.
Baseline characteristics of the 23 patients are shown in Table 1. The median number of prior lines of therapy was 3 (range 1-10). Twenty-two (96%) of the patients had received a second-generation ALK TKI. The remaining patient had only been treated with crizotinib because of lack of access to other ALK TKIs. Overall, the median number of prior ALK TKIs was 2 (range 1-4). Twenty (87%) patients had received the CNS-penetrant ALK TKIs, alectinib or brigatinib. Eight (35%) patients were previously treated with chemotherapy. The CNS was the only site of progression on the immediately preceding therapy for 22 (96%) patients. One patient had progression of both CNS and osseous disease. Nine (39%) patients had one or more sites of extracranial disease on baseline imaging, which were not measurable per RECIST v1.1. Apart from the patient with progressive bone metastases, none of the patients with nonmeasurable extracranial disease were progressing at these sites. Six (26%) patients had an extracranial lesion (all lung lesions) that was measurable but deemed inactive on the basis of prolonged stability by the treating investigator.
TABLE 1.
Baseline Characteristics of the Study Population
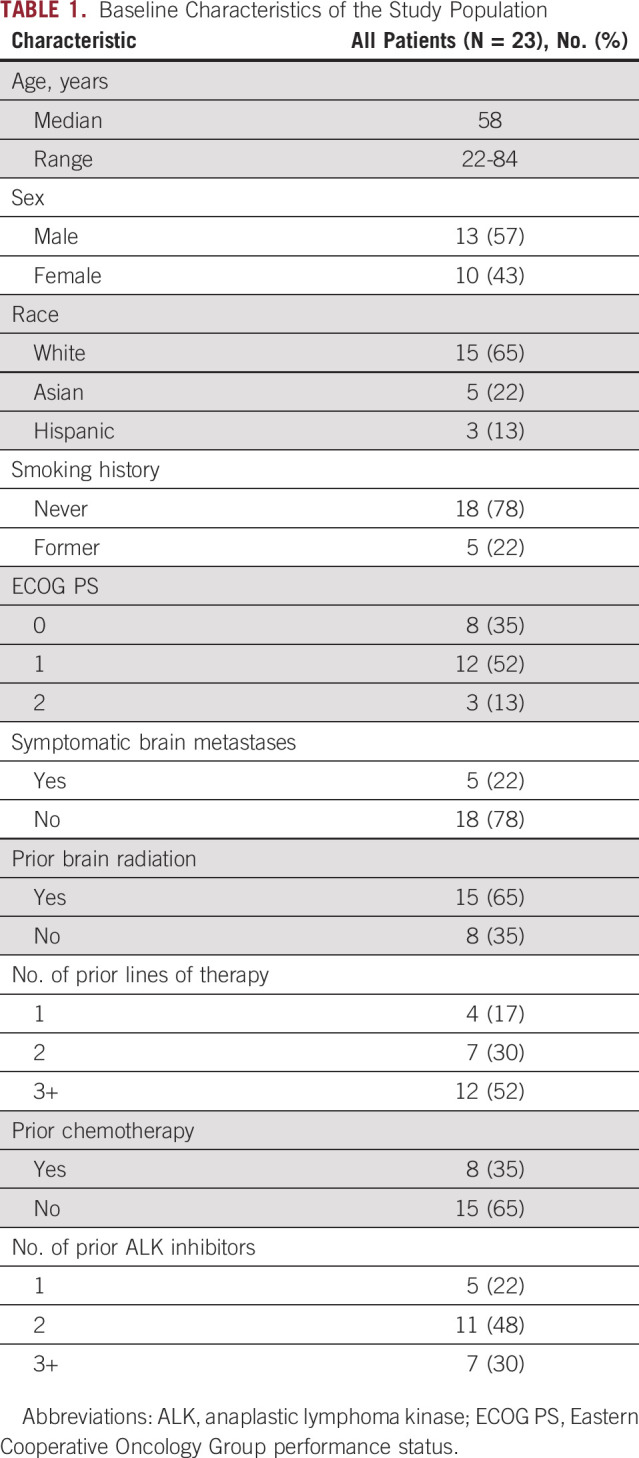
The majority (n = 19, 83%) of patients had only parenchymal brain metastases. The remaining four patients had both leptomeningeal and parenchymal involvement. Twenty (87%) patients had at least one brain metastases measuring ≥ 1 cm. Five (22%) patients were symptomatic from CNS disease at the time of enrollment. Five (22%) patients were on steroids to manage symptomatic CNS metastases at study entry. Fifteen (65%) patients had irradiated brain metastases, including eight who were previously treated with stereotactic radiosurgery alone and seven patients who had received partial or whole brain radiation. The median time between completion of brain radiation and initiation of lorlatinib was 20.2 (range 6.0-47.9) months. Six patients had undergone resection of brain metastases before enrollment, four of whom had subsequently received radiation.
Safety
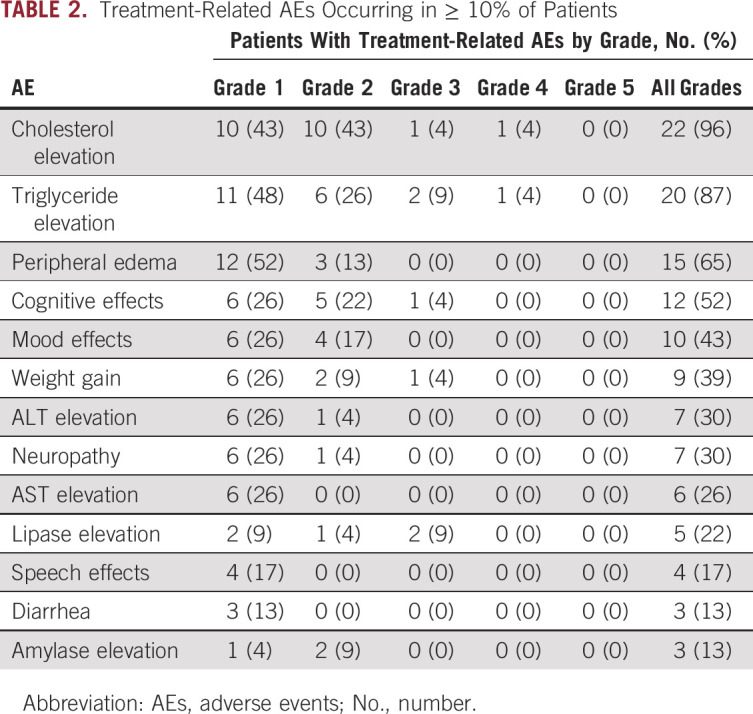
All 23 patients were evaluated for safety (Table 2). The most common treatment-related AEs of any grade were hypercholesterolemia (n = 22, 96%), hypertriglyceridemia (n = 20, 87%), edema (n = 15, 65%), cognitive effects (n = 12, 52%), irritability/mood changes (n = 10, 43%), and weight gain (n = 9, 39%). Other reported CNS AEs included slowed speech (n = 4, 17%), dizziness (n = 1, 4%), and hallucination (n = 1, 4%). CNS AEs were generally mild (grade ≤ 2). Nine grade 3-4 treatment-related AEs were observed, including triglyceride elevation (n = 3, 13%), cholesterol elevation (n = 2, 9%), asymptomatic lipase elevation (n = 2, 9%), cognitive effects (n = 1, 4%), and weight gain (n = 1, 4%). In addition, two patients experienced a grade 5 respiratory event. The first patient developed respiratory failure after 19 months on study with imaging findings suggestive of intrathoracic disease progression and superimposed pneumonitis. The treating investigators attributed the pneumonitis to lorlatinib. The second patient developed hypoxemic respiratory failure related to pulmonary hypertension after 24 months on lorlatinib. As pulmonary emboli and cardiac disease were excluded, the event was attributed to lorlatinib.
TABLE 2.
Treatment-Related AEs Occurring in ≥ 10% of Patients
Seventeen (74%) patients experienced an AE necessitating dose reduction. The median interval between initiating lorlatinib and dose reduction was 29 (range 4-883) days. Nine (39%) patients required ≥ 2 dose reductions. The mean lorlatinib dose intensity was 70 mg. One patient discontinued lorlatinib because of ongoing edema and dyspnea despite two dose reductions.
Efficacy
The efficacy analysis included 22 patients. As discussed above, one patient with nonmeasurable CNS disease at baseline was excluded. Thirteen (59%) patients had a confirmed objective intracranial response at 12 weeks, including three (14%) patients with complete intracranial response (Fig 1). Eight (36%) additional patients had stability of intracranial disease, yielding an intracranial DCR of 95% at 12 weeks. The intracranial DCR at 12 weeks exceeded the target threshold of 85%. Three (14%) patients converted from partial to complete intracranial response with further follow-up. Thus, six (27%) patients had complete intracranial response while on study. Best intracranial response and a representative image of intracranial response are presented in Appendix Figures A2 and A3. None of the patients with stable intracranial disease had an objective response during the follow-up period. The best response among patients with leptomeningeal disease was stable disease without either complete response or progression.
FIG 1.
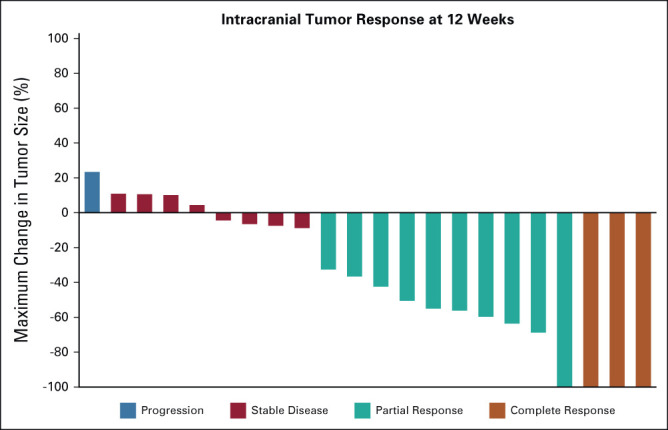
Intracranial antitumor activity of lorlatinib. The waterfall plot depicts intracranial tumor response at 12 weeks as assessed by modified RECIST version 1.1.
The intracranial PFS rate at 12 months was 79% (95% CI, 53 to 94). Patients with ongoing CNS disease control at a follow-up of > 1 year included several patients receiving dose-reduced lorlatinib (Fig 2A). With a median follow-up of 16.8 months from initiation of lorlatinib, nine (41%) of 22 patients experienced disease progression. Four (18%) patients developed progression of brain metastases, including one patient who also had progression of extracranial disease. Five patients had progression that was confined to extracranial sites. Four of five patients with extracranial-only progression continued treatment beyond progression given ongoing intracranial disease control. The median intracranial PFS was 24.6 months (95% CI, 20.2 to not reached; Fig 2B). Because of the limited number of progression events, the secondary efficacy end points of extracranial PFS and median intracranial duration of response could not be estimated.
FIG 2.
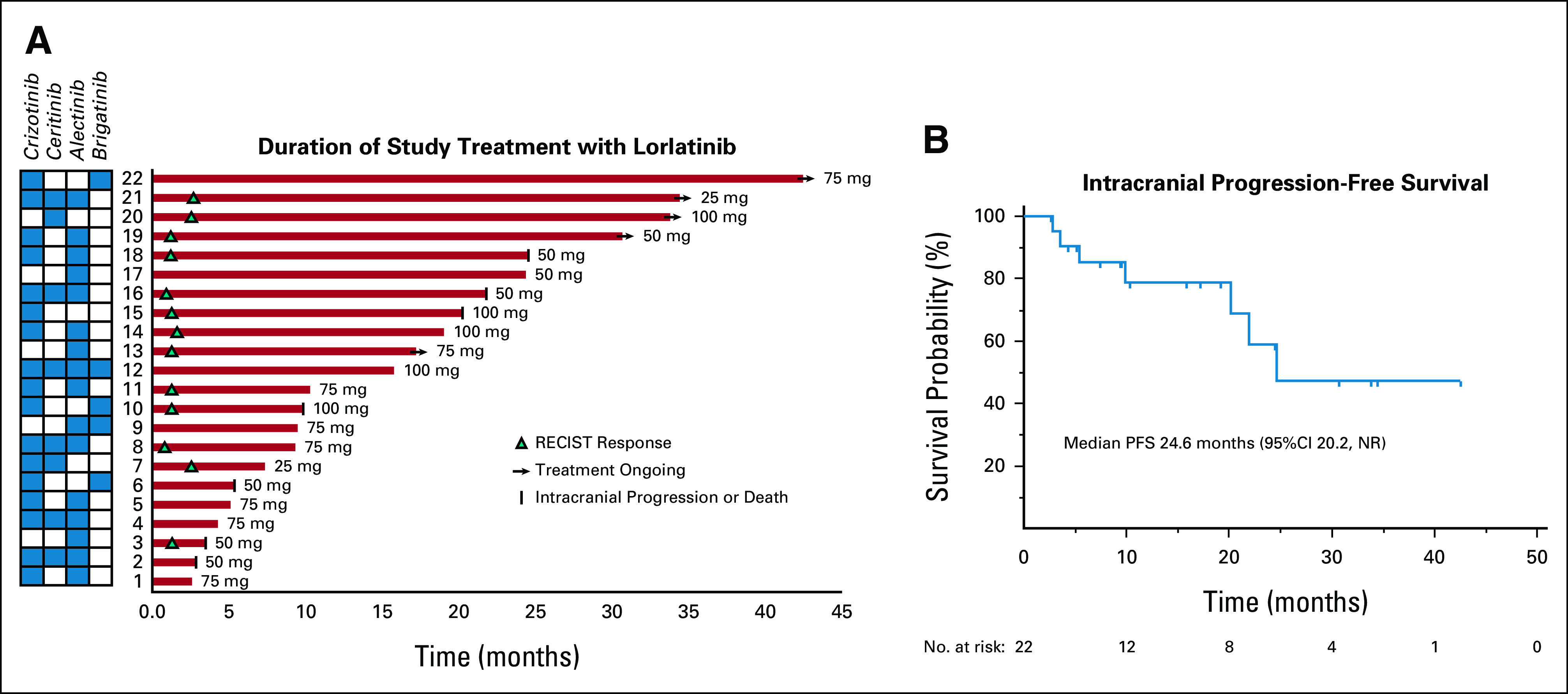
Durability of intracranial responses to lorlatinib. (A) The swimmer plot illustrates the duration of treatment with lorlatinib. Minimum dose of lorlatinib received is indicated next to each patient. Intracranial response to treatment, patient and brain metastasis status, and lorlatinib treatment status are indicated with symbols (see the legend). ALK inhibitors received before lorlatinib are indicated in the grid. (B) The Kaplan-Meier curve illustrates intracranial PFS with lorlatinib. NR, not reached; PFS, progression-free survival.
Of the four patients with intracranial progression on study, only one underwent resection of an enlarging brain metastasis. Molecular and histologic analysis demonstrated ALK+ lung adenocarcinoma that did not harbor ALK mutations but had low-level amplification of the MET gene. There was no prelorlatinib biopsy for comparison. None of the 22 patients evaluated for efficacy pursued optional CSF sampling during the study period.
Patient Disposition
Sixteen patients discontinued lorlatinib during the follow-up period. For six patients, lorlatinib was stopped as a result of disease progression. Three patients discontinued lorlatinib for toxicity, including one patient with intolerable edema and two patients with fatal respiratory events as described above. One patient with nonmeasurable intracranial disease discontinued treatment because of functional decline related to underlying disease. As a result of worsening functional status from pre-enrollment radiation necrosis and persistent neurologic symptoms that predated study entry, respectively, two other patients elected to stop lorlatinib. The remaining four patients left the study because of financial hardship (n = 1) or transitioning to commercial lorlatinib (n = 3).
Prevalence of CNS-Only Progression on Second-Generation ALK TKIs
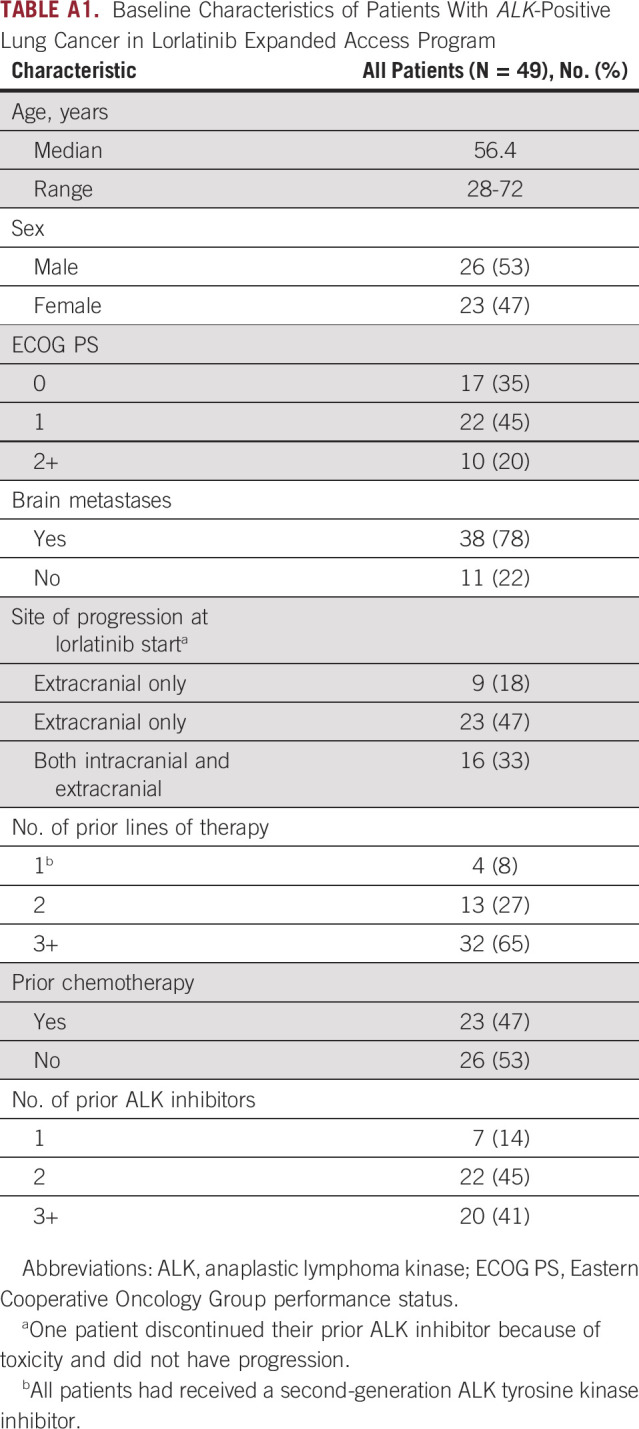
Estimates of the prevalence of CNS-only progression on second-generation ALK TKIs are lacking. To better understand the relevance of the approach explored in this trial in the context of the ALK therapeutic landscape, we reviewed records of unselected patients with ALK+ NSCLC who were enrolled in the lorlatinib expanded access program (ClinicalTrials.gov identifier: NCT03178071) at Massachusetts General Hospital and Dana-Farber Cancer Institute to determine the frequency of CNS-only progression. The expanded access program was selected as the representative data set as patients with nonmeasurable disease and those with CNS-only progression were permitted to enroll. In total, 49 patients were enrolled between February 2018 and November 2018 (Appendix Table A1). All patients had received a second-generation ALK TKI, and 98% had been exposed to alectinib and/or brigatinib. Overall, nine (18%) had experienced CNS-only progression on a second-generation ALK TKI.
DISCUSSION
In this investigator-initiated, single-arm phase II study, we evaluated the intracranial activity of the third-generation ALK TKI lorlatinib in patients with metastatic ALK+ NSCLC who had CNS-only relapse on earlier-generation ALK TKIs. Among this group of patients, lorlatinib demonstrated potent intracranial activity, with an intracranial objective response rate and an intracranial DCR of 59% and 95%, respectively, at 12 weeks. Our findings support lorlatinib as a highly effective therapeutic strategy for the subset of patients with isolated CNS progression on other ALK TKIs.
Early studies of resistance to ALK TKIs identified the CNS as a common site of relapse and a routine site of involvement at diagnosis.11,16 Therefore, current management strategies for ALK+ NSCLC place emphasis on CNS-penetrant, next-generation ALK TKIs. Despite substantial intracranial activity of the second-generation ALK TKIs, alectinib and brigatinib, 10% of patients treated with these drugs in the first-line setting experience intracranial progression within the initial year of treatment.3,17 In a retrospective analysis, we identified CNS-only relapse events in 18% of patients progressing on a second-generation ALK TKI. Baseline CNS disease is a strong predictor of future CNS progression. Indeed, the intracranial PFS rate at 2 years among patients receiving first-line brigatinib was 48% for those with pretreatment brain metastases compared with 74% for patients without brain involvement.3 In addition to demonstrating that second-generation ALK TKIs do not completely eliminate the propensity for CNS metastases to beget further CNS progression events, the pattern of failure analyses suggests that the CNS is vulnerable even among patients who do not have CNS metastases at diagnosis. Thus, there is ongoing need for therapeutic strategies that can be initiated to salvage CNS progression.
Lorlatinib is a potent, next-generation ALK TKI with the broadest spectrum of activities against ALK mutations. In phase I/II testing, lorlatinib induced significant responses in ALK+ tumors that developed resistance to second-generation ALK TKIs as a result of acquired ALK kinase domain mutations.14 Here, we observed that nearly all (95%) patients who received lorlatinib in our study experienced control of CNS disease and a majority (59%) of patients met criteria for intracranial objective response. As 87% of patients in our study had received alectinib or brigatinib before initiating lorlatinib, this raises the possibility that ALK-dependent mechanisms might have accounted for some CNS relapses. As escalating the dose of alectinib or brigatinib can overcome CNS progression,18,19 it is also conceivable that ALK is incompletely inhibited in the CNS at standard dosing of both drugs. Considering the challenges of distinguishing between resistance and pharmacokinetic failure, our study supports empiric use of lorlatinib for patients with CNS-confined progression on second-generation ALK TKIs. Furthermore, the finding that lorlatinib significantly reduced risk of developing brain metastases in the CROWN study provides compelling rationale for considering lorlatinib for first-line treatment,10 particularly among patients with baseline CNS disease.
The safety profile for lorlatinib in our study was similar to previous studies.7,20 Specifically, the most common AEs were lipid abnormalities, edema, cognitive and mood effects, and weight gain. In addition to these toxicities, we observed fatal pulmonary toxicity at a rate (9%) that exceeded previous reports.7 The over-representation of this rare toxicity in our study may be an artifact of the small sample size. Interestingly, certain expected toxicities were also over-represented in our study, including cognitive and mood effects, which were observed in 52% and 43% of patients, respectively. The rate of these AEs was double the frequency of similar events reported for all-comers treated with lorlatinib in the global phase I-III studies.7,10 It is possible that traits enriched in our study population compared with other studies—brain metastases, prior CNS radiation (65%)—may increase likelihood of developing neurocognitive toxicity or enhance vigilance for this class of toxicities. Additional studies are needed to assess for correlation between brain metastases and local CNS therapies received and the spectrum and frequency of lorlatinib-related toxicities.
In our study, the rate of dose reduction was also higher than that anticipated from previous studies. Thus, in addition to potentially enhancing risk of certain toxicities, the unique attributes of the population enrolled might have affected tolerance of side effects. Dose adjustment occurred early and often in our study, with 74% of patients requiring dose interruption and reduction and a median time to dose reduction of 29 days. The majority of dose reductions were driven by neurocognitive toxicity or edema. Importantly, dose reduction had a mitigating effect and did not compromise durability of intracranial response to lorlatinib (Fig 2A), suggesting that the dose decrease did not result in subtherapeutic dosing. These findings should reassure practitioners that CNS efficacy can be maintained in patients requiring dose reduction.
Our study had several important limitations. The sample size was intentionally small as we primarily recruited a subset of patients with ALK+ NSCLC. We justified the small sample size by targeting a dramatic signal of intracranial activity (DCR of 85%) that would confidently support the hypothesis that CNS-specific relapses do not forfeit ALK dependence. The sample size was further limited by the fact that three patients discontinued treatment early to transition to commercial lorlatinib, affecting our analysis of durability of response. Despite sample size limitations, we developed our study as a platform for providing insight into the biologic underpinnings of CNS-specific relapses on next-generation ALK TKIs. To this end, the study included optional CSF analyses, but none of the patients in the study elected to pursue this procedure. As a result, our assumptions of ALK dependence are based on clinical outcomes and are not confirmed by molecular studies.
In summary, in this phase II study, we observed robust intracranial activity of lorlatinib in patients with isolated CNS progression on second-generation ALK TKIs. The intracranial DCR of 95% with lorlatinib in this context suggests that brain metastases from patients with CNS-only progression remain ALK-dependent.
ACKNOWLEDGMENT
The authors would like to thank all patients; their families and caregivers; and the coinvestigators, study staff, and research coordinators at the two participating centers.
APPENDIX
FIG A1.
Study schema. The schematic describes the study design and key eligibility criteria. *Treatment beyond progression permitted at investigator discretion. AJCC, American Joint Committee on Cancer; ALK, anaplastic lymphoma kinase; FISH, fluorescence in situ hybridization; NGS, next-generation sequencing; NSCLC, non–small-cell lung cancer; TKI, tyrosine kinase inhibitor.
FIG A2.
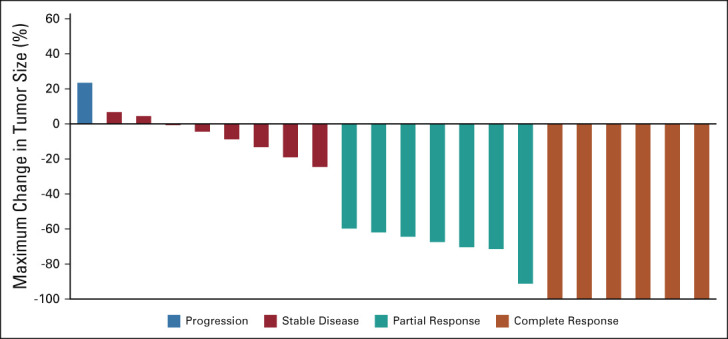
Best intracranial response to lorlatinib. The waterfall plot depicts best intracranial tumor response as assessed by modified RECIST version 1.1.
FIG A3.
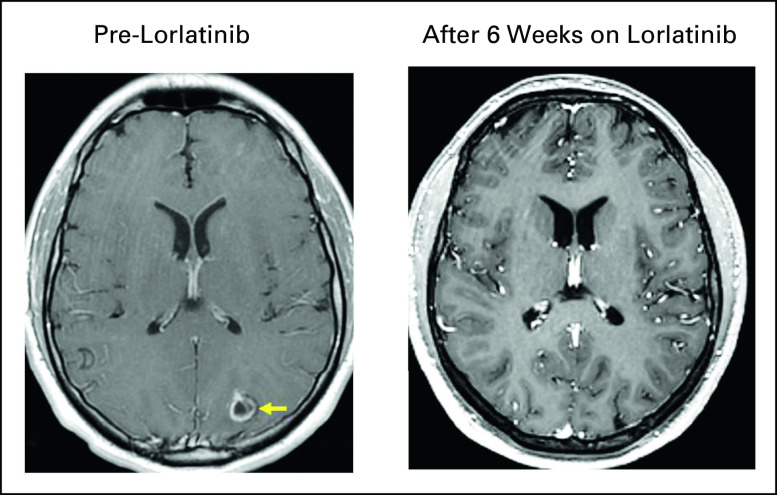
Resolution of brain metastasis on lorlatinib. Serial brain imaging demonstrates complete resolution of a left occipital metastasis (A) pre-lorlatinib (yellow arrow) and (B) after 6 weeks on lorlatinib. Complete intracranial response in this lesion and all pre-lorlatinib brain metastases was maintained for 23 months. The patient had previously received crizotinib and alectinib.
TABLE A1.
Baseline Characteristics of Patients With ALK-Positive Lung Cancer in Lorlatinib Expanded Access Program
Ibiayi Dagogo-Jack
Honoraria: American Academic Health System, Total Health Conferencing, DAVA Oncology, Creative Educational Concepts, Medscape, ASCO Post, OncLive/MJH Life Sciences
Consulting or Advisory Role: Boehringer Ingelheim, AstraZeneca, Xcovery, Catalyst Pharmaceuticals, Pfizer, Syros Pharmaceuticals, Novocure, BostonGene, Bayer, Genentech, Sanofi, Janssen
Research Funding: Pfizer (Inst), Array BioPharma (Inst), Novartis (Inst), Genentech (Inst), Calithera Biosciences (Inst), Vivace Therapeutics (Inst)
Travel, Accommodations, Expenses: Pfizer, Array BioPharma
Geoffrey R. Oxnard
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Makenzi Evangelist
Consulting or Advisory Role: Takeda, AstraZeneca, Mirati Therapeutics, Regeneron
Subba R. Digumarthy
Honoraria: Siemens
Travel, Accommodations, Expenses: Siemens Healthcare Diagnostics
Other Relationship: provides independent image analysis for hospital-contracted clinical research trials programs for several companies including Merck, Pfizer, Bristol Mayer Squibb, Novartis, Roche, Polaris, Cascadian, AbbVie, Gradalis, Clinical Bay, Zai Laboratories. R (Inst)
Jessica J. Lin
Honoraria: Pfizer, OncLive
Consulting or Advisory Role: C4 Therapeutics, Genentech, Nuvalent Inc, Blueprint Medicines, Turning Point Therapeutics, Bayer, Mirati Therapeutics, Novartis, Elevation Oncology
Research Funding: Hengrui Therapeutics (Inst), Turning Point Therapeutics (Inst), Novartis (Inst), Neon Therapeutics (Inst), Relay Therapeutics (Inst), Elevation Oncology (Inst), Bayer (Inst), Roche (Inst), Linnaeus Therapeutics (Inst), Nuvalent Inc (Inst)
Travel, Accommodations, Expenses: Pfizer
Justin F. Gainor
Employment: Ironwood Pharmaceuticals (I)
Stock and Other Ownership Interests: Ironwood Pharmaceuticals (I)
Honoraria: Merck, Novartis, Pfizer, Takeda, BeiGene
Consulting or Advisory Role: Genentech, Bristol Myers Squibb, Takeda, Amgen, Merck, Jounce Therapeutics, Blueprint Medicines, Gilead Sciences, Lilly, Moderna Therapeutics, Karyopharm Therapeutics, ITeos Therapeutics, Pfizer, Mirati Therapeutics, Nuvalent Inc, EMD Serono, Silverback Therapeutics, Novartis, BeiGene
Research Funding: Merck (Inst), Novartis (Inst), Genentech, Bristol Myers Squibb (Inst), Adaptimmune (Inst), AstraZeneca (Inst), Jounce Therapeutics (Inst), Blueprint Medicines (Inst), Moderna Therapeutics (Inst), Tesaro (Inst), Alexo Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/775917
Michael S. Rabin
Stock and Other Ownership Interests: Acuity Bio
Rebecca S. Heist
Honoraria: Chugai/Roche
Consulting or Advisory Role: Novartis, Daiichi Sankyo, EMD Serono/Merck, AbbVie
Research Funding: AbbVie (Inst), Novartis (Inst), Roche (Inst), Incyte (Inst), Celgene (Inst), Mirati Therapeutics (Inst), Peregrine Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Debiopharm Group (Inst), Corvus Pharmaceuticals (Inst), Daiichi Sankyo (Inst), Agios (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Turning Point Therapeutics (Inst)
Alice T. Shaw
Employment: Novartis Institutes for BioMedical Research
Stock and Other Ownership Interests: Novartis Institutes for BioMedical Research
No other potential conflicts of interest were reported.
SUPPORT
Supported in full by Pfizer. I.D.-J. received funding from the National Cancer Institute Career Development Award (K12CA087723-16).
AUTHOR CONTRIBUTIONS
Conception and design: Ibiayi Dagogo-Jack, Alona Muzikansky, Alice T. Shaw
Provision of study materials or patients: Ibiayi Dagogo-Jack, Geoffrey R. Oxnard, Makenzi Evangelist, Jessica J. Lin, Justin F. Gainor, Michael S. Rabin, Rebecca S. Heist
Collection and assembly of data: Geoffrey R. Oxnard, Makenzi Evangelist, Subba R. Digumarthy, Jessica J. Lin, Justin F. Gainor, John F. Murphy, Michael S. Rabin, Rebecca S. Heist, Alice T. Shaw
Data analysis and interpretation: Ibiayi Dagogo-Jack, Subba R. Digumarthy, Jessica J. Lin, Justin F. Gainor, John F. Murphy, Rebecca S. Heist, Alona Muzikansky, Alice T. Shaw
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ibiayi Dagogo-Jack
Honoraria: American Academic Health System, Total Health Conferencing, DAVA Oncology, Creative Educational Concepts, Medscape, ASCO Post, OncLive/MJH Life Sciences
Consulting or Advisory Role: Boehringer Ingelheim, AstraZeneca, Xcovery, Catalyst Pharmaceuticals, Pfizer, Syros Pharmaceuticals, Novocure, BostonGene, Bayer, Genentech, Sanofi, Janssen
Research Funding: Pfizer (Inst), Array BioPharma (Inst), Novartis (Inst), Genentech (Inst), Calithera Biosciences (Inst), Vivace Therapeutics (Inst)
Travel, Accommodations, Expenses: Pfizer, Array BioPharma
Geoffrey R. Oxnard
This author is a member of the JCO Precision Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Employment: Foundation Medicine
Stock and Other Ownership Interests: Roche
Makenzi Evangelist
Consulting or Advisory Role: Takeda, AstraZeneca, Mirati Therapeutics, Regeneron
Subba R. Digumarthy
Honoraria: Siemens
Travel, Accommodations, Expenses: Siemens Healthcare Diagnostics
Other Relationship: provides independent image analysis for hospital-contracted clinical research trials programs for several companies including Merck, Pfizer, Bristol Mayer Squibb, Novartis, Roche, Polaris, Cascadian, AbbVie, Gradalis, Clinical Bay, Zai Laboratories. R (Inst)
Jessica J. Lin
Honoraria: Pfizer, OncLive
Consulting or Advisory Role: C4 Therapeutics, Genentech, Nuvalent Inc, Blueprint Medicines, Turning Point Therapeutics, Bayer, Mirati Therapeutics, Novartis, Elevation Oncology
Research Funding: Hengrui Therapeutics (Inst), Turning Point Therapeutics (Inst), Novartis (Inst), Neon Therapeutics (Inst), Relay Therapeutics (Inst), Elevation Oncology (Inst), Bayer (Inst), Roche (Inst), Linnaeus Therapeutics (Inst), Nuvalent Inc (Inst)
Travel, Accommodations, Expenses: Pfizer
Justin F. Gainor
Employment: Ironwood Pharmaceuticals (I)
Stock and Other Ownership Interests: Ironwood Pharmaceuticals (I)
Honoraria: Merck, Novartis, Pfizer, Takeda, BeiGene
Consulting or Advisory Role: Genentech, Bristol Myers Squibb, Takeda, Amgen, Merck, Jounce Therapeutics, Blueprint Medicines, Gilead Sciences, Lilly, Moderna Therapeutics, Karyopharm Therapeutics, ITeos Therapeutics, Pfizer, Mirati Therapeutics, Nuvalent Inc, EMD Serono, Silverback Therapeutics, Novartis, BeiGene
Research Funding: Merck (Inst), Novartis (Inst), Genentech, Bristol Myers Squibb (Inst), Adaptimmune (Inst), AstraZeneca (Inst), Jounce Therapeutics (Inst), Blueprint Medicines (Inst), Moderna Therapeutics (Inst), Tesaro (Inst), Alexo Therapeutics (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/775917
Michael S. Rabin
Stock and Other Ownership Interests: Acuity Bio
Rebecca S. Heist
Honoraria: Chugai/Roche
Consulting or Advisory Role: Novartis, Daiichi Sankyo, EMD Serono/Merck, AbbVie
Research Funding: AbbVie (Inst), Novartis (Inst), Roche (Inst), Incyte (Inst), Celgene (Inst), Mirati Therapeutics (Inst), Peregrine Pharmaceuticals (Inst), Exelixis (Inst), Millennium (Inst), Debiopharm Group (Inst), Corvus Pharmaceuticals (Inst), Daiichi Sankyo (Inst), Agios (Inst), Exelixis (Inst), Pfizer (Inst), Lilly (Inst), Turning Point Therapeutics (Inst)
Alice T. Shaw
Employment: Novartis Institutes for BioMedical Research
Stock and Other Ownership Interests: Novartis Institutes for BioMedical Research
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gainor JF, Tseng D, Yoda S, et al. : Patterns of metastatic spread and mechanisms of resistance to crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis Oncol 10.1200/PO.17.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pacheco JM, Gao D, Smith D, et al. : Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol 14:691-700, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camidge DR, Kim HR, Ahn MJ, et al. : Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: Second interim analysis of the phase III ALTA-1L trial. J Clin Oncol 38:3592-3603, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok T, Camidge DR, Gadgeel SM, et al. : Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 31:1056-1064, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Gadgeel S, Peters S, Mok T, et al. : Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 29:2214-2222, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou HY, Li Q, Engstrom LD, et al. : PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci USA 112:3493-3498, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon BJ, Besse B, Bauer TM, et al. : Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol 19:1654-1667, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Felip E, Shaw AT, Bearz A, et al. : Intracranial and extracranial efficacy of lorlatinib in patients with ALK-positive non-small-cell lung cancer previously treated with second-generation ALK TKIs. Ann Oncol 32:620-630, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Bauer TM, Shaw AT, Johnson ML, et al. : Brain penetration of lorlatinib: Cumulative incidences of CNS and non-CNS progression with lorlatinib in patients with previously treated ALK-positive non-small-cell lung cancer. Target Oncol 15:55-65, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw AT, Bauer TM, de Marinis F, et al. : First-line lorlatinib or crizotinib in advanced. N Engl J Med 383:2018-2029, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Costa DB, Shaw AT, Ou SH, et al. : Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 33:1881-1888, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng MM, Li YS, Jiang BY, et al. : Clinical utility of cerebrospinal fluid cell-free DNA as liquid biopsy for leptomeningeal metastases in ALK-rearranged NSCLC. J Thorac Oncol 14:924-932, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Aldea M, Hendriks L, Mezquita L, et al. : Circulating tumor DNA analysis for patients with oncogene-addicted NSCLC with isolated central nervous system progression. J Thorac Oncol 15:383-391, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Shaw AT, Solomon BJ, Besse B, et al. : ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol 37:1370-1379, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long GV, Trefzer U, Davies MA, et al. : Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol 13:1087-1095, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Gainor JF, Sherman CA, Willoughby K, et al. : Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol 10:232-236, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters S, Camidge DR, Shaw AT, et al. : Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377:829-838, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Urbanska EM, Santoni-Rugiu E, Melchior LC, et al. : Intracranial response of ALK+ non-small-cell lung cancer to second-line dose-escalated brigatinib after alectinib discontinuation due to drug-induced hepatitis and relapse after whole brain radiotherapy followed by stereotactic radiosurgery. Clin Lung Cancer 22:e528-e532, 2021 [DOI] [PubMed] [Google Scholar]
- 19.Gainor JF, Chi AS, Logan J, et al. : Alectinib dose escalation reinduces central nervous system responses in patients with anaplastic lymphoma kinase-positive non-small cell lung cancer relapsing on standard dose alectinib. J Thorac Oncol 11:256-260, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw AT, Felip E, Bauer TM, et al. : Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 18:1590-1599, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]