Abstract
The Pedra Branca Forest is in a highly urbanised region of the central portion of Rio de Janeiro City and comprises the largest urban forest in the world (> 12,000 ha). The local flora and fauna are protected by three conservation units and the Estação Biológica Fiocruz Mata Atlântica (EFMA), which comprises 462 hectares on the east side of the remnant. The local biodiversity is still little known compared to other Atlantic Forest remnants from the Rio de Janeiro State. Here, we provide results of a survey of medium- and large-sized terrestrial mammals from the EFMA. In addition, we analysed the distribution of this fauna along three habitat types defined as Peridomicile, Transitional Forest and Forest Core. Sampling was performed from 2017 to 2020 and comprised a camera-trap survey, interviews with residents and local workers and occasional records. Results include occurrence records for 16 autochthonous and one allochthonous (Callithrix sp.) wild mammals, which are distributed into 14 families and seven orders, in addition to the presence of free-ranging domestic dogs and cats. Four species are in some category of threat of extinction at national or global levels. Amongst them, Leontopithecusrosalia (first record for the Rio de Janeiro City in more than a century) and Leopardusguttulus are classified as Vulnerable by IUCN. Most wild native species were registered in the three habitat types, but with differences in the frequency of records. Our results indicate that the presence of domestic dogs and cats influenced the species composition in each area, with Nasuanasua, Dasyproctaleporina and Didelphisaurita less frequent in places where domestic dogs and cats are more frequent. This is the first systematic effort to understand the occurrence and distribution of mid- and large-sized mammals in the Pedra Branca Forest.
Keywords: camera traps, conservation, diversity, domestic dog, habitat use, Pedra Branca Forest, species richness
Introduction
Three Atlantic Forest remnants—Pedra Branca, Tijuca and Gericinó-Mendanha—are present in Rio de Janeiro City. The Pedra Branca Forest is the largest remnant of urban forest in the world and is located in a highly urbanised region of the central portion of the City. This remnant is partially connected to the Tijuca Forest by small forest fragments separated by highways and both are isolated from the Gericinó-Mendanha Forest by a matrix of urban densification. The flora and fauna of Pedra Branca are protected by the Parque Estadual da Pedra Branca (PEPB; Pedra Branca State Park), Parque Natural Municipal da Prainha, Reserva Biológica de Guaratiba and the Estação Biológica Fiocruz Mata Atlântica (EFMA; Fiocruz Atlantic Forest Biological Station). Most of the territory is preserved by the PEPB, which comprises areas above 100 m a.s.l. (ca. 12,000 hectares). The EFMA is on the east side of the remnant, in an area under high human pressure, whose biological diversity, including mammals, is still poorly known compared to other localities in the Municipality and in the State of Rio de Janeiro—for example, Tijuca Forest (Freitas et al. 2006, Silva 2017, Silva et al. 2018) and Serra dos Órgãos (Cronemberger et al. 2019), respectively.
The EFMA is adjacent to six communities with high social vulnerability and precarious sanitation conditions. These communities extend through the forest edge, putting domestic and wild animals, insect vectors and humans in potential contact, which constitutes a favourable environment for the circulation of zoonotic and non-zoonotic pathogens (White and Razgour 2020). From the viewpoint of wildlife surveillance, the scenario deserves special attention, since outbreaks of emerging and re-emerging infectious zoonotic diseases are associated with interactions between pathogens and potential hosts (usually mammals) and anthropogenic changes in the environment, including habitat loss, socioeconomic factors and demographic increase (Daszak et al. 2012, Jones et al. 2008).
Studies with mammals in the Pedra Branca Forest that used systematised sampling were only conducted for the small-sized species, including bats, rodents and marsupials (Gentile et al. 2018, Tavares et al. 2021). Thus, the few records of mid- and large-sized mammals that exist for this region are the result of occasional observations. We carried out a survey of medium and large-sized mammals and analysed the distribution of this fauna from the peridomicile to the forest core, as part of a project to understand the ecological interfaces that may favour the circulation of zoonotic and non-zoonotic pathogens amongst humans, wildlife and domestic animals in the EFMA territory.
Data resources
Individualised records of medium- and large-sized mammals from Fiocruz Atlantic Forest Biological Station, Rio de Janeiro, south-eastern Brazil, registered by camera trap, are available in Suppl. material 1. Results from SIMPER analysis with the contribution of each species to overall dissimilarities amongst sampling areas are available in Suppl. material 2.
Material and Methods
Study area
The study was carried out at the Estação Biológica Fiocruz Mata Atlântica (EFMA; central coordinates 22°56'25" S, 43°24'18" W, Fig. 1), located on the eastern slope of the Pedra Branca Forest, Municipality of Rio de Janeiro, Brazil. The EFMA comprises 462 ha, of which 262 ha (57%) overlap with the Parque Estadual da Pedra Branca (PEPB). It is made up of remnants of both the Lowland Dense Ombrophilous Forest (50 m a.s.l.) and the Submontane Dense Ombrophilous Forest (50–500 m a.s.l.). The lowland forest is composed of different habitat types impacted by the presence of six communities with high social vulnerability and precarious sanitation conditions. These communities are connected at different levels to densely populated neighbourhoods in the Jacarepaguá region. In addition to a high demographic occupation, other anthropogenic impacts are present, such as small-scale agricultural and poaching activities (Domingues and Rodrigues 2007).
Figure 1.
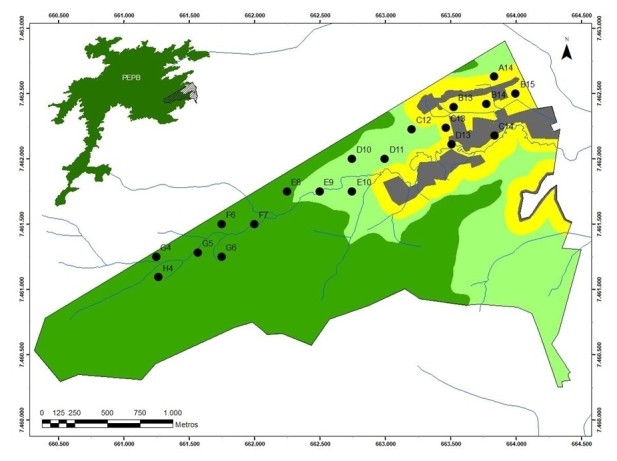
The location of the Estação Biológica Fiocruz Mata Atlântica (EFMA) on the east side of Pedra Branca Forest (left, above), Rio de Janeiro; and the distribution of camera-traps at EFMA, in areas defined as Peridomicile (yellow), Transition Forest (light green) and Forest Core (dark green). Combinations of letters and numbers refer to camera identifications. See Table 1 for coordinates, period and effort at each point.
The areas used for the mammal survey are distributed along a gradient of anthropogenic intervention, where each area defined for sampling represents a type of habitat along this gradient: (i) Peridomicile, which consists of areas up to 100 m adjacent to the communities (ca. 30–35 m a.s.l.) and is characterised by the presence of backyards and orchards, with vegetation dominated by exotic species; (ii) Transitional Forest, which extends from the end of the peridomicile area to 100 m.a.s.l., with a prevalence of native plants, but with dense understorey and low canopy; and (iii) Forest Core, including all areas above 100 m a.s.l. and comprising preserved areas, without human constructions, with little human activities, more open understorey, high canopy and great presence of epiphytic plants, streams and rock formations on a sloping topography.
Sampling design and data collection
The species survey was conducted using three different methods: (i) sampling with camera-traps; (ii) interviews with residents who live around the EFMA; and (iii) occasional records made directly by researchers during field activities. Only mammals whose species-level identification can be performed, based on external morphology, were considered in the results. This procedure allowed the inclusion of all mammals with a body weight of > 1.0 kg (Chiarello 2008), as well as representatives of other taxa that could be reliably identified in the sampled area (e.g. Didelphis, Sylvilagus). The nomenclature used for xenarthrans and marsupials followed different authors in Gardner (2008). For the others, the nomenclature follows Wilson and Reeder (2005).
For the camera trap survey, Trophy Cam trail cameras (Bushnell, Overland Parks, KS, USA) were distributed at 19 points along the areas defined as peridomicile, transitional forest and forest core (Table 1, Fig. 1). The sampling grid followed a minimum distance of 250 m between the points, including the altitudinal gradient, covering much of the environmental heterogeneity of the EFMA. The study was conducted from June 2018 to May 2020, with one camera positioned at each point (Table 1). These cameras were installed ca. 40 cm above the ground. They were installed randomly in spots where animals are expected to pass, such as trails, forest clearings and near fruit trees. Camera traps remained operational from 17 to 306 days throughout the study period and were checked every 30 days to change memory cards and batteries when needed. The images of all individuals of the same species detected by the same camera trap within a 1-h interval were treated as a single record.
Table 1.
Sampling sites with camera traps in the Estação Biológica Fiocruz Mata Atlântica, Rio de Janeiro.
| Point | Habitat | Setting | Removal | Sampling effort (days) |
| A14 | Peridomicile | 14/01/2019 | 04/07/2019 | 171 |
| B13 | Peridomicile | 30/11/2018 | 10/07/2019 | 222 |
| B14 | Peridomicile | 10/12/2018 | 10/07/2019 | 212 |
| B15 | Peridomicile | 10/12/2018 | 10/07/2019 | 212 |
| C13 | Peridomicile | 27/11/2018 | 09/01/2019 | 43 |
| C14 | Peridomicile | 13/11/2018 | 04/07/2019 | 233 |
| D13 | Peridomicile | 13/11/2018 | 04/07/2019 | 233 |
| C12 | Transition forest | 29/06/2018 | 16/07/2018 | 17 |
| D10 | Transition forest | 29/06/2018 | 16/07/2018 | 17 |
| D11 | Transition forest | 14/01/2019 | 15/05/2020 | 285 |
| E09 | Transition forest | 29/06/2018 | 16/07/2018 | 17 |
| E10 | Transition forest | 16/01/2019 | 30/08/2019 | 226 |
| E08 | Forest core | 11/09/2018 | 11/10/2018 | 30 |
| F06 | Forest core | 11/09/2018 | 11/10/2018 | 30 |
| F07 | Forest core | 11/09/2018 | 11/10/2018 | 30 |
| G04 | Forest core | 24/01/2019 | 12/08/2019 | 200 |
| G05 | Forest core | 24/01/2019 | 26/11/2019 | 306 |
| G06 | Forest core | 11/09/2018 | 11/10/2018 | 30 |
| H04 | Forest core | 24/01/2019 | 12/07/2019 | 169 |
The interviews to survey the species that occur in the region were conducted with residents, Fiocruz employees involved in the maintenance of EFMA trails and researchers who work with the local fauna and flora. Occasional records of mammals made by our staff or by other Fiocruz researchers were also considered.
Data analyses
Effort for the camera trap survey was calculated by multiplying the number of cameras installed by the number of days remaining active (unit: camera-days; Srbek-Araujo and Chiarello 2007), totalling 2,683 camera-days. Sampling sufficiency was verified by the rarefaction curve (Mao Tau) of the accumulated richness as a function of the days sampled (Soberón and Llorente 1993, Colwell et al. 2004). Estimated richness was calculated using Jackknife-2 and Chao-2 indexes (Zahl 1977, Chao 1984). An Analysis of Variance (ANOVA) was used to test the statistical significance of differences between the averages of the records/camera-days between the sampling areas in the EFMA. The Shapiro-Wilk test was used to confirm the normal distribution of the data. These analyses were all performed using PAST 4.01 software.
The homogeneity of multivariate dispersions was tested following Anderson (2006). We explored the patterns of similarity of records between areas using multivariate permutational analysis of variance (PERMANOVA) and non-metric multidimensional scaling analysis (nMDS) on Jaccard distances from the presence and absence matrix. Average similarity amongst sampling points was achieved by performing a Hierarchical agglomerative clustering using Ward's method and cluster adequacy was accessed through a cluster-wise cluster stability assessment by bootstrap resampling (Hennig 2007). A similarity percentage (SIMPER) analysis was performed to compare the contributions of the species between sampling sites. SIMPER analysis is based on the Bray-Curtis Index for estimating the average dissimilarity between pairs of sample groups and determining the contributions of each species to the average between-group Bray-Curtis dissimilarity (Clarke 1993). To assess whether the presence of records of domestic dogs and cats influenced the composition of wild animal records, we also used PERMANOVA to test differences between the sampling points with and without the domestic species, considering only the matrix of presence and absence of wild species. All analyses were performed using the “vegan”, "cluster" and "fpc" packages in the R platform (Oksanen et al. 2020, R Core Team 2021).
The conservation status of each species on a global and national level was derived respectively from the IUCN Red List of Threatened Species (version 2021.3) and the Red Book of Threatened Brazilian Fauna (Instituto Chico Mendes de Conservação da Biodiversidade 2018). We also report the status of each species in the list of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (Convention on International Trade in Endangered Species of Wild Fauna and Flora 2021).
Results
In addition to domestic dogs and cats, the camera trap survey, interviews and other sporadic records revealed the presence of 17 species of medium- and large-sized autochthonous (16 spp.) and allochthonous (Callithrix sp.) wild mammals, which are organised into 14 families and seven orders (Table 2).
Table 2.
Mid- and large-sized mammal species recorded by camera-traps (1), interviews (2) and occasional observation (3) in the Estação Biológica Fiocruz Mata Atlântica, Rio de Janeiro, including their conservation status at national (Instituto Chico Mendes de Conservação da Biodiversidade 2018) and global (IUCN 2021) scales (LC = Least Concern, NT = Near Threatened, VU = Vulnerable, EN = Endangered, NE = Not Evaluated). Restrictions on international trade due to degrees of threat follow the Convention on International Trade in Endangered Species of Wild Fauna and Flora (2021): Appendix I (high risk of extinction [AP1]), Appendix II (moderate risk of extinction [AP2]) and Appendix III (species protected in at least one country [AP3]).
| Taxa | English name | Record | Conservation status (IUCN/ICMBio/CITES) |
| Didelphimorphia | |||
| Didelphidae | |||
| Didelphisaurita | Big-eared opossum | 1, 2, 3 | LC / LC / - |
| Cingulata | |||
| Dasypodidae | |||
| Dasypusnovemcinctus | Nine-banded armadillo | 1, 2 | LC / LC / - |
| Chlamyphoridae | |||
| Cabassoustatouay | Greater naked-tailed armadillo | 2 | LC / LC / - |
| Euphractussexcinctus | Six-banded armadillo | 2 | LC / LC / - |
| Pilosa | |||
| Bradypodidae | |||
| Bradypusvariegatus | Brown-throated sloth | 2, 3 | LC / LC / AP2 |
| Myrmecophagidae | |||
| Tamanduatetradactyla | Southern tamandua | 1, 2, 3 | LC / LC / - |
| Primates | |||
| Callithrichidae | |||
| Callithrix sp. | Common marmoset | 2, 3 | Hybrid |
| Leontopithecusrosalia | Golden lion tamarin | 2, 3 | EN / EN / AP2 |
| Cebidae | |||
| Sapajusnigritus | Black capuchin | 2, 3 | NT / - / - |
| Carnivora | |||
| Canidae | |||
| Cerdocyonthous | Crab-eating fox | 1, 2 | LC / LC / AP2 |
| Canislupusfamiliaris | Domestic dog | 1 | Domestic |
| Procyonidae | |||
| Nasuanasua | Coati | 1, 2, 3 | LC / LC / - |
| Procyoncancrivorus | Crab-eating raccoon | 1, 2 | LC / LC / - |
| Felidae | |||
| Leopardusguttulus | Southern little spotted cat | 1, 2 | VU / VU / AP1 |
| Feliscatus | Domestic cat | 1 | Domestic |
| Rodentia | |||
| Erethizontidae | |||
| Coendouspinosus | Paraguayan hairy dwarf porcupine | 2, 3 | LC / LC / LC |
| Cuniculidae | |||
| Cuniculuspaca | Lowland paca | 1, 2 | LC / LC / AP3 |
| Dasyproctidae | |||
| Dasyproctaleporina | Red-rumped agouti | 1, 2 | LC / LC / - |
| Lagomorpha | |||
| Leporidae | |||
| Sylvilagustapetillus | Coastal tapeti | 1, 2 | VU / NE / - |
The sampling effort with camera-traps provided 1,189 records of 12 species of wild mammals and domestic dogs and cats. Eighteen species were recorded by interviews, of which nine were not recorded by camera traps (Bradypusvariegatus, Cabassoustatouay, Dasypusseptemcinctus, Dicotylestajacu, Eirabarbara, Euphractussexcinctus, Galictiscuja, Leontopithecusrosalia and Sapajusnigritus). Amongst them, the following six were included in our list: B.variegatus, Leontopithecusrosalia, S.nigritus and T.tetradactyla, which were confirmed by direct observation; and C.tatouay and E.sexcinctus, whose occurrence is confirmed for Tijuca Forest—an Atlantic Forest remnant geographically close to EFMA. Although the occurrence of D.tajacu has been confirmed for the Mendanha Forest—another Atlantic Forest remnant geographically close to EFMA—we chose not to include it in our list due to the rarity of the species in the Municipality of Rio de Janeiro. Thus, the records of Eirabarbara, Galictiscuja, Dicotylestajacu and Dasypusseptemcinctus obtained from interviews were considered dubious. Eight species were recorded by direct observation, none exclusively.
Of the 17 wild mammal species recorded, all are autochthonous, except Callithrix sp., which is a hybrid of Callithrixjacchus and Callithrixpenicillata, widely distributed in the City of Rio de Janeiro. The record of Leontopithecusrosalia is the first of the current distribution of the species to the Municipality of Rio de Janeiro. Leontopithecusrosalia is coded as Endangered in the Red Book of Threatened Brazilian Fauna (Instituto Chico Mendes de Conservação da Biodiversidade 2018) and IUCN (Ruiz-Miranda et al. 2015). Leopardusguttulus is classified as Vulnerable in the Red Book of Threatened Brazilian Fauna (Instituto Chico Mendes de Conservação da Biodiversidade 2018) and IUCN (Oliveira et al. 2014). Sapajusnigritus is coded as Near Threatened in the Red Book of Threatened Brazilian Fauna (Instituto Chico Mendes de Conservação da Biodiversidade 2018), but the species has not been evaluated by the IUCN. Leontopithecusrosalia and Leopardusguttulus are included in Appendix I of CITES; Bradypusvariegatus and Cerdocyonthous are in Appendix II; and Cuniculuspaca is listed in Appendix III of CITES.
Considering only the results of camera-traps, the highest concentration of records occurred in the peridomicile (N = 519, 0.391/camera-days), followed by the forest core (N = 375, 0.472/camera-days) and transitional forest (N = 295, 0.525/camera-days; Table 3). Of 12 species recorded by camera-traps, 10 occurred in the Peridomicile and Transitional Forest and nine in the Forest Core. The most recorded species in camera-traps was Didelphisaurita (N = 370, 0.138/camera-days), followed by Cuniculuspaca (N = 279, 0.104/camera-days), Canislupusfamiliaris (N = 174, 0.065/camera-days) and Dasyproctaleporina (N = 164, 0.065/camera-days; Table 3).
Table 3.
Absolute records (left) and camera-day records (right) of mid- and large-sized mammal species recorded by camera-traps per sampling area in the Estação Biológica Fiocruz Mata Atlântica, Rio de Janeiro.
| Species | Peridomicile | Transition | Forest Core | Total | ||||
| C.l.familiaris | 122 | 0.092 | 21 | 0.037 | 31 | 0.039 | 174 | 0.065 |
| C.thous | 30 | 0.023 | 10 | 0.018 | – | 0.000 | 40 | 0.015 |
| C.paca | 162 | 0.122 | 6 | 0.011 | 111 | 0.140 | 279 | 0.104 |
| D.leporina | 1 | 0.001 | 66 | 0.117 | 97 | 0.122 | 164 | 0.061 |
| D.novemcinctus | 11 | 0.008 | 5 | 0.009 | 26 | 0.033 | 42 | 0.016 |
| D.aurita | 146 | 0.110 | 166 | 0.295 | 58 | 0.073 | 370 | 0.138 |
| F.catus | 21 | 0.016 | – | 0.000 | – | 0.000 | 21 | 0.008 |
| L.guttulus | – | 0.000 | 5 | 0.009 | 5 | 0.006 | 10 | 0.004 |
| N.nasua | – | 0.000 | 2 | 0.004 | 16 | 0.020 | 18 | 0.007 |
| P.cancrivorus | – | 0.007 | 10 | 0.018 | 23 | 0.029 | 42 | 0.016 |
| S.tapetillus | 8 | 0.006 | – | 0.000 | – | 0.000 | 8 | 0.003 |
| T.tetradactyla | 9 | 0.007 | 4 | 0.007 | 8 | 0.010 | 21 | 0.008 |
| Total | 519 | 0.391 | 295 | 0.525 | 375 | 0.472 | 1.189 | 0.443 |
In the peridomicile, the most frequent species were Cuniculuspaca (N = 162, 0.122/camera-days), Didelphisaurita (N = 146, 0.110/camera-days) and Canislupusfamiliaris (N = 122, 0.092/camera-days); in the transitional forest, were Didelphisaurita (N = 166, 0.295/camera-days), Dasyproctaleporina (N = 66 records, 0.117/camera-days) and Canislupusfamiliaris (N = 21, 0.037/camera days); while in the forest core, the predominant species were Cuniculuspaca (N = 111, 0.140/camera-days), Dasyproctaleporina (N = 97, 0.122/camera-days) and Didelphisaurita (N = 58, 0.073/camera-days; Table 3).
Didelphisaurita showed the highest frequency of occurrence, found in 89% of the sampling points, followed by Canislupusfamiliaris, with 63%, and Tamanduatetradactyla with 58%. The domestic dog was recorded at all peridomicile points, in addition to records in some transitional forest and forest core points. However, domestic cats, Feliscatus and Sylvilagustapetillus were only found in the peridomicile points. On the other hand, Leopardusguttulus and Nasuanasua were recorded only in the Transitional Forest and Forest Core. Cerdocyonthous was recorded in the Peridomicile and Transitional Forest (Table 4).
Table 4.
Distribution of mid- and large-sized mammal species recorded by camera-trap in the Estação Biológica Fiocruz Mata Atlântica, Rio de Janeiro.
| Species | Peridomicile | Transition | Forest core | ||||||||||||||||
| A14 | B13 | B14 | B15 | C13 | C14 | D13 | C12 | D10 | D11 | E09 | E10 | E08 | F06 | F07 | G04 | G05 | G06 | H04 | |
| C.l.familiaris | 16 | 17 | 49 | 8 | 5 | 22 | 5 | – | – | 9 | – | 12 | – | – | 2 | – | 5 | – | 24 |
| C.thous | 1 | 4 | 7 | 1 | 17 | – | – | – | – | 10 | – | – | – | – | – | – | – | – | – |
| C.paca | – | 56 | – | – | – | 3 | 103 | – | – | 4 | – | 2 | – | – | – | 31 | 44 | – | 36 |
| D.leporina | – | – | – | 1 | – | – | – | – | 1 | 51 | – | 14 | 1 | 2 | – | 35 | 39 | – | 20 |
| D.novemcinctus | 8 | 2 | – | 1 | – | – | – | – | – | 3 | – | 2 | – | – | – | 15 | 10 | – | 1 |
| D.aurita | 24 | 30 | 36 | 23 | – | 2 | 31 | 6 | 5 | 51 | 5 | 99 | 6 | 6 | – | 34 | 7 | 1 | 4 |
| F.catus | 3 | 7 | 2 | 1 | 2 | 3 | 3 | – | – | – | – | – | – | – | – | – | – | – | – |
| L.guttulus | – | – | – | – | – | – | – | – | – | 3 | – | 2 | – | – | – | – | 3 | – | 2 |
| N.nasua | – | – | – | – | – | – | – | – | – | – | – | 2 | 3 | 1 | 1 | 2 | 5 | 1 | 3 |
| P.cancrivorus | – | 5 | 1 | – | – | 2 | 1 | – | – | 7 | – | 3 | – | – | – | 19 | 2 | – | 2 |
| S.tapetillus | – | 2 | – | – | – | – | 6 | – | – | – | – | – | – | – | – | – | – | – | – |
| T.tetradactyla | 2 | 2 | 1 | 3 | 1 | – | – | – | – | 2 | – | 2 | – | – | – | 2 | 4 | 1 | 1 |
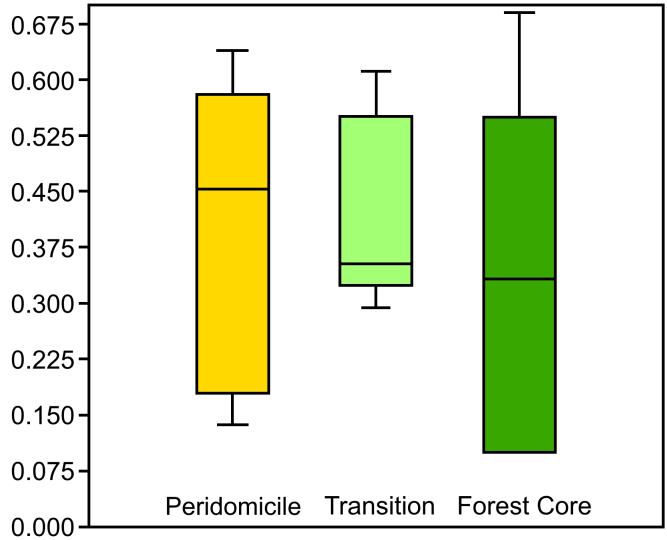
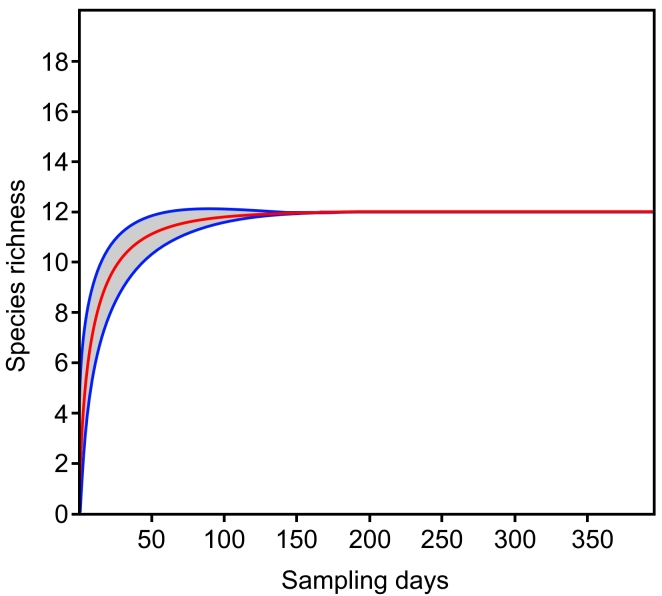
Considering the three sampling areas (Peridomicile, Transitional Forest and Forest Core) as independent populations, we did not find significant differences between the averages of camera-day records for these areas (F = 0.238; p = 0.792; Fig. 2). The Shapiro-Wilk test indicated the normality of the data (p = 0.949). The rarefaction curve (Mao Tao) of the accumulated richness by the sampling days reached its asymptote with 12 species recorded between 100 and 150 sampling days (from a total of 395 days; Fig. 3). The observed richness was the same as estimated by the Jackknife-2 and Chao-2, within the 95% confidence interval of the observed species.
Figure 2.
Analysis of variance (ANOVA) for records/camera-days for each sampling area (Peridomicile, Transitional Forest, Forest Core) for mid- and large-sized mammals from Estação Biológica Fiocruz Mata Atlântica, Rio de Janeiro.
Figure 3.
Rarefaction curve (Mao Tao) of accumulated richness by sampling days for mid- and large-sized mammals from Estação Biológica Fiocruz Mata Atlântica, Rio de Janeiro. Blue lines correspond to the 95% confidence interval.
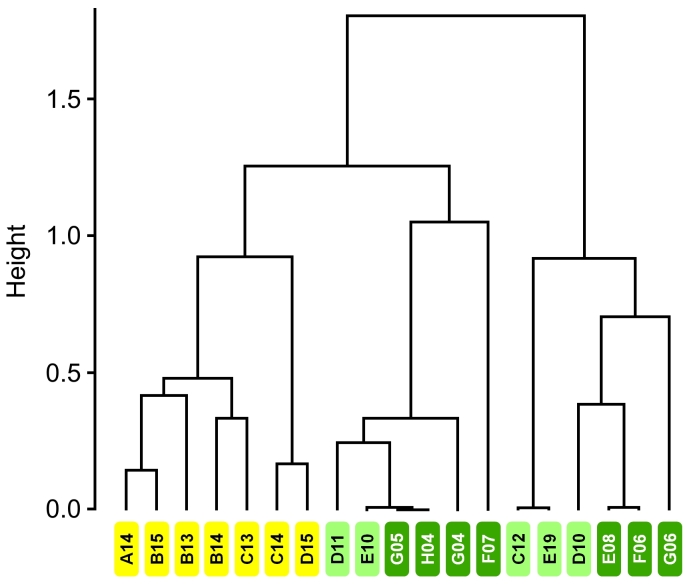
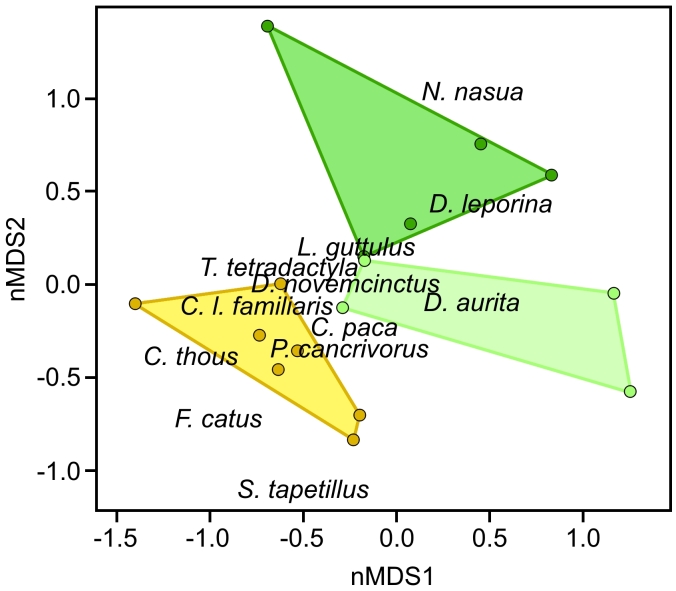
The areas showed homogeneity of multivariate dispersion (F = 1.29, p = 0.29) and differed significantly in relation to the similarity of the records (F = 4.75, p < 0.001, Fig. 4). The nMDS analysis (stress = 0.12, r2 = 0.98; Fig. 5) revealed no overall overlap between the areas, indicating a difference in the composition of records in each area. In general, most species were recorded in the Peridomicile, but the results indicate that Nasuanasua and Dasyproctaleporina are more associated with the Forest Core. Indeed, pairwise comparisons of the three areas show that N.nasua plays a important role in distinguishing Forest Core from the other two areas (Suppl. material 2). Even though L.guttulus was not recorded in Peridomicile, the species was not important to differentiate this area from the others. Still, records of L.guttulus significantly differed between the Transitional Forest and the Forest Core (Suppl. material 2). Records of C.thous were more abundant in Peridomicile and SIMPER analyses showed its importance in differentiating this area from the other two (Suppl. material 2). Didelphisaurita was recorded in almost all points, but with a highest concentration in the Transitional Forest. Considering only wild species, our results revealed that the presence of domestic dogs (F = 5.14, p = 0.001) and domestic cats (F = 3.25, p = 0.008) influenced species composition, with Nasuanasua, Dasyproctaleporina and Didelphisaurita less frequently registered in the points where domestic dogs and cats were most frequent. Additionally, records of domestic animals were important to differentiate Peridomicile from Transitional Forest and Forest Core (Suppl. material 2) with a higher number of records for these species near to more anthropogenised areas.
Figure 4.
Dendrogram produced by cluster analysis (Ward method) from the similarity (Jaccard distance) between points in the peridomicile (yellow), transitional forest (light green) and forest core (dark green), considering the species richness of medium and large-sized mammals in the Estação Biológica Fiocruz Mata Atlântica, Rio de Janeiro.
Figure 5.
Plot of the nMDS analysis, based on Jaccard distances showing sampling points and species coordinates. The convex polygons delimit the peridomicile (yellow), transitional forest (light green) and forest core (dark green) in the Estação Biológica Fiocruz Mata Atlântica, Rio de Janeiro.
Our data indicated the existence of three stable groups (mean bootstrapped Jaccard similarities > 0.85 and overall cluster instability < 0.06 for all three clusters). Peridomicile records clearly differentiate this area from the other two (i.e. Transitional Forest and Forest Core) as all sample points defined as Peridomicile were grouped into a single cluster (Fig. 4). The other two groups included points from both Transitional Forest and Forest Core and this analysis did not allow the differentiation of these two areas (Fig. 4).
Discussion
Species richness
About 60 spp. of mammals from different orders are known to occur in the region of the Pedra Branca Forest (Secretaria do Meio Ambiente 2013, Gentile et al. 2018, Pontes et al. 2021, Tavares et al. 2021). However, standardised efforts were focused on bats, rodents and marsupials (Gentile et al. 2018, Tavares et al. 2021). Records of medium- and large-sized mammals were based on occasional observations. Thus, this is the first standardised sampling effort to survey medium and large mammals in the region. To EFMA, we considered valid the records of 17 species of autochthonous and allochthonous wild mammals distributed in 14 families and seven orders, in addition to domestic dogs and cats. All species recorded in EFMA are known to occur in other protected areas in the State of Rio de Janeiro (e.g. Modesto et al. 2008, Delciellos et al. 2012, Silva 2017, Silva et al. 2018). In the Parque Nacional da Tijuca, which is the geographically closest Atlantic Forest remnant to EFMA, Silva et al. (2018) recorded 16 species of medium and large mammals, with the dominance of Nasuanasua, Didelphisaurita and Cuniculuspaca. Some species, registered in the Tijuca Forest using camera traps, such as Cabassoustatouay and Sapajusnigritus, were not recorded by this method in the EFMA, but were included in the list, based on the results of interviews (Cabassoustatouay and Sapajusnigritus) and subsequent observation (Sapajusnigritus). Recently, Pontes et al. (2021) recorded the occurrence of Pumaconcolor on the west side of Pedra Branca Forest and surroundings. Despite the rarity of the species in the metropolitan region of Rio de Janeiro, we do not rule out the possibility of its occurrence within the limits of the EFMA, considering its connectivity with the rest of the remnant.
Despite differences in habitat structure and composition amongst the Peridomicile area, Transitional Forest and Forest Core, differences were not observed in the abundance of medium and large mammals amongst these areas in the EFMA. The survey focused mainly on the use of camera traps because they favour records of species that are difficult to detect through active search and because the method requires less effort when compared to active search or capture (Carbone et al. 2001, Santos-Filho and Silva 2002, Silveira et al. 2003, Trolle 2003, Srbek-Araujo and Chiarello 2005). Surveys of mid- and large-sized mammals using camera traps have already been carried out in several protected areas in the State of Rio de Janeiro, including Parque Nacional da Tijuca (Silva et al. 2018), Parque Nacional da Restinga de Jurubatiba (Xavier 2016), Parque Nacional de Itatiaia, Parque Nacional da Serra dos Órgãos (Aximoff et al. 2015), Reserva Ecológica de Guapiaçu (Carvalho et al. 2014), Parque Nacional da Serra da Bocaina (Delciellos et al. 2012), Parque Estadual da Ilha Grande (Lessa 2012) and Parque Estadual do Desengano (Modesto et al. 2008).
In surveys with camera-traps conducted in the mountainous region of Rio de Janeiro, which comprises the largest remnant of continuous Atlantic Forest in the State, more than 20 species of medium and large mammals were recorded, including some threatened, such as Pumayagouaroundi, Pumaconcolor, Dicotylestajacu and Tayassupecari (e.g. Carvalho et al. 2014, Aximoff et al. 2015), which are either almost extinct or rare in the Municipality of Rio de Janeiro, such as Dicotylestajacu and Pumaconcolor (Martins and Pontes 2021, Pontes et al. 2021).
Conservation remarks
Domestic dogs and cats, which showed high frequency of occurrence in the EFMA, also showed high frequency in Parque Nacional da Tijuca (Silva et al. 2018). The record of domestic animals in protected areas is frequent, especially in metropolitan areas, where the presence of residences in the surroundings or even within these areas is common. According to Silva (2017), the records of domestic dogs in the Tijuca Forest are concentrated during the day, indicating that they are domiciled animals, spending the night in their residences. The activity of dogs in the EFMA is not limited to areas with high human presence (Peridomicile) and overlaps with some species also recorded in the present study, such as N.nasua and D.leporina (q.v. Silva et al. 2018). The records of dogs in more preserved areas (i.e. Transitional Forest and Forest Core), although at low frequency, agree with findings of Silva et al. (2018). It is important to highlight here that these animals are, in general, domiciled, but raised freely in the territory.
Domestic dogs and cats exert different types of pressure on local biodiversity. Amongst the 17 wild species recorded in the EFMA, there are predation records by dogs for eight species, competition records for six and pathogen transmission reports for two (Lessa et al. 2016). In the Jardim Botânico do Rio de Janeiro, which is adjacent to the Tijuca Forest, Rangel and Neiva (2013) report predation of D.aurita, T.tetradactyla and P.cancrivorus, all recorded at EFMA. C.thous is reported as a susceptible species for disease transmission and competition from domestic dogs (Lessa et al. 2016). Our results indicate a significant relationship between domestic dogs and C.thous to less preserved areas, thus increasing the risk of those threats emerging in EFMA’s territory. For domestic cats, predation pressure on wild mammals is greater when Feliscatus individuals are feral (Oliveira et al. 2010, Loss et al. 2013). The shared land use between domestic cats and wild felid species, such as L.guttulus (present in EFMA), is also a threat due to possible niche overlap (Ferreira et al. 2019). Domestic cats might transmit pathogens to wild mammals, such as the rabies virus and pose threats to local wildlife (Gerhold and Jessup 2012). Our results, however, do not indicate the occurrence of domestic and wild cats in the same area, with the records of domestic cats restricted to the Peridomicile and the records of L.guttulus in the Transition Forest and Forest Core. L.guttulus is commonly reported as a forest dwelling species able to tolerate some degree of human disturbance (Oliveira et al. 2010). Our result is in accordance with this previous report as it shows the relationship of L.guttulus to forest areas with intermediate anthropogenic pressure. The diet of L.guttulus includes small mammals, even comprising exotic rodent species (Rinaldi et al. 2015, Tortato et al. 2021). High frequencies of synanthropic marsupial and rodent species in anthropogenised areas have already been reported for the area (Gentile et al. 2018), potentially influencing the occurrence of L.guttulus in areas with intermediate human disturbance. Results for N.nasua are opposed to observations in protected areas that receive tourists, such as Jardim Botânico do Rio de Janeiro (Brazil) and Parque Nacional Iguazú (Argentina) where this species is commonly observed near areas with high human activity (Hirsch 2009, Cunha 2010). Thus, depending on the characteristics of the area, such as tourist visitation and prey abundance, species occurrence and abundance inside an anthropogenic gradient may vary between localities.
In relation to primates, it is also worth mentioning the record of the golden-lion-tamarin, Leontopithecusrosalia, in the EFMA and adjacent areas. One individual of this species was recorded by direct observation in 2017, living with a group of Callithrix. Subsequently, the species was also reported by residents and Fiocruz employees, who reported the presence of more than one individual, with at least one female and a juvenile. We have records of at least three individuals that co-exist with Callithrix in the surroundings of EFMA. The species is endemic to the southeast Atlantic Forest, originally occurring in coastal lowland forests of the States of Rio de Janeiro and southern Espírito Santo (Coimbra-Filho 1969, Kleiman and Rylands 2002). Apparently, in the 1960s, it was already extinct in 17 municipalities, including Rio de Janeiro, remaining restricted to the São João River Basin, with its occurrence limited to the Municipalities of Silva Jardim, Araruama, Cabo Frio and Saquarema (Kierulff 1993). Recently, it was also recorded in forest fragments in the Municipality of Duque de Caxias (Burity et al. 2007). The origin of these individuals in the EFMA and adjacent areas is still uncertain and may be related to the existence of illegal breeding sites in the region.
Peridomicile is a distinct area inside EFMA’s territory concerning medium- large-sized mammal communities, probably by the effects of the high level of anthropogenic disturbances. Habitat loss, introduction of exotic species and poaching are the main threats to mammals in the Pedra Branca Forest (Secretaria do Meio Ambiente 2013). These pressures are the target of mitigation actions in EFMA's territory. Regarding habitat loss, we highlight the control over irregular constructions within the Fiocruz area (Sector 1 of Colônia Juliano Moreira), including the prohibition of new buildings and land expansions. For free-ranging domestic animals, there are actions to raise awareness about responsible custody to reduce the number of unaccompanied animals; inspection actions to reduce the abandonment of animals in the territory; castration and vaccination of abandoned animals; and referral of untrained animals for adoption. These actions to minimise contact between domestic and wild animals aim to reduce predation of wild birds and mammals and minimise the risk of pathogen spill-over from domestic to wild mammals, such as the Canine Distemper Virus (Megid et al. 2013) and the fungus Sporothrix spp., which causes sporotrichosis, a zoonotic disease that mainly affects cats, but can also affect humans (Almeida-Paes et al. 2014). Regarding poaching in EFMA, there are campaigns to raise awareness of the legislation that prohibits hunting and articulation with the Environmental Police for actions aimed at curbing the action. These actions apparently reduced the local habitat loss and curbed the poaching activity at EFMA. Despite this, it is still necessary to keep the monitoring of native mammals and develop more research focused on the impact of domestic animals and poaching on the species composition and population dynamics of wild mammals. This was the first study with systematic sampling of the mammals from Pedra Branca Forest and the results are the basis for understanding the ecological interfaces that favour contact between wildlife and humans, directly or indirectly through domestic animals.
Supplementary Material
Individualised records of mammals from Fiocruz Atlantic Foresthttps://doi.org/10.3897/BDJ.10.e86756.suppl1
Iuri Veríssimo, Beatriz Maria da Silva Jorge
Data type
Occurrence records
File: oo_717025.xlsx
SIMPER analysis results
Cecília Andreazzi
Data type
average between-group Bray-Curtis dissimilarity
Brief description
SIMPER analysis results: Contribution of each species to overall dissimilarities amongst sampling areas.
File: oo_711742.xlsx
Acknowledgements
Mylena Borges, Carolina Lacorte Rangel and Stephany Nardi for fieldwork assistance. RLMN received financial support from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil, process E-26/204.243/2021). RM received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil, process 313963/2018-5) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, Brazil, processes E-26/203.274/2017, E-26/210.254/2018, E-202.487/2018, E-26/200.967/2021).
Hosting institution
Fundação Oswaldo Cruz - Fiocruz
Conflicts of interest
The authors declare that there is no conflict of interests.
Author contributions
RM, IV, CSA and SFCN designed the project; IV, GC, BMSJ, JAT and RLMN performed fieldwork; IV, GC, BMSJ and RLMN identified specimens; IV, GC, RLMN, CSA and RM contributed to data analyses; all authors wrote the first draft, read and approved the final version.
Conflicts of interest
The authors declare that there is no conflict of interests.
References
- Almeida-Paes Rodrigo, de Oliveira Manoel Marques Evangelista, Freitas Dayvison Francis Saraiva, do Valle Antônio Carlos Francesconi, Zancopé-Oliveira Rosely Maria, Gutierrez-Galhardo Maria Clara. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrixbrasiliensis Is associated with atypical clinical presentations. PLOS Neglected Tropical Diseases. 2014;8(9) doi: 10.1371/journal.pntd.0003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics. 2006;62:245–253. doi: 10.1111/j.1541-0420.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- Aximoff Izar, Cronemberger Cecilia, Pereira Fabiane de Aguiar. Long-term survey by camera traps of non-volant mammals in two national parks in Rio de Janeiro State. Oecologia Australis. 2015;19(01):215–231. doi: 10.4257/oeco.2015.1901.14. [DOI] [Google Scholar]
- Burity Carlos Henrique de Freitas, Cruz Leandro Duarte da, Rocha Vera Lucia, Conceição Nelson Barroso da, Luz Daniel Eduardo da, Santos Durval da Silva, Campos Devyhon da Costa, Pissinatti Alcides. Golden lion tamarins, Leontopithecusrosalia (Linnaeus, 1766) in the Taquara Municipal Natural Park (Duque De Caxias, RJ): A southern extension of the known range. Neotropical Primates. 2007;14(1):30–31. doi: 10.1896/044.014.0107. [DOI] [Google Scholar]
- Carbone C., Christie S., Conforti K., Coulson T., Franklin N., Ginsberg J. R., Griffiths M., Holden J., Kawanishi K., Kinnaird M., Laidlaw R., Lynam A., Macdonald D. W., Martyr D., McDougal C., Nath L., O'Brien T., Seidensticker J., Smith D. J. L., Sunquist M., Tilson R., Shahruddin W. N. The use of photographic rates to estimate densities of tigers and other cryptic mammals. Animal Conservation. 2001;4(1):75–79. doi: 10.1017/s1367943001001081. [DOI] [Google Scholar]
- Carvalho Israel Dias, Oliveira Rildo, Pires Alexandra Santos. Medium and large-sized mammals of the Reserva Ecológica de Guapiaçú, Cachoeiras de Macacu, RJ. Biota Neotropica. 2014;14(3) doi: 10.1590/1676-06032014007414. [DOI] [Google Scholar]
- Chao A. Non-parametric estimation of the classes in a population. Scandinavian Journal of Statistics. 1984;11:265–270. [Google Scholar]
- Chiarello Adriano G. Density and population size of mammals in remnants of Brazilian Atlantic Forest. Conservation Biology. 2008;14(6):1649–1657. doi: 10.1111/j.1523-1739.2000.99071.x. [DOI] [PubMed] [Google Scholar]
- Clarke K. R. Non-parametric multivariate analyses of changes in community structure. Austral Ecology. 1993;18(1):117–143. doi: 10.1111/j.1442-9993.1993.tb00438.x. [DOI] [Google Scholar]
- Coimbra-Filho AF. Mico-leão, Leontideusrosalia (Linnaeus, 1766), situação atual da espécie no Brasil. Anais da Academia Brasileira de Ciências. 1969;41 (Suppl.):29–42. [Google Scholar]
- Colwell Robert K., Mao Chang Xuan, Chang Jing. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology. 2004;85(10):2717–2727. doi: 10.1890/03-0557. [DOI] [Google Scholar]
- Flora Convention on International Trade in Endangered Species of Wild Fauna and. Appendices. https://cites.org/eng/app/appendices.php. [2022-05-02T00:00:00+03:00]. https://cites.org/eng/app/appendices.php [DOI] [PubMed]
- Cronemberger Cecilia, Delciellos Ana Cláudia, Barros Camila dos Santos de, Gentile Rosana, Weksler Marcelo, Braz Alan Gerhardt, Teixeira Bernardo Rodrigues, Loretto Diogo, Vilar Emmanuel Messias, Pereira Fabiane Aguiar, Santos Jayme Roberto Cirilo dos, Geise Lena, Pereira Luciana Guedes, Aguieiras Marcia, Vieira Marcus Vinícius, Estrela Pedro Cordeiro, Junger Raquel Batista, Honorato Reginaldo dos Santos, Moratelli Ricardo, Vilela Roberto do Val, Guimarães Roger Rodrigues, Cerqueira Rui, Costa-Neto Sócrates Fraga da, Cardoso Thiago dos Santos, Nascimento Jorge Luiz do. Mamíferos do Parque Nacional da Serra dos Órgãos: atualização da lista de espécies e implicações para a conservação. Oecologia Australis. 2019;23(02):191–214. doi: 10.4257/oeco.2019.2302.02. [DOI] [Google Scholar]
- Cunha AA. Negative effects of tourism in a Brazilian Atlantic forest National Park. Journal for Nature Conservation. 2010;18:291–295. doi: 10.1016/j.jnc.2010.01.001. [DOI] [Google Scholar]
- Daszak Peter, Zambrana-Torrelio Carlos, Bogich Tiffany L., Fernandez Miguel, Epstein Jonathan H., Murray Kris A., Hamilton Healy. Interdisciplinary approaches to understanding disease emergence: The past, present, and future drivers of Nipah virus emergence. Proceedings of the National Academy of Sciences. 2012;110:3681–3688. doi: 10.1073/pnas.1201243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delciellos Ana Cláudia, Novaes Roberto Leonan Morim, Loguercio Mariana Fiuza de Castro, Geise Lena, Santori Ricardo Tadeu, Souza Renan De França, Papi Bernardo Silveira, Raíces Daniel, Vieira Nadjha Rezende, Felix Saulo, Detogne Nathalia, Da Silva Cleber Christianes Souza, Bergallo Helena De Godoy, Rocha-Barbosa Oscar. Mammals of Serra da Bocaina National Park, state of Rio de Janeiro, southeastern Brazil. Check List. 2012;8(4) doi: 10.15560/8.4.675. [DOI] [Google Scholar]
- Domingues LCSM, Rodrigues C. Campus Fiocruz da Mata Atlântica: o desafio de implantação de um novo Campus associando a promoção da conservação ambiental e o desenvolvimento socioeconômico em uma área de fronteira junto ao Parque Estadual da Pedra Branca, Município do Rio de Janeiro. In: FAUUSP, editor. Anais do Seminário Nacional sobre o Tratamento de Áreas de Preservação Permanente em Meio Urbano e Restrições Ambientais ao Parcelamento do Solo – APP Urbana; 2007.2007.
- Ferreira Giovanne A., Nakano-Oliveira Eduardo, Andriolo Artur, Genaro Gelson. Spatial overlap between domestic cats and wild felines in an insular Atlantic Forest remnant. Animal Biology. 2019;69(2):157–172. doi: 10.1163/15707563-17000110. [DOI] [Google Scholar]
- Freitas S. R., Neves C. L., Chernicharo P. Tijuca National Park: two pioneering restorationist initiatives in Atlantic forest in southeastern Brazil. Brazilian Journal of Biology. 2006;66(4):975–982. doi: 10.1590/s1519-69842006000600004. [DOI] [PubMed] [Google Scholar]
- Gardner AL. Mammals of South America. Vol. 2. The University of Chicag Press; Chicago: 2008. 690. [DOI] [Google Scholar]
- Gentile Rosana, Cardoso Thiago S., Costa-Neto Sócrates F., Teixeira Bernardo R., D'Andrea Paulo S. Community structure and population dynamics of small mammals in an urban-sylvatic interface area in Rio de Janeiro, Brazil. Zoologia. 2018;35:1–12. doi: 10.3897/zoologia.35.e13465. [DOI] [Google Scholar]
- Gerhold R. W., Jessup D. A. Zoonotic diseases associated with free-roaming cats. Zoonoses and Public Health. 2012;60(3):189–195. doi: 10.1111/j.1863-2378.2012.01522.x. [DOI] [PubMed] [Google Scholar]
- Hennig Christian. Cluster-wise assessment of cluster stability. Computational Statistics & Data Analysis. 2007;52(1):258–271. doi: 10.1016/j.csda.2006.11.025. [DOI] [Google Scholar]
- Hirsch Ben T. Seasonal Variation in the Diet of Ring-Tailed Coatis (Nasuanasua) in Iguazu, Argentina. Journal of Mammalogy. 2009;90(1):136–143. doi: 10.1644/08-mamm-a-050.1. [DOI] [Google Scholar]
- Biodiversidade Instituto Chico Mendes de Conservação da. In: Livro Vermelho da Fauna Brasileira Ameaçada de Extinção. Biodiversidade Instituto Chico Mendes de Conservação da., editor. Vol. 2. ICMBio; Brasília: 2018. Mamíferos.622 [Google Scholar]
- IUCN The IUCB Red List of threatened species. https://www.iucnredlist.org/ [2022-05-02T00:00:00+03:00]. https://www.iucnredlist.org/
- Jones Kate E., Patel Nikkita G., Levy Marc A., Storeygard Adam, Balk Deborah, Gittleman John L., Daszak Peter. Global trends in emerging infectious diseases. Nature. 2008;451(7181):990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierulff MCM. Uma avaliação das populações silvestres de mico-leão-dourado, Leontopithecusrosalia, e uma proposta de estratégia para a conservação da espécie. Universidade Federal de Minas Gerais; Belo Horizonte: 1993. [Google Scholar]
- Kleiman DG, Rylands AB. Lion tamarins: biology and conservation. Smithsonian Institution Press; Washington, DC: 2002. 405 [Google Scholar]
- Lessa Isadora, Corrêa Seabra Guimarães Tainah, de Godoy Bergallo Helena, Cunha André, M. Vieira Emerson. Domestic dogs in protected areas: a threat to Brazilian mammals? Natureza & Conservação. 2016;14(2):46–56. doi: 10.1016/j.ncon.2016.05.001. [DOI] [Google Scholar]
- Lessa Isadora Cristina Motta. Os mamíferos de médio porte e suas respostas à fatores ambientais, físicos e antrópicos, sobre diferentes perspectivas, no Parque Estadual da Ilha Grande RJ. Universidade do Estado do Rio de Janeiro; Rio de Janeiro: 2012. 83 [Google Scholar]
- Loss Scott R., Will Tom, Marra Peter P. The impact of free-ranging domestic cats on wildlife of the United States. Nature Communications. 2013;4(1) doi: 10.1038/ncomms2380. [DOI] [PubMed] [Google Scholar]
- Martins Rafael Andrada de Araújo, Pontes Jorge Antônio Lourenço. Registro da ocorrência de Pecari tajacu(Linnaeus, 1758), uma espécie que era declaradaextinta no município do Rio de Janeiro, estadodo Rio de Janeiro, Sudeste do Brasil. Boletim da Sociedade Brasileira de Mastozoologia. 2021;88:58–61. [Google Scholar]
- Megid Jane, Teixeira Carlos R., Cortez Adriana, Heinemann Marcos B., Antunes João M. A.P., Fornazari Felipe, Rassy Fabricio B., Richtzenhain Leonardo J. Canine distemper virus infection in a lesser grison (Galictiscuja): first report and virus phylogeny. Pesquisa Veterinária Brasileira. 2013;33(2):247–250. doi: 10.1590/s0100-736x2013000200018. [DOI] [Google Scholar]
- Modesto Thiago Carvalho, Pessôa Flávia Soares, Enrici Maria Carlota, Attias Nina, Jordão-Nogueira Tássia, Costa Luciana de Moraes, Albuquerque Hermano Gomes, Bergallo Helena de Godoy. Mamíferos do Parque Estadual do Desengano, Rio de Janeiro, Brasil. Biota Neotropica. 2008;8(4):153–159. doi: 10.1590/s1676-06032008000400015. [DOI] [Google Scholar]
- Oksanen Atte, Kaakinen Markus, Latikka Rita, Savolainen Iina, Savela Nina, Koivula Aki. Regulation and Trust: 3-month follow-up study on COVID-19 mortality in 25 European Countries. JMIR Public Health and Surveillance. 2020;6(2) doi: 10.2196/19218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira T, Trigo T, Tortato M, Paviolo A, Bianchi R, Leite-Pitman MRP. Leopardusguttulus . IUCN Red List of Threatened Species. 2014 doi: 10.2305/iucn.uk.2016-2.rlts.t54010476a54010576.en. [DOI]
- Oliveira T. G., Tortato M. A., Silveira L., Kasper C. B., Mazim F. D., Lucherini M., Jácomo A. T., Bonifácio J., Soares G., Marques R. V., Sunquist M. In: Biology and Conservation of Wild Felids. D Macdonald, A Loveridge., editors. Oxford University Press; 2010. Ocelot ecology and its effect on the small-felid guild in the lowland neotropics. [Google Scholar]
- Pontes Jorge Antônio Lourenço, Martins Rafael Andrada de Araújo, Regio Luiz Eduardo Mendonça, Soares Mário Luiz Gomes, Chaves Filipe de Oliveira, Bergallo Helena Godoy. The reappearance of Pumaconcolor (Linnaeus, 1771) (Mammalia, Carnivora, Felidae) in the city of Rio de Janeiro, Brazil. Check List. 2021;17(5):1353–1358. doi: 10.15560/17.5.1353. [DOI] [Google Scholar]
- Rangel Cristiane Hollanda, Neiva Carla Helena Mendes Bunn. Predação de vertebrados por cães Canislupusfamiliaris (Mammalia: Carnivora) no Jardim Botânico do Rio de Janeiro, RJ, Brasil. Biodiversidade Brasileira. 2013;3(2):261–269. [Google Scholar]
- Team R Core. R Foundation for Statistical Computing; 2021. R: A language and environment for statistical computing. [Google Scholar]
- Rinaldi Alcides Ricieri, Rodriguez Flávia Heloísa, Carvalho Anderson Luiz, Passos Fernando Camargo. Feeding of small Neotropical felids (Felidae: Carnivora) and trophic niche overlap in antropized mosaic landscape, South Brazilian. Biotemas. 2015;28(4) doi: 10.5007/2175-7925.2015v28n4p155. [DOI] [Google Scholar]
- Ruiz-Miranda C. R., Pissinatti A., Kierulff M. C.M., Oliveira L. C., Mittermeier R. A., Valença-Montenegro M. M., de Oliveira P., Jerusalinsky L. Leontopithecusrosalia . IUCN Red List of Threatened Species. 2015 doi: 10.2305/iucn.uk.2021-1.rlts.t11506a192327291.en. [DOI]
- Santos-Filho Manoel dos, Silva Maria Nazareth Ferreira da. Uso de habitats por mamíferos em área de Cerrado do Brasil Central: um estudo com armadilhas fotográficas. Revista Brasileira de Zoociências. 2002;4(1):57–73. [Google Scholar]
- Ambiente Secretaria do Meio. Plano de manejo do Parque Estadual da Pedra Branca (PEPB) 2013. http://www.femerj.org/wp-content/uploads/Plano-de-manejo-do-Parque-Estadual-da-Pedra-Branca-PEPB-2.pdf http://www.femerj.org/wp-content/uploads/Plano-de-manejo-do-Parque-Estadual-da-Pedra-Branca-PEPB-2.pdf
- Silva KV. Ocorrência, tamanho populacional e atividade do cão doméstico (Canislupusfamiliaris) no Parque Nacional da Tijuca, RJ. Universidade Federal Rural do Rio de Janeiro; Seropédica: 2017. 52 [Google Scholar]
- Silva Katyucha Von Kossel de Andrade, Kenup Caio Fittipaldi, Kreischer Catharina, Fernandez Fernando A. S., Pires Alexandra S. Who let the dogs out? Occurrence, population size and daily activity of domestic dogs in an urban Atlantic Forest reserve. Perspectives in Ecology and Conservation. 2018;16(4):228–233. doi: 10.1016/j.pecon.2018.09.001. [DOI] [Google Scholar]
- Silveira Leandro, Jácomo Anah T. A., Diniz-Filho José Alexandre F. Camera trap, line transect census and track surveys: a comparative evaluation. Biological Conservation. 2003;114(3):351–355. doi: 10.1016/s0006-3207(03)00063-6. [DOI] [Google Scholar]
- Soberón Jorge, Llorente Jorge. The use of species accumulation functions for the prediction of species richness. Conservation Biology. 1993;7(3):480–488. doi: 10.1046/j.1523-1739.1993.07030480.x. [DOI] [Google Scholar]
- Srbek-Araujo Ana C., Chiarello Adriano G. Armadilhas fotográficas na amostragem de mamíferos: considerações metodológias e comparação de equipamentos. Revista Brasileira de Zoologia. 2007;24(3):647–656. doi: 10.1590/s0101-81752007000300016. [DOI] [Google Scholar]
- Srbek-Araujo Ana Carolina, Chiarello Adriano Garcia. Is camera-trapping an efficient method for surveying mammals in Neotropical forests? A case study in south-eastern Brazil. Journal of Tropical Ecology. 2005;21(1):121–125. doi: 10.1017/s0266467404001956. [DOI] [Google Scholar]
- Tavares Jonatas, Novaes Roberto Leonan Morim, Veríssimo Iuri, Kuzel Maria Alice, da Costa-Neto Sócrates, Rangel Caroline, Borges Mylena, Medrado Helena, Alves Bruno, Souza Renan, Pinto Menezes Ana Carolina, Menezes-Júnior Luis Fernando, Dias Daniela, de Andreazzi Cecilia, Gentile Rosana, Moratelli Ricardo. Bats from the Pedra Branca Forest, Rio de Janeiro, Brazil. Biodiversity Data Journal. 2021;9 doi: 10.3897/bdj.9.e77400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortato Marcos Adriano, Oliveira-Santos Luiz Gustavo Rodrigues, Moura Maurício Osvaldo, de Oliveira Tadeu Gomes. Small prey for small cats: the importance of prey-size in the diet of southern tiger cat Leopardusguttulus in a competitor-free environment. Studies on Neotropical Fauna and Environment. 2021:1–12. doi: 10.1080/01650521.2021.1902202. [DOI]
- Trolle Mogens. Mammal survey in the southeastern Pantanal, Brazil. Biodiversity and Conservation. 2003;12(4):823–836. doi: 10.1023/a:1022489426920. [DOI] [Google Scholar]
- White Rebekah J., Razgour Orly. Emerging zoonotic diseases originating in mammals: a systematic review of effects of anthropogenic land‐use change. Mammal Review. 2020;50(4):336–352. doi: 10.1111/mam.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DE, Reeder DM. Mammal species of the world: a taxonomic and geographic reference. 3. Johns Hopkins University Press; 2005. 2142 [Google Scholar]
- Xavier Mariana Sampaio. Mamíferos terrestres de médio e grande porte do Parque Nacional da Restinga de Jurubatiba: riqueza de espécies e vulnerabilidade local. Universidade Federal do Rio de Janeiro; Rio de Janeiro: 2016. 101 [Google Scholar]
- Zahl Samuel. Jackknifing An Index of Diversity. Ecology. 1977;58(4):907–913. doi: 10.2307/1936227. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individualised records of mammals from Fiocruz Atlantic Foresthttps://doi.org/10.3897/BDJ.10.e86756.suppl1
Iuri Veríssimo, Beatriz Maria da Silva Jorge
Data type
Occurrence records
File: oo_717025.xlsx
SIMPER analysis results
Cecília Andreazzi
Data type
average between-group Bray-Curtis dissimilarity
Brief description
SIMPER analysis results: Contribution of each species to overall dissimilarities amongst sampling areas.
File: oo_711742.xlsx