Treatment with immune checkpoint inhibitors (ICIs) has shown remarkable clinical response for many cancers. This response is, however, limited to approximately 15%-20% of patients, raising a need for reliable response biomarkers, especially biomarkers that apply to many tumor types to achieve maximum clinical benefits.1 A biomarker increasingly referenced in clinical use is the tumor mutational burden (TMB), which is a measure of the total number of mutations in the coding region of the genome.2,3 A prospective biomarker analysis of the basket trial KEYNOTE-158, in which 1,066 patients with solid tumor across 10 cancer types were treated with pembrolizumab, demonstrated that oncology patients with high TMB, defined as ≥ 10 mut/Mb on the FoundationOne CDx assay, showed a higher frequency of response to anti-programmed cell death protein 1 (PD1) treatment versus non–high TMB (< 10 mut/Mb). The US Food and Drug Administration (FDA) subsequently approved the TMB ≥ 10 mut/Mb as a biomarker for administering anti-PD1 therapy for advanced solid tumors that have progressed from prior treatment.4 However, recent studies have suggested that the TMB levels, strength of immune selection, and response to ICI treatment differ between male and female patients with melanoma.5-7 These sex differences motivated us to examine whether usage of the 10 mut/Mb threshold for both sexes could introduce an unwarranted sex bias when selecting patients for anti-PD1 treatment.
To study this question, we mined the largest publicly available data set of ICI-treated patient responses with TMB and demographic information.3 This data set includes 1,286 patients across nine different cancer types treated with anti-PD1/Programmed death-ligand 1 (PDL1), 99 patients treated with anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and 255 patients treated with an anti-PD1 and anti-CTLA4 combination. Among the 130 patients with melanoma available in this cohort, we first observe a higher median TMB in male versus female patients with melanoma (median TMB = 11.81 v 6.51, respectively, Wilcoxon rank-sum test P < .10; Fig 1A top group), in concordance with previous reports.5 We next asked whether the difference in survival of patients with high versus non–high TMB is dependent on the sex of the patient. We find that using the ≥ 10 mut/Mb threshold identifies female patients with melanoma with markedly better overall survival (hazard ratio [HR] = 0.19, P < .03) but fails to do so for male patients (HR = 0.94, P < .85; Fig 1B top group). The HR observed in male patients is thus five times higher than female patients (P interaction between sex and TMB via log-rank test < .03; Fig 1B top group).
FIG 1.
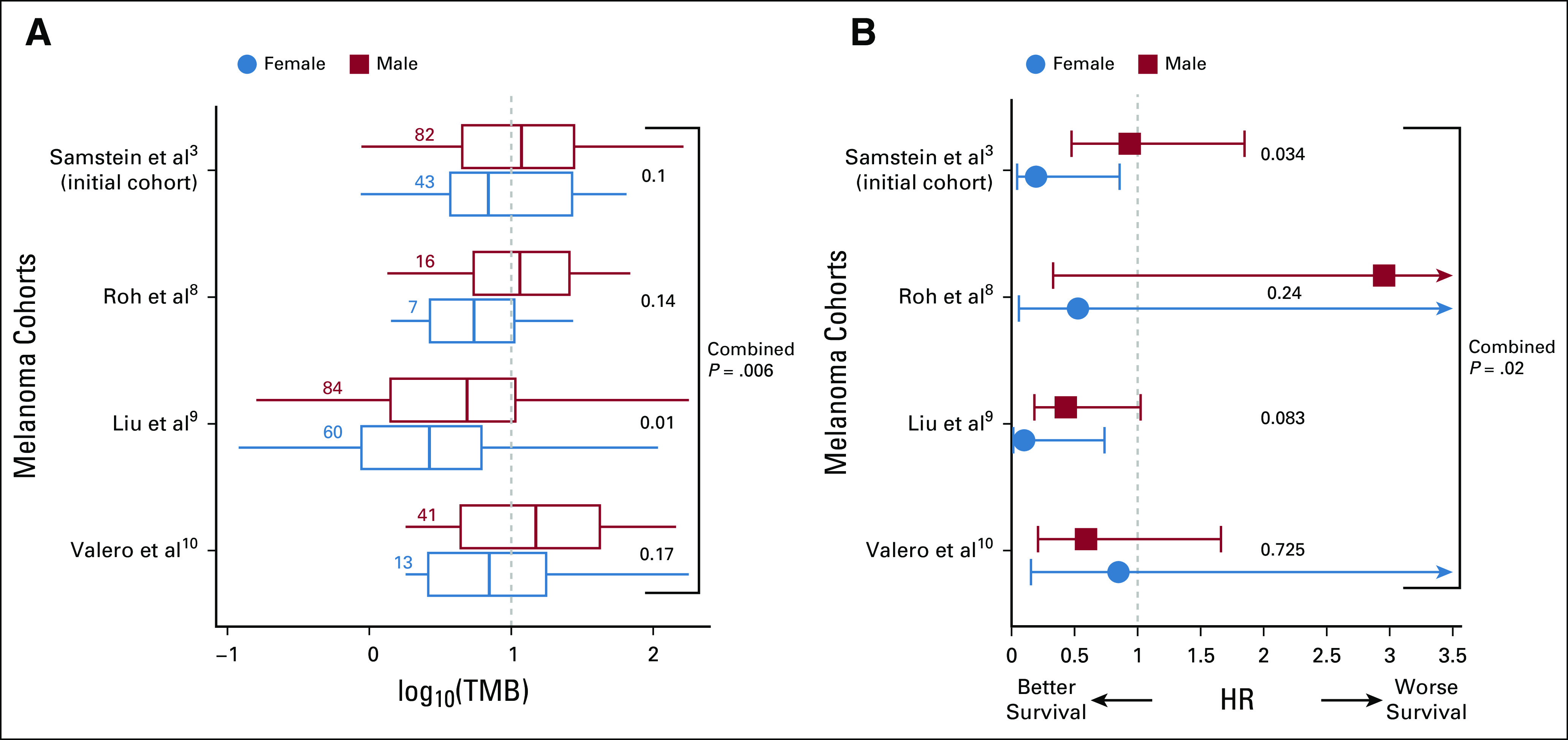
The association between high TMB status and survival of melanoma patients after anti-PD1/PDL1 treatment is dependent on the sex of the patients. (A) The distribution of log10(TMB) and the number of single nucleotide variants per megabase of sequenced genome (x-axis) for male and female patients for four different melanoma cohorts (Samstein et al,3 Roh et al,8 Liu et al,9 and Valero et al10; y-axis). The blue-dotted vertical line denotes the FDA-approved TMB threshold for pembrolizumab of 10 mut/Mb. The number of samples in each group is provided alongside the respective box plots. The center line, box edges, and whiskers denote the median, interquartile range, and the rest of the distribution in respective order, additionally showing outliers. P values of TMB differences are calculated using a one-tail Wilcoxon rank-sum test and provided on the right-hand side of each box plot. (B) HRs for male (red) and female (blue) patients with high TMB (≥ 10 mutation/Mb) versus the rest (x-axis) in four different melanoma cohorts (y-axis). Bars represent the standard 95% CIs. The significance of difference in male versus female hazard ratios is computed using a Wald test for the contribution of the coefficient of the interaction between TMB threshold and sex in a Cox proportional-hazards model. FDA, US Food and Drug Administration; HR, hazard ratio; PD1, programmed cell death protein 1; PDL1, programmed death-ligand 1; TMB, tumor mutational burden.
To test the robustness of these findings, we repeated the above analysis in all additional publicly available melanoma cohorts treated with anti-PD1 where overall survival, TMB, and patient demographics are available (Roh et al8 [N = 23], Liu et al9 [N = 144], and Valero et al10 [N = 56]). Consistently, we observed a higher median TMB in male versus female patients with melanoma in each of these three cohorts (Fig 1A bottom three groups) and found a lower HR in female than male patients in two out of three cohorts (Fig 1B bottom three groups). A combined meta-analysis (weighted z test) of all the four cohorts together shows a higher median TMB in male versus female patients (combined P = .006) and a lower HR in female versus male patients (combined P = .027). We note that these findings have limited immediate clinical implications as high TMB is not currently an FDA prerequisite for treating metastatic melanoma patients with anti-PD1.11 However, as clinicians may still take this threshold into account while considering therapies for a patient given the central role of TMB as a biomarker in general (and in ongoing clinical trials, eg, NCT04187833 and NCT02553642), we think it is important to take note of this potential bias.
We next tested whether the sex bias observed above extends to other ICI and non-ICI treatments in melanoma. To this end, we mined the survival and TMB information of patients with melanoma in three additional patient cohorts: the first treated with anti-CTLA4 (N = 17412,13), the second treated with an anti-PD1/PDL1 and anti-CTLA4 combination (N = 1153), and the third treated with different chemotherapies (N = 32214). We did not observe a significant difference in HR between male and female patients in any of these cohorts (P < .14, P < .8, and P < .4, in respective order), indicating that the sex bias observed in melanoma is specific to anti-PD1/PDL1 treatments.
We next asked whether the sex bias is present in other cancer types treated with anti-PD1/PDL1. Analyzing patient data across additional seven different cancer types from Samstein et al (2019), we first charted the distribution of TMB values in tumors from female and male patients in each of these cancer types (Fig 2A). We observed considerable differences in the HR values between female and male patients in glioblastoma (N = 114, females v males HR = 0.50 v 0.89, P interaction < .59; Fig 2B) and in cancers of unknown origin (N = 88, females v males HR = 1.03 v 0.15, P interaction < .06; Fig 2B). Notably, the HR is higher for males in glioblastoma patients and for females in cancer of unknown patients. The effect found in glioblastoma remained consistent when merging two additional small glioblastoma cohorts treated with anti-PD1 (Zhao et al [N = 15], Lombardi et al [N = 12])15,16 with our initial cohort (N = 141, females v males HR = 0.56 v 1.19, P interaction < .36).
FIG 2.
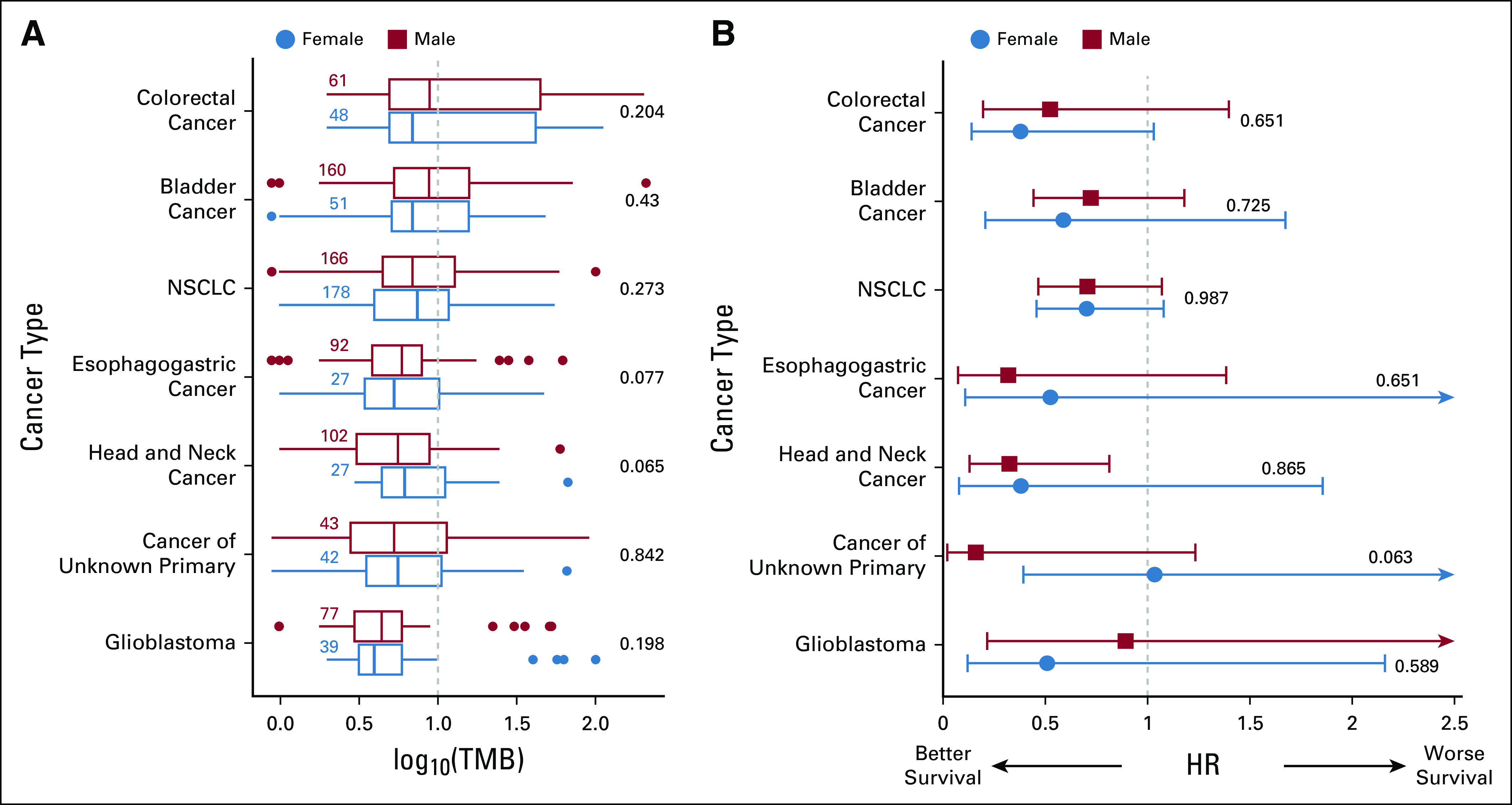
The association between high TMB status and survival after anti-PD1/PDL1 treatment for male and female patients separately in seven cancer types. (A) Standard box plots displaying the distribution of log10(TMB) (x-axis) for male and female patients across cancer types (y-axis) in a similar manner to Figure 1A. (B) HRs of patients with high TMB (≥ 10 mutation/Mb) versus the rest (x-axis) in each cancer type (y-axis), sex color code as in (A), displayed in a similar manner to that of Figure 1B. Renal cell carcinoma is not reported in our analysis as its HR cannot be computed confidently. HR, hazard ratio; NSCLC, non–small-cell lung cancer; PD1, programmed cell death protein 1; PDL1, programmed death-ligand 1; TMB, tumor mutational burden.
To test whether the small sizes of the glioblastoma and cancer of unknown origin data sets may impede the discovery of potentially significant sex-dependent effects, we down-sampled the melanoma anti-PD1/PDL1 treatment cohort to the size of the glioblastoma and cancer of unknown origin cohorts (N = 114 and N = 88, respectively3). We repeated the down-sampling analysis 5,000 times, keeping the respective female-to-male ratio as in these cohorts. In these down-sampled melanoma cohorts, we find a large but statistically insignificant difference between HR in male and female patients: mean HR = 0.20 and 0.95 for females and males, respectively; P = .51 for a set size equal to that of glioblastoma cohort and a mean HR = 0.20 and 1.04 for females and males, respectively; and P = .46 for a set size equal to that of cancer of unknown origin cohort. These results suggest that the small size of the glioblastoma and cancer of unknown origin may hinder our ability to identify significant trends and calls for further testing in larger cohorts. Interestingly, we note that although the size of the non–small-cell lung cancer (NSCLC) cohort is substantial (N = 329), we do not observe any notable difference in HR between male and female patients with NSCLC (female v male HR = 0.70 v 0.69, P interaction < .99; Fig 2B), which is further confirmed in another cohort (N = 16, P interaction < .24).17
In summary, our findings indicate that the FDA-approved threshold of high TMB for selecting patients for anti-PD1/PDL1 treatment is informative for stratifying female but not male patients with metastatic melanoma. These findings may be of future relevance given ongoing clinical trials investigating the role of higher TMB as a biomarker for anti-PD1/PDL1 in melanoma (ClinicalTrials.gov identifiers: NCT04187833 and NCT02553642). Interestingly, in NSCLC, we did not observe notable differences in HR between male and female patients despite the large size of the cohort. Furthermore, our findings suggest that usage of this high TMB biomarker may introduce a sex bias in glioblastoma and cancers of unknown origin, which needs to be carefully tested further in larger data sets, as has been suggested by others for a variety of clinical findings regarding immunotherapy and immunology that may have a sex bias.18
Eytan Ruppin
Stock and Other Ownership Interests: Medaware Ltd, Metabomed Ltd
Patents, Royalties, Other Intellectual Property: Patent applications have been submitted by Tel-Aviv University, University of Maryland, and NCI for synthetic lethality and expression of deconvolution computational analysis approaches
Uncompensated Relationships: Pangea Therapeutics
No other potential conflicts of interest were reported.
SUPPORT
This research used the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov). This research was supported by the Intramural Research Program of the National Institutes of Health, NCI, Center for Cancer Research. S.S. is supported by the NCI-UMD Partnership for Integrative Cancer Research Program.
PREPRINT VERSION Preprint version available on https://www.biorxiv.org/content/10.1101/2021.05.28.446208v1.
N.S. and S.S. are co-first authors.
DATA SHARING STATEMENT
Scripts and data used in the study are provided to reproduce each step of results and plots in this GitHub repository.
AUTHOR CONTRIBUTIONS
Conception and design: Neelam Sinha, Sanju Sinha, Ayelet Erez, Alejandro A. Schäffer, Kenneth Aldape, Eytan Ruppin
Collection and assembly of data: Neelam Sinha, Sanju Sinha, Eytan Ruppin
Data analysis and interpretation: Neelam Sinha, Sanju Sinha, Kuoyuan Cheng, Sanna Madan, Bríd M. Ryan, Kenneth Aldape, Eytan Ruppin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eytan Ruppin
Stock and Other Ownership Interests: Medaware Ltd, Metabomed Ltd
Patents, Royalties, Other Intellectual Property: Patent applications have been submitted by Tel-Aviv University, University of Maryland, and NCI for synthetic lethality and expression of deconvolution computational analysis approaches
Uncompensated Relationships: Pangea Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Jardim DL, Goodman A, de Melo Gagliato D, et al. : The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell 39:154-173, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litchfield K, Reading JL, Puttick C, et al. : Meta-analysis of tumor and T cell intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 184:596-614.e14, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samstein RM, Lee CH, Shoushtari AN, et al. : Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51:202-206, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marabelle A, Fakih M, Lopez J, et al. : Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21:1353-1365, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Ye Y, Jing Y, Li L, et al. : Sex-associated molecular differences for cancer immunotherapy. Nat Comm 11:1179, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro A, Pyke RM, Zhang X, et al. : Strength of immune selection in tumors varies with sex and age. Nat Comm 11:4128, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li CH, Haider S, Shiah Y-J, et al. : Sex differences in cancer driver genes and biomarkers. Cancer Res 78:5527-5537, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Roh W, Chen P-L, Reuben A, et al. : Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med 9:eaah3560, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Schilling B, Liu D, et al. : Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med 25:1916-1927, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valero C, Lee M, Hoen D, et al. : The association between tumor mutational burden and prognosis is dependent on treatment context. Nat Genet 53:11-15, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration . FDA approves pembrolizumab for adjuvant treatment of melanoma. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adjuvant-treatment-melanoma
- 12.Van Allen EM, Miao D, Schilling B, et al. : Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350:207-211, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder A, Makarov V, Merghoub T, et al. : Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 371:2189-2199, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J, Chen AX, Gartrell RD, et al. : Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat Med 25:462-469, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lombardi G, Barresi V, Indraccolo S, et al. : Pembrolizumab activity in recurrent high-grade gliomas with partial or complete loss of mismatch repair protein expression: A monocentric, observational and prospective pilot study. Cancers 12:2283, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizvi NA, Hellmann MD, Snyder A, et al. : Cancer immunology: Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124-128, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein S, Morgan R: The impact of sex and gender on immunotherapy outcomes. Biol Sex Differ 11:24, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Scripts and data used in the study are provided to reproduce each step of results and plots in this GitHub repository.


