Abstract
Asian weaver ants (Oecophyllasmaragdina) are an important biocontrol agent in agricultural habitats. We conducted surveys in oil palm plantations in Riau, Indonesia for an obligate myrmecophilous butterfly larvae, Liphyrabrassolis (Lepidoptera, Lycaenidae), that is known to consume weaver ant larvae in other habitat types. We found L.brassolis larvae in five of the twenty nests surveyed, with larval presence not being related to weaver ant nest size. We also observed L.brassolis larvae in a weaver ant mass rearing facility. This is the first report of L.brassolis from oil palm plantations and may have implications for the use of weaver ants as biological control agents.
Keywords: Formicidae, parasitism, biocontrol, bagworm, Riau
Introduction
The Asian weaver ant, Oecophyllasmaragdina (Fabricius, 1775), is widespread from western Asia to northern Australia (Wetterer 2017) and maintains arboreal territories through aggressive behaviour. Worker ants patrol tree branches and attack other animals because they are generalist predators, preying on insects and other arthropods and also attack humans who disturb their nests (Crozier et al. 2010). The Asian weaver ant has several ecological and economic functions regarding its ecological interactions: enhancing potential nitrogen (Pinkalski et al. 2015, Vidkjær et al. 2015, Vidkjær et al. 2016), shaping plant-pollinator interactions (Rodríguez-Gironés et al. 2013) and acting as biocontrol agent (Peng et al. 1999, Peng and Christian 2005, Peng and Christian 2013, William et al. 2015, Anato et al. 2017). Weaver ants are commonly used as biocontrol agents in several agricultural systems with potential to control major pests such as true bugs (Coreidae and Miridae), beetles (Chrysomelidae), aphids (Aphididae), caterpillars (Lepidoptera), leaf miners (Coleoptera), leafrollers (Lepidoptera), fruit flies (Drosophilidae), leafhoppers (Cicadellidae) and shoot borers (Lepidoptera) (Van Mele 2007).
The majority of the species in the butterfly family Lycaenidae are associated with ants, either mutualistically or parasitically, with interactions either being facultative or obligate (Pierce et al. 2002). Parasitic interactions usually involve myrmecophagy, with butterfly larvae consuming the ant brood. In the family Lycaenidae, 74 species show this behaviour during some stage of their life cycle (Pierce 1995). These parasitic-myrmecophagy interactions can be found between Africa and northern Australia (Fiedler 1991, Eastwood and Fraser 1999, Pierce et al. 2002, Terblanche and Van Hamburg 2003, Crozier et al. 2010, Table 1).
Table 1.
List of lycaenid lepidopteran with myrmecophagy association
| Family | Subfamily | Tribe/Subtribe | Species | Associated ant | Distribution | References |
| Lycaenidae | Miletinae | Liphyrini | Liphyrabrassolis | Oecophyllasmaragdina | Australia, Thailand, Malaysia, Indonesia | Fiedler (1991), Eastwood and Fraser (1999), Pierce et al.(2002), Crozier et al. (2010) |
| Lycaenidae | Miletinae | Liphyrini | Liphyragrandis | Oecophylla spp. | Papua, Australia, Pacific islands | Pierce et al.(2002), Crozier et al. (2010) |
| Lycaenidae | Miletinae | Lachnocnemini | Lachonocnemabibulus | Camponotus spp., Crematogaster spp., Pheidole spp. | Africa continent | Pierce et al. (2002) |
| Lycaenidae | Miletinae | Lachnocnemini | Lachonocnemabrimo | Camponotus spp., Crematogaster spp., Pheidole spp. | Africa continent | Pierce et al. (2002) |
| Lycaenidae | Miletinae | Lachnocnemini | Lachonocnemadurbani | Camponotus spp., Crematogaster spp., Pheidole spp. | Africa continent | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Theclini/ Arhopalitii | Arhopalawildei | Polyrachis spp. | Papua, Northern Australia | Eastwood and Fraser (1999), Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Theclini/ Luciti | Acrodipsasaurata | Crematogaster spp., Papyrius spp. | Australia | Eastwood and Fraser (1999), Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Theclini/ Luciti | Acrodipsasbrisbanensis | Crematogaster spp., Papyrius spp. | Australia | Eastwood and Fraser (1999), Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Theclini/ Luciti | Acrodipsascuprea | Crematogaster spp., Papyrius spp. | Australia | Fiedler (1991), Eastwood and Fraser (1999), Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Theclini/ Luciti | Acrodipsasillidgei | Crematogaster spp., Papyrius spp. | Australia | Fiedler (1991), Eastwood and Fraser (1999), Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Theclini/ Luciti | Acrodipsasmyrmecophila | Crematogaster spp., Papyrius spp., Iridomyrmex spp. | Australia | Fiedler (1991), Eastwood and Fraser (1999), Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Theclini/ Zesiiti | Zesiuschrysomallus | Oecophylla spp. | South Asia | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Theclini/ Ogyriti | Ogyrisidmo | Camponotus spp. | Australia | Fiedler (1991), Eastwood and Fraser (1999) |
| Lycaenidae | Lycaeninae | Aphnaeini | Cigaritis [Apharitis] acamas | Crematogaster spp. | Africa continent, Japan | Fiedler (1991), Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Aphnaeini | C. [Spindasis] japanesenyassae | Crematogaster spp. | Africa continent, Japan | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Aphnaeini | C. [Spindasis] takanonis | Crematogaster spp. | Africa continent, Japan | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Aphnaeini | Trimeniaagyroplaga | Anoplolepis spp. | Africa continent | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Aphnaeini | T.wallengrenii | Anoplolepis spp. | Africa continent | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Aphnaeini | T. [Argyrocupha] malagrida | Anoplolepis spp. | Africa continent | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Aphnaeini | Oxychaetadicksoni | Crematogaster spp., Myrmicaria spp. | South Africa | Fiedler (1991), Terblanche and Van Hamburg (2003) |
| Lycaenidae | Lycaeninae | Polyommatini/ Polyommatiti | Phengarisdaitozana | Myrmica spp. | Asia | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Polyommatini/ Polyommatiti | P.atroguttata | Myrmica spp. | Asia | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Polyommatini/ Polyommatiti | Lepidochrysops spp. | Camponotus spp. | Africa continent | Pierce et al. (2002) |
| Lycaenidae | Lycaeninae | Polyommatini/ Polyommatiti | Maculinea spp. | Myrmica spp., Aphaenogaster spp. | Europe, Asia | Pierce et al. (2002) |
Liphyrabrassolis (Westwood, 1864), is obligately myrmecophilous, with larvae being dependent on weaver ants (Oecophyllasmaragdina) and eating the ant brood (Dodd 1902, Eastwood and Fraser 1999, Pierce et al. 2002). As L.brassolis feeds on the larvae of weaver ants, it might have the potential to affect ant populations. Hence, it is important to understand its occurrence in widespread crops, such as oil palm plantation, where O.smaragdina has the potential to control pests, such as leaf-eating caterpillars.
Materials and Methods
Sampling of weaver ant nests was conducted in an oil palm plantation located in the Province of Riau in Sumatra, Indonesia (0°56'35.3"N, 101°11'56.4"E) in August 2021. A localised outbreak of Claniatertia Templeton, 1847 (Lepidoptera, Pyschidae) bagworm was ongoing when the sampling was conducted. Twenty active weaver ant nests were chosen randomly on twenty individual palms. Only nests in which ants were present (confirmed using binoculars), constructed from live green palm leaflets, were sampled. The nest was collected, wrapped in a plastic bag and all connecting leaflets and fronds were cut using machete and pruning shears. Nest dimensions (length, width and depth) were measured. On the ground, nests were thoroughly dissected and checked for the presence of L.brassolis larva. Dissected nests were removed from bags and replaced at the base of the palm to allow ants to return.
During this period, as part of another study, we were conducting mass-rearing of weaver ants with the aim of propagation and release as biocontrol agent against leaf-eating caterpillar pests. All the colonies were fed on a 30% sugar solution and live insects or fish as a protein source. Observations of L.brassolis were conducted in this mass-rearing activity in 25 ant colonies that were being propagated in plastic bottles (Offenberg 2014) for integrated pest management (IPM).
Liphyrabrassolis larvae, found in ant nests, were identified morphologically (Crozier et al. 2010, Eastwood et al. 2010, Dupont et al. 2016). The larva of this species is distinguished by its carapace-like structure with a hard cuticle both dorsally and laterally (Fig. 1), which is used to defend against ants attacks (Dupont et al. 2016). All speciments of L.brassolis larvae and O.smaragdina were housed in Smart Research Institute, Riau, Indonesia.
Morphology of Liphyrabrassolis that were collected from weaver ant nests during field observations in an oil palm plantation in Riau Province, Indonesia.
Figure 1a.
dorsal side of larva
Figure 1b.
ventral side of larva
Figure 1c.
pupal stage
Figure 1d.
pupal exuvia
A generalised linear model (GLM) with binomial errors was used to investigate the relationship between the weaver ant nest size and L.brassolis occurrence. The occurrence of L.brasollis larvae was used as a binary response variable with one nest sample per observation. Nest size was calculated as an ellipsoid volume using the three measured nest dimensions. Correction for overdispersion was conducted using the quasibinomial family since the residual deviance was larger than the degree of freedom (Crawley 2015). All statistical analyses were performed using the R statistical program version 4.0.2 (R Core Team 2020) in the R Studio version 1.1.423 (RStudio Team 2020) environment. Graphs were created using “ggplot2” and “tidyverse” R packages (Wickham et al. 2019). The generalised linear model was performed using the "lme4" package (Bates et al. 2015). The dataset of observation is deposited in GBIF, Global Biodiversity Information Facility (https://doi.org/10.15468/upzkak).
Results and Discussion
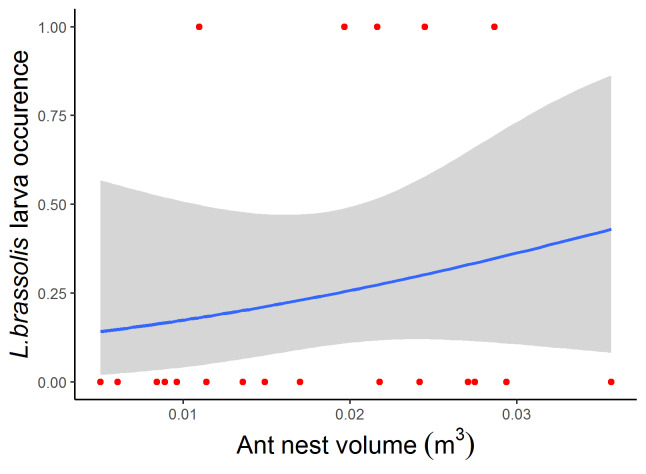
Liphyrabrassolis larvae were present inside both weaver ant nests from the field (5 of 20 nests sampled) and mass-rearing observations (2 of 25 nests). In all seven nests with L.brassolis larvae present, only a single larvae was found (Suppl. material 1). A similar rate of occurrence of L.brassolis was reported during the ant harvesting season in Khao Chong, Thailand (this study: 16%, Eastwood et al. 2010: 21%). We found no evidence for a relationship between ant nest volume and L.brassolis larva presence (GLM, binomial errors, d.f. = 19, t = 0.78, p = 0.44; Fig. 2). This contrasts with a previous anecdotal observation mentioning that L.brassolis was found in larger weaver ant nests (Itterbeeck 2014).
Figure 2.
Relationship between Liphyrabrassolis larvae occurrence and weaver ant nest volume. Fitted line comes from a GLM with binomial errors.
We observed the larvae in the mass-rearing facility actively following the weaver ants during migration from the original disturbed leaf nest into the plastic bottle used to house the colony (Fig. 3). The presence of L.brassolis larvae in mass-rearing facilities should be avoided since consumption will reduce the number of ant brood. Mass-rearing operators should be trained to notice the L.brassolis larvae, so that larvae can be removed before the ant colony becomes established in the new nest. Education is needed here, since often local people misidentify L.brassolis larvae as a large ant or queen ant (Eastwood et al. 2010) . This is important because the weaver ant mass-rearing is commonly used to produce ant brood for use as bird feed (Prayoga 2015), a protein source for human consumption (Sribandit et al. 2008) or as an augmentation strategy for integrated pest management (IPM) programmes (Peng et al. 2014).
Figure 3.
Presence of Liphyrabrassolis (Lepidoptera: Lycaenidae), an obligate-parasitic myrmecophily larva, inside a weaver ant (Oecophyllasmaragdina) colony in the mass-rearing facility.
Although adult L.brassolis have been reported in West Java, Indonesia (Peggie 2012), this is the first report of this species in an oil palm plantation to our knowledge, despite multiple other butterfly surveys in this habitat in Sumatra (Panjaitan et al. 2020, Panjaitan et al. 2021). In Thailand, the larva of L.brassolis is commonly reported in weaver ant nests on orchard plantations, being first documented during entomophagy by local people (Eastwood et al. 2010). Furthermore, the presence of L.brassolis larva in weaver ant nests has not yet been reported during brood harvesting activity by local people in Indonesia (Césard 2004).
Implementation of biological control against leaf-eating caterpillars in an oil plantation is needed to support sustainable palm oil practices. However, only a small number of studies have investigated the ecological function of O.smaragdina as a biological control agent in oil palm plantation (Pierre and Idris 2013). With specific interaction with O.smaragdina, the butterfly L.brassolis can be a bioindicator for weaver ant colony in oil palm plantations. Liphyrabrassolis larvae are only ever found associated with O.smaragdina. However, other Lycaenid larvae have been observed in the nests of Oecophylla spp, such as Liphyragrandis and Zesiuschrysomallus (Pierce et al. 2002).
Our finding indicates that further investigations of weaver ant symbionts in oil palm plantations could be useful, in particular, because this ant species has potency for controlling leaf-eating caterpillar pests. It would be worth conducting larger-scale observations over greater numbers of colonies in order to measure any potential impacts of L.brassolis larvae on colony fitness, both in the field and in mass-rearing facilities. Furthermore, this observation represents an additional data point for lepidopteran biodiversity in Sumatra, especially in oil palm landscapes.
Supplementary Material
L.brassolis field observation
Andreas Dwi Advento
Data type
Observation data
Brief description
These data are an observational census for L.brassolis occurrence in an oil palm plantation. They cover date sampling, location (plantation block), time sampling, nest dimension, larva number per nest and larva condition. (https://doi.org/10.15468/upzkak)
File: oo_683034.xlsx
Acknowledgements
ADA and KMY were supported by a Malaysian Ministry of Higher Education Fundamental Research Grant (FRGS/1/2014/STWN10/UMS/02/1). TMF was supported by a Czech Science Foundation Standard Grant (19-14620S). We would also like to thank to PT. Smart Tbk for supporting ADA to pursue his Masters study in the Universiti Malaysia Sabah and all SMARTRI staff for their support.
Grant title
Malaysian Ministry of Higher Education Fundamental Research Grant (FRGS/1/2014/STWN10/UMS/02/1); Czech Science Foundation Standard Grant (19-14620S)
Hosting institution
Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah; Smart Research Institute, PT Smart Tbk, Indonesia
Ethics and security
The data in the paper were collected as part of an ongoing leaf-eating caterpillar pest and its natural enemy monitoring in the plantations, following standard industry operating procedures.
Conflicts of interest
The authors declare no conflict of interest.
Contributor Information
Andreas Dwi Advento, Email: adadvento@gmail.com.
Kalsum M Yusah, Email: kalsum@ums.edu.my.
Author contributions
ADA, KMY and TMF contributed to the conception and design of the study. ADA collected the data. ADA, KMY and TMF wrote the first draft of the manuscript. All authors contributed to manuscript revision and have read and approved the submitted version.
Conflicts of interest
The authors declare no conflict of interest.
References
- Anato F. M., Sinzogan A. A. C., Offenberg J., Adandonon A., Wargui R. B., Deguenon J. M., Ayelo P. M., Vayssières J. - F., Kossou D. K. Oecophyllalonginoda (Hymenoptera: Formicidae) lead to increased cashew kernel size and kernel quality. Journal of Economic Entomology. 2017;110(3):1133–1137. doi: 10.1093/jee/tox054. [DOI] [PubMed] [Google Scholar]
- Bates Douglas, Mächler Martin, Bolker Ben, Walker Steve. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Césard Nicolas. In: Forest products, Livelihoods and Conservation. Case Studies of Non-Timber Forest Product Systems. Kusters Koen, Belcher Brian., editors. 1. Asia. Center for International Forestry Research; Bogor: 2004. Harvesting and commercialisation of kroto (Oecophylla smaragdina) in the Malingping area, West Java, Indonesia.10. [Google Scholar]
- Crawley Michael J. Statistics: An Introduction Using R. 2nd edition. John Wiley and Sons; Chichester: 2015. 357. [Google Scholar]
- Crozier Ross H., Newey Philip S., Schluns Ellen A., Robson Simon K. A., et al. A masterpiece of evolution - Oecophylla weaver ants (Hymenoptera: Formicidae) ISSN 1997-3500 Myrmecological News, 2010;13(May):57–71. [Google Scholar]
- Dodd F. P. Contribution to the life history of Liphyrabrassolis, Westw. Entomologist. 1902;35:153–188. [Google Scholar]
- Dupont Steen T., Zemeitat Dany S., Lohman David J., Pierce Naomi E. The setae of parasitic Liphyrabrassolis butterfly larvae form a flexible armour for resisting attack by their ant hosts (Lycaenidae: Lepidoptera) Biological Journal of the Linnean Society. 2016;117(3):607–619. doi: 10.1111/bij.12656. [DOI] [Google Scholar]
- Eastwood Rod, Fraser A. M. Associations between lycaenid butterflies and ants in Australia. Australian Journal of Ecology. 1999;24(5):503–537. doi: 10.1046/j.1440-169x.1999.01000.x. [DOI] [Google Scholar]
- Eastwood Rod, Kongnoo Pitoon, Reinkaw Manus. Collecting and eating Liphyrabrassolis (Lepidoptera: Lycaenidae) in southern Thailand. The Journal of Research on the Lepidoptera. 2010;43(January):19–22. doi: 10.5962/p.266505. [DOI] [Google Scholar]
- Fiedler Konrad: Systematic, evolutionary, and ecological implications of myrmecophily within the Lycaenidae (Insecta: Lepidoptera: Papilionoidea) Bonnerzoologische Monographien. Vol. 13. Zoologisches Forschungsinst. und Museum Alexander Koenig; Bonn: 1991. 211. [Google Scholar]
- Itterbeeck Joost Van. Prospects of semi-cultivating the edible weaver ant Oecophyllasmaragdina. Wageningen University; Wageningen: 2014. 112. [Google Scholar]
- Offenberg Joachim. The use of artificial nests by weaver ants: a preliminary field observation. ISSN 1985-1944 Asian Myrmecology, 6(1) 2014;6(1):119–128. [Google Scholar]
- Panjaitan Rawati, Drescher Jochen, Buchori Damayanti, Djunijanti Peggie, Harahap Idham Sakti, Scheu Stefan, Hidayat Purnama. Diversity of butterflies (Lepidoptera) across rainforest transformation systems in Jambi, Sumatra, Indonesia. Biodiversitas Journal of Biological Diversity. 2020;21(11) doi: 10.13057/biodiv/d211117. [DOI] [Google Scholar]
- Panjaitan Rawati, Hidayat Purnama, Peggie Djunijanti, Buchori Damayanti, Scheu Stefan, Drescher Jochen. The Butterflies of Jambi (Sumatra, Indonesia): An EFForTS Field Guide. LIPI Press; Jakarta: 2021. 101. [DOI] [Google Scholar]
- Peggie Djunijanti. A list of the butterflies of Ujung Kulon National Park, Java, Indonesia. Treubia. 2012;39(December):67–76. [Google Scholar]
- Peng Renkang, Christian Keith. Do weaver ant (Hymenoptera: Formicidae) marks affect mango internal quality and storage life? Journal of Economic Entomology. 2013;106(1):299–304. doi: 10.1603/ec12162. [DOI] [PubMed] [Google Scholar]
- Peng Renkang, Lan La Pham, Christian Keith. Weaver Ant Role in Cashew Orchards in Vietnam. Journal of Economic Entomology. 2014;107(4):1330–1338. doi: 10.1603/ec14039. [DOI] [PubMed] [Google Scholar]
- Peng R. K., Christian K., Gibb K. The effect of colony isolation of the predacious ant, Oecophyllasmaragdina (F.) (Hymenoptera: Formicidae), on protection of cashew plantations from insect pests. International Journal of Pest Management. 1999;45(3):189–194. doi: 10.1080/096708799227789. [DOI] [Google Scholar]
- Peng R. K, Christian K. Integrated pest management in mango orchards in the Northern Territory Australia, using the weaver ant, Oecophyllasmaragdina, (Hymenoptera: Formicidae) as a key element. International Journal of Pest Management. 2005;51(2):149–155. doi: 10.1080/09670870500131749. [DOI] [Google Scholar]
- Pierce Naomi E. Predatory and parasitic lepidoptera: carnivores living on plants. Journal of the Lepidoterists’ Society. 1995;49(4):412–453. [Google Scholar]
- Pierce Naomi E., Braby Michael F., Heath Alan, Lohman David J., Mathew John, Rand Douglas B., Travassos Mark A. The Ecology and Evolution of Ant Association in the Lycaenidae (Lepidoptera) Annual Review of Entomology. 2002;47(1):733–771. doi: 10.1146/annurev.ento.47.091201.145257. [DOI] [PubMed] [Google Scholar]
- Pierre Exelis Moise, Idris Azarae Hj. Studies on the predatory activities of Oecophyllasmaragdina (Hymenoptera: Formicidae) on Pteromapendula (Lepidoptera: Psychidae) in oil palm plantations in Teluk Intan, Perak (Malaysia) ISSN 1985-1944 Asian Mrymecology. 2013;5:163–176. [Google Scholar]
- Pinkalski Christian, Damgaard Christian, Jensen K_M. V, Peng Renkang, Offenberg Joachim. Quantification of ant manure deposition in a tropical agroecosystem: implications for host plant nitrogen acquisition. Ecosystems. 2015;18(8):1373–1382. doi: 10.1007/s10021-015-9906-5. [DOI] [Google Scholar]
- Prayoga Bayu. Kupas Tuntas Budidaya Kroto Cara Modern. 4th Edition. Penebar Swadaya; Jakarta: 2015. 124. [Google Scholar]
- Team R Core. Vienna, Austria; 2020. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rodríguez-Gironés M. A, Gonzálvez F. G, Llandres A. L, Corlett R. T, Santamaría Luis. Possible role of weaver ants, Oecophyllasmaragdina, in shaping plant-pollinator interactions in South-East Asia. Journal of Ecology. 2013;101(4):1000–1006. doi: 10.1111/1365-2745.12100. [DOI] [Google Scholar]
- Team RStudio. Boston, MA; 2020. RStudio: Integrated Development Environment for R. [Google Scholar]
- Sribandit Wissanurak, Wiwatwitaya Decha, Suksard Santi, Offenberg Joachim. The importance of weaver ant (Oecophyllasmaragdina Fabricius) harvest to a local community in Northeastern Thailand. Asian Myrmecology. 2008;2(1):129–138. doi: 10.20362/am.002015. [DOI] [Google Scholar]
- Terblanche R. F, Van Hamburg H. The taxonomy, biogeography and conservation of the myrmecophilous Chrysoritis butterflies (Lepidoptera: Lycaenidae) in South Africa. Koedoe. 2003;46(2):65–81. [Google Scholar]
- Van Mele Paul. A historical review of research on the weaver ant Oecophylla in biological control. Agricultural and Forest Entomology. 2007;10(1):13–22. doi: 10.1111/j.1461-9563.2007.00350.x. [DOI] [Google Scholar]
- Vidkjær N. H, Wollenweber Bernd, Gislum René, Jensen K. MV, Fomsgaard I. S. Are ant feces nutrients for plants? A metabolomics approach to elucidate the nutritional effects on plants hosting weaver ants. Metabolomics. 2015;11(4):1013–1028. doi: 10.1007/s11306-014-0757-4. [DOI] [Google Scholar]
- Vidkjær N. H, Wollenweber Bernd, Jensen K. MV, Ambus P. L, Offenberg Joachim, Fomsgaard I. S. Urea in weaver ant feces: quantification and investigation of the uptake and translocation of urea in Coffeaarabica. Journal of Plant Growth Regulation. 2016;35(3):803–814. doi: 10.1007/s00344-016-9586-1. [DOI] [Google Scholar]
- Wetterer James K. Geographic distribution of the weaver ant Oecophyllasmaragdina. Asian Myrmecology. 2017;Volume 9 doi: 10.20362/am.009004. [DOI] [Google Scholar]
- Wickham Hadley, Averick Mara, Bryan Jennifer, Chang Winston, McGowan Lucy, François Romain, Grolemund Garrett, Hayes Alex, Henry Lionel, Hester Jim, Kuhn Max, Pedersen Thomas, Miller Evan, Bache Stephan, Müller Kirill, Ooms Jeroen, Robinson David, Seidel Dana, Spinu Vitalie, Takahashi Kohske, Vaughan Davis, Wilke Claus, Woo Kara, Yutani Hiroaki. Welcome to the Tidyverse. Journal of Open Source Software. 2019;4(43) doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- William J. G, Hella J, Lars E, Offenberg J, Mwatawala M, Rwegasira G, et al. Benefit-cost analysis of alternative insect pests management in cashew and mango orchards in Tanzania. Quarterly Journal of Econometrics Research. 2015;1(2):32–44. doi: 10.18488/journal.88/2015.1.2/88.1.32.44. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
L.brassolis field observation
Andreas Dwi Advento
Data type
Observation data
Brief description
These data are an observational census for L.brassolis occurrence in an oil palm plantation. They cover date sampling, location (plantation block), time sampling, nest dimension, larva number per nest and larva condition. (https://doi.org/10.15468/upzkak)
File: oo_683034.xlsx