Abstract
This work demonstrates that gp96 preparations isolated from cells infected with intracellular bacteria induce cytotoxic T-lymphocyte responses and confer protection. Our findings extend previous reports on the immunogenicity of gp96-associated peptides to antigens derived from intracellular bacteria. Immunization with gp96 may therefore represent a promising vaccination strategy against bacterial pathogens.
Acquired resistance against numerous intracellular bacteria depends on CD8+ T cells (14). This has meant major hurdles to conventional vaccination strategies. Recent approaches to overcome such obstacles include immunizations with naked DNA and with recombinant live bacterial or viral vectors (12). An alternative strategy is vaccination with heat shock protein (hsp)-peptide complexes. The immunological relevance of the peptide-binding capacity of the hsp gp96 was first described for the tumor system (25). It is reported that gp96 isolated from tumor cells induces anti-tumor-specific cytotoxic T lymphocytes (CTL) against cells from the parent tumor (30). Recent studies have extended the feasibility of eliciting immune responses against antigens from different sources using gp96-peptide complexes (26). Immunization with gp96 induced potent CTL responses to peptides of viral antigens (5, 11, 20, 28), model antigens (2, 21), and minor histocompatibility antigens (2). These findings, together with the capacity of gp96 to bind a broad array of peptides, provide the conceptual framework for vaccination strategies with gp96.
The hsp gp96 is localized in the endoplasmic reticulum (ER), where it seems to play a role in processing of antigens in the major histocompatibility complex (MHC) class I pathway. Cytoplasmic proteins are cleaved into peptides by proteasomes and transported into the ER lumen by the transporter associated with antigen processing. In the ER these peptides associate with gp96 molecules and are then loaded onto MHC class I molecules (17, 19, 24, 27). Although it is accepted that a broad range of peptides can bind to gp96 (17, 24), the peptide- binding characteristics of gp96 molecules are still incompletely understood. Recent findings suggest that peptides can be accommodated in a hydrophobic pocket of gp96 (22). The promiscuous binding specificity of gp96 suggests that the variety of peptides bound by gp96 molecules reflects virtually the entire peptide repertoire in the ER (1).
It has been speculated that gp96 targets peptides to antigen-presenting cells (APC) and thereby improves their immunogenicity. This is consistent with the finding that APC express cell surface receptors for hsp (3, 23) which promote internalization via endocytosis and processing through the MHC class I pathway (7). Recently, the receptor for gp96 has been identified as CD91 (alpha-2-macroglobulin receptor) (4).
We tested the capacity of gp96 isolated from cells infected with the intracellular bacteria Listeria monocytogenes and Mycobacterium tuberculosis to induce a protective immune response in mice. gp96 preparations were generated from mice infected intravenously (i.v.) with 5 × 103 L. monocytogenes strain EGD organisms or 2 × 105 to 5 × 105 M. tuberculosis strain H37Rv organisms. Spleens and livers were harvested on day 5 (Listeria-infected mice) or day 28 (Mycobacterium-infected mice) and homogenized with a laboratory blender (Seward Medical, Oxford, United Kingdom). gp96 was purified as described previously (2). Immediately after centrifugation, cell pellets from livers and spleens (from approximately 15 to 20 mice) were homogenized in 200 ml of hypotonic buffer (30 mM NaHCO3, containing a protease inhibitor cocktail [Boehringer Mannheim, Germany] [pH 7.1]). Following centrifugation (35,000 × g at 4°C for 30 min), the supernatants were filter sterilized, applied to a concanavalin A (Con A)-Sepharose column and eluted with phosphate-buffered saline (PBS) (pH 7.2). Con A-bound material was eluted with PBS containing 10% α-methyl-mannoside. The eluate was separated by fast-performance liquid chromatography using a MonoQ column (5/5; Pharmacia) with an NaCl gradient from 0 to 1 M. Fractions were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis for gp96 by using a monoclonal antibody specific for gp96 (anti-grp94, SPA-850, clone 9G10; Stressgen, Victoria, British Columbia, Canada). Only fractions containing gp96 as their major content and devoid of other detectable contaminating proteins were used for vaccination experiments. The gp96 content in each fraction was quantified by measuring the optical density at 280 nm with an extinction coefficient of 1.0. Mass spectrometry analysis revealed that the gp96 preparations used in these studies did not contain microbial contaminants.
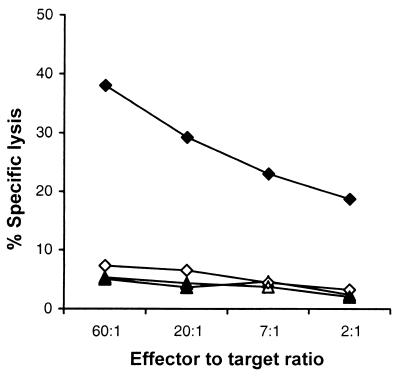
To determine the capacity of gp96 preparations to induce CD8+ CTL responses, C57BL/6 mice were immunized subcutaneously (s.c.) at the base of the tail with 30 μg of gp96 in 100 to 200 μl of PBS. gp96 was isolated from spleens and livers from uninfected (control) or L. monocytogenes-infected C57BL/6 mice. After 10 days, splenocytes were stimulated with lipopolysaccharide-induced blasts as APC (1 × 106 cells/ml) and heat-killed L. monocytogenes (HKL) (5 × 106 organisms/ml). Three days later, the medium was replaced with fresh culture medium supplemented with 10% interleukin 2-enriched Con A (Sigma)–rat spleen cell supernatant (16). A significant expansion of CD8+ T cells (>80% of gated lymphocytes), as assessed by CD8 and CD4 staining in fluorescence-activated cell sorting analysis, was observed on day 6 in cultures from mice immunized with gp96 isolated from L. monocytogenes-infected organs. In contrast, cultures from mice immunized with the control gp96 preparation showed only marginal growth of CD8+ T cells (<20%) (results not shown). On day 7, cultures were restimulated with lipopolysaccharide-induced blasts, HKL, and Con A-rat spleen cell supernatant. Cultures were tested using RMA cells as targets on day 12 in a standard 51Cr release assay as described previously (31). Only cultures from mice immunized with gp96 isolated from L. monocytogenes-infected organs exhibited specific CTL activity against listerial antigens (Fig. 1). Hence, gp96 from Listeria-infected cells induced Listeria-specific CD8+ CTL in the absence of adjuvants.
FIG. 1.
gp96 isolated from organs of L monocytogenes-infected mice prime CD8+ CTL. Ten days after s.c. immunization of C57BL/6 mice with 30 μg of gp96 from Listeria-infected organs (closed symbols) or noninfected organs (open symbols), the spleens were removed and splenocytes were stimulated as described before. On day 12, cultures were tested for CTL activity on RMA cells alone (triangles) or RMA cells pulsed with HKL antigens (diamonds) in a standard 51Cr release assay.
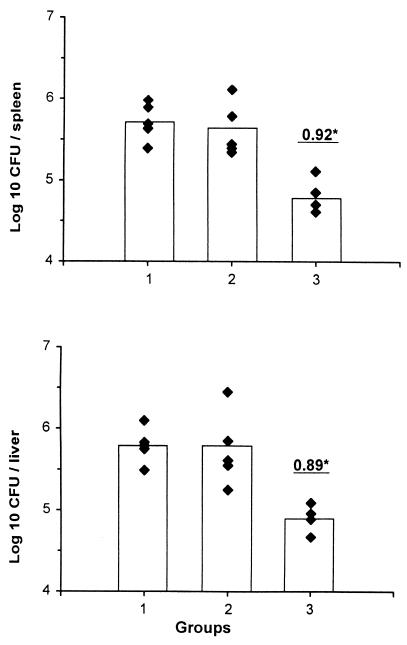
Next, we tested whether gp96 isolated from L. monocytogenes-infected cells induces protective immunity. C57BL/6 mice were immunized (s.c.) with gp96 isolated from livers and spleens of infected C57BL/6 mice and noninfected C57BL/6 mice (control). Ten days after immunization, the mice were infected i.v. with 6.4 × 103 live L. monocytogenes organisms in PBS. Five days later, the organs were homogenized and appropriate dilutions were plated on trypticase soy plates. After 1 day of culture at 37°C, the numbers of CFU were determined. Pretreatment of mice with gp96 reduced the bacterial load in spleens (change in value of the number of CFU per organ [Δlog], 0.92; P < 0.001) and livers (Δlog, 0.89; P < 0.001) significantly (Fig. 2). In contrast, treatment of mice with gp96 from control mice did not affect bacterial numbers. Protection induced by i.v. immunization with gp96 from L. monocytogenes-infected organs was comparable to that shown in Fig. 2 (data not shown). Importantly, different batches of gp96 preparations resulted in comparable data. The highest level of protection was observed when mice were immunized 10 days prior to infection with bacteria. Multiple injections with gp96 did not substantially improve protection levels. Altogether, these findings demonstrate that immunization with gp96 stimulates CD8+ CTL and effectively induces protection against L. monocytogenes, as demonstrated previously using other vaccination protocols, such as those using naked DNA (9) or killed bacteria (29).
FIG. 2.
Protection against listerial infection by gp96 from infected mice. C57BL/6 mice were treated with PBS alone (group 1) or immunized s.c. with gp96 (30 μg) from organs of noninfected C57BL/6 mice (group 2) or gp96 (30 μg) from organs of L. monocytogenes-infected C57BL6 mice (group 3). Ten days after immunization, mice were infected i.v. with L. monocytogenes organisms. On day 5, the numbers of CFU in spleens and livers were determined. Underlined numbers indicate differences (log 10) between the average numbers of CFU observed for experimental groups and those observed for PBS-treated controls. The individual numbers of CFU are illustrated by the closed diamonds, and the bars illustrate the mean number of CFU per group. The experiment, with 5 animals per group, has been repeated with comparable results. ∗, significant protection (P < 0.001, Mann-Whitney test).
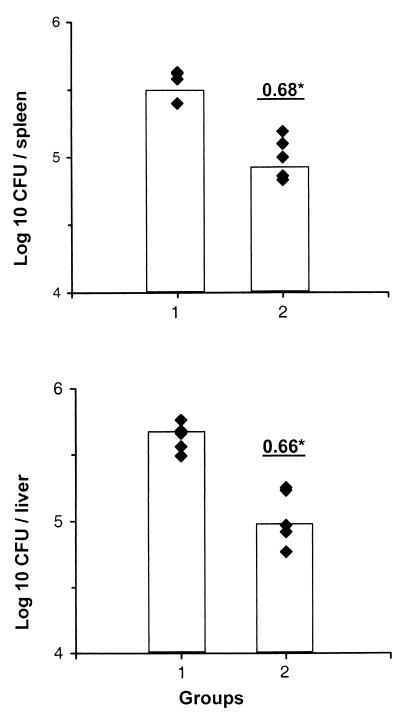
Next, we asked whether gp96 from M. tuberculosis-infected organs is capable of inducing protective immunity in mice against M. tuberculosis. In the mouse, effective protection against M. tuberculosis requires both CD4+ and CD8+ T cells, although the precise role of these T-cell subsets in the control of tuberculosis is still incompletely understood (10, 14, 18). C57BL/6 mice were immunized with gp96 isolated from livers and spleens of M. tuberculosis-infected or noninfected C57BL/6 mice. After 10 days, mice were infected i.v. with 1 × 105 to 2 × 105 live M. tuberculosis organisms in PBS. On day 28 after infection, spleens and livers were homogenized and appropriate dilutions were plated on Middlebrook agar plates supplemented with oleic acid-albumin-dextrose-catalase enrichment (Difco, Detroit, Mich.). After 3 to 4 weeks of culture at 37°C, the numbers of CFU were determined. A single dose of 30 μg of gp96 from M. tuberculosis-infected organs significantly reduced the numbers of CFU of M. tuberculosis both in spleens (Δlog, 0.68; P < 0.001) and in livers (Δlog; 0.66; P < 0.001) compared with controls (Fig. 3). Although vaccination with Mycobacterium bovis BCG was not included in these experiments, in our hands BCG consistently induced 1 to 1.4 log protection. Thus, in mycobacterial infections microbial peptides are also complexed with gp96, and vaccination of mice with these complexes induces partial protection against M. tuberculosis.
FIG. 3.
gp96 complexes isolated from organs of M. tuberculosis-infected mice induce protective immunity against tuberculosis in mice. C57BL/6 mice were immunized s.c. with gp96 (30 μg) from organs of noninfected C57BL/6 mice (group 1) or from organs of M. tuberculosis-infected mice (group 2). Mice were infected 10 days after immunization with M. tuberculosis strain H37Rv. The numbers of CFU in spleens and livers of 5 animals per group were determined on day 28 after infection. Numbers indicate differences (log 10) between the average numbers of CFU of experimental groups and that of the PBS-treated control. The experiment was repeated with comparable results. ∗, significant protection (P < 0.001, Mann-Whitney test).
We show, for the first time, the feasibility of vaccinating with gp96-peptide complexes against intracellular bacteria. Our experiments reveal that gp96 from organs of mice infected with intracellular bacteria induces a specific CD8+ CTL response. We therefore assume that during infection, bacterial peptides comprising epitopes for CD8+ T cells associate with gp96. Moreover, our results demonstrate that vaccination of mice with these gp96-peptide complexes induces protection against intracellular bacteria. Our findings reveal that gp96-peptide complexes are themselves potential vaccine candidates for the control of intracellular bacteria, even when the identity of the specific microbial antigenic epitope is unknown. We do, nevertheless, consider it important to identify the protective epitopes complexed to gp96. Immunodominant peptides have been eluted from gp96 molecules, and their sequences have been identified for some systems (6, 13, 20). As the sequence of the genome of M. tuberculosis is now available (8), sequence determination of peptides eluted from gp96 may directly lead to the respective protein antigen. Thus, subunit or DNA vaccination approaches based on novel antigens could become feasible. Currently, we are attempting to isolate and sequence M. tuberculosis-derived peptides from gp96 molecules in order to design novel vaccine candidates against tuberculosis, which remains one of the major health threats to humans to this day (15).
Acknowledgments
This work was supported by the DFG (KA 573/4-1) and the BMBF (‘Mykobakterielle Infektionen’).
We thank Caitlin McCoull and Lucia Lom-Terborg for critically reading the manuscript and for their helpful comments. Many thanks also to Carmen Blum for expert technical assistance.
REFERENCES
- 1.Arnold D, Wahl C, Faath S, Rammensee H G, Schild H. Infuences of transporter associated with antigen processing (TAP) on the repertoire of peptides associated with the endoplasmic reticulum-resident stress protein gp96. J Exp Med. 1997;186:461–466. doi: 10.1084/jem.186.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold D, Faath S, Rammensee H-G, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee H G, de la Salle H, Schild H. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- 4.Binder R J, Han D K, Srivastava P K. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–153. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 5.Blachere N E, Li Z, Chandawarkar R Y, Suto R, Jaikaria N S, Basu S, Udono H, Srivastava P K. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breloer M, Marti T, Fleischer B, von Bonim A. Isolation of processed H-2Kb-binding ovalbumin-derived peptides associated with the stress proteins hsp70 and gp96. Eur J Immunol. 1998;28:1016–1021. doi: 10.1002/(SICI)1521-4141(199803)28:03<1016::AID-IMMU1016>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 7.Castellino F, Boucher P E, Eichelberg K, Maythew M, Rothman J E, Houghton A N, Germain R N. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Sulston J E, Taylor K, Whitehead S, Barrel B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Fensterle J, Grode L, Hess J, Kaufmann S H E. Effective DNA vaccination against listeriosis by prime/boost inoculation with the gene gun. J Immunol. 1999;163:4510–4518. [PubMed] [Google Scholar]
- 10.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heikema A, Agsteribbe E, Wilschut J, Huckriede A. Generation of heat shock protein-based vaccines by intracellular loading of gp96 with antigenic peptides. Immunol Lett. 1997;57:69–74. doi: 10.1016/s0165-2478(97)00048-5. [DOI] [PubMed] [Google Scholar]
- 12.Hess J, Schaible U, Raupach B, Kaufmann S H E. Exploiting the immune system: towards new vaccines against intracellular bacteria. Adv Immunol. 2000;75:1–88. doi: 10.1016/s0065-2776(00)75001-2. [DOI] [PubMed] [Google Scholar]
- 13.Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, Hizuta A, Tanaka N, Srivastava P K, Nakayama E. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162:1303–1309. [PubMed] [Google Scholar]
- 14.Kaufmann S H E. Immunity to intracellular bacteria. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Lippincott-Raven; 1998. pp. 1345–1381. [Google Scholar]
- 15.Kaufmann S H E. Is the development of a new tuberculosis vaccine possible? Nat Med. 2000;6:955–960. doi: 10.1038/79631. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann S H E, Hahn H. Biological function of T cell lines with specificity for the intracellular bacteria Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982;155:1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lammert E, Arnold D, Nijenhuis M, Momburg F, Hämmerling G J, Brunner J, Stefanovic S, Rammensee H G, Schild H. The endoplasmic reticulum-resident stress protein gp96 binds peptides translocated by TAP. Eur J Immunol. 1997;27:923–927. doi: 10.1002/eji.1830270418. [DOI] [PubMed] [Google Scholar]
- 18.Müller I, Cobbold S P, Waldmann H, Kaufmann S H E. Impaired resitance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicchitta C V. Biochemical, cell biological and immunological issues surrounding the edoplasmic reticulum chaperone GRP94/gp96. Curr Opin Immunol. 1998;10:103–109. doi: 10.1016/s0952-7915(98)80039-3. [DOI] [PubMed] [Google Scholar]
- 20.Nieland T J, Tan M C, Monne-Van Muijen M, Koning F, Kruisbeek A M, Van Bleek G M. Isolation of an immunodominant viral peptide that is endogenously bound to the stress protein GP96/GRP94. Proc Natl Acad Sci USA. 1996;93:6135–6139. doi: 10.1073/pnas.93.12.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roman E, Moreno C. Synthetic peptide non-covalently bound to bacterial hsp70 elicit peptide-specific T-cell responses in vivo. Immunology. 1996;88:487–492. doi: 10.1046/j.1365-2567.1996.d01-697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sastry S, Linderoth N. Molecular mechanisms of peptide loading by the tumor rejection antigen/heat shock chaperone gp96 (GRP94) J Biol Chem. 1999;274:12023–12035. doi: 10.1074/jbc.274.17.12023. [DOI] [PubMed] [Google Scholar]
- 23.Singh-Jashuja H, Toes R E M, Spee P, Munz C, Hilf N, Schoenberger S P, Riccardi-Castagnoli P, Neefjes J, Rammensee H G, Arnold-Schild D, Schild H. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor mediated endocytosis. J Exp Med. 2000;191:1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spee P, Neefjes J. TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and calrecticulin. Eur J Immunol. 1997;27:2441–2449. doi: 10.1002/eji.1830270944. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava P K, DeLeo A B, Old L J. Tumor rejection antigen of chemically induced tumors of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava P K, Menoret A, Basu S, Binde R J, McQuade K L. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava P K, Udono H, Blachere N E, Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 28.Suto R, Srivastava P K. A mechanism of the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 29.Szalay G, Ladel C H, Kaufmann S H E. Stimulation of protective CD8+ T lymphocytes by vaccination with nonliving bacteria. Proc Natl Acad Sci USA. 1995;92:12389–12392. doi: 10.1073/pnas.92.26.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura Y, Peng P, Liu K, Daou M, Srivastava P K. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 31.Zügel U, Schoel B, Yamamoto S, Hengel H, Morein B, Kaufmann S H E. Crossrecognition by CD8 T cell receptor αβ cytotoxic T lymphocytes of peptides in the self and mycobacterial hsp60 which share intermediate sequence homology. Eur J Immunol. 1995;25:451–458. doi: 10.1002/eji.1830250222. [DOI] [PubMed] [Google Scholar]