Abstract
Background:
There is a need to accurately identify pregnant women at risk for preterm birth as early as possible. Recent developments in technology enable the recording of uterine electrical activity (electrohysterogram) from the anterior abdominal wall in a non-invasive way.
Objective:
To investigate whether uterine activity recorded under resting conditions at a gestational age of 34 weeks could identify a risk of preterm birth.
Study design:
A commercial antenatal holter device with its dedicated software was used to record and store raw data of the maternal and fetal electrocardiograms and uterine activity for the Safe Passage Study. Uterine activity was recorded under resting conditions from 34 weeks’ gestation in epochs of 250 ms (millisecond) for at least 30 min. From this database the raw data, recorded at a mean gestational age of 34 weeks, of 50 women who had preterm deliveries were selected for comparison with data of women who had term deliveries. Mean uterine activity, expressed in microvolt (μV)/epoch, was used for the comparison.
Results:
After exclusion of 25 participants where labour was induced or augmented and another three for other reasons, 36 remained in each group. The participants in each group were comparable in respect of maternal age, gravidity, parity, gestational age at recruitment and duration of recording. Uterine activity in the preterm group (60.3 μV/epoch) differed significantly (p<0.01) from that of the comparison group (52.4 μV/epoch). Using a cut-off point of 52.3 μV/epoch as obtained from receiver operator characteristic curves (area under the curve 0.72), the sensitivity and specificity of identifying risks of preterm labour were 81% and 50% respectively.
Conclusion:
Results of this small study are promising but need to be confirmed in larger studies and preferably at earlier gestational age.
Keywords: Electrohysterography, Fetal monitoring, Monica AN24, Preterm birth, Uterine activity
Introduction
Preterm labour, especially in early gestation, is a major cause of death and disability [1]. In a recent community-based study, it was found that in 70% of all fetal, neonatal and infant deaths, from a gestation of 22 weeks to the age of 1 year, birth had occurred before 37 weeks [2]. One of the top 15 United Kingdom research priorities for preterm birth is to determine which interventions are most effective to predict or prevent preterm birth [3]. Despite advances in neonatal care, preterm birth is still a main cause of death in the United States, especially among blacks [4]. Although home uterine monitoring may result in fewer admissions to neonatal intensive care units, it leads to more unscheduled antenatal visits and tocolytic treatment without any impact on perinatal mortality or the incidence of preterm birth [5]. The use of tocodynamometry for the detection of uterine contractions in obese women seems to be inaccurate [6]. In addition, measurement of the frequency of uterine contractions is not useful for predicting preterm delivery [7]. However, in recent years, noninvasively Ectrohysterography (EHG) has become available for the prediction of preterm delivery [8,9]. In addition, it has been shown that EHG is superior to tocodynamometry in the detection of uterine activity, especially in obese women [10]. The Safe Passage Study (SPS) of the Prenatal Alcohol in SIDS and Stillbirth (PASS) Network was developed to test the hypothesis that prenatal alcohol exposure is associated with increased risk for stillbirth and/or sudden infant death syndrome [11]. For the collection of raw data on the maternal and fetal Electrocardiogram (ECG) and uterine activity we used the AN24 mobile Holter device (Monica Healthcare, Nottingham, UK) during three periods before the onset of labour [12]. For this study we compared the uterine activity, recorded at 34 weeks’ gestation, of women who delivered before 37 weeks with that of women who delivered at term.
Materials and Methods
Study design
From 1st August 2007 to 31st January 2015, 6866 singleton pregnancies were recruited at the Tygerberg Hospital (Cape Town, South Africa) site of the SPS. As part of the study, the Fetal Heart Rate (FHR) and uterine contractions were monitored for at least 50 min under resting conditions in a quiet room.
Preparations for monitoring
Participants were positioned in a 15° right or left lateral position, with a wedge placed under one of the gluteal areas to prevent supine hypotension. The skin was washed with soap and water if the participant had used skin ointment recently, and gentle exfoliation with a medical abrasive paper strip removed superficial dry squamous cells to ensure lower electrode impedance. For the recordings at 34 weeks to 38 weeks gestation, four electrodes were placed in a diamond-shaped pattern on the anterior abdominal wall, one 5 cm above the umbilicus, one just above the pubic hairline, and the other two 5 cm to the left and 10 cm to the right of the umbilicus respectively, but at the same level as the umbilicus. The fifth electrode, for reference, was placed just lateral to the one on the right side. After application, the five electrodes were connected to the AN24 device which is attached to the abdominal wall with an elastic band to prevent it from falling down and to keep the devices in similar position across all studies. The electrical activity of the uterus was sampled every 250 ms (milliseconds). At the end of the recording, the device was removed and connected to a laptop for downloading of the raw data. During these sessions, tracings of the FHR and uterine contractions were not seen by the person doing the recording and both participant and operator were kept blinded from the results of all analyses.
Study population
For this study the raw recorded data of 50 participants who delivered live babies before a gestational age of 37 weeks were selected at random to compare with a group of participants who delivered at 37 weeks or later. Dates on which the recordings of the comparison group were done were selected to be as close as possible to the date of the recording of the participant who delivered prematurely. We also tried to match the gestational age of the comparison participant as closely as possible, to avoid the effect that a higher gestational age of the comparison group at the time of recording could have on the uterine activity. If the patient selected for the comparison group had also delivered before 37 weeks or if the recording was of poor quality, the next participant was selected from the list of recordings in a similar way.
The DK 2.2.0.0 programme was used to analyse the stored raw data of the selected participants. The total electrical activity, as calculated from all the individual measurements captured every 250 ms (milliseconds), was then divided by the number of measurements for that specific participant to express the uterine activity in microvolts (μV) per 250 ms (milliseconds) epoch. The two groups were then compared, in respect of general information on the pregnancy, uterine activity at 34 weeks, test delivery interval, gestational age at delivery and birth weight.
Statistical analyses
Statistical analyses were done with STATISTICA (Dell Inc. (2015), Dell Statistica (data analysis software system), version 13. software.dell.com). Continuous variables, such as birth weight and gestational age in the two different groups were compared with Analysis of Variance (ANOVA). Bonferroni multiple comparisons were used to identify significant differences among more than two means. A logistic regression analysis was done to determine the odds ratio for preterm against the comparison group relative to the influence of uterine activity and a Receiver Operating Characteristic curve (ROC curve) was used to determine the cut-off value of uterine activity between the preterm and comparison groups and a categorised box plot illustrate uterine activity between the two groups.
Ethical approval has been obtained from the Health Research Ethics Committee of Stellenbosch University (Ethics approval number: N06/10/210).
Results
The data of three participants were excluded from the analyses. In one participant some of the electrodes on the abdominal wall became detached and spurious uterine activity was recorded. The other two were excluded from the preterm labor part of the study as the recording of the uterine activity was done before 34 weeks and therefore outside the selected time. Data from an additional 25 participants were excluded as labor was induced or augmented (Figure 1). Clear recordings of uterine activity were obtained (Figure 2).
Figure 1:
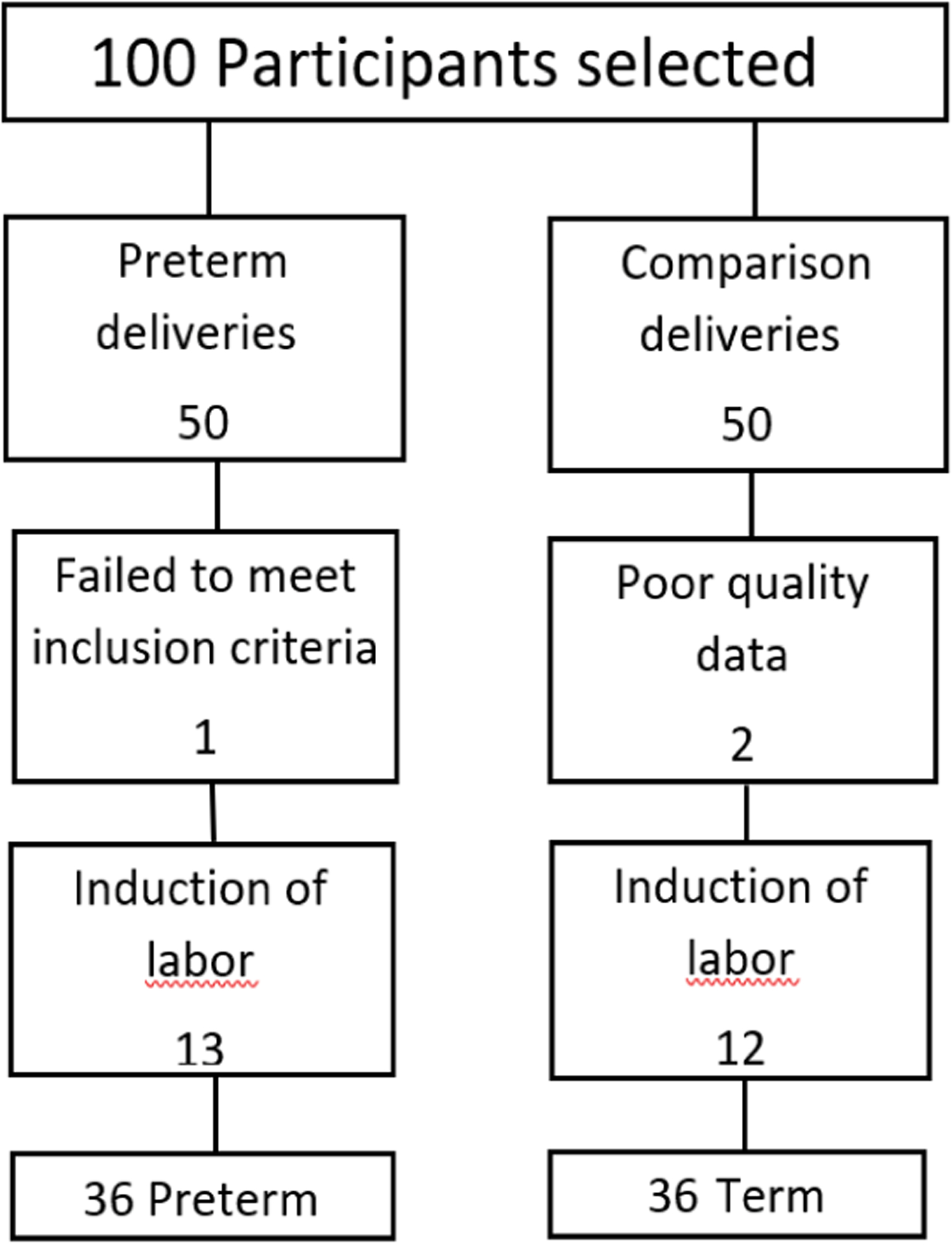
Study profile.
Figure 2:
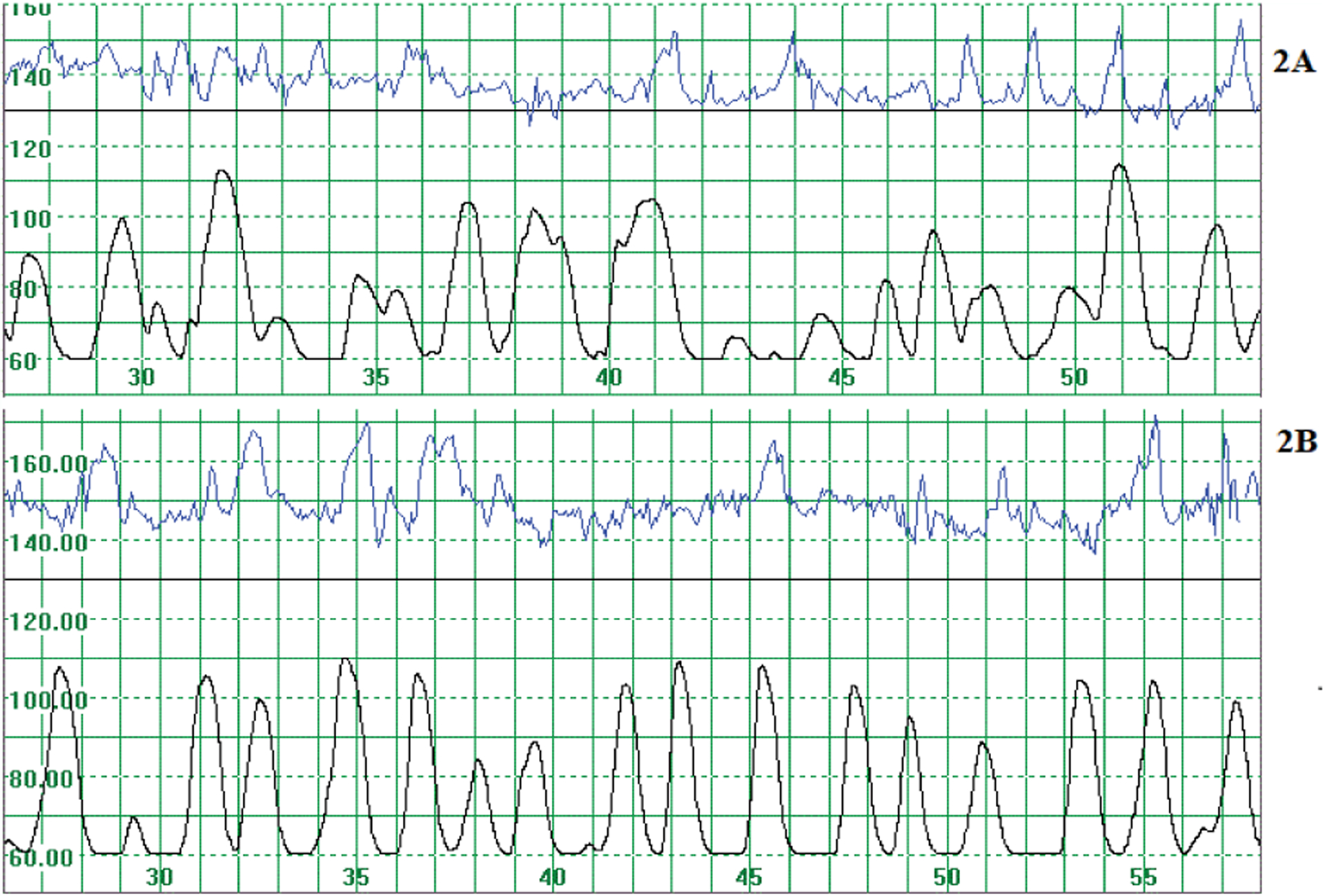
Recording of the fetal heart rate (upper tracing) and uterine activity (lower tracing). The scale on the horizontal axis indicates time; one horizontal block indicates one minute. The fetal heart rate is indicated on the vertical axis in bpm where 100 bpm relates to about 39 μV. 2A: Uterine activity of 73.2 μV/epoch of 250 milliseconds, 6 days before birth. 2B: Uterine activity of 72.1 μV/epoch of 250 ms (milliseconds) 1 day before birth.
The preterm and comparison groups were very similar in respect of maternal age, gravidity, parity and gestational ages at recruitment to the study and at the recording and duration of the recording (Table 1). The mean gestational age at delivery of the preterm birth group was 247 (Standard Deviation (SD) 4.0) days in contrast to the 275 (SD 7.7) of the comparison group (p< 0.01). The mean recording-delivery-time also differed significantly (p<0.001); 6 (SD 4.2) days and 34 (SD 8.2) days for the preterm and comparison groups respectively. The mean birth weights were 2,481 (SD 381) g and 3,046 (SD 464) g in the preterm and comparison groups respectively (p< 0.01).
Table 1:
Background information on patients.
| Preterm birth group (N=36) | Comparison group (N=36) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | P* | |
| Age (years) | 25.4 | 5.3 | 23.7–27.2 | 24.4 | 5.4 | 22.6–26.2 | 0.41 |
| Gravidity | 2.3 | 1.1 | 1.9–2.6 | 1.9 | 1.1 | 1.5–2.2 | 0.11 |
| Parity | 1.2 | 1.1 | 0.9–1.6 | 0.9 | 1.1 | 0.5–1.2 | 0.16 |
| GA at recruitment (days) | 143 | 59 | 123–164 | 141 | 52 | 124–159 | 0.74 |
| GA at recording (days) | 241 | 3.7 | 240–242 | 241 | 3.9 | 240–243 | 0.83 |
| Duration of recording (min) | 54 | 5.1 | 52–56 | 55 | 3.5 | 53–56 | 0.83 |
| Uterine activity (μV/epoch) | 60.3 | 8.8 | 57.4–63.3 | 52.4 | 10.3 | 48.9–55.8 | <0.01 |
| Recording delivery intervals | 6 | 4.2 | 5–8 | 34 | 8.2 | 31–36 | <0.01 |
| GA at delivery (days) | 247 | 4 | 246–249 | 275 | 7.7 | 272–277 | <0.01 |
| Birth weight (g) | 2481 | 381 | 2352–2609 | 3046 | 464 | 2889–3203 | <0.01 |
GA: Gestational Age;
p from Mann-Whitney U test
One participant delivered 6.6 hours after the test. Uterine activity was 62.9 μV/epoch. When she was admitted for diabetes and pregnancy-induced hypertension, decelerations of the FHR were noted at the beginning of labor for which an emergency caesarean section was done. Six participants delivered on the following day, between 15.0 hours and 30.6 hours after the recording. Uterine activity in this group ranged between 49.7 μV/epoch and 65.9 μV/epoch. The remaining preterm births occurred later.
The mean uterine activity in the preterm group was 60.3 μV/epoch in contrast to 52.4 μV/epoch in the comparison group (Mann-Whitney U, p<0.01). However, there was also overlap between the two groups (Figure 3). Higher uterine activity was associated with preterm birth, but there were cases where a high uterine activity was not followed by preterm delivery. When the medical records were examined, no obvious clinical cause could be found to explain why the increased uterine activity was not followed by preterm delivery.
Figure 3:
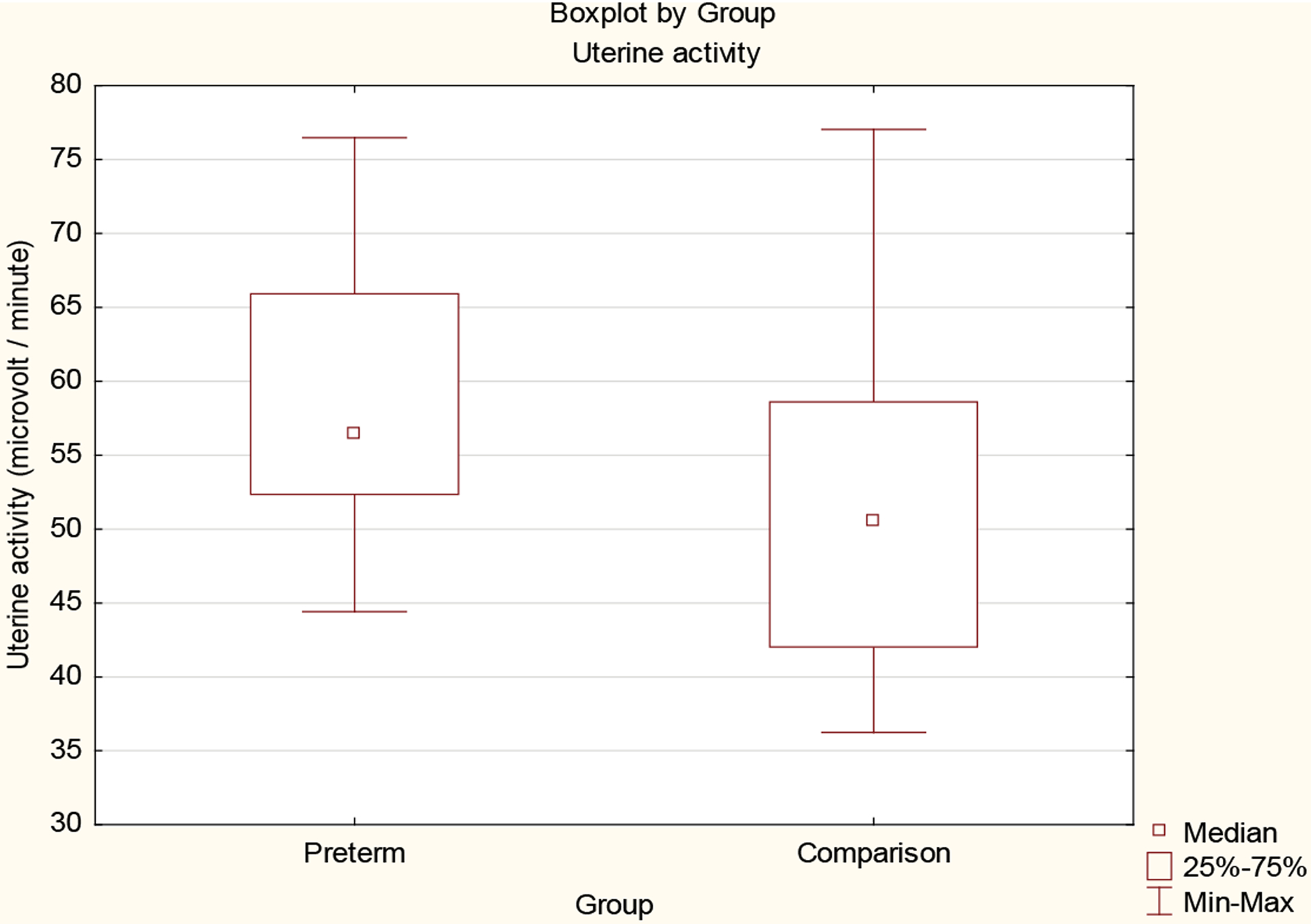
Box plots of uterine activity (μV/epoch) for preterm and comparison groups.
In the logistic regression analysis, the likelihood ratio test, the score test and the Wald test all show that the outcomes are significantly affected by uterine activity. The odds ratio for preterm birth was equal to 1.09 with a 95% confidence interval for the true odds ratio given by [1.03, 1.15]. Thus, the odds for preterm birth increased significantly (p=0.001) for each unit increase in the value of uterine activity. When the ROC curve was used, the area under the ROC curve was 0.72 (0.60–0.84) (Figure 4). The optimal cut off value was 52.27 μV/epoch, giving a sensitivity and specificity of 81% and 50% respectively.
Figure 4:
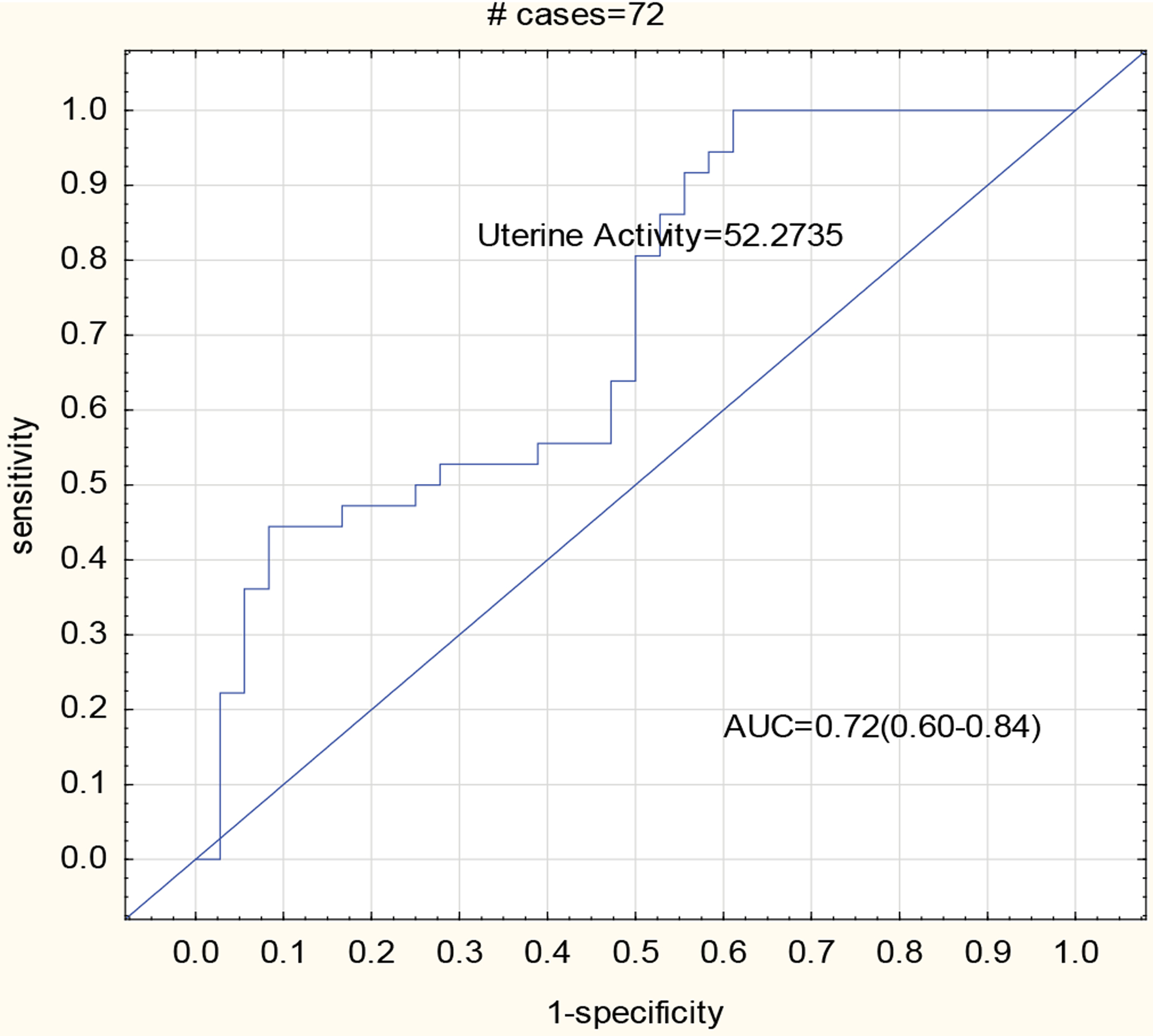
Receiver operator characteristics curve of the study.
Comment
Principle findings
Uterine EHG activity at 34 weeks’ gestation of women who gave birth before 37 weeks was significantly higher than that of women who gave birth at 37 weeks or later [3]. In addition to the gestational age at the time of the recording, the two groups were comparable in respect of age, gravidity, parity, gestational age at recruitment, and duration of the recording.
Results
Our results are promising as there is an urgent need to identify pregnant women with a risk of preterm birth early, not only to prevent the birth but also to administer steroids to enhance lung maturity should preterm birth occur [1,13]. A previous meta-analysis of home uterine activity monitoring demonstrated that the risks of preterm delivery in singleton pregnancies were reduced [14]. More recently a larger review also found that women using home uterine monitoring were less likely to experience birth before 34 weeks. However, the significant difference was no longer evident after a sensitivity analysis, restricting the analysis to studies at low risk of bias based on study quality [5]. As no impact on maternal or perinatal outcomes was found and as it may lead to unscheduled antenatal visits, home uterine monitoring was not recommended.
One of the reasons for the poor performance of home uterine activity monitoring may be failure to accurately detect uterine activity in obese women as the correct placement of the external to-co transducer on the fundus of the uterus is crucial for the detection of uterine contractions [6]. As changes in uterine muscle activity precede onset of labour and as it is now possible to detect uterine electrical activity in a non-invasive way, measurement of the EHG has great potential [15].
Different methods have been described regarding how to assess the EHG, such as the power density spectrum integral, [16] nonlinear correlation among channels in a multichannel recorder [17], velocity propagation of EHG signals [15], peak frequency 15 and median frequency [18]. However, most of these studies were done in research environments while we were using a device available for general use.
Using a cut-off value of 52.3 μV/epoch a sensitivity of 81% and specificity of 50% was found. However, there was also overlap between the two groups as illustrated by (Figure 2). We therefore wondered why participants with high uterine activity did not go into labour sooner and why some with low uterine activity delivered prematurely. There are several possible explanations for this finding as the initiation of labour is also associated with cervical conditions.
Iams et al. [7], assessed the frequency of uterine contractions to indicate a risk for spontaneous preterm delivery. Although the likelihood of preterm delivery increased with increased frequency of contractions, its measurement was not clinically useful for the predicting of preterm delivery. They found significant associations between prematurity and cervical length, Bishop Score and fibronectin in cervicovaginal secretions. The status of the cervix and conditions influencing it is therefore another important aspect of the initiation of preterm birth.
Clinical implications
For the first time a commercial monitor was used to assess the use of the EHG to identify the risks of preterm labour. We found that total uterine activity, expressed as μV/epoch, was higher in pregnant women at 34 weeks who had preterm birth when compared with term deliveries [3]. Uterine activity as such did not identify 50% of women at risk for preterm labour. However, it should be remembered that preterm labour is regarded as a syndrome, a condition with many different causes [19]. A dual approach to the selection of preterm labour, assessing uterine activity and cervical status [20] at the same time, could be more efficient in identifying pregnant women at risk (Figure 5). In addition, further research is needed to determine whether our interesting finding is also present at earlier gestational ages as the prevention of preterm labour is more crucial at earlier stages of pregnancy. Future research on uterine activity should become more feasible as a new technique on uterine electromyography becomes available [21–23]. It seems that short-burst and burst uterine electromyographic activity is more frequently observed in mid-trimester women with short cervical lengths [24].
Figure 5:
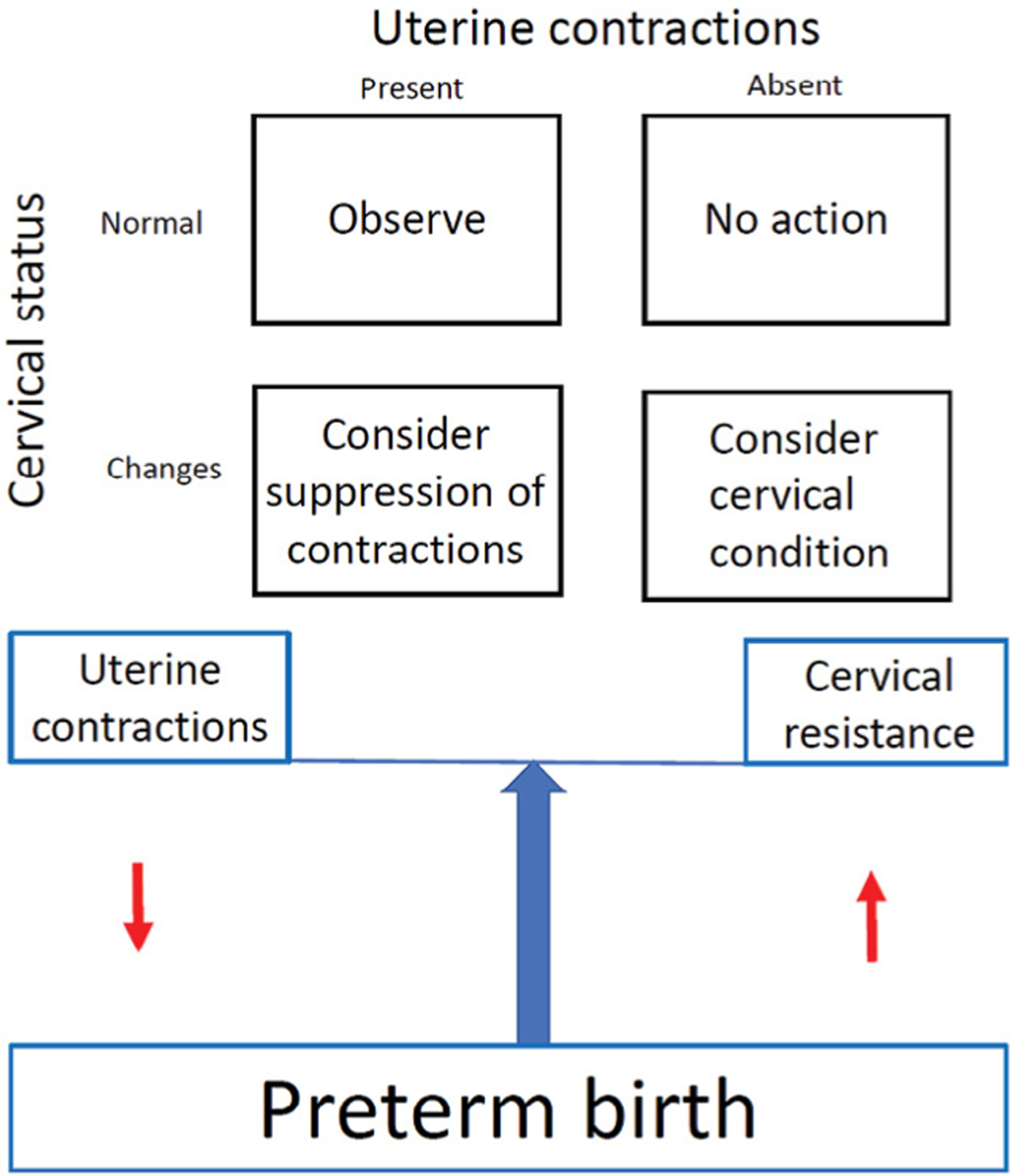
Management of preterm labour according to cervical status and uterine activity.
Research implications
As it has become possible to noninvasively record uterine activity before the onset of labour, as obtained from electrodes on the anterior abdominal wall of the mother, increased activity could be ascertained at different gestational ages. As uterine contractions differ in frequency, duration, and amplitude, it would be necessary to ascertain which of these have the best predictive values for preterm labour. As progress of labour is a balance between forces and resistance, the best identification of the risks of preterm birth would probably be a combination of monitoring uterine activity and assessing the cervical condition.
Strengths and limitations
One of the defects of the study is that the cyclic cranial-caudal movement of the uterus, as caused by respiratory movements, was not assessed. It has been shown that such movements could cause movement-induced artefacts in the interpretation of the EMG but it is still uncertain how very precise interpretation of the electrical signal will influence its clinical use [25].
Conclusion
An association has been found between increased uterine activity as recorded at 34 weeks’ gestation and birth before 37 completed weeks. This finding opens the door for larger studies to further investigate the use of electrohysterography in the identification of pregnant women at risk for preterm birth.
Condensation
Non-invasive assessment of uterine activity from the abdominal wall at 34 weeks’ gestation has a sensitivity of 81% to predict preterm birth.
Acknowledgement
We wish to thank Lucy Brink who helped with the preparation of the figures.
Funding
The study was funded by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute on Deafness and other Communication Disorders: U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991, and U01 AA016501. The funding body had no role in conducting the research or writing the paper.
References
- 1.Jørgensen JS, Jacobsson B, Vinter CA, Lamont RF, Maršál K. Ingemar ingemarsson memorial symposium on preterm delivery at the XXI FIGO world congress. Acta Obstet Gynecol Scand. 2016;95(5):495–500. [DOI] [PubMed] [Google Scholar]
- 2.Boyd TK, Wright CA, Odendaal HJ, Elliott AJ, Sens MA, Folkerth RD, et al. The stillbirth classification system for the safe passage study. Pediatr Dev Pathol. 2017;20(2):120–32. [DOI] [PubMed] [Google Scholar]
- 3.Duley L, Uhm S, Oliver S; Preterm Birth Priority Setting Partnership Steering Group. Top 15 UK research priorities for preterm birth. Lancet. 2014;383(9934):2041–2. [DOI] [PubMed] [Google Scholar]
- 4.Iams JD. Prevention of preterm parturition. N Engl J Med. 2014;370(3):254–61. [DOI] [PubMed] [Google Scholar]
- 5.Urquhart C, Currell R, Harlow F, Callow L. Home uterine monitoring for detecting preterm labour. Cochrane Database Syst Rev. 2017;2(2):CD006172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mumuney AA, Hwang K, Sunwoo N, Burd I, Blakemore K. The impact of maternal body mass index and gestational age on the detection of uterine contractions by tocodynamometry: A retrospective study. Reprod Sci. 2016;23(5):638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iams JD, Newman RB, Thom EA, Goldenberg RL, Heubach EM, Moawad A, et al. Frequency of uterine contractions and the risk of spontaneous preterm delivery. N Engl J Med. 2002;346(4):250–5. [DOI] [PubMed] [Google Scholar]
- 8.Lucovnik M, Maner WL, Chambliss LR, Blumrick R, Balducci J, Novak-Antolic Z, et al. Noninvasive uterine electromyography for prediction of preterm delivery. Am J Obstet Gynecol. 2011;204(3):228.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marque CK, Terrien J, Rihana S, Germain G. Preterm labour detection by use of a biophysical marker: the uterine electrical activity. BMC Pregnancy Childbirth. 2007;7(1):S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Euliano TY, Nguyen MT, Darmanjian S, McGorray SP, Euliano N, Onkala A, et al. Monitoring uterine activity during labor: a comparison of 3 methods. Am J Obstet Gynecol. 2013;208(1):66.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GDV, et al. The safe passage study: design, methods, recruitment, and follow-up approach. Paediatr Perinat Epidemiol. 2014;28(5):455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofmeyr F, Groenewald CA, Nel DG, Myers MM, Fifer WP, Signore C, et al. Fetal heart rate patterns at 20 to 24 weeks’ gestation as recorded by fetal electrocardiography. J Matern Fetal Neonatal Med. 2014;27(7):714–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liggins GC. Fetal lung maturation. ANZJOG. 1994;34(3):247–50. [DOI] [PubMed] [Google Scholar]
- 14.Colton T, Kayne HL, Zhang Y, Heeren T. A metaanalysis of home uterine activity monitoring. Am J Obstet Gynecol. 1995;173(5):1499–505. [DOI] [PubMed] [Google Scholar]
- 15.Lucovnik M, Kuon RJ, Chambliss LR, Maner WL, Shi SQ, Shi L, et al. Use of uterine electromyography to diagnose term and preterm labor. Acta Obstet Gynecol Scand. 2011;90(2):150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bregar TA, Lucovnik M, Verdenik I, Jager F, Gersak K, Garfield RE. Uterine electromyography during active phase compared with latent phase of labor at term. Acta Obstet Gynecol Scand. 2016;95(2):197–202. [DOI] [PubMed] [Google Scholar]
- 17.Hassan M, Terrien J, Muszynski C, Alexandersson A, Marque C, Karlsson B. Better pregnancy monitoring using nonlinear correlation analysis of external uterine electromyography. IEEE Trans Biomed Eng. 2013;60(4):1160–6. [DOI] [PubMed] [Google Scholar]
- 18.Verdenik I, Pajntar M, Leskošek B. Uterine electrical activity as predictor of preterm birth in women with preterm contractions. Eur J Obstet Gynecol Reprod Biol. 2001;95(2):149–53. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagan KO, To M, Tsoi E, Nicolaides KH. Preterm birth: the value of sonographic measurement of cervical length. BJOG. 2006;113 Suppl 3:52–6. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz N, Mhajna M, Moody HL, Zahar Y, Shkolnik K, Reches A, et al. Novel uterine contraction monitoring to enable remote, self-administered nonstress testing. Am J Obstet Gynecol. 2022;226(4):554.e1–554.e12. [DOI] [PubMed] [Google Scholar]
- 22.Esgahaldo F, Batista AG, Mourino H, Russo S, dos Reis CRP, Serrano F, et al. Automatic contraction detection using uterine electromyography. Appl Sci. 2020;10(20):7014. [Google Scholar]
- 23.Esgalhado F, Batista AG, Mouriño H, Russo S, dos Reis CRP, Serrano F, et al. Uterine contractions clustering based on electrohysterography. Comput Biol Med. 2020;123:103897. [DOI] [PubMed] [Google Scholar]
- 24.Marinescu PS, Young RC, Miller LA, Llop JR, Pressman EK, Seligman NS. Mid-trimester uterine electromyography in patients with a short cervix. Am J Obstet Gynecol. 2022;227(1):83.e1–83.e17. [DOI] [PubMed] [Google Scholar]
- 25.De Lau H, Rabotti C, Haazen N, Oei SG, Mischi M. Towards improving uterine electrical activity modeling and electrohysterography: ultrasonic quantification of uterine movements during labor. Acta Obstet Gynecol Scand. 2013;92(11):1323–6. [DOI] [PubMed] [Google Scholar]


