Abstract
Vaccination against COVID-19 reduces infection-related mortality. Unfortunately, reports of vaccine-induced immune thrombotic thrombocytopenia (VITT) in individuals administered adenovirus-vector-based vaccines (ChAdOx1 nCoV-19 and Ad26.COV2.S) have spurred side effect concerns. To address vaccine hesitancy related to this, it is essential to determine the incidence of VITT (defined by a 50% decrease in platelet count and positive anti-PF4 immunoassay within 4–28 days after vaccination) among patients administered two doses of an mRNA-based COVID-19 vaccination. We identified a retrospective cohort of 223,345 patients in the Cleveland Clinic Enterprise administered a COVID-19 vaccine at any location in Northeast Ohio and Florida from 12/4/2020 to 6/6/2021. 97.3% of these patients received an mRNA-based vaccination. Patients with: (1) a serial complete blood count both before and after vaccination and (2) a decrease in platelet count of ≥ 50% were selected for chart review. The primary outcome was the incidence of thrombotic events, including venous thromboembolism (VTE) and arterial thrombosis, 4–28 days post vaccination. Of 74 cohort patients with acute thrombosis, 72 (97.3%) demonstrated clear etiologies, such as active malignancy. Of two patients with unprovoked thrombosis, only one had findings concerning for VITT, with a strongly positive anti-PF4 antibody assay. In this large, multi-state, retrospective cohort, of 223,345 patients (97.2% of whom received the mRNA-based mRNA-1273 or BNT162b2 vaccines), we detected a single case that was concerning for VITT in a patient who received an mRNA vaccine. The overwhelming majority of patients with a thrombotic event 4–28 days following vaccination demonstrated clear etiologies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-022-02764-9.
Keywords: COVID-19, Vaccine induced thrombotic thrombocytopenia, mRNA vaccination, Thrombosis
Key points
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is recognized as a triad of (1) arterial/venous thrombosis in the setting of (2) thrombocytopenia (with initial case series reporting counts ranging from 10 to 113 × 109/L) and (3) a positive anti-PF4 immunoassay in the 4-28 days after vaccination.
We reviewed a multi-state, prospective, observational registry of patients administered a COVID-19 mRNA vaccination for thrombotic events in the setting of thrombocytopenia in the 4-28 days after their vaccination.
The overwhelming majority of patients with a thrombotic event 4-28 days following vaccination demonstrated very clear etiologies for thrombosis, the most common of which was active malignancy (40.5%).
In a large, multi-state, retrospective analysis of a prospective, observational registry, only a single case was concerning for VITT.
This research supports the safety of mRNA-based vaccines for the prevention of SARS-CoV-2 in a large, multi-state cohort.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11239-022-02764-9.
Introduction
Since their recommendation by the Advisory Committee on Immunization Practices (ACIP) as a two-dose series, mRNA-based vaccinations (mRNA-1273 [Moderna] or BNT162b2 [Pfizer-BioNTech]) against the coronavirus disease 2019 (COVID-19) remain the most promising avenue to containing the pandemic. Though a body of literature and post-administration surveillance has supported the high efficacy and safety of the available vaccines, reports of a rare prothrombotic syndrome (vaccine-induced immune thrombotic thrombocytopenia, VITT) in a few individuals administered an adenovirus-vector-based vaccine (ChAdOx1 nCoV-19 [AstraZeneca] and Ad26.COV2.S [Johnson and Johnson]) has spurred concerns globally for vaccine safety in other products – particularly among the relatively novel mRNA-based vaccines [1–3].
Evidence has emerged supporting VITT as a PF4-dependent syndrome, in which complexes of vaccine fragments and platelet factor 4 (PF4) evoke pathogenic antiplatelet immunoglobulin that is capable of invoking antibody receptor-mediated platelet degranulation, with subsequent platelet aggregation, thrombosis, and thrombocytopenia [4, 5]. Within 4–28 days of vaccination, VITT presents as a triad of (1) arterial/venous thrombosis in the setting of (2) thrombocytopenia (with initial case series reporting counts ranging from 10 to 113 × 109/L) and (3) a positive anti-PF4 immunoassay [6, 7]. Patients have presented with extremity deep vein thromboses (DVT), pulmonary embolism (PE), and arterial thromboses – though thrombotic episodes in cerebral, splanchnic (e.g. mesenteric, portal), and other atypical vascular beds have also been reported [8].
Published studies of VITT following administration of adenoviral vector vaccines—although potentially life-threatening—suggest that this is an exquisitely rare phenomenon, with an estimated incidence of one per 100,000 individuals [9]. Among mRNA vaccines, the incidence is estimated to be even lower. Using data from VAERS, See et al. generated a case series of thrombosis with thrombocytopenia syndrome (TTS) post COVID-19 vaccination, finding rates of TTS among mRNA vaccines of 0.00855 per million, consistent with background incidence [8]. Observational data from VAERS and v-safe, indicate that mild and short-lived adverse effects (for example, injection site pain, headache, fatigue), are more common among mRNA vaccines [10]. In this study, we evaluated the incidence of this rare prothrombotic syndrome and other acute thrombotic events in a large multi-state cohort of 223,345 patients after administration of mRNA-based COVID-19 vaccines (either the mRNA-1273 [Moderna] or BNT162b2 [Pfizer-BioNTech] vaccine). Compared with previous studies utilizing data from active and passive surveillance systems—v-safe and VAERS, respectively—our study invites the possibility of quantifying cases of VITT not necessarily caught with such infrastructure.
Methods
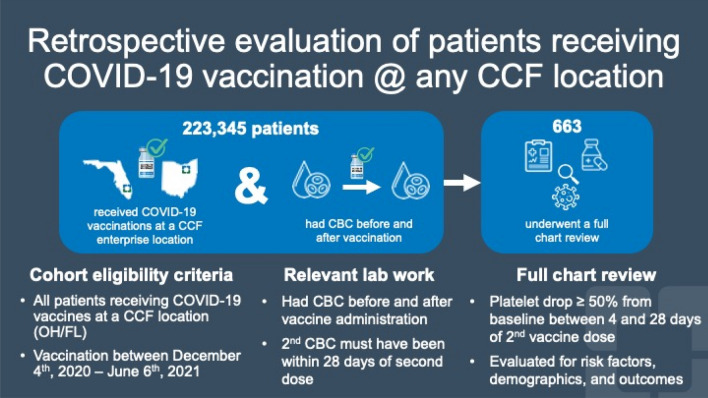
This study was a retrospective analysis of a prospective, observational registry of all patients receiving a COVID-19 vaccine at any Cleveland Clinic location in Northeast Ohio or Florida and was approved by the Cleveland Clinic Institutional Review Board. Eligible subjects were patients who: received a COVID-19 vaccination from 12/4/2020 to 6/6/2021; had a complete blood count (CBC) before and after vaccine administration; and had a platelet drop of ≥ 50% from baseline between 4 and 28 days after their second dose of mRNA-based vaccination or after one dose of an adenovirus-based vaccination – thus all patients in this cohort were considered ‘fully vaccinated’ at inclusion. Subjects who fulfilled these eligibility criteria were selected for full chart review, which entailed evaluation of their medical notes, determining their clinical course, extracting active conditions, and considering active medications at the time of serial blood sampling (Fig. 1; Table 1).
Fig. 1.
Graphical flowchart of the prospective, observational registry
Table 1.
Overall summaries for the retrospective cohort
| Total (N = 663) |
||
|---|---|---|
| Factor | Not missing | Count present (Statistics) |
| Age At Immunization | 663 | 68.2 ± 13.2 |
| Gender | 663 | |
| Female | 303 (45.7) | |
| Male | 360 (54.3) | |
| Manufacturer | 663 | |
| J&J | 18 (2.7) | |
| Moderna | 239 (36.0) | |
| Pfizer | 406 (61.2) | |
| Platelet % Decrease | 663 | − 62.7 ± 11.6 |
| Dead (at time of chart review) | 663 | 115 (17.3) |
| Race (patient reported) | 657 | |
| Asian | 9 (1.4) | |
| Black | 80 (12.2) | |
| Multiracial/Multicultural | 11 (1.7) | |
| Other | 2 (0.30) | |
| White | 555 (84.5) | |
| Ethnicity (patient reported) | 656 | |
| Hispanic | 26 (4.0) | |
| Non-Hispanic | 630 (96.0) | |
| DVT | 663 | 43 (6.5) |
| Acute DVT | 663 | 36 (5.4) |
| PE | 663 | 31 (4.7) |
| Stroke or TIA | 663 | 14 (2.1) |
| MI | 663 | 7 (1.06) |
| ITP | 663 | 26 (3.9) |
| Troponin + or STEMI | 663 | 165 (24.9) |
| Cerebral Venous Sinus Thrombosis | 663 | 0 (0.00) |
| Splanchnic Vein Thrombosis | 663 | 0 (0.00) |
| PF4 ELISA Conducted | 663 | 16 (2.4) |
| Serotonin Release Assay Conducted | 663 | 2 (0.30) |
| HIV (0/1) | 661 | 5 (0.76) |
| Adenovirus (0/1) | 116 | 3 (2.6) |
| Parvovirus (0/1) | 25 | 8 (32.0) |
| RSV (0/1) | 148 | 7 (4.7) |
| EBV | 116 | 76 (65.5) |
| COVID infection prior to vaccination | 663 | 85 (12.8) |
| Omeprazole | 663 | 129 (19.5) |
| Cimetidine | 663 | 0 (0.00) |
| Ranitidine | 663 | 4 (0.60) |
| Rifampin | 663 | 3 (0.45) |
| Quinidine | 663 | 0 (0.00) |
| Carbamazepine | 663 | 1 (0.15) |
| Pantoprazole | 663 | 255 (38.5) |
| Bactrim | 663 | 77 (11.6) |
| Aspirin | 663 | 287 (43.3) |
| Clopidogrel | 663 | 43 (6.5) |
| Ticagrelor | 663 | 5 (0.75) |
| Prednisone | 663 | 120 (18.1) |
| Hydrochlorothiazide | 663 | 79 (11.9) |
| Vancomycin | 663 | 95 (14.3) |
| Spironolactone | 663 | 56 (8.4) |
| Chemo | 663 | 321 (48.4) |
Statistics presented as Mean ± SD, N (column %)
Patient medical records were reviewed for thrombotic events (e.g. deep vein thrombosis, pulmonary embolism, stroke/transient ischemic attack, cerebral venous sinus thrombosis, and splanchnic vein thrombosis); risk factors for thrombocytopenia (e.g. spironolactone, omeprazole, vancomycin); and markers of VITT (e.g. platelet count and anti-PF4 titers). A composite of thrombotic events (which included all DVTs, PEs, MIs, and stroke/TIAs) was created in order to improve our power to evaluate high risk predictors, as it was assumed that PF4/IgG complexes would mediate an increase in thrombosis risk at all sites.
Patient medical records were also evaluated for the presence of competing risks for thrombosis. These included common thrombogenic medications (e.g. prednisone, vancomycin) and viruses (e.g. HIV, parvovirus). Additionally, cycle information and timing were extracted for patients undergoing chemotherapy for a concomitant malignancy. Any laboratory tests to determine anti-platelet antibodies, such as in the workup for Heparin-induced thrombocytopenia (HIT) or VITT, were also reviewed (Table 2). In patients with a thrombotic event within 4–28 days of vaccination, narrative elements of the chart were interrogated to determine specific etiology (Table 3). In patients with a documented thrombotic event, a vascular medicine physician evaluated the images obtained to validate the chronicity (acute/chronic) of the thrombosis.
Table 2.
Summaries of the high risk predictors by whether a composite event occurred, excluding chronic DVTs
| No composite event (N = 589) |
Composite event (N = 74) |
||||
|---|---|---|---|---|---|
| Factor | N | Statistics | N | Statistics | p-value |
| HIV (0/1) | 587 | 5 (0.85) | 74 | 0 (0.00) | 0.99d |
| Adenovirus (0/1) | 100 | 3 (3.0) | 16 | 0 (0.00) | 0.99d |
| Parvovirus (0/1) | 22 | 7 (31.8) | 3 | 1 (33.3) | 0.99d |
| RSV (0/1) | 130 | 7 (5.4) | 18 | 0 (0.00) | 0.60d |
| EBV (0/1) | 101 | 69 (68.3) | 15 | 7 (46.7) | 0.10c |
| Previous COVID | 589 | 75 (12.7) | 74 | 10 (13.5) | 0.85c |
| Omeprazole (0/1) | 589 | 113 (19.2) | 74 | 16 (21.6) | 0.62c |
| Cimetidine (0/1) | 589 | 0 (0.00) | 74 | 0 (0.00) | |
| Ranitidine (0/1) | 589 | 4 (0.68) | 74 | 0 (0.00) | 0.99d |
| Rifampin (0/1) | 589 | 3 (0.51) | 74 | 0 (0.00) | 0.99d |
| Quinidine (0/1) | 589 | 0 (0.00) | 74 | 0 (0.00) | |
| Carbamazepine (0/1) | 589 | 1 (0.17) | 74 | 0 (0.00) | 0.99d |
| Pantoprazole (0/1) | 589 | 228 (38.7) | 74 | 27 (36.5) | 0.71c |
| Bactrim (0//1) | 589 | 69 (11.7) | 74 | 8 (10.8) | 0.82c |
| Aspirin (0/1) | 589 | 257 (43.6) | 74 | 30 (40.5) | 0.61c |
| Clopidogrel (0/1) | 589 | 35 (5.9) | 74 | 8 (10.8) | 0.13d |
| Ticagrelor (0/1) | 589 | 4 (0.68) | 74 | 1 (1.4) | 0.45d |
| Prednisone (0/1) | 589 | 107 (18.2) | 74 | 13 (17.6) | 0.90c |
| Hydrochlorothiazide (0/1) | 589 | 72 (12.2) | 74 | 7 (9.5) | 0.49c |
| Vancomycin (0/1) | 589 | 83 (14.1) | 74 | 12 (16.2) | 0.62c |
| Spironolactone (0/1) | 589 | 53 (9.0) | 74 | 3 (4.1) | 0.15c |
| Chemo (0/1) | 589 | 282 (47.9) | 74 | 39 (52.7) | 0.43c |
Statistics presented as N (column %)
p-values: c = Pearson’s chi-square test, d = Fisher’s Exact test
Table 3.
Etiologies of all thrombotic events in cohort
| Etiology | N (%) |
|---|---|
| Malignancy | 30 (40.5) |
| Chemotherapy associated with increased thrombosis risk | 24 (32.4) |
| Hypercoagulable state | 7 (9.5) |
| Catheter-related | 6 (8.1) |
| Recent surgery | 6 (8.1) |
| Reduced mobility | 5 (6.8) |
| Cardiovascular risk factors | 13 (17.6) |
| Diagnosed/suspected ITP | 3 (3.9) |
| Sepsis | 2 (2.7) |
| Unprovoked PE with thrombocytopenia | 1 (1.4) |
| Suspicious for VITT | 1 (1.4) |
This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Categorical factors were summarized with frequencies and percentages, while continuous measures were described with means, standard deviations, medians, and quartiles. To compare groups defined by acute DVT status on categorical factors, Pearson chi-square and Fisher exact tests were used. Continuous measures were compared using two-sample t-tests and Wilcoxon rank sum tests. An alpha of 0.05 was used to denote statistical significance. Analysis was performed in SAS software (version 9.4; Cary, NC).
Results
Subjects were considered for selection from 779,000 patients who received a COVID-19 vaccination at any Cleveland Clinic location in Northeast Ohio and Florida from 12/4/2020 to 6/6/2021. Of these, 223,345 had a serial CBC both before and after the date of their vaccination. Of the 223,345, we found 663 subjects with a platelet drop of ≥ 50% from baseline between 4 and 28 days after their second vaccination, fulfilling one of the criteria for VITT. These patients were selected for further chart review.
Of these 663 patients, 303 (45.7%) were female with an average age of 68.2 years at the time of immunization. Most patients (97.3%) received an mRNA-based vaccine – with 406 (61.2%) receiving the BNT162b2 (Pfizer-BioNTech) vaccine and 239 (36.0%) receiving the mRNA-1273 (Moderna) vaccine. At the time of review, 115 (17.3%) of these patients had died (Table 1). 18 (15.7%) of these deaths occurred among individuals with thrombotic events within our specified follow-up period post-vaccination. Importantly, none of these deaths were secondary to unprovoked thrombosis. Leading causes of death included malignancy (5, 27.8%), respiratory failure (4, 22.2%), and shock (3, 16.7%).
Competing risks for thrombosis, such as thrombogenic medications or pathogens, were extracted from the electronic medical record and evaluated. 85 patients (12.8%) had been infected with SARS-CoV-2, as determined by laboratory testing, prior to vaccination. Many patients were taking medications known to increase the risk for thrombocytopenia, such as proton-pump inhibitors omeprazole (129, 19.5%) or pantoprazole (255, 38.5%), prednisone (120, 18.1%), or vancomycin (95, 14.3%). A plurality of patients (321, 48.4%) were undergoing chemotherapy as a treatment for malignancy (Table 1).
Thrombotic events were ascertained through chart review. Of 663 charts reviewed, 43 patients (6.5%) were noted to have a deep vein thrombosis (DVT) of any temporality – either acute or chronic. Of these, 36 were categorized as acute onset upon independent second review by a vascular medicine physician certified in vascular ultrasound and not involved in care of each patient. Additionally, 31 (4.7%) patients developed a pulmonary embolism (PE), and 14 (2.1%) had a stroke or transient ischemic attack. A surprisingly high proportion of patients (165, 24.9%) had an elevated blood cardiac troponin, but only 7 (1.1%) met criteria for acute myocardial infarction as determined by a cardiologist not involved in the study (Table 1). Importantly, no patients in this cohort had either a cerebral venous sinus thrombosis or splanchnic vein thrombosis – two unusual sites for thrombosis associated with VITT in published literature [5].
Among our cohort subjected to intense chart review, 80 patients had documented thrombosis (defined as a DVT of any temporality, PE, MI, and/or stroke/TIA) and 2 had a positive anti-PF4 antibody – each fulfilling the other criteria for the triad of VITT described in the introduction. However, among individuals with documented acute thrombosis, anti-platelet antibodies were not associated with thrombosis. Only 2 individuals in this subset of patients with thrombosis received an adenovirus-based vaccine (which has previously been associated with thrombosis); neither of these patients had a positive PF4 antibody, and both had recent surgery. The remainder of these patients received an mRNA-based vaccine. The plurality of thrombocytopenia in our sample was associated with concomitant chemotherapy (48.4%). In this analysis, no high-risk factors were significant.
Finally, we sought to determine if any predictors were associated with a composite outcome using all thrombotic events (acute and chronic DVTs, PEs, MIs, and stroke/TIAs). Table 2 summarizes high-risk predictors by whether a composite event occurred. With the definition of composite using all events, there were no significant differences between groups. Nearly 83% of patients had a single event, while 16% had 2 events, and 1% had 3 events. By sensitivity analysis, the same test was run with only acute thrombotic events, explicitly excluding chronic thrombotic events (see Table S1 and S2). No risk factors were significant in either analysis.
Discussion
In this large, multi-state, retrospective analysis of a prospective, observational registry, we evaluated the incidence of VITT in 223,345 patients (97.2% of whom received the mRNA-1273 [Moderna] or BNT162b2 [Pfizer-BioNTech] vaccines). We identified 663 subjects with a decrease in platelets greater than or equal to 50% from baseline within 28 days after their second vaccine dose and ascertained if there was a thrombotic event through detailed chart review. Of 74 patients with acute thrombosis, 72 (97.3%) demonstrated clear etiologies. Many cases of thrombocytopenia in the month following COVID-19 vaccination were associated with a concurrent chemotherapy regimen. Thirty patients (40.5%) had active malignancies, of which 24 were being treated with chemotherapy associated with increased thrombosis risk. 7 patients (9.5%) were considered hypercoagulable, 6 (8.1%) had catheter-related thrombosis, 6 (8.1%) had recent surgery, 5 (6.8%) had reduced mobility, 13 (17.6%) had cardiovascular risk factors, 3 (3.9%) had diagnosed/suspected immune thrombocytopenia, and 2 (2.6%) were septic. Two patients had thrombosis with unclear risk factors (unprovoked).
Of the two patients with unprovoked thrombosis, only one had findings concerning for VITT. This patient received the BNT162b2 vaccine one day prior to presenting with a pulmonary embolism. At that time, they had positive screening tests for HIT, a negative serotonin release assay, and a positive antiphospholipid serology. At four months follow up, they no longer had a positive serology.
Limitations of our study arise from its observational nature. Unfortunately, only 16 of 74 patients with both a significant drop in platelet count and a thrombotic event were subjected to a PF4 ELISA assay. As such, it is certainly possible that VITT played a role in some of the thrombotic events and was simply not diagnosed due to lack of a PF4 test. Further, due to our single indetermine case of VITT, it is difficult to provide an accurate or precise estimate of incidence in our cohort. Though VITT may occur in the setting of an mRNA-based vaccine, this is an exquisitely rare phenomenon. In this study, we detected a single case that was concerning for VITT among 223,345 patients (97.2% of whom received the mRNA-based mRNA-1273 [Moderna] or BNT162b2 [Pfizer-BioNTech] vaccines). The overwhelming majority of patients with a thrombotic event in the 28 days following vaccination demonstrated clear etiologies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The two lead authors would like to thank Cleveland Clinic Lerner College of Medicine of Case Western Reserve University for sponsorship to conduct this study and present at an international conference.
Funding
The following grants supported this study: R01HL158801 and LRPHL120200 (to SJC).
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to the inability to adequately blind data but are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
None of the authors have financial or personal conflicts to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
William M. Patterson and Brady D. Greene are co-first authors.
References
- 1.Solís Arce JS, Warren SS, Meriggi NF, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27:1385–1394. doi: 10.1038/s41591-021-01454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly M, Jones A, Robinson E. Public Trust and willingness to vaccinate against COVID-19 in the US from October 14, 2020, to March 29, 2021. JAMA. 2021;325:2397–2399. doi: 10.1001/jama.2021.8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Nishikawa M, Kanno H, et al. Long-term effects of Pfizer-BioNTech COVID-19 vaccinations on platelets. Cytometry A. 2022 doi: 10.1002/cyto.a.24677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Hundelshausen P, Lorenz R, Siess W, Weber C. Vaccine-induced immune thrombotic thrombocytopenia (VITT): targeting pathomechanisms with bruton tyrosine kinase inhibitors. Thromb Haemost. 2021;121:1395–1399. doi: 10.1055/a-1481-3039. [DOI] [PubMed] [Google Scholar]
- 5.Tefera L, Cameron SJ. SVM Communications: vaccine-induced immune thrombotic thrombocytopenia (VITT) – what the vascular medicine physician should know. Vasc Med. 2021;26:579–581. doi: 10.1177/1358863X211030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325:2448. doi: 10.1001/jama.2021.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenblum HG, Gee J, Liu R, et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the vaccine adverse event reporting system and v-safe. Lancet Infect Dis. 2022;22:802–812. doi: 10.1016/S1473-3099(22)00054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to the inability to adequately blind data but are available from the corresponding author upon reasonable request.