Abstract
Nepenthespudica, a new species from North Kalimantan, Indonesia, is described and illustrated. The species belongs to the N.hirsuta group (sensu Cheek and Jebb 1999) but exhibits some characters that are unique within the group or even within the genus. Above all, it produces underground, achlorophyllous shoots with well-developed, ventricose lower pitchers that form in soil cavities or directly in the soil. No lower pitchers are formed above ground. The main part of its prey are ants, besides other litter- and soil-inhabiting species of invertebrates. A number of infaunal species were found in both aerial and underground pitchers, mainly Diptera and nematodes. Nepenthespudica is known only from a few neighbouring localities in the Mentarang Hulu district of North Kalimantan, where it grows on ridgetops at an elevation of 1100–1300 m. Its discovery underlines the natural richness of Borneo’s rainforest and the necessity to preserve this important ecosystem with its enormous and still undiscovered biodiversity.
Keywords: Borneo, carnivorous plant, Caryophyllales, Mentarang Hulu, prey composition, taxonomy, underground trap
Introduction
Nepenthes L. is a genus of more than 160 species (Golos et al. 2020) primarily distributed in tropical and subtropical Southeast Asia, with centres of diversity in Borneo, Sumatra, and the Philippines. A small number of species occur in outlying areas, including Madagascar, Seychelles, Sri Lanka, northeastern India, southern China, northeastern Australia, and various islands of the western Pacific Ocean (McPherson et al. 2009). The Nepenthes flora of Borneo, with around 40 recognised species, is one of the most species-rich of all. Although the island is still partially covered with extensive primary forest, its area has been rapidly decreasing in recent decades (Miettinen et al. 2011). Commercial logging and subsequent land conversion (mostly for oil palm plantations) drastically reduced the area of pristine old-growth forest from 55.8 Mha in 1973 to 20.6 Mha in 2015 (Gaveau et al. 2016), making the Borneo rainforest one of the most rapidly vanishing ecosystems in the world. The island is botanically relatively well explored in the northern part, i.e. Malaysian Borneo (Sarawak and Sabah) and Brunei, where only remnants of untouched rainforest exist, usually protected as national parks and reserves. In contrast, Indonesian Borneo (Kalimantan) is one of the world’s least explored and most threatened biodiversity hotspots, still with vast areas of relatively intact forest (Raes et al. 2009). However, besides the expansion of oil palm plantations, the announced establishment of the new capital of Indonesia, Nusantara, in East Kalimantan might have a serious impact on the vulnerable biota of Borneo (e.g. Teo et al. 2020). The Nepenthes flora of Kalimantan is poorly known compared to that of Malaysian and Bruneian Borneo, with relatively few modern records. Thus, the new discoveries that have emerged recently after expeditions to certain remote areas of Kalimantan (Robinson et al. 2019; Golos et al. 2020) are not surprising.
Here we describe a new species of Nepenthes from lower montane rainforest in North Kalimantan, Indonesia, which produces well-developed, fully functional and effective underground traps – a strategy as yet unknown in any species of carnivorous plant with pitfall traps. While the majority of carnivorous plants produce their traps above ground or in water, underground traps have up till now been recorded only in the genera Genlisea Benth. & Hook.f., Philcoxia P.Taylor & V.C.Souza and Utricularia L. These genera use three different trapping mechanisms. While Utricularia employs actively working sucking utricles (i.e. Poppinga et al. 2016), Genlisea employs passive ‘lobster-pot’ type traps (Taylor 1991; Płachno et al. 2008). The adhesive leaves of Philcoxia are shallowly buried in sand to receive just enough light to maintain their photosynthetic ability (Pereira et al. 2012). On the other hand, pitfall traps (i.e. traps that rely on gravity) produced from wholly subterranean shoots that have evolved specifically to function underground have not been recorded in carnivorous plants so far (see, e.g. Darnowski et al. 2018).
Materials and methods
This study is based on plants found in February 2012 in the Mentarang Hulu district of North Kalimantan province, Indonesia. A total of 17 plants were examined across five different sites. Plants were photographed, sampled and subsequently thoroughly compared with original drawings and descriptions given in protologues of morphologically allied Nepenthes species. Specimens of the Nepentheshirsuta group were examined in the herbaria BO, K and L (see Suppl. material 1) and the type material was deposited in BO (herbarium codes according to Thiers 2022).
For scanning electron microscopy (SEM), the representative trap parts were fixed in ethanol and later dehydrated and subjected to critical-point drying using liquid CO2. They were then sputter-coated with gold and examined at an accelerating voltage of 20 kV using a Hitachi S-4700 SEM (Hitachi, Tokyo, Japan), which is housed in the Institute of Geological Sciences, Jagiellonian University in Kraków.
Material for prey investigation was sampled from both underground (tree-root cavities) and aboveground pitchers. The entire contents of five lower pitchers and one aerial rosette pitcher was poured out through a 25 μm sieve, immediately fixed in 4% formaldehyde at circa 80 °C, and stored for 14–21 days, before insects and acarids including also larvae were separated and fixed again. The fine content including nematodes, annelids and organic detritus was transferred into glycerine according to De Grisse (1969) and finally mounted onto wax-glycerine slides and examined. Fixed specimens were identified under a light microscope and documented. All individuals that showed signs of digestion were considered prey. Individuals without signs of digestion were identified and assessed as either prey or infauna based on their biology and present life stages (e.g. larvae were mostly considered infauna). All insect and mite preparations are deposited at the Department of Entomology of Moravian Museum Brno. All the nematodes are deposited in the Department of Forest Protection and Wildlife Management in Brno. Permanent slides of Pristinaarmata (Naididae) are deposited at the National Museum in Prague, Czech Republic (Schenková and Čermák 2013).
Taxonomic treatment
. Nepenthes pudica
Dančák & Majeský sp. nov.
FC85A024-B0C6-5506-A71B-081479ADBEC1
urn:lsid:ipni.org:names:77300236-1
Figure 1.
NepenthespudicaA juvenile rosette pitcher B upper pitchers (4 on the right; each from a different plant), intermediate pitcher (1 on the left) C habitat with mature plant D habitat with lower pitchers excavated from the soil. Photographs by M. Dančák.
Figure 2.
NepenthespudicaA detail of lower pitchers excavated from the soil B lower pitchers in a cavity under tree roots–note greening of phyllodia formed in presence of low light C lower pitchers revealed under a moss mat D lower pitchers extracted from a cavity–note achlorophyllous shoot and reduced phyllodia formed in total darkness. Photographs by M. Dančák.
Figure 3.
NepenthespudicaA male flowers B male plant with inflorescence C infructescence D female plant with infructescence. Photographs by M. Dančák.
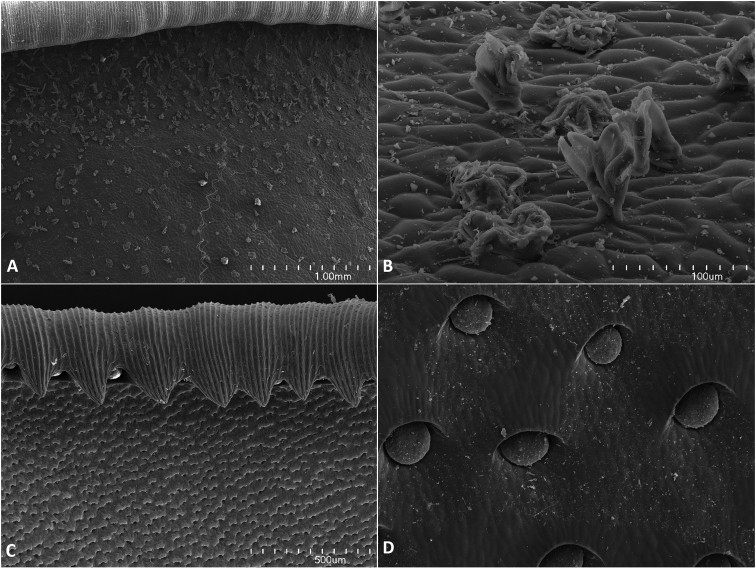
Figure 4.
Nepenthespudica, SEM images of lower pitcher A outer wall with outer margin of peristome B detail of trichome on the outer wall C inner wall and inner margin of peristome showing eglandular zone covered with lunate cells and peristome teeth with peristomal glands D inner wall showing glandular zone with digestive glands. SEMs by B.J. Płachno.
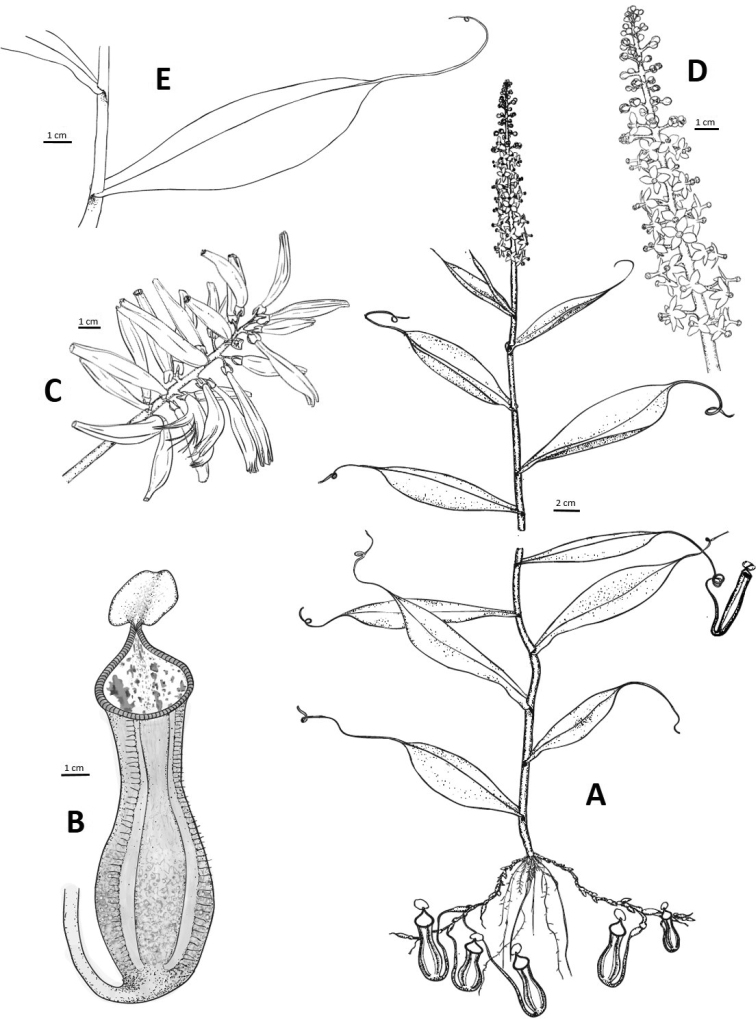
Figure 5.
NepenthespudicaA habit B lower pitcher C infructescence D male inflorescence E detail of climbing stem with a leaf. Drawn by Kateřina Janošíková.
Diagnosis.
Nepenthespudica differs from N.hispida Beck in producing short basal underground (vs. aboveground) shoots; ± glabrous (vs. hairy) stems; petiolate (vs. sessile) climbing shoot leaves with auriculate, shortly decurrent (vs. decurrent-amplexicaul) bases; rare (vs. common) upper pitchers; red (vs. green or red blotched) lower pitchers; ± glabrous (vs. hairy) mature pitchers; ventricose (vs. ovoid-ellipsoid) lower pitchers; infundibular (vs. subcylindrical, tapering) upper half of the lower pitcher; 3–5.5 cm (vs. 1.5–3 cm) wide lower pitchers; male flowers in pairs (vs. single or rarely in pairs) and androphore c. 4 mm (vs. 1.5–2 mm) long.
Type.
Indonesia. North Kalimantan: Malinau Regency, c. 1110 m a.s.l., 2 February 2012, W. Tjiasmanto, M. Paris & M. Dančák s.n. (BO, holotype BO1985840, isotype BO1985839).
Description.
Terrestrial climber producing climbing shoot and underground basal shoots. Climbing shoots up to c. 20 m long, stem glabrous, c. 4–6 mm thick, internodes c. 4 cm long. Underground basal shoots short, with reduced, partially or completely achlorophyllous leaves (nanophylls) bearing well-developed lower pitchers, not observed to branch or develop roots. Rosette leaves chartaceous, subsessile to shortly petiolate, oblanceolate, up to 16 cm long, up to 4 cm wide, apex subobtuse or acute to acuminate, base auriculate, shortly decurrent, glabrous on both sides but densely hairy with short brown hairs on the margins, tendril up to 16 cm long, uncoiled. Leaves of climbing shoots coriaceous, shortly petiolate, oblanceolate, up to 20 cm long, up to 4.5 cm wide, with 2–4 inconspicuous longitudinal veins on each side of the midrib, apex acute, base auriculate, shortly decurrent, glabrous on both sides, margins glabrous, tendril coiling. Rosette pitchers produced only briefly on aboveground rosettes, up to 9 cm high, up to 3 cm wide, thin-chartaceous, subcylindrical to ovoid in the lower part. Lower pitchers produced exclusivel on underground basal shoots, 7–11 cm high, 3–5.5 cm wide, thin-coriaceous, becoming thicker-walled and markedly sturdier when produced at depth, arising abruptly from the uncoiled tendril, ventricose, broadly ovoid to globose in the lower half, infundibular above, clearly widening towards the mouth; eglandular zone of the inner surfaces extending from the mouth almost to the middle of the pitcher; inner surface near the mouth white, conspicuously red blotched, outer surface red-purple, faintly blotched, occasionally entirely off-white when produced at depth; two fringed wings running from the bottom of the pitcher to the mouth at the front; mouth round, rising at the rear into a short neck; peristome cylindrical in section, up to 2 mm wide, inner surface with distinct teeth up to 0.8 mm long, ribs up to 0.5 mm apart, up to 0.2 mm wide; lid broadly ovate, c. 20–30 mm long, c. 20 mm wide, with short spur; large, craterlike nectar glands ± elliptic in outline, up to 0.35 mm long, scattered densely in the middle of the lower surface. Upper pitchers rarely produced, up to 9 cm high, up to 2 cm wide, thin-coriaceous, arising gradually from the tendril, narrowly infundibular at the base, subcylindrical above; eglandular zone of the inner surfaces covering upper 1/3 of the pitcher; outer surface green, inner surface near the mouth yellowish; two fringed wings running from the middle of the pitcher to the mouth at the front; mouth round, with or without very short neck; peristome cylindrical, up to 1.5 mm wide, inner surface with very short teeth, ribs up to 0.25 mm apart, c. 0.1 mm wide; lid broadly ovate, 11–16 mm long, 9–13 mm wide, with curved spur; craterlike nectar glands as in lower pitchers, up to 0.3 mm long. Male inflorescence a raceme, peduncle c. 14 cm, rachis c. 13 cm, partial peduncles 2-flowered, bracts absent, pedicels 4–7 mm long, tepals elliptic, up to 6 by 3 mm; androphore c. 4 mm long, anther head 2.5 by 1.5 mm. Female inflorescence unknown. Infructescence racemose. Fruit a fusiform capsule, reddish brown at maturity, conspicuously glossy, valves of fruits c. 45 by 4 mm. Seeds 20–25 mm long.
Habitat and ecology.
The species occurs on ridgetops over sandstone rocks in lower montane rainforest. The known elevational range is 1100–1300 m a.s.l. The plants frequently grow near trees whose branched roots form cavities covered with a moss layer. Lower pitchers are then copiously produced inside these cavities. If no cavities are available, the pitchers are produced directly in soil, deep litter or under moss cushions. At some sites, Nepenthestentaculata Hook.f. and N.stenophylla Mast. grew sympatrically with N.pudica, while a species from the N.fusca species complex was spotted growing epiphytically in at least one locality.
The subterranean growth habit of Nepenthespudica was consistently observed across the five studied sites but was not shared by the sympatric Nepenthes species, demonstrating that it was not simply the result of unusual local conditions. The underground shoots of N.pudica had no obstacles preventing them from growing upwards, suggesting that they are not negatively gravitropic as is typical of stems. Neither did they show signs of growing towards light, even when concealed only under a soft moss cushion or already slightly chlorophyllous (Fig. 2B). Based on this and their generally lateral character, it might be supposed that they are negatively phototropic rather than positively gravitropic.
Distribution.
The species is known only from a few adjoining localities in the western part of the Mentarang Hulu district of North Kalimantan, Indonesia. The exact locations have been withheld in order to prevent poaching by unscrupulous commercial collectors.
Etymology.
The specific epithet pudica (bashful, shy), is a feminine adjective and alludes to the fact that lower pitchers remain concealed from direct view.
Conservation status.
Nepenthespudica is endemic to Borneo. It is known from five closely situated sites, which represent a single location (IUCN 2022). Both the extent of occurrence (EOO) and minimal area of occupancy (AOO) of N.pudica are estimated to be less than 4 km2. There is uncertainty as to whether the species occurs within Kayan Mentarang National Park, as its borders were not marked in the field at the time of discovery. However, the available maps suggest all the sites are actually located outside the national park, thus legally unprotected. Due to its restricted distribution, small population size and possible habitat loss, the species qualifies to be assigned preliminary conservation status as critically endangered (CR), based on criteria B1 ab(iii) and D of the IUCN Red List categories and criteria (IUCN 2012).
Prey composition and infauna.
We found 1785 invertebrate individuals belonging to 40 different taxa (Tables 2, 3) in suspensions sampled from five underground pitchers (found in a tree-root cavity) and one aerial rosette pitcher (growing 2 metres above the soil surface and arising from an offshoot of a fallen climbing stem). Necromass of the prey consisted of sclerites of highly digested invertebrates. It contained mainly litter- and soil-inhabiting species as well as a large amount of plant detritus. Among soil- and litter-inhabiting species we observed mites (mostly from the family Oribatidae), leaf litter–inhabiting beetles (families Scydmaenidae, Pselaphidae, Liodidae, Carabidae) and a single ant of the genus Anochetus (subfamily Ponerinae). These taxa are mostly mycophagous, detritophagous, or predators. However, the main and the essential prey component was a species of ant from the subfamily Myrmicinae, probably a species of the genus Crematogaster, which is closely associated with Nepenthes (Bonhomme et al. 2011). A number of individuals of an ant from the genus Polyrhachis were found in the aboveground rosette pitcher in contrast with their rare occurrence in underground traps.
Table 2.
Prey composition of Nepenthespudica based on analysis of five underground pitchers and one aerial pitcher.
| Prey composition in traps | traps from root cavity | abovegr. | total | ||||
|---|---|---|---|---|---|---|---|
| trap 1 | trap 2 | trap 3 | trap 4 | trap 5 | trap 6 | ||
| Acarina, Oribatidae spp. | 1 | 3 | 14 | c. 100 | 20 | c. 138 | |
| Acarina div. | 1 | 1 | 25 | 27 | |||
| Araneae, cf. Lycosidae | 1 | 1 | |||||
| Araneae: cf. Dysderidae | 1 | 1 | |||||
| Araneae | 1 | 1 | |||||
| Arachnoidea, g. sp. | 1 | 1 | 2 | ||||
| Coleoptera, Aphodiidae g. sp. | 1 | 1 | |||||
| Coleoptera, Carabidae g. sp. | 2 | 2 | |||||
| Coleoptera, cf. Leiodidae | 6 | 6 | |||||
| Coleoptera, Pselaphidae g. sp. | 1 | 1 | |||||
| Coleoptera, Scydmaenidae g. sp. | 2 | 7 | 4 | 2 | 15 | ||
| Coleoptera, g. sp. 1 | 3 | 3 | |||||
| Coleoptera, g. sp. 2 | 2 | 2 | |||||
| Diptera, Phoridae g. sp. | 1 | 1 | |||||
| Diptera, Nematocera g. sp. | 2 | 3 | 5 | ||||
| Diptera, g. sp. | 1 | 1 | |||||
| Hemiptera, Derbidae g. sp. | 1 | 1 | |||||
| Hymenoptera, Chalcidoidea g. sp. | 1 | 1 | |||||
| Hymenoptera, Formicinae: Camponotuscf.gigas | 4 | 4 | |||||
| Hymenoptera, Formicinae: Polyrhachis sp. | 3 | 1 | 1 | 17 | 22 | ||
| Hymenoptera, Formicinae g. sp. | 3 | 1 | 4 | ||||
| Hymenoptera, Myrmicinae g. sp. 1 | c. 500 | 11 | c. 100 | c. 50 | c. 700 | c. 1361 | |
| Hymenoptera, Myrmicinae g. sp. 2 | 1 | 1 | 25 | 27 | |||
| Hymenoptera, Ponerinae: Anochetus sp. | 1 | 1 | |||||
| Hymenoptera, Sphecidae g. sp. | 2 | 1 | 3 | ||||
| Sum of individuals | c. 509 | 31 | c. 151 | c. 164 | c. 754 | 22 | c. 1631 |
| Sum of taxa | 8 | 10 | 11 | 7 | 8 | 3 | 25 |
Table 3.
Infauna composition of Nepenthespudica based on analysis of five underground pitchers and one aerial pitcher. (abovegr. = aerial pitcher; L1, L2, L3, L4 – larval stages).
| Infauna composition in traps | traps from root cavity | abovegr. | total | ||||
|---|---|---|---|---|---|---|---|
| trap 1 | trap 2 | trap 3 | trap 4 | trap 5 | trap 6 | ||
| Diptera, Stratiomyidae (larvae) | 1 | 6 | 7 | ||||
| Diptera, Culicidae: Uranotaenia sp. 1 | 2 L1,1 L3,4 L4 | 4 L3,11 L4 | 22 | ||||
| Diptera, Culicidae: Uranotaenia sp. 2 | 9 L3 | 5 L2,1 L3,2 L4 | 1 L1,3 L2,7 L4 | 28 | |||
| Diptera, Culicidae: Culex sp. | 4 L4 | 4 L2,1 L4 | 3 L4 | 1 L4 | 13 | ||
| Diptera, Acalyptrata | 2 L2 | 1 L1, 4 L2 | 8 L1 | 15 | |||
| Annelida, Naididae: Pristinaarmata | 6 | 6 | |||||
| Nematoda, Cephalobidae: Heterocephalobus sp. | 8 | 8 | |||||
| Nematoda, Aphelenchida: Aphelenchoides sp. 1 | 1 | 1 | |||||
| Nematoda, Aphelenchida: Aphelenchoides sp. 2 | 1 | 1 | |||||
| Nematoda, Panagrolaimidae: Propanagrolaimus sp. | 8 | 8 | |||||
| Nematoda, Wilsonematinae: Ereptonema sp. | 1 | 1 | |||||
| Nematoda, Plectidae: Plectus sp. | 1 | 1 | |||||
| Nematoda, Diplogasteridae: Pristionchus sp. | 27 | 27 | |||||
| Nematoda, Rhabditidae (dauer larvae) | 16 | 16 | |||||
| Sum of individuals | 20 | 14 | 13 | 30 | 26 | 51 | 154 |
| Sum of taxa | 4 | 2 | 3 | 8 | 3 | 3 | 14 |
Surprisingly, we found relatively numerous infauna, especially larvae of mosquitoes, nematodes and annelids in both aboveground and underground pitchers (Table 3). We identified three species of mosquitoes from two genera, Uranotaenia and Culex. Identified nematodes belong to seven families: Aphelenchoididae, Cephalobidae, Diplogastridae, Panagrolaimidae, Plectidae, Rhabditidae (dauer larvae) and Wilsonematidae. The most abundant were members of families Rhabditidae and Diplogastridae detected in the aboveground trap, which were previously recorded from the pitcher fluid of Nepenthesmirabilis (Lour.) Druce (Bert et al. 2011). In underground traps, nematodes were rare and in different compositions compared to the aboveground trap. The most abundant were members of the families Cephalobidae (Heterocephalobus) and Panagrolaimidae (Panagrolaimus). One of the most interesting inquilines found in the underground pitchers was a new species of annelid worm, Pristinaarmata (family Naididae), described previously by Schenková and Čermák (2013).
Selected specimens examined.
See Suppl. material 1.
Discussion
Nepenthespudica is the first carnivorous species confirmed to use pitfall traps specifically in the subterranean environment. It produces almost exclusively underground pitchers that are well developed and fully functional. Although in some species of Nepenthes pitchers are occasionally reported to develop in plant litter or directly in the soil (Salmon 1993; Nerz et al. 1998; Clarke 2001; Ghazalli et al. 2020), no species that specifically targets this environment to this extent has been documented to date. This is not surprising, as pitchers are generally much larger than other types of traps and are rather fragile due to their hollow character. Therefore, they are generally unsuitable for the soil environment, where considerable pressure is needed to form a cavity. As the pitchers of N.pudica are of a typical size for the genus, they are by far the biggest underground traps among all known carnivorous plants. While the other genera of carnivorous plants that produce underground traps are, due to the small size of their traps, capable of catching only microscopic or very small prey (Seine et al. 2002; Pereira et al. 2012), the pitchers of N.pudica catch prey of the same size as other pitcher plants.
The traps of carnivorous plants are complex and metabolically costly organs that must be produced at the expense of tissues optimised for photosynthesis (Givnish et al. 1984; Pavlovič and Saganová 2015). In pitcher plants, this trade-off often manifests in the separation of primary prey- and light-harvesting structures spatially–e.g. on an intra-leaf level as in most Nepenthes—and also temporally, as in the seasonal production of solely photosynthetic leaves by Cephalotus Labill. and some Sarracenia L., both examples of separation on an inter-leaf but intra-shoot level (McPherson and Schnell 2011; Cross et al. 2019). In N.pudica, this ‘division of labour’ is unusually displayed at the level of the shoots. This strategy is analogous to that of certain strongly shoot-dimorphic aquatic Utricularia, such as U.intermedia Hayne, whose specialised carnivorous shoots penetrate a loose organic sediment while the green stems seek sunlight in clear water near the surface (Adamec 2007).
Each leaf of a typical Nepenthes comprises an entirely photosynthetic lamina-like phyllodium and a predominantly carnivorous and only marginally photosynthetic pitcher (Pavlovič et al. 2007, 2009; Karagatzides and Ellison 2009). The unusual architecture of N.pudica (Fig. 5A) appears to have largely freed it from the phylogenetic constraint of having functional phyllodia and pitchers in close physical proximity, and thereby allowed it to exploit a novel source of prey in the form of the subterranean environment, limiting competition with sympatric congeners. However, this body plan is likely to come with certain costs. Subterranean pitchers, by virtue of having to displace surrounding substrate as they grow, might be expected to have significantly thicker walls and a higher concentration of structural compounds (e.g. lignin) than those produced above ground. Preliminary observations indicate that underground pitchers are indeed markedly thicker-walled and sturdier (M. Dančák & M. Golos, pers. observ.). All else being equal, this would increase their construction costs, partly offsetting benefits from carnivory, and likely dictate longer pitcher lifespans, reflecting a greater ‘payback time’ for recovery of these costs (see Osunkoya et al. 2008). And this does not even consider the additional stem biomass needed for dimorphic shoots. Moreover, the greater separation of the two types of assimilatory organs in N.pudica must presumably necessitate two-way exchange of nutrients and photosynthates over much greater mean distances than in species with typical pitcher–phyllodium pairs (see Osunkoya et al. 2007). All told, the benefit from subterranean carnivory must be significant to make up for these additional costs and this is perhaps the reason this strategy is not seen more widely across the genus.
Among Nepenthes, the species that come closest to this degree of shoot specialisation are perhaps those in which pitchers produced in low-light conditions near ground level are borne on crowded, greatly reduced phyllodia (the latter sometimes termed ‘nanophylls’; Cheek 2015). The best known of these, N.ampullaria Jack, additionally produces largely or entirely pitcherless climbing stems (Tan and Wong 1996), mirroring the situation in N.pudica, though the latter’s production of solely carnivorous shoots appears to be unique among Nepenthes and indeed among all pitcher plants. Also of note is the comparatively little-known N.rhombicaulis Sh.Kurata of Sumatra, which rarely if ever produces upper pitchers and has been speculated to target underground prey, though until now its lower pitchers have only been documented to develop within dense moss and detritus rather than being truly subterranean (Salmon 1993; Schmid-Hollinger 1994; Clarke 1997a, 2001). This species, which appears to occupy a similar ecological niche to members of the N.hirsuta group in Borneo, would be a prime candidate for further investigation in this regard.
Since the discovery of Nepenthespudica, field observations in the Berau region of East Kalimantan (M. Golos, pers. observ. June 2019) have revealed a similar taxon that likewise produces achlorophyllous subterranean shoots bearing nanophylls with reddish pitchers (Fig. 6). This taxon also produces few aerial traps, though it notably differs from the type population of N.pudica in growing at considerably lower elevations. Its precise taxonomic affinities have yet to be determined.
Figure 6.
Nepenthes sp. with excavated underground traps (bottom left) from a locality in the Berau region of East Kalimantan. Photograph by M.R. Golos.
As was demonstrated above, the prey of Nepenthespudica consists of various species of soil- and litter-inhabiting fauna. With 25 different taxa, the diversity of identified prey was rather high, which is typical for species growing at higher elevations (Adam 1997). However, ants were the main prey component found in both aerial (subfamily Formicinae) and lower pitchers (subfamily Myrmicinae). At this point, we can assume that N.pudica is predominantly an ant specialist, as are the majority of Nepenthes species.
Consistently with other Nepenthes species, N.pudica harbours relatively numerous and diverse infauna in both types of pitchers (154 individuals and 14 identified taxa). Besides mosquitoes, which are commonly associated with pitcher plants (Vong et al. 2021), larvae of aquatic Diptera (family Stratiomyidae and subsection Acalyptrata) were detected as well. The insect-trapping structures of pitcher plants (especially Nepenthaceae and Sarraceniaceae) frequently harbour dipteran larvae, which utilize the food niche in pitchers (Adlassnig et al. 2011). Members of the family Stratiomyidae are true aquatic organisms inhabiting many kinds of phytotelmata such as tree holes, leaf axils and modified leaves (Greeney 2000); however, their presence in pitchers is not as common in comparison with members of other dipteran families such as Syrphidae, Ceratopogonidae or Chiromidae (Kitching 2000). Rather surprising is the fact that lower pitchers of N.pudica also contained abundant dipteran infauna, including mosquitoes. This indicates that the tree-root cavities from which samples were taken were accessible to the outside-living invertebrates. Therefore, even the hidden lower pitchers can serve as a stable and permanent water habitat (phytotelma) similar to other Nepenthes species or other plants, e.g. unrelated Bromeliaceae (Thorp and Rogers 2015), and play an essential role in the development of these symbionts, especially during dry periods. However, underground pitchers produced in compacted substrate (Fig. 2A) would presumably not be similarly accessible to ovipositing insects.
Nematodes formed the other large group of infauna. Identified individuals belonged to families Aphelenchoididae, Cephalobidae, Diplogastridae, Panagrolaimidae, Plectidae, Rhabditidae (dauer larvae) and Wilsonematidae. The most abundant were members of the genus Pristionchus (Diplogastridae), detected only in the aboveground trap and obviously associated with the main prey, an ant species of the genus Polyrhachis. Species of the genus Pristionchus feed selectively on bacteria and fungi decomposing insect carcasses (Rae et al. 2008), including various genera of ants, e.g. Formica, Lasius and Myrmica (Wahab 1962; Ishaq et al. 2021). The nematodes detected in lower pitchers were members of genera generally living in soil and water environments and feeding on bacteria and fungi decomposing organic material (Bongers 1990). The only exception was the genus Halicephalobus, the species of which are aquatic but occur in extreme environments (Borgonie et al. 2011; Geraert et al. 1988), various phytotelmata (Andrassy 1952; Körner 1954) or as parasites (Stefanski 1954).
Probably the most interesting species living in the pitchers of Nepenthespudica was the annelid worm Pristinaarmata (Naididae), which was described from and found so far only in its lower pitchers. For the description and discussion on its relation to N.pudica, see Schenková and Čermák (2013).
The living strategy of Nepenthespudica can be viewed as an advantageous evolutionary adaptation. As carnivorous plants are highly dependent on prey for organic nutrients essential for reproductive success (Zamora et al. 1997), strong selective pressures may have acted on traits related to prey capture (Ellison et al. 2001). Hence, the potentially strong competition for prey and possible environmental limitations in the forest understorey (e.g. dryness affecting ridgetops) might be avoided by moving the traps underground.
Nepenthespudica belongs to the N.hirsuta group, which is endemic to Borneo and includes at least two putative close relatives: N.hirsuta Hook.f. and N.hispida. Another two species are sometimes considered members of this group, namely the Bornean N.macrovulgaris J.R.Turnbull & A.T.Middleton and N.philippinensis Macfarl. from the island of Palawan (Cheek and Jebb 1999). However, the recent phylogeny of the genus (Murphy et al. 2020), while proving the close relationships of N.hirsuta and N.hispida, does not support the close affinities of N.macrovulgaris and N.philippinensis, either mutually or to N.hirsuta and N.hispida. Nepentheshirsuta and N.hispida share a combination of traits that distinguishes this group from the rest of the genus. These are especially the growth form (well-developed rosetted, non-climbing phase), hairy stem, more or less ovoid shape of the lower pitchers, oblique pitcher mouth, ± cylindrical peristome, lid without appendages and flowers usually in pairs (Cheek and Jebb 1999). Nepenthespudica, while possessing most of these characteristics, shows several unique traits. These are namely a) underground basal shoots (the other species form aboveground basal shoots); b) upper pitchers are only rarely produced in lower parts of the climbing stem; c) lower pitchers are produced exclusively underground; d) the shape of the lower pitchers is ventricose with the lower half ovoid to globose and the upper half infundibular. Another possible member of the N.hirsuta group, Nepenthesleptochila Danser, was described from northern North Kalimantan (Mt. Djempanga; Danser 1928), but this name is usually considered a heterotypic synonym of N.hirsuta (Clarke 1997b; Jebb and Cheek 1997; Cheek and Jebb 2001; Phillipps et al. 2008; McPherson et al. 2009). Nevertheless, the original description mentions several significant differences compared to N.hirsuta (e.g. a well-developed eglandular zone inside the pitchers, glabrous stems and pitchers, and much smaller pitchers) so its identity is at least questionable. Nepenthesleptochila also bears considerable resemblance to N.pudica, especially in being rather glabrous. However, the two taxa differ in all the four previously mentioned characters typical for N.pudica and therefore we do not consider them conspecific. For a comparison of critical diagnostic characters of N.hirsuta (excluding N.leptochila), N.hispida, N.leptochila and N.pudica, see Table 1.
Table 1.
Main morphological differences between Nepenthespudica and related species, including N.leptochila, which is not recognised by most researchers. The characters that best differentiate N.pudica from the other species are in bold.
| Characteristic | N.hirsuta | N.hispida | N.pudica | N.leptochila |
|---|---|---|---|---|
| short basal shoots | aboveground | aboveground | underground | aboveground |
| stem indumentum | hairy | hairy | ±glabrous | ±glabrous |
| stem colour | brown | purplish grey | brownish green to purplish | reddish |
| climbing shoot leaf shape | obovate | oblanceolate to oblong | oblanceolate | obovate-lanceolate |
| climbing shoot leaf width | 3–6 cm | 1.8–3.3 | up to 4.5 cm | 2.5–5.5 cm |
| climbing shoot leaf | petiolate | sessile | petiolate | shortly petiolate |
| climbing shoot leaf base | semi-amplexicaul | decurrent-amplexicaul | auriculate, shortly decurrent | auriculate, hardly decurrent |
| climbing shoot leaf texture | thin-coriaceous | thin-coriaceous | coriaceous | chartaceous |
| climbing shoot leaf apex | acute or rounded | acuminate to obtuse | acute | acute, obtuse or rounded |
| longitudinal veins | 3–4 | 3 | 2–4 not prominent | 5 |
| tendril indumentum | hairy | hairy | hairy or glabrous | glabrous? |
| upper pitchers | few | common | rare | present |
| lower pitcher colour | green | green or red blotched | red | ? |
| adult pitcher indumentum | hairy | hairy | ±glabrous | glabrous? |
| lower pitcher shape | ovoid | ovoid-ellipsoid | ventricose | ovoid-ellipsoid |
| lower half of lower pitcher | ovoid | ovoid-ellipsoid | ovoid to globose | ovoid to globose |
| upper half of lower pitcher | conical | subcylindrical, tapering | infundibular | ~cylindrical, tapering |
| lower pitcher length | up to 15 cm | 5–8.5 cm | 7–11 cm | up to 8 cm |
| lower pitcher width | up to 7 cm | 1.5–3 cm | 3–5.5 cm | up to 3 cm |
| eglandular zone | almost absent | nearly 1/2 of the surface | nearly 1/2 of the surface | 1/3 of the surface |
| peristome width | up to 6 mm | 0.5–1.2 mm | up to 2 mm | up to 1.5 mm |
| peristome in section | cylindrical or flattened | cylindrical | cylindrical | cylindrical or flattened |
| male flowers | in pairs | single or rarely in pairs | in pairs | ? |
| androphore length | 3.5–6 mm | 1.5–2 mm | ~4 mm | ? |
| ecology | ridgetops | heath forest | ridgetops | ? |
| elevational distribution | 0–1000 m | 100–800 m | 1100–1300 m | ~300 m |
Supplementary Material
Acknowledgements
We are grateful to Jiří Schlaghamerský and Jana Schenková (Annelida), Igor Malenovský and Pavel Lauterer (Insecta), Rudolf Rozkošný (larvae of inquilines) and Walter Sudhaus (Nematoda) for help with the determination of prey and to Petr Heřman for comments on Diptera. Kateřina Janošíková kindly prepared the drawing. For facilitating access to herbarium and type materials, we thank Ida Haerida, Yasper Michael Mambrasar, Joeni Setijo Rahajoe and Rugayah (BO); Martin Cheek (K); and Roxali Bijmoer and Nicolien Sol (L). We thank also Kartini Kramadibrata and Muhammad Mansur from Bogor herbarium for help with depositing the type specimens. Alastair Robinson is thanked for valuable comments and suggestions that helped to improve the manuscript. Finally, M. Golos would like to pay tribute to the late Mamed Bin Anwar, without whose generous help the Berau taxon would not be documented herein. Martin Dančák was supported by the internal fund of Palacký University IGA PrF-2022-013.
Citation
Dančák M, Majeský Ľ, Čermák V, Golos MR, Płachno BJ, Tjiasmanto W (2022) First record of functional underground traps in a pitcher plant: Nepenthes pudica (Nepenthaceae), a new species from North Kalimantan, Borneo. PhytoKeys 201: 77–97. https://doi.org/10.3897/phytokeys.201.82872
Funding Statement
Palacký University
Supplementary materials
List of examined specimens
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Martin Dančák, Ľuboš Majeský, Václav Čermák, Michal R. Golos, Bartosz J. Płachno, Wewin Tjiasmanto
Data type
docx file
Explanation note
Nepentheshirsuta (including N.leptochila) and Nepentheshispida.
References
- Adam JH. (1997) Prey spectra of Bornean Nepenthes species (Nepenthaceae) in relation to their habitat. Pertanika. Journal of Tropical Agricultural Science 20: 121–134. [Google Scholar]
- Adamec L. (2007) Investment in carnivory in Utriculariastygia and U.intermedia with dimorphic shoots. Preslia 79(2): 127–139. [Google Scholar]
- Adlassnig W, Peroutka M, Lendl T. (2011) Traps of carnivorous pitcher plants as a habitat: Composition of the fluid, biodiversity and mutualistic activities. Annals of Botany 107(2): 181–194. 10.1093/aob/mcq238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrassy I. (1952) Freilebende Nematoden aus dem Bükk-Gebirge. Annales Historico-Naturales Musei Nationalis Hungarici 2: 13–65. [Series Nova] [Google Scholar]
- Bert W, De Ley IT, van Driessche R, Segers H, De Ley P. (2011) Baujardiamirabilis gen. n., sp. n. from pitcher plants and its phylogenetic position within Panagrolaimidae (Nematoda: Rhabditida). Nematology 5: 405–420. 10.1163/156854103769224395 [DOI] [Google Scholar]
- Bongers T. (1990) The maturity index, an ecological measure of environmental disturbance based on nematode species composition. Oecologia 83(1): 14–19. 10.1007/BF00324627 [DOI] [PubMed] [Google Scholar]
- Bonhomme V, Gounand I, Alaux C, Jousselin E, Barthélémy D, Gaume L. (2011) The plant-ant Camponotusschmitzi helps its carnivorous host-plant Nepenthesbicalcarata to catch its prey. Journal of Tropical Ecology 27(1): 15–24. 10.1017/S0266467410000532 [DOI] [Google Scholar]
- Borgonie G, García-Moyano A, Litthauer D, Bert W, Bester A, van Heerden E, Möller C, Erasmus M, Onstott TC. (2011) Nematoda from the terrestrial deep subsurface of South Africa. Nature 474(7349): 79–82. 10.1038/nature09974 [DOI] [PubMed] [Google Scholar]
- Cheek MR. (2015) Nepenthes (Nepenthaceae) of Halmahera, Indonesia. Blumea 59(3): 215–225. 10.3767/000651915X689091 [DOI] [Google Scholar]
- Cheek M, Jebb M. (1999) Nepenthes (Nepenthaceae) in Palawan, Philippines. Kew Bulletin 54(4): 887–895. 10.2307/4111166 [DOI] [Google Scholar]
- Cheek M, Jebb M. (2001) Flora Malesiana. Series I – Seed plants. Volume 15: Nepenthaceae. Nationaal Herbarium Nederland, Leiden.
- Clarke CM. (1997a) Another nice trip to Sumatra. Carnivorous Plant Newsletter 26(1): 4–10. [Google Scholar]
- Clarke CM. (1997b) Nepenthes of Borneo. Natural History Publications (Borneo), Kota Kinabalu, 207 pp. [Google Scholar]
- Clarke CM. (2001) Nepenthes of Sumatra and Peninsular Malaysia. Natural History Publications (Borneo), Kota Kinabalu, 326 pp. [Google Scholar]
- Cross A, Kalfas N, Nunn R, Conran J. (2019) Cephalotus: the Albany Pitcher Plant. Redfern Natural History Productions, Poole.
- Danser BH. (1928) The Nepenthaceae of the Netherlands Indies. Bulletin du Jardin Botanique de Buitenzorg 9: 249–438. [Google Scholar]
- Darnowski D, Bauer U, Méndez M, Horner J, Plachno BJ. (2018) Prey selection and specialization by carnivorous plants. In: Ellison AM, Adamec L. (Eds) Carnivorous Plants-Physiology, ecology and evolution.Oxford University Press, 285–293. 10.1093/oso/9780198779841.003.0021 [DOI]
- De Grisse A. (1969) Redescription ou modifications de quelques techniques utilisées dans l`étude des nématodes phytoparasitaires. Mededelingen Rijksfaculteit der Landbouwwetenschappen Gent 34: 351–369. [Google Scholar]
- Ellison A, Gotelli N, Brewer S, Cochran-Stafira L, Kneitel J, Miller T, Worley A, Zamora R. (2001) The evolutionary ecology of carnivorous plants. Advances in Ecological Research 33: 1–74. 10.1016/S0065-2504(03)33009-0 [DOI] [Google Scholar]
- Gaveau DL, Sheil D, Salim MA, Arjasakusuma S, Ancrenaz M, Pacheco P, Meijaard E. (2016) Rapid conversions and avoided deforestation: Examining four decades of industrial plantation expansion in Borneo. Scientific Reports 6(1): e32017. 10.1038/srep32017 [DOI] [PMC free article] [PubMed]
- Geraert E, Sudhaus W, Lenaerts L, Bosmans E. (1988) Halicephalobuslaticauda sp. n., a nematode found in a Belgian coal mine (Nematoda, Rhabditida). Annales de la Société Royale Zoologique de Belgique 118: 5–12. [Google Scholar]
- Ghazalli MN, Tamizi AA, Nikong D, Besi EE, Mat Esa MI, Mohd Esa AR, Latiff A, Zaini AZ, Shakri MA. (2020) Nepentheslatiffiana and N.domei (Nepenthaceae), two new species of pitcher plants from Terengganu, Peninsular Malaysia. Webbia 75(1): 5–28. 10.36253/jopt-7950 [DOI] [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. (1984) Carnivory in the bromeliad Brocchiniareducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. American Naturalist 124(4): 479–497. 10.1086/284289 [DOI] [Google Scholar]
- Golos MR, Robinson AS, Barer M, Dančák M, de Witte J, Limberg A, Sapawi NBM, Tjiasmanto W. (2020) Nepenthesfractiflexa (Nepenthaceae), a new Bornean pitcher plant exhibiting concaulescent metatopy and a high degree of axillary bud activation. Phytotaxa 432(2): 125–143. 10.11646/phytotaxa.432.2.3 [DOI] [Google Scholar]
- Greeney HF. (2000) The insects of plant-held waters: A review and bibliography. Journal of Tropical Ecology 17(2): 241–260. 10.1017/S026646740100116X [DOI] [Google Scholar]
- Ishaq LS, Hotopp A, Silverbrand S, Dumont EJ, Michaud A, MacRae JD, Stock SP, Groden E. (2021) Bacterial transfer from Pristionchusentomophagus nematodes to the invasive ant Myrmicarubra and the potential for colony mortality in coastal Maine. iScience 24(6): 1–24. 10.1016/j.isci.2021.102663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN (2012) IUCN Red List Categories and Criteria. Version 3.1, 2nd ed. IUCN, Gland, Switzerland and Cambridge, UK. https://portals.iucn.org/library/node/10315 [accessed 2 May 2022]
- IUCN (2022) Guidelines for using the IUCN Red List Categories and Criteria. Version 15. Prepared by the Standards and Petitions Committee. https://nc.iucnredlist.org/redlist/content/attachment_files/RedListGuidelines.pdf [accessed 2 May 2022]
- Jebb M, Cheek M. (1997) A skeletal revision of Nepenthes (Nepenthaceae). Blumea 42(1): 1–106. [Google Scholar]
- Karagatzides JD, Ellison AM. (2009) Construction costs, payback times, and the leaf economics of carnivorous plants. American Journal of Botany 96(9): 1612–1619. 10.3732/ajb.0900054 [DOI] [PubMed] [Google Scholar]
- Kitching RL. (2000) Food webs and container habitats: the natural history and ecology of phytotelmata. Cambridge University Press, 431 pp. 10.1017/CBO9780511542107 [DOI]
- Körner H. (1954) Die Nematodenfauna des vergehenden Holzes and ihre Beziehung zu Insekten. Zoologische Jahrbucher 82: 345–533. [Google Scholar]
- McPherson S, Schnell D. (2011) Sarraceniaceae of North America. Redfern Natural History Productions, Poole 170(1): 133–133. [Google Scholar]
- McPherson S, Fleischmann A, Robinson A. (2009) Pitcher plants of the Old World (Vol. 1). Redfern Natural History Productions, Poole 161(4): 449–450. 10.1111/j.1095-8339.2009.01023.x [DOI] [Google Scholar]
- Miettinen J, Shi C, Liew SC. (2011) Deforestation rates in insular Southeast Asia between 2000 and 2010. Global Change Biology 17(7): 2261–2270. 10.1111/j.1365-2486.2011.02398.x [DOI] [Google Scholar]
- Murphy B, Forest F, Barraclough T, Rosindell J, Bellot S, Cowan R, Golos M, Jebb M, Cheek M. (2020) A phylogenomic analysis of Nepenthes (Nepenthaceae). Molecular Phylogenetics and Evolution 144: e106668. 10.1016/j.ympev.2019.106668 [DOI] [PubMed]
- Nerz J, Mann P, Alt T, Smith T. (1998) Nepenthessibuyanensis, a new Nepenthes from Sibuyan, a remote island of the Philippines. Carnivorous Plant Newsletter 27(1): 18–23. 10.55360/cpn271.jn393 [DOI] [Google Scholar]
- Osunkoya OO, Daud SD, Di Giusto B, Wimmer FL, Holige TM. (2007) Construction costs and physico-chemical properties of the assimilatory organs of Nepenthes species in northern Borneo. Annals of Botany 99(5): 895–906. 10.1093/aob/mcm023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunkoya OO, Daud SD, Wimmer FL. (2008) Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Annals of Botany 102(5): 845–853. 10.1093/aob/mcn162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Saganová M. (2015) A novel insight into the cost–benefit model for the evolution of botanical carnivory. Annals of Botany 115(7): 1075–1092. 10.1093/aob/mcv050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Masarovičová E, Hudák J. (2007) Carnivorous syndrome in Asian pitcher plants of the genus Nepenthes. Annals of Botany 100(3): 527–536. 10.1093/aob/mcm145 [DOI] [PMC free article] [PubMed]
- Pavlovič A, Singerová L, Demko V, Hudák J. (2009) Feeding enhances photosynthetic efficiency in the carnivorous pitcher plant Nepenthestalangensis. Annals of Botany 104(2): 307–314. 10.1093/aob/mcp121 [DOI] [PMC free article] [PubMed]
- Pereira CG, Almenara DP, Winter CE, Fritsch PW, Lambers H, Oliveira RS. (2012) Underground leaves of Philcoxia trap and digest nematodes. Proceedings of the National Academy of Sciences of the United States of America 109(4): 1154–1158. 10.1073/pnas.1114199109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipps A, Lamb AL, Lee CC. (2008) Pitcher Plants of Borneo, 2nd edn. Natural History Publications (Borneo), Kota Kinabalu.
- Płachno BJ, Kozieradzka-Kiszkurno M, Świątek P, Darnowski DW. (2008) Prey attraction in carnivorous Genlisea (Lentibulariaceae). Acta Biologica Cracoviensia. Series; Botanica 50: 87–94. [Google Scholar]
- Poppinga S, Weisskopf C, Westermeier AS, Masselter T, Speck T. (2016) Fastest predators in the plant kingdom: Functional morphology and biomechanics of suction traps found in the largest genus of carnivorous plants. AoB Plants 8: plv140. 10.1093/aobpla/plv140 [DOI] [PMC free article] [PubMed]
- Rae R, Riebesell M, Dinkelacker I, Wang Q, Herrmann M, Weller AM, Dietrich C, Sommer RJ. (2008) Isolation of naturally associated bacteria of necromenic Pristionchus nematodes and fitness consequences. The Journal of Experimental Biology 211(12): 1927–1936. 10.1242/jeb.014944 [DOI] [PubMed] [Google Scholar]
- Raes N, Roos MC, Slik JWF, van Loon EE, ter Steege H. (2009) Botanical richness and endemicity patterns of Borneo derived from species distribution models. Ecography 32(1): 180–192. 10.1111/j.1600-0587.2009.05800.x [DOI] [Google Scholar]
- Robinson AS, Golos MR, Barer M, Sano Y, Forgie JJ, Garrido D, Gorman CN, Luick AO, McIntosh NWR, McPherson SR, Palena GJ, Pančo I, Quinn BD, Shea J. (2019) Revisions in Nepenthes following explorations of the Kemul Massif and the surrounding region in north-central Kalimantan, Borneo. Phytotaxa 392(2): 97–126. 10.11646/phytotaxa.392.2.1 [DOI] [Google Scholar]
- Salmon B. (1993) Some observations on the trapping mechanisms of Nepenthesinermis and N.rhombicaulis. Carnivorous Plant Newsletter 23(1–2): 11–12.
- Schenková J, Čermák V. (2013) Description of Pristinaarmata n. sp. (Clitellata: Naididae: Pristininae) from a carnivorous plant (Nepenthes sp.) in Borneo, Indonesia. Zootaxa 3686(5): 587–592. 10.11646/zootaxa.3686.5.7 [DOI] [PubMed] [Google Scholar]
- Schmid-Hollinger R. (1994) More knowledge about Nepenthesrhombicaulis. Carnivorous Plant Newsletter 23(3): 62–63.
- Seine R, Porembski S, Balduin M, Theisen I, Wilbert N, Barthlott W. (2002) Different prey strategies of terrestrial and aquatic species in the carnivorous genus Utricularia (Lentibulariaceae). Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 124(1): 71–76. 10.1127/0006-8152/2002/0124-0071 [DOI] [Google Scholar]
- Stefanski W. (1954) Rhabditisgingivalis sp. n. parasite trouvé dans un granulome de la gencive chez un cheval. Acta Parasitologica Polonica 1: 329–333. [Google Scholar]
- Tan WK, Wong CL. (1996) Aerial pitchers of Nepenthesampullaria. Nature Malaysiana 21(1): 12–14.
- Taylor P. (1991) The genus Genlisea. Carnivorous Plant Newsletter 20(1–2): 20–26.
- Teo HC, Lechner AM, Sagala S, Campos-Arceiz A. (2020) Environmental impacts of planned capitals and lessons for Indonesia’s new capital. Land (Basel) 9(11): 438. 10.3390/land9110438 [DOI] [Google Scholar]
- Thiers B. (2022) Index Herbariorum: a global directory of public herbaria and associated staff. New York botanical Garden’s virtual herbarium. http://sweetgum.nybg.org/ih/ [accessed 6 February 2022]
- Thorp JH, Rogers DC. (2015) Thorp and Covich’s Freshwater Invertebrates (4th edn. ), Ecology and General Biology. Academic Press, 1118 pp. 10.1016/B978-0-12-385026-3.01002-0 [DOI] [Google Scholar]
- Vong V, Ali A, Onsanit S, Thitithanakul S, Noon-Anant N, Pengsakul T. (2021) Larval mosquito (Diptera: Culicidae) abundance in relation with environmental conditions of pitcher plants Nepenthesmirabilisvar.mirabilis in Songkhla Province, Thailand. Songklanakarin Journal of Science and Technology 43(2): 431–438. [Google Scholar]
- Wahab A. (1962) Untersuchungen über Nematoden in den Drüsen des Kopfes der Ameisen (Formicidae). Zeitschrift fur Morphologie und Oekologie der Tiere 52(1): 33–92. 10.1007/BF00446341 [DOI] [Google Scholar]
- Zamora R, Gómez JM, Hódar JA. (1997) Responses of a carnivorous plant to prey and inorganic nutrients in a Mediterranean environment. Oecologia 111(4): 443–451. 10.1007/s004420050257 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of examined specimens
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Martin Dančák, Ľuboš Majeský, Václav Čermák, Michal R. Golos, Bartosz J. Płachno, Wewin Tjiasmanto
Data type
docx file
Explanation note
Nepentheshirsuta (including N.leptochila) and Nepentheshispida.