Abstract
Following recent mimosoid phylogenetic and phylogenomic studies demonstrating the non-monophyly of the genus Albizia, we present a new molecular phylogeny focused on the neotropical species in the genus, with much denser taxon sampling than previous studies. Our aims were to test the monophyly of the neotropical section Arthrosamanea, resolve species relationships, and gain insights into the evolution of fruit morphology. We perform a Bayesian phylogenetic analysis of sequences of nuclear internal and external transcribed spacer regions and trace the evolution of fruit dehiscence and lomentiform pods. Our results find further support for the non-monophyly of the genus Albizia, and confirm the previously proposed segregation of Hesperalbizia, Hydrochorea, Balizia and Pseudosamanea. All species that were sampled from section Arthrosamanea form a clade that is sister to a clade composed of Jupunba, Punjuba, Balizia and Hydrochorea. We find that lomentiform fruits are independently derived from indehiscent septate fruits in both Hydrochorea and section Arthrosamanea. Our results show that morphological adaptations to hydrochory, associated with shifts into seasonally flooded habitats, have occurred several times independently in different geographic areas and different lineages within the ingoid clade. This suggests that environmental conditions have likely played a key role in the evolution of fruit types in Albizia and related genera. We resurrect the name Pseudalbizzia to accommodate the species of section Arthrosamanea, except for two species that were not sampled here but have been shown in other studies to be more closely related to other ingoid genera and we restrict the name Albizia s.s. to the species from Africa, Madagascar, Asia, Australia, and the Pacific. Twenty-one new nomenclatural combinations in Pseudalbizzia are proposed, including 16 species and 5 infraspecific varietal names. In addition to the type species Pseudalbizziaberteroana, the genus has 17 species distributed across tropical regions of the Americas, including the Caribbean. Finally, a new infrageneric classification into five sections is proposed and a distribution map of the species of Pseudalbizzia is presented.
Keywords: Arthrosamanea , hydrochory, monophyly, Neotropics, phylogeny, taxonomy
Introduction
The genus Albizia Durazz. has a complicated taxonomic history but has generally been treated as a pantropical genus with 120–140 species, of which 36 are endemic to Africa, with c. 30 species in Madagascar, of which c. 24 are endemic, c. 35 species in Asia, one in Australia, and 22 in tropical America (Lewis and Rico Arce 2005; Rico Arce et al. 2008). All species are woody, forming trees of variable stature and inhabit a wide range of lowland tropical biomes (Figs 1 and 2), including rain forests, seasonally dry tropical forests, and savannas, with one species, Albiziajulibrissin Durazz., the type species of the genus, in subtropical and warm temperate forests in Asia. However, Albizia remains poorly defined; its delimitation remains one of the most challenging taxonomic problems in the legume family, and it is currently considered the main “dustbin” genus in tribe Ingeae (Koenen et al. 2020). In the past, the most problematic genus of tribe Ingeae was Pithecellobium Mart., but its taxonomy has been gradually clarified (Barneby and Grimes 1996). Resolution of the taxonomic status of Albizia has lagged behind that of Pithecellobium and only really started at the end of the twentieth century. For example, several new neotropical genera have been segregated from Albizia: Balizia Barneby & J.W. Grimes; Hesperalbizia Barneby & J.W. Grimes, and Hydrochorea Barneby & J.W. Grimes. Barneby and Grimes (1996) also re-established the genus Pseudosamanea Harms, which previously had been treated as a synonym within Albizia (Table 1). However, at the time they were established, the monophyly of these new and re-established genera had not been tested using phylogenetic analyses of molecular data.
Figure 1.
Morphology of Albizia s.l. showing selected members of the genera Albizia and Pseudalbizziaa–cAlbiziaferruginea (Guill. & Perr.) Benth. in Congo a detail of leaf rachis and gland between terminal pinnae b detail of leaflets of a terminal pinna c seed and funiculus attached to the valve dAlbiziaglaberrima (Schumach. & Thonn.) Benth. in Malawi, detail of inflorescence eAlbiziaanthelmintica Brongn. in Malawi, habit fAlbiziaadianthifolia (Schumach.) W. Wight in Congo, habit gAlbiziaglaberrima in Malawi, branches and inflorescences hAlbiziachinensis (Osbeck) Merr. in Thailand, inflorescences iAlbiziaodoratissima (L. f.) Benth. in Thailand, fruits jAlbiziaprocera (Roxb.) Benth. in Thailand, fruits kAlbiziasplendens Miq. in Thailand, woody fruit lPseudalbizziamultifloravar.multiflora in Ecuador, woody fruit m, nPseudalbizziapistaciifolia (Willd.) E.J.M Koenen & Duno in Ecuador m habit n woody fruit. Photos: a, b David J. Harris / With permission from RBG Edinburgh c Claude Boucher Chisale d–f Günter Baumann g Jos Stevens h Natcha Sutjaritjai i–k Prateep Panyadee l–n Xavier Cornejo.
Figure 2.
Habit, flower and fruit variation in the genus PseudalbizziaaP.adinocephala pods (Hughes 1913) bP.coripatensis inflorescence (Hughes 2433) cP.coripatensis pods (Hughes 2433) dP.inundata pods (JRI Wood 26530) eP.multiflora habit (Hughes 2214) fP.multiflora leaves and pods (Hughes 2214) gP.pistaciifolia leaves and inflorescence (Cornejo 8426, GUAY) hP.niopoides habit (Hughes 419) iP.niopoides pods (Rivera 2245) jP.polycephala inflorescence (de Queiroz 15515) kP.tomentosa inflorescence (Hughes 1143) lP.sinaloensis pods (Hughes 1576) mP.tomentosa habit (Hughes 1335) nP.tomentosa pods (Hughes 1307). All photos by Colin Hughes except g, Xavier Cornejo.
Table 1.
Main taxonomic changes related to Albizia, Ingeae tribe [1981–2008]. Modified from Rico Arce et al. (2008).
| Nielsen (1981) | Barneby and Grimes (1996) | Lewis and Rico Arce (2005) | Rico Arce et al. (2008) | Iganci et al. 2015 |
|---|---|---|---|---|
| Albizia | Albizia | Albizia | Albizia | Albizia |
| Balizia | Balizia | |||
| Cathormion | Cathormion | Cathormion | * | * |
| Hydrochorea | * | Hydrochorea | ||
| Hesperalbizia | Hesperalbizia | |||
| Pseudosamanea | Pseudosamanea |
* Not explicitly mentioned in the study.
Barneby and Grimes (1996) placed the remaining New World species of Albizia in their section Arthrosamanea (Britton & Rose) Barneby & J.W. Grimes. They characterized this section as forming a group that is homogeneous in most respects, but diverse in the late developmental stages of the fruit, which vary in: 1) fruit opening type: dehiscent, indehiscent, or breaking, 2) lateral shape: flat to conspicuously raised above the seed chambers, 3) texture and consistency of the valves: papery, chartaceous or woody, 4) longitudinal shape: straight to weakly falcate (Barneby and Grimes 1996) (Figs 1, 2 and 4). Within section Arthrosamanea, four series were proposed by Barneby and Grimes (1996): series Paniculatae with papery, plano-compressed, inertly dehiscent pods with continuous valves (13 species); series Arthrosamanea comprising 3 species with lomentiform, plano-compressed pods, where the ripe valves crack transversely between seeds but the wiry sutures persist at maturity; series Multiflorae (2 species) characterized by lomentiform fruits only reluctantly separating into articles, the thick-textured valves and sutural keels breaking transversely under pressure; and the monospecific series Inundatae which bears crypto-lomentiform pods, dehiscent through the sutures and with the valves differentiating into a continuous exocarp and a segmented endocarp separating into 1-seeded segments (Barneby and Grimes 1996).
Figure 4.
Phylogeny of the Jupunba clade redrawn from an ASTRAL species tree analysis by Soares et al. (2022) that utilizes data from Ringelberg et al. (2022) showing the evolutionary transitions from ancestrally papery, plano-compressed fruits to septate indehiscent fruits and subsequently to lomentiform hydrochorous fruits associated with species growing in seasonally inundated habitats in Pseudalbizzia and similar parallel transitions in Balizia and Hydrochorea. Photos of Pseudalbizziainundata, P.multiflora, P.niopoidesP.tomentosa and Baliziapedicellaris, by Colin Hughes, of Hydrochoreamarginata, Jupunbabarbouriana and J.leucophylla, by Erik Koenen.
Series Paniculatae is widespread across Mexico, Central and South America, occurring mainly in seasonally dry forests, grasslands, and less often in humid forests (in South America). All species of series Paniculatae have papyraceous, dehiscent fruits with one exception, A.berteroana (DC.) Fawc. & Rendle (the earlier combination A.berteroana (DC.) M. Gómez was invalidly published due to incorrect citation of the basionym, see Barneby and Grimes 1996), whose fruits are indehiscent and fall to the ground entire. In contrast, the other three series are distributed from Panama to South America and are most diverse in the Amazon basin (Barneby and Grimes 1996), and usually have more or less woody fruits, which are articulated and indehiscent, some dividing into monospermous segments through the grooves of the valves, considered to be an adaptation for hydrochory, i.e., seed dispersal in riparian and seasonally inundated forests (e.g., A.inundata (Mart.) Barneby & J.W. Grimes, A.pistaciifolia (Willd.) Barneby & J.W. Grimes, and A.subdimidiata (Splitg.) Barneby & J.W. Grimes).
The segregate genera established by Barneby and Grimes (1996) have not been universally accepted. For example, in the most recent taxonomic treatment of Albizia for Mexico and Central America (Rico Arce et al. 2008), the genera Balizia, Hesperalbizia, and Pseudosamanea were not recognized (Table 1). However, subsequent phylogenetic analyses have confirmed that these genera were rightfully segregated as distinct evolutionary lineages, Hesperalbizia being more closely related to Lysiloma Benth. (Duno de Stefano et al. 2021), and the closely related Balizia and Hydrochorea (the former reduced to synonymy of the latter, Soares et al. 2022) being placed as the sister-group of Jupunba Britton & Rose (Iganci et al. 2015; Soares et al. 2021, 2022). Phylogenomic analysis of the mimosoid clade, based on DNA sequences of 964 targeted nuclear genes confirmed these findings and, furthermore, showed that the species from the Americas (i.e., sect. Arthrosamanea) form a separate lineage from the African, Madagascan and Asian species (Koenen et al. 2020). Koenen et al. (2020) also showed that Pseudosamanea, although difficult to place in any clade, is not closely related to either Albizia s.s. or section Arthrosamanea and is perhaps most closely related to Samanea Merr. and Chloroleucon Britton & Rose ex Record.
While the data of Koenen et al. (2020) provided a robust phylogenomic backbone for the mimosoid and ingoid clades, and clearly demonstrated the non-monophyly of Albizia by sampling 25 species of that genus, only three of the 19 species from Central and South American sect. Arthrosamanea were included in that study, leaving doubts about the monophyly of that section and whether it should be segregated under the circumscription of Barneby and Grimes (1996), or whether there are further potential segregates, given the possibility that some of these species are more closely related to other neotropical genera. That possibility was suggested by the occurrence of lomentiform fruits in some species of section Arthrosamanea as reflected in the classification into separate series by Barneby and Grimes (1996). Similar lomentiform fruits also occur in Hydrochorea (Barneby and Grimes 1996; Soares et al. 2022) and Albizia s.s. (Albiziadolichadena (Kosterm.) I.C. Nielsen, Albiziamoniliformis (DC.) F. Muell., Albiziarosulata (Kosterm.) I.C. Nielsen and Albiziaumbellata (Vahl) E.J.M. Koenen), as well as a few other ingoid lineages (Barneby and Grimes 1996: 204; Koenen 2022a). For this reason, several species of neotropical Albizia have homotypic synonyms in Arthrosamanea Britton & Rose, Samanea or Cathormion Hassk., genera which previously had been recognized and defined based mainly on characters of fruit texture and dehiscence (Barneby and Grimes 1996). Barneby and Grimes (1996: 204) considered these fruit types to have arisen multiple times in parallel in different genera, and this was confirmed by subsequent phylogenetic (Iganci et al. 2015; Soares et al. 2021) and phylogenomic studies (Koenen et al. 2020), although the neotropical lomentiform Albizia species were not included in these studies, or remained unresolved.
Here we investigate whether Albiziasect.Arthrosamanea is monophyletic and thereby provide a more rigorous basis for recognizing its evolutionary distinctiveness from Albizia s.s. as a segregate genus. We infer a new phylogeny with emphasis on the neotropical species and make use of further insights offered by the phylogenomic analysis of Ringelberg et al. (2022). In addition, we use a tree topology inferred from data from the latter study to evaluate whether lomentiform fruits in Albiziasect.Arthrosamanea are independently derived from other lineages in which this fruit type occurs. Based on our phylogenetic results and the recent findings of Koenen et al. (2020) and Ringelberg et al. (2022), we update the taxonomy of neotropical Albizia by resurrecting the genus Pseudalbizzia Britton & Rose.
Materials and methods
We used the nuclear ribosomal External and Internal Transcribed Spacer (ETS and ITS) regions that previously been used to study sister-group relationships within tribe Ingeae (Brown et al. 2008; Iganci et al. 2015; Souza et al. 2016). Our combined dataset included 123 accessions, of which 50 are from Genbank and 73 are newly sequenced here, including 25 species of Albizia s.l. sequenced for the first time. The outgroup, Vachelliafarnesiana (L.) Wight & Arn., was designated to root the tree (Table 2). The plastid trnK region was initially explored but preliminary analyses suggested it is not sufficiently phylogenetically informative and these data were excluded from this study.
Table 2.
Voucher information of taxa included in the phylogenetic analysis with their corresponding GenBank accession numbers.
| Accessions ITS | Accessions ETS |
|---|---|
| Albiziaadianthifolia (Schumach.) W. Wight, MW699934, BGRO 001 | Albiziaadianthifolia, MW699372, BGRO 001 |
| Albiziaamara (Roxb.) Boivin, MW699936, BGRO 003 | Albiziaamara, MW699374, BGRO 003 |
| Albiziaanthelmintica Brongn., MW699937, BGRO 004 | Albiziaanthelmintica, MW699375, BGRO 004 |
| Albiziaarenicola R. Vig., MW699938, R. Randrianaivo 642, MO | Albiziaantunesiana Harms, MW699376, S.H.C.P. 966, MO |
| Albiziabrevifolia Schinz, MW699940, BGRO 005 | Albiziaarenicola, MW699377, R. Randrianaivo 642, MO |
| Albiziaglaberrima Hutch. & Dalziel, MW699943, R.E. Gereau 6203, MA | Albiziabrevifolia, MW699378, BGRO 005 |
| Albiziagummifera (J.F. Gmel.) C.A. Sm., MW699944, J.E. Lawesson 5094, AAU | Albiziachinensis (Osbeck) Merr., MW69379, A. Ntemi & A. Athumani 478, MO |
| Albiziaharveyi E. Fourn., MW699945, BGRO 006 | Albiziacrassiramea Lace, MW699380, K. Larsen et al. 46378, AAU |
| Albiziajulibrissin Durazz., MW699946, BGRO 007 | Albiziaferruginea (Guill. & Perr.) Benth., MW699382, C.H. Jongkind 2098, MA |
| Albiziakalkora (Roxb.) Prain, MW699947, E. Bouflord 26356, MO | Albiziaglaberrima, MW699383, R.E. Gereau 6203, MA |
| Albizialebbeck (L.) Benth., MW699948, C. Chan 7539, CICY | Albiziagummifera, MW699384, J.E. Lawesson 5094, AAU |
| Albiziapetersiana (Bolle) Oliv., MW699950, BGRO 008 | Albiziaharveyi, MW699385, BGRO 006 |
| Albiziaprocera (Roxb.) Benth., MW699953, BGRO 009 | Albiziajulibrissin, MW699387, BGRO 007 |
| Albiziaretusa Benth., MW699954, K. Yasuda 1804, MO | Albiziakalkora, MW699388, E. Bouflord 26356, MO |
| Albiziatanganyicensis Baker f., MW699956, BGRO 010 | Albizialebbeck (L.) Benth., MW699389, C. Chan 7539, CICY |
| Albiziaumbellata (Vahl) E. J. M. Koenen, EF638182.1 | Albizialebbekioides (DC.) Benth., MW699390, H. Balslev 9333, AAU |
| Balizialeucocalyx (Britton & Rose) Barneby & J.W. Grimes, MW699959, S. Aguilar & F. Aguilar 1833, M | Albizialucidior (Steud.) I.C. Nielsen ex H. Hara, MW699391, J.F. Maxwell 95–259, MO |
| Lysilomaacapulcense (Kunth) Benth., MW699960, H. Gómez D. 2003, MO | Albiziapetersiana (Bolle) Oliv., MW699394, BGRO 008 |
| Lysilomalatisiliquum (L.) Benth., MW699961, P. Simá 2287, CICY | Albiziaprocera, MW699396, BGRO 009 |
| Pseudalbizziaadinocephala (Donn. Sm.) E.J.M. Koenen & Duno, MW699935, BGRO 002 | Albiziaretusa, MW699397, K. Yasuda 1804, MO |
| MW699958, J.L. Linares 5406, FCME | Albiziasahafariensis Capuron, MW699398, R. Randrianaivo et al. 1387, MO |
| Pseudalbizziaberteroana (Balb. Ex DC.) Britton & Rose, MW699939, A. Jimenez 2113, MO | Albiziatanganyicensis, MW699400, BGRO 010 |
| Pseudalbizziaedwallii (Hoehne) E.J.M. Koenen & Duno, MW699942, J.M. Silva & L.M. Abe 4237, MEXU | Albiziaumbellata, EF638157.1 |
| Pseudalbizziamultiflora (Kunth) E.J.M. Koenen & Duno, MW699949, X. Cornejo 1922, GUAY | Balizialeucocalyx, MW699403, S. Aguilar & F. Aguilar 1833, M |
| Pseudalbizziapistaciifolia (Willd.) E.J.M. Koenen & Duno, MW699951, X. Cornejo 5323, GUAY | Baliziapedicellaris (DC.) Barneby & J.W. Grimes, MW699404, P.R. House 1880, MA |
| Pseudalbizziapolycephala (Benth.) E.J.M. Koenen & Duno, MW699952, L.P. Queiroz 9578, MEXU | Havardiamexicana, MW699405, S. Foldi s.n., CICY |
| Pseudalbizziasinaloensis (Britton & Rose) E.J.M. Koenen & Duno, MW699955, C.E. Hughes et al. 1576, FCME | Hesperalbiziaoccidentalis, MW699406, J.G. Hernandez Oria 21, FCME |
| Pseudalbizziatomentosa (Micheli) E.J.M. Koenen & Duno, MW699957, A. Dorantes et al. 165, CICY | Lysilomaacapulcense, MW699407, H. Gómez D. 2003, MO |
| Pseudosamaneacubana (Britton & P. Wilson) Barneby & J.W. Grimes, MW699941, GHBG 001 | Lysilomalatisiliquum, MW699408, P. Simá 2287, CICY |
| Pseudosamaneaguachapele (Kunth) Harms, MW699962, BGRO 011 | Paraseriantheslophantha, MW699409, H. Balslev et al. 62450, AAU |
| Zapotecaformosa (Kunth) H.M. Hern., MW699963, R. Duno s.n. CICY. Additional accessions (ITS): Acaciaacradenia F.Muell., AF487765.1 | Pithecellobiumdiversifolium, MW699410, J.F.B. Pastore & R.M. Harley 2599 MO |
| Acacialongifolia (Andrews) Willd., HM007655.1 | Pithecellobiumexcelsum, MW699411, G. P. Lewis et al 2339, MO |
| Acaciellaangustissima (Mill.) Britton & Rose, EF638169.1 | Pseudalbizziaadinocephala, MW699373, BGRO 002 |
| Baliziapedicellaris (DC.) Barneby & J.W. Grimes, JX870657.1 | MW699402, J.L. Linares 5406, FCME |
| Calliandradysantha Benth., JX870684.1 | Pseudalbizziaedwallii, MW699381, J.M. Silva & L.M. Abe 4237, MEXU |
| Calliandrafoliosa Benth., EF638181.1 | Pseudalbizziainundata (Mart.) E.J.M. Koenen & Duno, MW699386, H. Balslev et al. 97355, AAU |
| Cojobaarborea (L.) Britton & Rose, JX870758.1 | Pseudalbizziamultiflora (Kunth) E.J.M. Koenen & Duno, MW699392, X. Cornejo & T. Andres 8705, GUAY |
| Cojobaundulatomarginata L. Rico, EF638187.1 | Pseudalbizzianiopoides (Spruce ex Benth.) E.J.M. Koenen & Duno, MW699393, J.R. Grande 374, VEN |
| Ebenopsisebano (Berland.) Barneby & J.W. Grimes, JX870759.1 | PseudalbizziapolycephalaMW699395, L.P. Queiroz 9578, MEXU |
| Enterolobiumcontortisiliquum (Vell.) Morong, EF638190.1 | Pseudalbizziasinaloensis, MW699399, C.E. Hughes et al. 1576, FCME |
| Enterolobiumcyclocarpum (Jacq.) Griseb., EF638191.1 | Pseudalbizziatomentosa, MW699401, A. Dorantes et al. 165, CICY |
| Enterolobiumtimbouva Mart., JX870760.1 | Pseudosamaneacubana (Britton & P. Wilson) Barneby & J.W. Grimes, MW699412, BJ FTGH 2000 |
| Faidherbiaalbida (Delile) A. Chev., EU812008.1 | Pseudosamaneaguachapele, MW699413, BGRO 011 |
| Havardiamexicana (Rose) Britton & Rose, JX870762.1 | Samaneatubulosa (Benth.) Barneby & J.W. Grimes, MW699414, G.A. Parada & V.D. Rojas 2480, MO. Additional accessions: Acaciaacradenia, EF638116.1 |
| Havardiapallens (Benth.) Britton & Rose, KF921656.1 | Acacialongifolia, EF638115.1 |
| Hesperalbiziaoccidentalis (Brandegee) Barneby & J.W. Grimes, EF638195.1 | Acaciellaangustissima EF638082.1 |
| Hycrochoreacorymbosa (Rich.) Barneby & J.W. Grimes, JX870763.1 | Pseudalbizziaadinocephala EF638144.1 |
| Jupunbatrapezifolia (Vahl.) Moldenke, EF638166.1 | Albiziakalkora EF638158.1 |
| Mariosousacoulteri (Benth.) Seigler & Ebinger, EF638198.1 | Albizialebbeck EF638155.1 |
| Mariosousadolichostachya (S.F. Blake) Seigler & Ebinger, EF638199.1 | Albiziasaponaria (Lour.) Blume, EF638085.1 |
| Paraseriantheslophantha (Willd.) I.C. Nielsen, EF638204.1 | Archidendropsisbasaltica (F. Muell.) I.C. Nielsen, EF638141.1 |
| Pithecellobiumdiversifolium Benth., JX870768.1 | Archidendropsisthozetiana (F. Muell.) I.C. Nielsen, EF638140.1 |
| Pithecellobiumdulce (Roxb.) Benth., EF638207.1 | Calliandradysantha EF638121.1 |
| Pithecellobiumexcelsum (Kunth) Mart., EF638208.1 | Calliandrafoliosa EF638122.1 |
| Samaneasaman (Jacq.) Merr., JX870770.1 | Cojobaarborea EF638095.1 |
| Samaneatubulosa (Benth.) Barneby & J.W. Grimes, EF638212.1 | Cojobaundulatomarginata EF638096.1 |
| Pseudosamaneaguachapele (Kunth) Harms, JX870769.1 | Ebenopsisconfinis (Standl.) Britton & Rose, EF638100.1 |
| Senegaliaberlandieri (Benth.) Britton & Rose, KY688777.1 | Ebenopsisebano EF638101.1 |
| Sphingaacatlensis (Benth.) Barneby & J.W. Grimes, EF638214.1 | Enterolobiumcontortisiliquum EF638151.1 |
| Vachelliacampechiana (Mill.) Seigler & Ebinger, EF638215.1 | Enterolobiumcyclocarpum EF638149.1 |
| Vachelliafarnesiana (L.) Wight & Arn., EF638219.1 | Faidherbiaalbida EF638163.1 |
| Viguieranthusambongensis (R. Vig.) Villiers, JX870773.1 | Havardiapallens EF638146.1 |
| Viguieranthusdensinervus Villiers, JX870774.1 | Hesperalbiziaoccidentalis EF638139.1 |
| Viguieranthusmegalophyllus (R. Vig.) Villiers, JX870776.1 | Hycrochoreacorymbosa EF638138.1 |
| Viguieranthussubauriculatus Villiers, JX870778.1 | Jupunbatrapezifolia (Vahl.) Moldenke, EF638110.1 |
| Zapotecatetragona (Willd.) H.M. Hern., JX870784.1 | Mariosousacoulteri (Benth.) Seigler & Ebinger, EF638124.1 |
| Mariosousadolichostachya EF638084.1 | |
| Pararchidendronpruinosum (Benth.) I.C. Nielsen, EF638129.1 | |
| Paraserianthestoona (Bailey) I.C. Nielsen, EF638106.1 | |
| Pithecellobiumdulce EF638142.1 | |
| Pseudosamaneaguachapele EF638160.1 | |
| Samaneasaman EF638136.1 | |
| Samaneatubulosa EF638135.1 | |
| Senegaliaberlandieri EF638162.1 | |
| Sphingaacatlensis EF638145.1 | |
| Vachelliafarnesiana EF638128.1 | |
| Viguieranthusambongensis KR997873.1 | |
| Viguieranthusdensinervus JX870891.1 | |
| Viguieranthusmegalophyllus KR997871.1 | |
| Viguieranthussubauriculatus KR997076.1 | |
| Zapotecaformosa EF638134.1 | |
| ZapotecatetragonaEF638133.1. |
Fresh leaf material collected in the field plus herbarium material from the Jardín Botánico Regional Roger Orellana (CICY) were used for DNA extraction. Herbarium specimens used in these analyses came from AAU, CICY, FCME, MA, MEXU, and MO (acronyms as in Thiers 2016). Additional sequences were downloaded from GenBank (Table 2).
DNA from leaf fragments was obtained using the DNeasy Plant Mini Kit (QIAGEN Inc., Valencia, California) following the manufacturer’s specifications. To assess concentration and relative quality of DNA, 3 µl of final volume plus 2 µl loading buffer were run for 30 minutes at 6 V cm-1 on a 1% agarose gel prepared with 0.5× TBE. The resulting gel was developed by immersion for 20–30 minutes in a 0.1 µg ml-1 ethidium bromide solution and later observed in a DigiDoc-It Imaging System (version 6.7.1; UVP, Inc., Cambridge, UK) transilluminator. DNA purity and concentration were quantified with a NanoDrop 2000c. Afterwards, DNA samples were standardized to 10 ng µl-1.
PCR amplifications were performed in an Applied Biosytems Veriti 96 Well Thermal Cycler. Volumes of reagents and conditions for the amplifications were as follows: ITS: 30 µL of mix containing 3 µl 10× Buffer, 2.5 µl MgCl2,, 0.6 µl (~10 ng) primer, 4 µl Q solution, 1 µl 1.25 mM L-1 dNTP, 0.2 µl (1 U) TAQ polymerase, 2 µl (~10 ng) DNA, then completed to volume (approx. 16.1 µl) with ultra-pure water. PCRs were conducted under the following protocol: 94 °C × 3 min + 30 cycles (94 °C × 1 min + 60.5 °C × 1 min + 72 °C × 2 min) + 72 °C × 7 min. Primers were S3 (AACCTGCGGAAGGATCATTG) (Käss and Wink 1997), and 26S (TAGAATTCCCCGGTTCGCTCGCCGTTAC) (Sun et al. 1994). ETS: 30 µl of mix containing 3 µl 10× Buffer, 2.5 µl MgCl2, 0.6 µl (~10 ng) primer, 4 µl Q solution, 1 µl 1.25 mM l-1 dNTP, 0.2 µl (1 U) TAQ polymerase, 2 µl (~10 ng) DNA, then completed to volume (approx. 16.1 µl) with ultra-pure water. PCR amplifications were conducted under the following protocol: 94 °C × 3 min + 30 cycles (94 °C × 1 min + 60.5 °C × 1 min + 72 °C × 2 min) + 72 °C × 7 min. Primers used were 18S-IGS (5’-GAGACAAGCATATGACTACTGGCAGGATCAACCAG-3’) and 26S-IGS (5’-GGATTGTTCACCCACCAATAGGGAACGTGAGCTG-3’) (Baldwin and Markos 1998).
The quality of the PCR products was evaluated by agarose electrophoresis (3 μl of final volume plus 2 μl of bromophenol blue, gel prepared with 0.5× TBE and 1% agarose, run at 120 volts and 25 amperes 30 min). PCR products were sequenced at Macrogen (http://www.macrogen.com/eng/) using the same amplification primers. The sequencing products were assembled and edited using the Sequencher v. 5.2.3. An initial automated alignment was conducted with MAFFT (Katoh et al. 2002) using the E-INS-i algorithm option, a 100PAM/k = 2 scoring matrix, a gap opening penalty of 1.3, and an offset value of 0.123. The alignments were visually inspected and manually edited for further improvement. The Akaike Information Criterion (AIC), implemented in jModeltest (Posada 2008) was used to select the best model of nucleotide substitution for each alignment. The selected models were TVM+I+G for ETS and GTR+I+G for ITS. Phylogenetic analyses were performed with MrBayes v.3.2.5 (Ronquist et al. 2012) separately for each dataset, and subsequently concatenated with each partition treated as independent and associated with its own evolutionary model. Analyses were performed using default parameters for 5 million generations. Two independent threads were run. Convergence was assessed with both MrBayes and Tracer (Rambaut et al. 2018). Posterior Probabilities (PP) ≤ 0.95 were considered weakly supported whereas PP of 0.95–1.0 were deemed strongly supported (Alfaro et al. 2003).
To examine the evolution of fruit types within New World Albizia we utilize a phylogeny derived from a new analysis based on data of Ringelberg et al. (2022) for the Jupunba clade, as described in Soares et al. (2022), because it is based on a large set of 560 nuclear exons and flanking non-coding regions, and therefore shows enhanced resolution within this clade compared to the ITS + ETS phylogeny.
Results
Alignments of our combined datasets recovered by MAFFT required few manual adjustments. The ETS sequences had 381 bp and, once aligned, 52% of the data were informative. In the case of ITS, the sequences were slightly longer, 551 bp but only 34% were informative.
None of the molecular-based analyses (ETS, ITS, and ETS+ITS) using Bayesian inference recovered the genus Albizia as monophyletic. The combined ETS + ITS phylogeny (Fig. 3) is used as the basis for discussing the results in detail. The outgroup Vachelliafarnesiana, plus Acaciellaangustissima (Mill.) Britton & Rose, Senegaliaberlandieri (Benth.) Britton & Rose and two species of Mariosousa Seigler & Ebinger form a paraphyletic grade subtending a fully supported clade (PP = 1) that includes all members of the tribe Ingeae as well as Acacia s.s.
Figure 3.
Phylogeny of the ingoid clade (sensu Koenen et al. 2020), i.e., the traditionally recognized tribes Ingeae + Acaciaeae (excl. Vachellia). Phylogram derived from Bayesian analysis in MrBayes of the combined ETS and ITS data for Albizia and related genera. Main clades are labeled A–F (see text). Posterior support values are indicated above branches.
The most relevant clade from the perspective of this study is highly supported (PP = 0.96) and includes all members of the genus Albizia and a few other genera of tribe Ingeae (clade A). The genus Albizia, as currently circumscribed, is non-monophyletic with species placed in two separate, strongly supported clades (Fig. 3). As in Koenen et al. (2020), species of Albiziasect.Arthrosamanea are placed in clade B (PP = 0.96), which equates to the Jupunba clade of Koenen et al. (2020). All species that were sampled from this section are included in this clade (Fig. 3 clade D), which received full support (PP = 1), and as in Koenen et al. (2020) this section is sister to clade C (PP = 1) comprising the genera Jupunba, Balizia and Hydrochorea.
Within sect. Arthrosamanea, three clades are well supported, one comprising Albiziapolycephala (Benth.) Killip ex Record and Albiziaedwallii (Hoehne) Barneby & J.W. Grimes of ser. Paniculatae, a second clade comprising species of ser. Paniculatae endemic to Mexico, Central America, and the Caribbean, and a third clade that includes Albizianiopoides (Benth.) Burkart (also ser. Paniculatae) and the species from the other three series. The phylogeny of Ringelberg et al. (2022) presented here in Fig. 4 based on a new analysis of the Jupunba clade accessions from Soares et al. (2022), has greater resolution within sect. Arthrosamanea and shows that ser. Paniculatae forms a well-resolved paraphyletic grade in which the other three series are nested. The other two non-monospecific series also appear to be non-monophyletic, with the monospecific ser. Inundatae nested inside ser. Arthrosamanea and these together in turn nested in ser. Multiflorae, although support for the paraphyly of ser. Multiflorae is only 0.72 pp.
The Old World species of Albizia form a monophyletic group (PP = 1) placed in clade E (PP = 0.96) (Fig. 3), with the genera Enterolobium Mart., Samanea, and Pseudosamanea. Within clade E Pseudosamanea (PP = 0.96) is sister to clade F which includes Enterolobium, Samanea and Old World Albizia. All these clades have high support (PP = 1). These analyses also support the transfer of Cathormionumbellatum Kosterm., which is placed in the Old World Albizia clade (Fig. 3), to Albizia, as proposed by Koenen et al. (2020).
Our analyses also confirm that the monotypic genus Hesperalbizia: H.occidentalis (Brandegee) Barneby & J.W. Grimes is sister to Lysiloma, in the Cojoba clade (sensu Koenen et al. 2020), unrelated to either New World Albizia (clade D) or Old World Albizia (clade F), as previously shown by Duno de Stefano et al. (2021). Furthermore, Pseudosamaneaguachapele (Kunth) Harms (previously Albiziaguachapele (Kunth) Dugand in Rico Arce et al. (2008)) is also unrelated to New World Albizia but is instead a member of clade E, sister to clade F which includes Enterolobium, Samanea and Old World Albizia.
In both Albiziasect.Arthrosamanea and the closely related Balizia and Hydrochorea, these phylogenies suggest that lomentiform fruits were independently derived from indehiscent fruits that are septate between the seeds, as species with the latter fruit type form paraphyletic grades to the lomentiform species in both cases (Fig. 4). In turn, these indehiscent septate fruits are nested within paraphyletic assemblages of species with fruits that dehisce along one or both sutures in both groups. Interestingly, in both cases, a single species with crypto-lomentiform fruits is found, but it is not clear whether these were derived from the same ancestral fruit type or not. In Hydrochorea this crypto-lomentiform species appears as an intermediate between indehiscent and lomentiform species, while in Albiziasect.Arthrosamanea the crypto-lomentiform-fruited A.inundata appears to be derived from a lomentiform-fruited ancestor. Another difference between these two groups is that the follicular dehiscence of Baliziapedicellaris (DC.) Barneby & J.W. Grimes fruits appears to be secondarily derived from indehiscent fruits, but we note that similar dehiscence is also found in a few species of Jupunba.
Discussion
This study addresses the non-monophyly of the genus Albizia and our results provide important insights into the evolutionary history of the neotropical species placed in sect. Arthrosamanea, with implications for their taxonomic classification. We show that sect. Arthrosamanea, with expanded taxon sampling relative to Koenen et al. (2020), and as also shown by the study of Ringelberg et al. (2022), is monophyletic with only two exceptions: Albizialeonardii Barneby & J.W. Grimes which is placed among taxa of the ‘senegalioid grade’ (Ringelberg et al. 2022; Terra et al. 2022) and Albiziacarbonaria Britton that is more closely related to Pseudosamanea (Koenen 2022b; Ringelberg et al. 2022).
The geographically-based splitting of a large genus in tribe Ingeae, such as Albizia, which occupies a pantropical distribution, is not unprecedented, nor unexpected, especially given the lack of pantropical monographic synthesis or geographically widely sampled phylogenies for the mimosoid clade. For example, the genus Pithecellobium, once the largest genus of tribe Ingeae, has been progressively divided during the last 50 years into multiple genera (see Brown et al. 2008 for a general history of the tribe). Another example is the genus Calliandra Benth. for which the New World species of Calliandra. ser. Laetevirentes were segregated into Zapoteca H.M. Hern. (Hernández 1986), and almost all Old World species allocated progressively to other segregated genera: Viguieranthus Villiers (Villiers 2002), Thailentadopsis Kosterm. (Lewis and Schrire 2003), Sanjappa E.R. Souza & M.V. Krishnaraj (Souza et al. 2016) and Afrocalliandra E.R. Souza & L.P. Queiroz (Souza et al. 2013). All these taxonomic rearrangements were supported by morpho-anatomical and molecular phylogenetic analyses. Molecular data have also demonstrated that Abarema is polyphyletic (Iganci et al. 2015), prompting reinstatement of the genera Jupunba and Punjuba Britton & Rose (Soares et al. 2021). Finally, neither Zygia P. Browne nor Marmaroxylon Killip are monophyletic, although a new generic classification for those genera has yet to be proposed (Ferm et al. 2019). The non-monophyly of Albizia documented here and elsewhere (Koenen et al. 2020; Ringelberg et al. 2022) is thus not a surprise and reflects the state of flux surrounding generic delimitation in mimosoids, especially within the ingoid clade.
Some of the taxonomic proposals of Barneby and Grimes (1996) relative to the American segregates of Albizia s.l. are confirmed by our results. The genera Balizia and Hydrochorea form part of the Jupunba clade (sensu Koenen et al. 2020) (Figs 3 and 4) (Iganci et al. 2015), although neither Hydrochorea nor Balizia are monophyletic in our phylogeny (Figs 3 and 4, see Soares et al. 2022). Two neotropical species, included in Albizia by Rico Arce et al. (2008) are also placed outside New World Albizia (Fig. 3): Hesperalbiziaoccidentalis is closely related to Lysiloma, in agreement with previous results (Iganci et al. 2015; Duno de Stefano et al. 2021); similarly, Pseudosamaneaguachapele, is also placed outside Albizia in our phylogeny, emerging, as expected, together with the other species Pseudosamaneacubana (Britton & Rose) Barneby & J.W. Grimes, although relationships within this clade are unresolved (Fig. 3).
Here we show that the dehiscent, papery, plano-compressed fruit type is ancestral within Albiziasect.Arthrosamanea (Fig. 4) and is associated with species growing predominantly in seasonally dry tropical forest and woodland, with successive shifts to septate indehiscent fruits and then lomentiform fruits with hydrochorous seed dispersal associated with species growing in seasonally inundated varzea forest, riverine habitats and low-lying margins of palm and white-sand savannas (Fig. 4). Interestingly, in the sister group of Albiziasect.Arthrosamanea, the mainly neotropical clade composed of Jupunba, Punjuba, Balizia, and Hydrochorea, a similar parallel evolutionary transition in fruit types is apparent. In Jupunba and Punjuba, fruits are always dehiscent, while a transition to septate indehiscent fruits occurred in Balizia, an exception being Baliziapedicellaris which has follicular dehiscence and a newly described species with crypto-lomentiform fruits (Fig. 4, and Soares et al. 2022). Nested within the paraphyletic Balizia is a clade comprising the genus Hydrochorea plus two African species of Cathormion, all species of which have indehiscent lomentiform fruits adapted for hydrochory and are found in riparian or other periodically flooded habitats in the Amazon basin, West Africa and the Congo basin (Fig. 4, and Soares et al. 2022).
Barneby and Grimes (1996) pointed out that a radiation of species with similarly heterogeneous fruit types to that seen in section Arthrosamanea occurs in Madagascan Albizia s.s. and that the association between lomentiform fruits, hydrochorous seed dispersal, and seasonally flooded habitats is also apparent in Old World Albizia s.s. For example, Albiziadolichadena, A.moniliformis, A.rosulata, and A.umbellata from Australasia also have lomentiform fruits and are distributed near streams or in riparian and swamp forests (Rico Arce et al. 2008). Furthermore, as indicated above and pointed out by Barneby and Grimes (1996), similar transitions to lomentiform fruits have occurred in parallel in several other lineages across the ingoid clade, including Cathormionaltissimum (Hook.f.) Hutch. & Dandy (sometimes referred to as Albiziaaltissima Hook.f.; Koenen 2022a) and Senegaliarostrata (Humb. & Bonpl. ex Willd.) Seigler & Ebinger (syn. Dugandiarostrata (Humb. & Bonpl. ex Willd.) Britton & Killip, syn. Manganaroaarticulata Speg.; Barneby and Grimes 1996: 204) in all cases apparently also closely associated with riparian and/or periodically inundated habitats. These repeated parallel derivations of similar, but not strictly homologous fruit types attest to the high evolvability of the mimosoid fruit more generally. In the light of phylogenetic evidence, it is now clear that these evolutionarily highly labile morphological adaptations of the fruit related to seed dispersal syndrome do not provide reliable characters for generic delimitation, supporting inclusion of the species that were placed in ser. Arthrosamanea, ser. Inundatae and ser. Multiflorae within Albiziasect.Arthrosamanea by Barneby and Grimes (1996), i.e., the clade of New World Albizia that is recovered in our analysis.
Taxonomic treatment
There are two validly published generic names – Pseudalbizzia of Britton and Rose (1928) and Arthrosamanea of Britton and Killip (1936) – that could be applied to the New World clade of Albizia. In accordance with Principle III of the International Code of Nomenclature (Turland et al. 2018), we reinstate Pseudalbizzia, the earlier name associated with this clade, and provide the corresponding new combinations for its constituent species.
Pseudalbizzia
Britton & Rose, N. Am. Fl. 23: 48. 1928.
2CAC816E-545E-5599-8431-91F617C81151
Type.
Pseudalbizziaberteroana Britton & Rose.
Arthrosamanea Britton & Rose, in Britton & Killip, Ann. New York Acad. Sci. 35: 128, 1936. AlbiziasectionArthrosamanea (Britton & Rose) Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 206. 1996. Type: Arthrosamaneapistaciifolia Britton & Rose.
Description.
Unarmed trees with sympodial growth, up to 30 m, rarely small treelets of c. 3 m, microphyllidious to macrophyllidious; trunk 35–120(–150) cm dbh; young stems and all leaves and inflorescence-axes more or less densely tomentellous to pilosulous; stipules puberulent to glabrous, deltate, narrowly triangular, triangular-ovate, narrowly ovate, or narrowly lanceolate, veinless or faintly 3-veined, falling early to tardily, perhaps sometimes obsolete and/or lacking on mature leaves. Leaves bipinnate, not sensitive, (1–)2–15(–19) pairs of pinnae; leaflets (2–)16–52(–63) pairs per pinna; a nectary immediately below first pair of pinnae, near or well below mid-petiole, sometimes lacking or reduced to a minute pore, round, elliptic or vertically elongate, either shallow-cupular or almost plane, thick-rimmed, sometimes immersed in petiolar groove or even obsolete, much smaller nectaries at some distal pinnae, at the tip of most pinnae, and between 1–2 furthest pairs of leaflets; leaflets gently decrescent toward each end of the rachis or toward the base of the rachis or sub-equilong, the first pair of leaflets often reduced to paraphyllidia, sometimes minute, sometimes absent or perhaps falling early, the blades of the remaining leaflets elliptic, elliptic-ovate, oblong-elliptic, narrowly oblong-elliptic, lance-oblong to linear-lanceolate, base obliquely truncate to shallowly semi-cordate, apex deltately subacute, deltately acute to subacute, obtuse or apiculate, the larger ones (1.5–)2–4(–6) times as long as wide, margin strongly to slightly revolute; venation generally palmate, of 2–4(–5) veins from the pulvinule, the nearly straight main vein a little forwardly displaced and giving rise on each side to 2–13 major secondary veins, the inner of 2(–3) posterior primary veins incurved-ascending to anastomose slightly beyond mid-blade, the outer posterior vein and sometimes a faint anterior one very short and weak, all venation immersed on upper face. Inflorescence primary axis up to 30 cm long; peduncles (1–)2–8(–10) per node of the capitulate or corymbose-umbellate inflorescence, capitula 8–26(–40)-flowered; bracts heteromorphic or homomorphic, ovate, oblong-obovate or spatulate, linear-spatulate, falling early or persistent, sessile or shortly pedicellate, the flowers moderately to strongly dimorphic, the terminal ones generally longer. Flowers 5-merous, rarely 6-merous, glabrous to densely pubescent externally. Peripheral flowers: calyx campanulate, turbinate, turbinate-campanulate or narrowly campanulate, sessile or short pedicellate, lobes very short, depressed-deltate, ovate or triangular, glabrous or puberulent; corolla narrowly trumpet-shaped, erect or recurved, lobes ovate to lance-ovate; androecium with 9–30(–32) stamens, up to 20 mm long, united at the base forming a clear stemonozone, the staminal tube as long or longer than the stemonozone; ovary sessile or shortly stipitate, slenderly ellipsoid, conical at apex, glabrous or pubescent; style a little longer than the stamens, slightly dilated at the stigma. Terminal flowers: sessile or almost so, calyx shallowly campanulate to broadly campanulate, corolla tubular; androecium with 16–38(–42) stamens, 8.5–11.5(–13) mm long, united at the base forming a clear stemonozone, staminal tube equalling or longer than the stemonozone. Fruits solitary, or rarely 2–4 per capitulum, sessile, subsessile or cuneately contracted at base into a short pseudo-stipe, the body linear, linear-elliptic, narrowly elliptic-oblong, straight or nearly straight, sometimes decurved, plano-compressed, apex rounded but minutely apiculate to obtuse, (8–)13(–15)-seeded; valves papery, coriaceous, or grossly ligneous, olivaceous, castaneous, fuscous-greenish, or brown becoming tan-brown, closely transverse venulose, minutely puberulous, tomentulose, glabrescent to glabrous, framed by straight sutures or dilated, sometimes 3-angulate but not winged, transversely or horizontally, dehiscence tardy to very tardy, inert, through both sutures or dehiscence 0, in the latter, the pod crypto-lomentiform, incipiently lomentiform or lomentiform, then the whole fruit long persistent on the tree, commonly falling entire and breaking on the ground into 8–12 individually indehiscent segments, funicle apically sigmoid or ribbon-like (not sigmoid), lentiform; seeds obliquely ascending or straight, disciform, oblong-ellipsoid, elliptic, strongly compressed, the translucent, brownish or greyish testa produced as a peripheral wing, adherent to the embryo, which does not fill the testa-cavity, the pleurogram small, inversely U-shaped or U-shaped.
Notes.
The genus forms a group that is homogeneous in most respects, but diverse in the late developmental stages of the fruit, including: 1) fruit opening type: dehiscent, indehiscent, or irregularly breaking, 2) lateral shape: flat to conspicuously raised over the seed chambers, 3) texture and consistency of the valves: papery, chartaceous to woody (Barneby and Grimes 1996). Figs 1, 2 and 4.
Pseudalbizzia (clade D) is the sister group of the Jupunba-Punjuba-Balizia-Hydrochorea clade (Fig. 3). Jupunba and Punjuba are markedly different morphologically, having spirally twisted dehiscent fruits with a red or ochre endocarp, reminiscent of the fruits of several other genera in tribe Ingeae (e.g., some Pithecellobium species, and some species of Archidendron F. Muell. and Cojoba Britton & Rose). The red or red-brown testa of the seeds of Jupunba and Punjuba are very distinctive, and are never black, and the embryo is nearly always aniline-blue due to the presence of delphinidin (an anthocyanidin). Punjuba is furthermore distinguished by its spicate inflorescences, which are not seen in Pseudalbizzia. Balizia has ligneous, indehiscent or tardily dehiscent pods, their seeds being released sometimes only after decay of the valves on the floor of terra firme forest, whereas in Hydrochorea the fruits are lomentiform, adapted to dispersal by water. The fruits of Hydrochorea recall some species of Pseudalbizzia adapted to similar riparian habitats. However, the species of Pseudalbizzia are markedly different in form of inflorescence, leaflet-venation, and shape of the ovary.
Two species previously placed in Albizia from the New World which were not included in our phylogenetic analysis, Albiziacarbonaria and A.leonardii, have since been shown to be placed outside the New World Albizia clade (Ringelberg et al. 2022; Koenen 2022b; Terra et al. 2022). Two other species, also not sampled here, nor by Ringelberg et al. (2022), are here tentatively included in Pseudalbizzia: Albiziabarinensis L. Cárdenas and Albiziabuntingii Barneby & J.W. Grimes (see below for discussion about the placement of these species). The genus Pseudalbizzia was published in the Flora of North America (Britton and Rose 1928) and included just a single species, P.berteroana. The original description of Pseudalbizzia closely matches Albizia and no characters distinguishing the two genera were discussed by Britton and Rose (1928). The generic name Arthrosamanea was also published by Britton & Rose, again with a single species, A.pistaciifolia (Willd.) Britton & Rose, in an account of the Mimosaceae and Caesalpiniaceae of Colombia (Britton and Killip 1936), but again no differences between the genus and Albizia or Pseudalbizzia were mentioned.
Pseudalbizzia as circumscribed here comprises 17 species and 5 varieties ranging in distribution from northwestern Mexico to northern Argentina and including the Greater Antilles (Figs 5 and 6). Full synonymy, detailed species descriptions, geographical distributions, representative samples of all species and keys for their identification can be found (under the name Albizia) in Barneby and Grimes (1996), Linares (2005) and Rico Arce et al. (2008). Finally, we propose a new sectional classification of Pseudalbizzia to account for the non-monophyly of the series of Barneby and Grimes (1996), based on the phylogenies (Figs 3 and 4) which sampled nearly all species. A key to the sections is provided.
Figure 5.
Distribution map of Pseudalbizzia sections Paniculata, Pseudalbizzia, Uninervia and Pseudalbizziabuntingii (incertae sedis), as per the legend.
Figure 6.
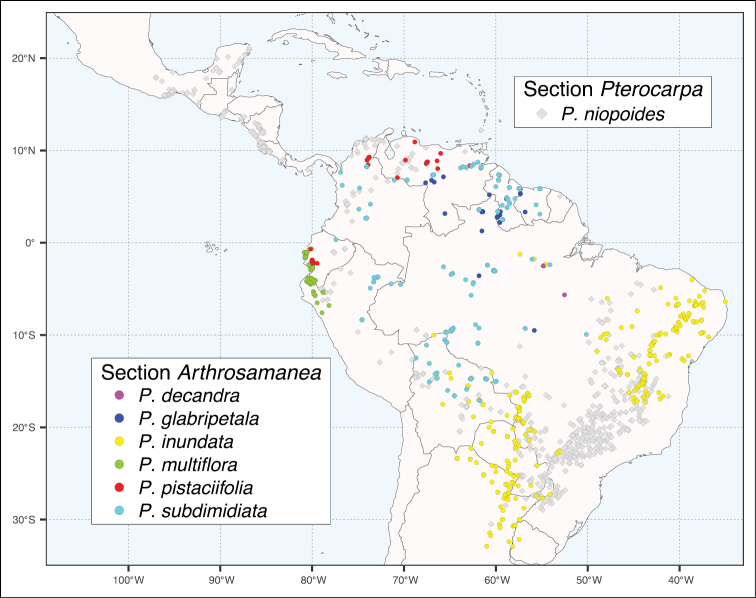
Distribution map of Pseudalbizzia sections Arthrosamanea and Pterocarpa, as per the legend.
Key to the sections of the genus Pseudalbizzia
| 1 | Leaflets with a single vein from the pulvinule | sect. Uninervia |
| – | Leaflets with 3–5 veins from the pulvinule | 2 |
| 2 | Fruits with a narrowly winged margin, seeds oblique, foliage microphyllidious | sect. Pterocarpa |
| – | Fruit margins not winged, or if winged, then foliage macrophyllidious and seeds straight | 3 |
| 3 | Fruits indehiscent and septate or lomentiform | sect. Arthrosamanea |
| – | Fruits dehiscent, plano-compressed, valves papery, not septate | 4 |
| 4 | Micro- to mesophyllidious foliage, distributed in South America | sect. Paniculata |
| – | Macro- or microphyllidious foliage, distributed in Mexico, Central America and the Caribbean | sect. Pseudalbizzia |
Pseudalbizzia sect. Paniculatae
(Benth.) E.J.M. Koenen & Duno stat. nov. and sect. nov.
51C280F7-9E14-54A5-A99D-9D3E63CC69FE
urn:lsid:ipni.org:names:77303802-1
Pithecellobium sect. Samanea ser. Paniculatae Benth. pro parte, London J. Bot. 3: 219. 1844.
Albizia sect. Arthrosamanea ser. Paniculatae (Benth.) Barneby & J.W. Grimes pro parte, Mem. New York Bot. Gard. 74(1): 208. 1996. Type species (designated by Barneby and Grimes, Mem. New York Bot. Gard. 74(1): 208. 1996.): Pithecellobiumpolycephalum Benth. = Pseudalbizziapolycephala (Benth.) E.J.M. Koenen & Duno.
Pithecellobium sect. Samanea ser. Parviflorae [sic] Benth. pro parte, Trans. Linn. Soc. London 30: 591 (exclus. sp. 77). 1875 & in Martius, Fl. Bras. 15(2): 445. 1876. Type species (designated by Barneby and Grimes, Mem. New York Bot. Gard. 74(1): 208. 1996.): Pithecellobiumpolycephalum Benth. = Pseudalbizziapolycephala (Benth.) E.J.M. Koenen & Duno.
Type.
Pithecellobiumpolycephalum Benth. = Pseudalbizziapolycephala (Benth.) E.J.M. Koenen & Duno.
Notes.
Micro- to mesophyllidious trees with paniculate compound inflorescences of efoliate pseudoracemes and dehiscent plano-compressed papery fruits. Four species of humid, semi-deciduous and seasonally dry tropical and extratropical forests and woodland in South America (Fig. 5).
Pseudalbizzia barinensis
(L. Cárdenas) E.J.M. Koenen & Duno comb. nov.
69D7200D-E6A5-5EC2-9482-4A99AA5F26F6
urn:lsid:ipni.org:names:77303803-1
Basionym.
Albiziabarinensis L. Cárdenas, Ernstia 21: 5, f. sn. 1983.
Type.
Venezuela. Barinas, muy cerca de Punta de Piedra, 3 Apr 1976, L. Cardenas de Guevara 2273 (holotype: MY; isotypes: BM!, F! [F0093839F], K! [K000527984], NY! [NY00001781], RB! [RB00539860], US! [US00385615], VEN).
Notes.
This species has not been included in any phylogenetic analysis, but its foliage, efoliate pseudoracemes and plano-compressed papery fruits leave little doubt that it should be placed in Pseudalbizzia. It is here included in section Paniculata based on these characters and its South American distribution.
Pseudalbizzia coripatensis
(Rusby) E.J.M. Koenen & Duno comb. nov.
0B73E401-51FE-5679-8C68-8B4BD54CB84E
urn:lsid:ipni.org:names:77303804-1
Basionym.
Pithecellobiumcoripatense Rusby, Bull. New York Bot. Gard. 4: 349. 1907.
Type.
Bolivia. La Paz, Sur Yungas, at Coripata, 6 May 1894, M. Bang 2176 (holotype: NY! [NY00334642]; isotypes: BM! [BM000952433], G-2! [G00364414, G00364429], GH-2! [GH00064010, GH00064011], M! [M0218258], K! [K000527985], MINN, MO! [MO-954213], US).
Pseudalbizzia edwallii
(Hoehne) E.J.M. Koenen & Duno comb. nov.
698DB3B9-86E0-5B91-9745-37BB74A055DB
urn:lsid:ipni.org:names:77303805-1
Basionym.
Pithecellobiumedwallii Hoehne, Bol. Inst. Brasil. Sci. 2: 243. 1926.
Type.
Brazil, São Paulo, G. Edwall 5608 (lectotype: SP, designated by Barneby and Grimes, Mem. New York Bot. Gard. 74(1): 209. 1996).
Pseudalbizzia polycephala
(Benth.) E.J.M. Koenen & Duno comb. nov.
AFACA64D-2A08-57CE-87AE-F8501CC2B269
urn:lsid:ipni.org:names:77303806-1
Basionym.
Pithecellobiumpolycephalum Benth., London J. Bot. 3: 219. 1844.
Type.
Brazil. Rio de Janeiro, J.B.E. Pohl 1420 (lectotype: K! (herb. Bentham) [K000528000], designated by Barneby and Grimes, Mem. New York Bot. Gard. 74(1): 208. 1996).
Pseudalbizzia sect. Uninervia
E.J.M. Koenen & Duno sect. nov.
85763601-3140-5932-A621-CC4521B16C7E
urn:lsid:ipni.org:names:77303807-1
Type.
Albiziaburkartiana Barneby & J.W. Grimes = Pseudalbizziaburkartiana (Barneby & J.W. Grimes) E.J.M. Koenen & Duno.
Notes.
Microphyllidious trees with the inflorescences of section Paniculata, but with a single vein from the pulvinule at the base of the leaflets. A single, narrowly endemic species in Paraná pine woodland and the Southern Mata Atlantica of Brazil (Fig. 5).
Pseudalbizzia burkartiana
(Barneby & J.W. Grimes) E.J.M. Koenen & Duno comb. nov.
7750A1A4-3BC0-5644-BEA1-054594AA514E
urn:lsid:ipni.org:names:77303808-1
Basionym.
Albiziaburkartiana Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 211–212. 1996.
Type.
Brazil. Santa Catarina, Capinzal, on upper Rio Uruguai, 700 m, 21 Dec 1973, P.R. Reitz & R. M. Klein 14359 (holotype: NY! [NY00001783]; isotype: US! [US00811452]).
Notes.
In the protologue the fruits were not described as these were not known at that time. This rare, locally endemic species has since been collected in fruit (Stival-Santos 678, BR), and we here provide a description of these. Fruits sessile but with a narrow pseudo-stipitate base, dehiscent along both slightly thickened sutures, the valves plano-compressed, papery in texture, light brown with finely prominent transverse veins, 6.5–12 × 1.2–1.6 cm, 7–12-seeded when well-fertilized.
Pseudalbizzia sect. Pseudalbizzia
.
5BE01C55-1AF6-500C-90AC-7AFE6176F62F
Notes.
Trees with micro- or macrophyllidious foliage, inflorescences composed of efoliate pseudoracemes arising singly from a leaf axil or sometimes the capitula solitary or paired in the leaf axils, or the pseudoracemes combined into a terminal panicle, fruits plano-compressed with papery valves, dehiscent along both sutures or more rarely indehiscent (in P.berteroana), sometimes with a winged margin, seeds straight. Four species predominantly of seasonally dry tropical forests in Mexico, Central America and the Caribbean (Fig. 5).
Pseudalbizzia adinocephala
(Donn. Sm.) E.J.M. Koenen & Duno comb. nov.
9481FEBE-D8DF-5829-935C-B582EED8281A
urn:lsid:ipni.org:names:77303809-1
Albizia xerophytica J. Linares, syn. nov., Revista Mex. Biodiversidad 76: 7. 2005. Type: Honduras. El Paraíso, Municipio Morocelí, orillas de Quebrada Grande c. 3.9 km al NE de Morocelí por el camino hacia El Plan. 2002. J.L. Linares et al. 5674 (holotype: MEXU! [MEXU01160777]; isotype: EAP).
Basionym.
Pithecellobiumadinocephalum Donn. Sm., Bot. Gaz. Crawfordsville. 57: 419. 1914.
Type.
Costa Rica. San José, Ad fundum La Verbena prope Alajuelita, 100 m, Aug 1894, A. Tonduz 8932 (US-3); Dec 1894 (lectotype: A. Tonduz 9077 [US-212774]!; isolectotypes: BR-3! [BR0000005189519, BR0000005189182, BR0000005189847], G! [G00364416], designated by Barneby and Grimes, Mem. New York Bot. Gard. 74(1): 218. 1996).
Notes.
Albiziaxerophytica was described from material from dry forest habitats in southern Honduras based on minor differences in leaf and fruit morphology, but we do not consider these to be significantly different from the range of variation that is observed in P.adinocephala and prefer the broader concept of the species as described in Barneby and Grimes (1996: 218–220). The difference in habitat (i.e., lower rainfall regions) also appears to be minor, as some specimens from wetter sites have been identified as A.xerophytica (see map in Rico Arce et al. 2008) while specimens of P.adinocephala have been collected across the full range of drier and wetter sites. Finally, the distribution of A.xerophytica is entirely enclosed by the much wider range of P.adinocephala.
Pseudalbizzia berteroana
(Balb. ex DC.) Britton & Rose, N. Amer. Fl. 23: 48. 1928.
8DFA2951-ABC6-58DB-9606-6EC6DDEDD7D4
Basionym.
Acaciaberteroana Balb. ex DC., Prodr. 2: 470. 1825.
Type.
Republica Dominicana, Sto. Domingo, C.L.G. Bertero, herb. Balbis s.n., 1821 (holotype: G; isotype: M! [M0218254]).
Pseudalbizzia sinaloensis
(Britton & Rose) E.J.M. Koenen & Duno comb. nov.
A29E728A-2240-5690-BD6D-285826FC85EE
urn:lsid:ipni.org:names:77303810-1
Basionym.
Albizia sinaloënsis in Britton & Rose, N. Amer. Fl. 23(1): 45. 1928.
Type.
Mexico. Sinaloa, vicinity of Fuerte, 26 March 1910, J.N. Rose, P.C. Standley & Russell 13559 (holotype: NY! [NY00001775]; isotype: US! [US00000483]).
Pseudalbizzia tomentosa
(M. Micheli) E.J.M. Koenen & Duno comb. nov.
40BDE739-729D-5963-8ACA-852EF9E64D0D
urn:lsid:ipni.org:names:77303811-1
Basionym.
Pithecellobiumtomentosum M. Micheli, Mém. Soc. Phys. Genève 34: 285, t. 28. 1903.
Type.
Mexico. Michoacán, rives de l’Espiritu Santo, 600 m, 19 April 1898 [E. Langlassé] 107 (G): Zilmatango, 30 m, aout 1898, n 280 (G). (lectotype: E. Langlassé 107 G-385667!; isolectotypes: K! [K000082098], NY (fragm.)! [NY00001777], designated by Standley, Contr. U.S. Natl. Herb. 23: 396. 1922).
Pseudalbizzia tomentosa var. nayaritensis
(Britton & Rose) E.J.M. Koenen & Duno comb. nov.
31D62C93-B409-5602-9EDB-FB676F83886F
urn:lsid:ipni.org:names:77303812-1
Basionym.
Albizzianayaritensis Britton & Rose, N. Amer. Fl. 23: 47. 1928.
Type.
Mexico. Nayarit; San Blas, La Palma, 20 m, 1923, J. González Ortega 90N (holotype: US! [US00918691]; isotypes: K! [K000082100], NY-2! [NY00001768, NY00001769]).
Pseudalbizzia tomentosa var. purpusii
(Britton & Rose) E.J.M. Koenen & Duno comb. nov.
081C2877-AA81-5327-8F84-B18849331B3E
urn:lsid:ipni.org:names:77303813-1
Basionym.
Albizziapurpusii Britton & Rose, N. Amer. Fl. 23: 45. 1928.
Type.
Mexico. Veracruz, Rancho Remudadero, 19°15'N, 96°34'W, April 1922, C.A. Purpus 8723 (holotype: NY! [NY00001773]; isotypes: GH! [GH00069252], MO! [MO-120564], UC! [UC214372], US! [US00000479]).
Pseudalbizziatomentosavar.tomentosa
Pseudalbizzia sect. Pterocarpa
E.J.M. Koenen & Duno sect. nov.
64902C4B-17E1-5620-9281-ADD482E16FC1
urn:lsid:ipni.org:names:77303814-1
Type.
Pithecellobiumniopoides Spruce ex Benth. = Pseudalbizzianiopoides (Spruce ex Benth.) E.J.M. Koenen & Duno.
Notes.
Microphyllidious trees with the inflorescence usually composed of axillary efoliate pseudoracemes, sometimes a partly or wholly terminal panicle (but not surpassing the foliage), the fruit with a narrowly winged margin and seeds oblique. A single widespread species found in deciduous seasonally dry forests, gallery forest, and evergreen forests in Mexico, Central and South America (Fig. 6).
Pseudalbizzia niopoides
(Spruce ex Benth.) E.J.M. Koenen & Duno comb. nov.
E1372BC2-152D-560D-B5A1-10A3BF1297C0
urn:lsid:ipni.org:names:77303815-1
Basionym.
Pithecellobiumniopoides Spruce ex Benth., Trans. Linn. Soc. London 30: 591. 1875.
Type.
Brazil, Pará, Santarem, Nov 1851, R. Spruce 1088, Herb. Bentham (holotype: K! [K000528013]).
Pseudalbizzia niopoides var. colombiana
(Britton) E.J.M. Koenen & Duno comb. nov.
3C0828F6-F254-508D-8F6C-B1C8EB40D791
urn:lsid:ipni.org:names:77303816-1
Albizia niopoides var. colombiana (Britton) Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 222. 1996.
Basionym.
Albizziacolombiana Britton, in Britton & Killip, Ann. New York Acad. Sci. 35: 131. 1936.
Type.
Colombia. Magdalena, near Bonda, Santa Marta, 3 August 1899, H.H. Smith 38 (holotype: NY! [NY00001784]; isotypes: BR! [BR0000005111176], E! [E00313853], K! [K000527990], NY!, U-2! [U0003354, U1253389]).
Pseudalbizzianiopoidesvar.niopoides
Pseudalbizzia sect. Arthrosamanea
(Britton & Rose) E.J.M. Koenen & Duno comb. nov.
F83EDACB-A757-557A-8FFE-7B78995BC5BA
urn:lsid:ipni.org:names:77303817-1
Arthrosamanea Britton & Rose, Ann. New York Acad. Sci. 35: 128, pro gen. 1936, sensu stricto.Albiziasect.Arthrosamanea (Britton & Rose) Barneby & J.W. Grimes pro parte, Mem. New York Bot. Gard. 74(1): 206. 1996. Type species: Arthrosamaneapistaciifolia (Willd.) Britton & Rose = Mimosapistaciifolia Willd. = Pseudalbizziapistaciifolia (Willd.) E.J.M. Koenen & Duno.
Albizia sect. Arthrosamanea ser. Multiflorae Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 234. 1996.
Albizia sect. Arthrosamanea ser. Inundatae Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 238. 1996.
Notes.
Micro- or macrophyllidious trees, usually the efoliate pseudoracemes arising singly and only rarely arranged in panicles, fruits indehiscent and septate, or lomentiform, one species crypto-lomentiform. Six species of usually humid, often seasonally inundated forest or riparian habitats in South America (Fig. 6).
Pseudalbizzia decandra
(Ducke) E.J.M. Koenen & Duno comb. nov.
C886C9E6-0DD6-554B-8591-77019E4D11BB
urn:lsid:ipni.org:names:77303818-1
Basionym.
Pithecellobiumdecandrum Ducke, Arch. Jard. Bot. Rio de Janeiro 5: 121. 1930.
Type.
Brazil. Pará, habitat in silvis non inundatis civitatis Pará circa Óbidos, A. Ducke (Herb. Amaz. Mus. Pará 15.724, et H.J.B.R. 10.174) et loco Serra do Dedal ad lacum Faro, A. Ducke (H.J.B.R. 20.198), ubi florebat Januario 1927, A. Ducke (lectotype: A. Ducke 10174 RB!; isolectotypes: G! [G00364418], K-2!: [K000527990, K000527998], U-2! [U0003349, U0003350], designated by Barneby and Grimes, Mem. New York Bot. Gard. 74(1): 234. 1996.).
Pseudalbizzia glabripetala
(H.S. Irwin) E.J.M. Koenen & Duno comb. nov.
5DB4921F-BAAA-53D4-B4ED-9845484E6CA0
urn:lsid:ipni.org:names:77303819-1
Basionym.
Pithecellobiumglabripetalum H.S. Irwin, in Mem. New York Bot. Gard. 15(1): 109. 1966.
Type.
Guyana. Orealla, Corantyne River, Oct 1879, G.S. Jenman 364 (holotype: NY! [NY00334664]; isotypes: BM!, P!).
Pseudalbizzia inundata
(Mart.) E.J.M. Koenen & Duno comb. nov.
4C06AB12-BD37-580B-8D43-94C8C87C9632
urn:lsid:ipni.org:names:77303820-1
Basionym.
Acaciainundata Mart., Spix & Mart. in Reise Bras. 1: 555. 1823.
Type.
Brazil. Minas Gerais, Rio Sao Francisco, 1818, C.F.P. von Martius 1659 (holotype: M! [M0218478]; isotypes: K! [K000797598], NY!).
Pseudalbizzia multiflora
(Kunth) E.J.M. Koenen & Duno comb. nov.
A8A22C6C-E33C-5EFC-A39A-51203902A971
urn:lsid:ipni.org:names:77303821-1
Basionym.
Acaciamultiflora Kunth, Nov. Gen. Sp. (quarto ed.) 6: 277–278. 1823.
Type.
Peru. Cajamarca, Prov. Jaén, San Felipe, 980 m, Aime Bonpland & F.W.H.A. von Humboldt 3562 (holotype: P! [P00679365]).
Pseudalbizziamultifloravar.multiflora
Pseudalbizzia multiflora var. sagasteguii
(Barneby & J.W. Grimes) E.J.M. Koenen & Duno comb. nov.
A92F7886-FF53-5102-A831-F852F3490F16
urn:lsid:ipni.org:names:77303822-1
Basionym.
Albiziamultifloravar.sagasteguii Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 237–238. 1996.
Type.
Peru. Cajamarca, Prov. Contumazá, in a quebrada near San Benito, A. Sagástegui 15410 (holotype: F! [F0042945F]; isotypes: MO! [MO-149743], NY!, US! [US00624358]).
Pseudalbizzia pistaciifolia
(Willd.) E.J.M. Koenen & Duno comb. nov.
469A491C-8055-526E-A64C-B76A0C7E09A0
urn:lsid:ipni.org:names:77303823-1
Basionym.
Mimosapistaciaefolia [sic] Willd., Sp. Pl. 4: 1028. 1806.
Type.
Venezuela. Caracas. F. Bredemeyer s.n., herb. Willdenow (holotype: B).
Pseudalbizzia subdimidiata
(Splitg.) E.J.M. Koenen & Duno comb. nov.
22F0902D-975E-5A6A-A042-02DFA0690D44
urn:lsid:ipni.org:names:77303824-1
Albizia subdimidiata (Splitg.) Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 234. 1996.
Basionym.
Acaciasubdimidiata Splitg. Tijdschr. Natuurl. Gesch. Physiol. 9: 112 (1842).
Type.
Suriname. “ad ripas fluminis Surinami superioris”, 27 April 1838. Splitgerber 917 (holotype: L [L0018505]).
Pseudalbizzia subdimidiata var. minor
(Barneby & J.W. Grimes) E.J.M. Koenen & Duno comb. nov.
29ACAF5B-D73B-54EC-AEA0-A3DF6CFAED32
urn:lsid:ipni.org:names:77303825-1
Basionym.
Albiziasubdimidiatavar.minor Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 234. 1996.
Type.
Guyana. Basin of Essequibo river, Kuyaliwak Falls, 1 Jan 1937, A.C. Smith 2156 (holotype: NY! [NY00001790]; isotypes: A! [A00069262], G! [G00364427], K! [K000528004], P, U! [U0003358]).
Pseudalbizziasubdimidiatavar.subdimidiata
Incertae sedis
Pseudalbizzia buntingii
(Barneby & J.W. Grimes) E.J.M. Koenen & Duno comb. nov.
C7F0C7C3-80B8-5C49-9F61-3D3BCD423547
urn:lsid:ipni.org:names:77303826-1
Basionym.
Albiziabuntingii Barneby & J.W. Grimes, Mem. New York Bot. Gard. 74(1): 223. 1996.
Type.
Venezuela. Zulia, alrededores de Casigua El Cubo, 100 m, al este del empalme de la via hacia Casigua con la carretera Machiques-La Fría, 25 Feb 1985, G.S. Bunting 13370 (holotype: NY! [NY00001782]).
Notes.
Fruits of this species are unknown and the species is only known from the type locality (Fig. 5), but it is similar in leaf and inflorescence morphology to several South American species of Pseudalbizzia, as described in the protologue. Especially the efoliate pseudoracemes point to this species most likely being correctly accommodated in Pseudalbizzia. Collection of fruits and/or inclusion of the species in phylogenetic studies is needed to confirm its generic and sectional placements.
Non-native species
Some cultivated and sometimes naturalized Old World Albizia species are found in the New World, including: A.procera (Roxb.) Benth., A.julibrissin, A.lebbeck (L.) Benth., and A.chinensis (Osbeck) Merr. For these species, Barneby and Grimes (1996) proposed AlbiziasectionAlbizia, now considered as Albizia s.s.
Author contributions
GAP, RR, GCFC, IVM, and RDD designed the study. GAP, LLCI, ELC, RDD contributed labwork. RDD, GCFC, IRM contributed data by supervising students in the lab. EJMK, RDD, XC, SM and CEH contributed taxonomic knowledge, JR contributed species distribution data and the maps. GAP, RDD, ITC, JRP, and RR undertook the phylogenetic analyses. EJMK, RDD, RR, GCFC, CEH and JR contributed to writing the manuscript.
Supplementary Material
Acknowledgements
We thank the National Council of Science and Technology (CONACYT) for financial support through project 81799 and the Centro de Investigación Científica de Yucatán A.C. (CICY). The mobility scholarship program for students of CONACYT supported the research stay by GAP at the Real Jardín Botánico-CSIC under the supervision of RR, and EJMK and JR were supported by the Swiss National Science Foundation (Early.Postdoc.Mobility fellowship P2ZHP3_199693 to EJMK, and grants 310003A_156140 and 31003A_182453/1 to CH). The visit to Aarhus University (Denmark) as well as Chiang Mai University (Thailand) was supported by a grant from the Carlsberg Foundation under the Flora of Thailand project (Dr. Henrik Balslev). We thank curators of the herbaria AAU, CICY, FCMEMEXU, MO, MA, and MEXU, who provided material for this study. All molecular work was done in the Laboratory of Molecular Markers (Unidad de Recursos Naturales). We remain especially grateful to Nestor Raigoza, Matilde Margarita Ortíz García, Verónica Limones Briones, and Silvia Hernandez for laboratory support. Finally, we thank Gwilym Lewis for comments and editorial input. An earlier version of this article was part of the Master thesis of GAP.
Citation
Aviles Peraza G, Koenen EJM, Riina R, Hughes CE, Ringelberg JJ, Carnevali Fernández-Concha G, Ramírez Morillo IM, Can Itza LL, Tamayo-Cen I, Ramírez Prado JH, Cornejo X, Mattapha S, Duno de Stefano R (2022) Re-establishment of the genus Pseudalbizzia (Leguminosae, Caesalpinioideae: mimosoid clade): the New World species formerly placed in Albizia. In: Hughes CE, de Queiroz LP, Lewis GP (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations. PhytoKeys 205: 371–400. https://doi.org/10.3897/phytokeys.205.76821
Funding Statement
National Council of Science and Technology (CONACYT) (grant 81799), Centro de Investigación Científica de Yucatán A.C. (CICY), Carlsberg Foundation, and Swiss National Science Foundation (grants 310003A_156140 and 31003A_182453/1)
References
- Alfaro ME, Zoller S, Lutzoni F. (2003) Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Molecular Biology and Evolution 20: 255–266. 10.1093/molbev/msg028 [DOI] [PubMed] [Google Scholar]
- Baldwin BG, Markos S. (1998) Phylogenetic Utility of the External Transcribed Spacer (ETS) of 18S–26S rDNA: Congruence of ETS and ITS trees of Calycadenia (Compositae). Molecular Phylogenetics and Evolution 10: 449–463. 10.1006/mpev.1998.0545 [DOI] [PubMed] [Google Scholar]
- Barneby RC, Grimes JW. (1996) Silk tree, Guanacaste, Monkey’s earring: A generic system for the synandrous Mimoseae of the Americas. Part I. Abarema, Albizia and allies. Memoirs of the New York Botanical Garden 74: 1–292. [Google Scholar]
- Britton NL, Killip EP. (1936) Mimosaceæ and Caesalpiniaceæ of Colombia. Annals of the New York Academy of Sciences 35: 101–228. 10.1111/j.1749-6632.1933.tb55366.x [DOI] [Google Scholar]
- Britton NL, Rose JN. (1928) North American Flora. Part I. (Rosales). Mimosaceae. 23: 1–194. [Google Scholar]
- Brown GK, Murphy DJ, Miller JT, Ladiges PY. (2008) Acacia s.s. and its relationship among tropical legumes, Tribe Ingeae (Leguminosae: Mimosoideae). Systematic Botany 33: 739–751. 10.1600/036364408786500136 [DOI] [Google Scholar]
- Duno de Stefano R, Tun Tun C, López Contreras JE, Carnevali Fernández-Concha G, Leopardi Verde CL, Ramírez-Prado JH, Can Itza LL, Tamayo Cen I. (2021) Phylogeny of Lysiloma (Fabaceae), a genus restricted to Megamexico with outliers in the West Indies and Florida. Acta Botánica Mexicana 128: e1728. 10.21829/abm128.2021.1782 [DOI]
- Ferm J, Korall P, Lewis GP, Ståhl B. (2019) Phylogeny of the Neotropical legume genera Zygia and Marmaroxylon and close relatives. Taxon 68: 661–672. 10.1002/tax.12117 [DOI] [Google Scholar]
- Hernández HM. (1986) Zapoteca: A new genus of Neotropical Mimosoideae. Annals of the Missouri Botanical Garden 73: 755–763. 10.2307/2399204 [DOI] [Google Scholar]
- Iganci JR, Soares MV, Guerra E, Morim MP. (2015) A preliminary molecular phylogeny of the Abarema alliance (Leguminosae) and implications for taxonomic rearrangement. International Journal of Plant Sciences 177: 34–43. 10.1086/684078 [DOI] [Google Scholar]
- Käss E, Wink M. (1997) Molecular phylogeny and phylogeography of Lupinus (Leguminosae) inferred from nucleotide sequences of the rbc L gene and ITS 1 + 2 regions of rDNA. Plant Systematics and Evolution 208: 139–167. 10.1007/BF00985439 [DOI] [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T. (2002) MAFFT: A novel method for rapid multiple sequence alignment based on Fourier transform. Nucleic Acids Research 30: 3059–3066. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen EJM. (2022a) On the taxonomic affinity of Albiziacarbonaria Britton (Leguminosae, Caesalpinioideae-mimosoid clade). In: Hughes CE, de Queiroz LP, Lewis GP. (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations.PhytoKeys 205: 363–370. 10.3897/phytokeys.205.82288 [DOI] [PMC free article] [PubMed]
- Koenen EJM. (2022b) Osodendron gen. nov. (Leguminosae, Caesalpinioideae), a new genus of mimosoid legumes of tropical Africa. In: Hughes CE, de Queiroz LP, Lewis GP. (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations.PhytoKeys 205: 453–470. 10.3897/phytokeys.205.82821 [DOI] [PMC free article] [PubMed]
- Koenen EJM, Kidner C, de Souza E, Simon MF, Iganci JR, Nicholls JA, Brown GK, Queiroz LP de, Luckow M, Lewis GP, Pennington T, Hughes CE. (2020) Hybrid capture of 964 nuclear genes resolves evolutionary relationships in the mimosoid legumes and reveals the polytomous origins of a large pantropical radiation. American Journal of Botany 107(12): 1710–1735. 10.1002/ajb2.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GP, Rico Arce L. (2005) Tribe Ingeae. In: Lewis G, Schrire B, Mackinder B, Lock M. (Eds) Legumes of the World.Royal Botanic Gardens, Kew, Richmond, 193–231.
- Lewis GP, Schrire BD. (2003) Thailentadopsis Kostermans (Leguminosae: Mimosoideae: Ingeae) resurrected. Kew Bulletin 58(2): 491–494. 10.2307/4120634 [DOI] [Google Scholar]
- Linares L. (2005) Especie nueva de Albizia (Leguminosae: Mimosoideae) de Centroamérica. Revista Mexicana de Biodiversidad 76: 7–10. 10.22201/ib.20078706e.2005.001.361 [DOI] [Google Scholar]
- Nielsen IC. (1981) Tribe 5. Ingeae. In: Polhill RM, Raven PH. (Eds) Advances in Legume Systematics.Part 1, Royal Botanic Gardens, Kew, Richmond, 173–190.
- Posada D. (2008) jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. 10.1093/molbev/msn083 [DOI] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67(5): 901–904. 10.1093/sysbio/syy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico Arce ML, Gale SL, Maxted N. (2008) A taxonomic study of Albizia (Leguminosae: Mimosoideae: Ingeae) in Mexico and Central America. Anales del Jardin Botanico de Madrid 65: 255–305. 10.3989/ajbm.2008.v65.i2.294 [DOI] [Google Scholar]
- Ringelberg JJ, Koenen EJM, Iganci JR, de Queiroz LP, Murphy DJ, Gaudeul M, Bruneau A, Luckow M, Lewis GP, Hughes CE. (2022) Phylogenomic analysis of 997 nuclear genes reveals the need for extensive generic re-delimitation in Caesalpinioideae (Leguminosae). In: Hughes CE, de Queiroz LP, Lewis GP. (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations.PhytoKeys 205: 3–58. 10.3897/phytokeys.205.85866 [DOI] [PMC free article] [PubMed]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MVB, Guerra E, Morim MP, Iganci JRV. (2021) Reinstatement and recircumscription of Jupunba and Punjuba (Fabaceae) based on phylogenetic evidence. Botanical Journal of the Linnean Society 196(4): 456–479. 10.1093/botlinnean/boab007 [DOI] [Google Scholar]
- Soares MVB, Koenen EJM, Iganci JRV, Morim MP. (2022) A new generic circumscription of Hydrochorea (Leguminosae, Caesalpinioideae, mimosoid clade) with an amphi-Atlantic distribution. In: Hughes CE, de Queiroz LP, Lewis GP. (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations.PhytoKeys 205: 401–438. 10.3897/phytokeys.205.82775 [DOI] [PMC free article] [PubMed]
- Souza ER de, Lewis GP, Forest F, Schnadelbach AS, van den Berg C, Queiroz LP de. (2013) Phylogeny of Calliandra (Leguminosae: Mimosoideae) based on nuclear and plastid molecular markers. Taxon 62: 1201–1220. 10.12705/626.2 [DOI] [Google Scholar]
- Souza ER de, Krishnaraj MV, Queiroz LP de. (2016) Sanjappa, a new genus in the tribe Ingeae (Leguminosae: Mimosoideae) from India. Rheedea 26: 1–12. [Google Scholar]
- Sun Y, Skinner DZ, Liang GH, Hulbert SH. (1994) Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theoretical and Applied Genetics 89: 26–36. 10.1007/BF00226978 [DOI] [PubMed] [Google Scholar]
- Terra V, Ringelberg JJ, Maslin B, Koenen EJM, Ebinger J, Seigler D, Hughes CE. (2022) Dilemmas in generic delimitation of Senegalia and allies (Caesalpinioideae, mimosoid clade): how to reconcile phylogenomic evidence with morphology and taxonomy? In: Hughes CE, de Queiroz LP, Lewis GP. (Eds) Advances in Legume Systematics 14. Classification of Caesalpinioideae Part 1: New generic delimitations.PhytoKeys 205: 261–278. 10.3897/phytokeys.205.79378 [DOI] [PMC free article] [PubMed]
- Thiers BM. (2016) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium, New York. http://sweetgum.nybg.org/ih/
- Turland NJ, Wiersema JH, Barrie FR, Greute W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF [Eds] (2018) International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Koeltz Botanical Books, Glashütten. 10.12705/Code.2018 [DOI]
- Villiers JF. (2002) Viguieranthus. In: Du Puy D, Labat J-N, Rabevohitra R, Villiers J-F, Bosser J, Moat J. (Eds) The Leguminosae of Madagascar.Royal Botanic Gardens, Kew, Richmond, 271–285.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.