Abstract
In this study, Pseudospermaarenarium is proposed as a new species, based on morphological, ecological, molecular and biochemical evidence. The new species grows on sandy ground under Populus and Pinussylvestris in north-western China and northern Europe, respectively. It is characterised by the combination of the robust habit, nearly glabrous pileus, large cylindrical basidiospores, thin-walled cheilocystidia and ecological associations with Populusalba × P.berolinensis and Pinussylvestris and unique phylogenetic placement. Additionally, a comprehensive toxin determination of the new species using ultra-high performance liquid chromatography-tandem mass spectrometry was conducted. Results showed that it was a muscarine-positive species. The content were approximately five times higher in the pilei [4012.2 ± 803.1–4302.3 ± 863.2 mg/kg (k = 2, p = 95%)] than in the stipes [850.4 ± 171.1–929.1 ± 184.2 mg/kg (k = 2, p = 95%)], demonstrating the severity of mushroom poisoning when patients consumed different parts of the poisonous mushroom. Amatoxins, phallotoxins, ibotenic acid, muscimol, psilocybin and psilocin were not detected.
Keywords: Agaricales, muscarine, mushroom toxin, new taxon, poisonous mushroom, ultra-high performance liquid chromatography-tandem mass spectrometry
Introduction
Inocybaceae is a family of agarics that contains many poisonous species. However, Kosentka et al. (2013) found that the most recent common ancestor of the family did not contain muscarine. Recognising its species diversity and detecting its toxins are essential to control and prevent poisoning incidents (Li et al. 2020; Deng et al. 2021a). According to the latest molecular phylogeny, seven genera were treated in Inocybaceae (Matheny et al. 2020). Pseudosperma, referred to as Inocybesect.Rimosae sensu stricto (Larsson et al. 2009) or Pseudosperma clade (Matheny 2009), is one of the muscarine-containing genera in the family with numerous cryptic and semi-cryptic species. It is characterised by rimulose to rimose pileus, furfuraceous to appressed furfuraceous stipe with flocculose apex, elliptic to sub-phaseoliform basidiospores, the absence of pleurocystidia and the presence of thin-walled cheilocystidia. Ninety-seven Pseudosperma taxa have been recorded in the IndexFungorum database (www.indexfungroum.org; retrieved 7 May 2022). Of these, more than 40 taxa have been reported or originally described in Europe (Bandini and Oertel 2020). Since the establishment of the genus in 2020, 16 new taxa have been discovered in Asia and Europe in the past 2 years alone (Bandini and Oertel 2020; Cervini et al. 2020; Jabeen and Khalid 2020; Saba et al. 2020; Yu et al. 2020; Bandini et al. 2021; Jabeen et al. 2021). However, the species diversity of Pseudosperma is still poorly explored in East Asia. In China, only six taxa have been verified, including three recently described species, viz., P.yunnanense, P.neoumbrinellum and P.citrinostipes (Bau and Fan 2018; Yu et al. 2020).
Ecologically, Pseudosperma species have an ectomycorrhizal symbiosis with various plants and are commonly found in north temperate forests dominated by Betula, Cedrus, Populus, Pinus, Picea, Quercus, Salix etc. During field surveys in north-western China, a poisonous Inocybaceae mushroom collected under Populus plantations caught the authors’ attention because of its strikingly robust habit. This stout Inocybaceae species has led to three poisoning incidents, with a total of seven patients in north-western China during the past 2 years. Two of these occurred in September in Ningxia and Shanxi in 2020 and another occurred in Ningxia in October 2021 (Li et al. 2021a, 2022). All patients from the three poisoning incidents suffered from classic parasympathetic nervous system stimulation syndromes. After microscopic examinations and molecular analyses, mushroom specimens obtained from poisoning locales, together with a European specimen, were proven as a new Pseudosperma species. Discussions on the distribution, relationships and distinction of the new species and its affinities are also provided. Additionally, to better understand the toxicity of the new species and contribute to their poisoning control and prevention, 11 major mushroom toxins, namely, two isoxazole derivatives (ibotenic acid and muscimol), two tryptamine alkaloids (psilocybin and psilocin), three amatoxins (α-, β- and γ-amanitin), three phallotoxins (phalloidin, phallacidin and phallisacin) and muscarine, were assayed.
Methods
Sampling, morphological observations and descriptions
The Chinese materials were collected in sandy poplar plantations from Ningxia Hui Autonomous Region and Shaanxi Province, where there is a temperate continental climate. The European material JV26578 was collected in a seashore forest from Estonia, in a hemiboreal zone. Macroscopic features were described, based on fresh materials and colour photographs. A small piece of the pileus, lamella or stipe tissue was mounted in 5% aqueous potassium hydroxide (KOH) on the slide and then examined using a light microscope when the tissue was completely rehydrated. Microscopic structures, including basidiospores, basidia, cheilocystidia, hymenophoral trama, caulocystidia, pileipellis and stipitipellis, were examined from rehydrated materials. The measurements of micro-structures follow Fan and Bau (2013) and Yu et al. (2020). The number of measured basidiospores is given as an abbreviation [n/m/p], which denotes n spores measured from m basidiomata of p collections. The measurements and Q values are given as (a)b–c(d), “b–c” covers a minimum of 90% of the measured values, “a” and “d” represent the extreme values; Q means the ratio of length/width in an individual basidiospore, Qm is the average Q of all basidiospores ± sample standard deviation (Ge et al. 2021; Na et al. 2022). Colour designations follow Kornerup and Wanscher (1978). Voucher specimens were deposited in the Herbarium of Herbarium of Changbai Mountain Nature Reserve (ANTU) with FCAS numbers and TUR-A.
DNA extraction, polymerase chain reaction, sequence amplification and data analysis
Genomic DNA was extracted from silica-dried materials using the NuClean Plant Genomic DNA Kit (ComWin Biotech, Beijing). The internal transcribed spacer (ITS) region, the nuclear large subunit (nLSU) and the RNA polymerase II second largest subunit (RPB2) sequences were amplified and sequenced separately by using primer pairs ITS1F/ITS4 (Gardes and Bruns 1993), LR0R/LR7 (Vilgalys and Hester 1990) and RPB2-6F/RPB2-7.1R (Matheny 2005). The PCR thermocycling protocol was 95 °C for 1 min at first, then followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 52 °C for 1 min, extension at 72 °C for 1 min and a final extension at 72 °C for 8 min (Wang et al. 2021). Sequencing work was done by Sangon Biotech (Shanghai) Co., Ltd. Sequences of related taxa in Pseudosperma, retrieved from previous studies, were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/) for phylogenetic analysis (Suppl. material 1). Mallocybeterrigena (Fr.) Matheny, Vizzini & Esteve-Rav. was used for the outgroup. The sequence data matrix for each locus was aligned by Mafft online service (https://mafft.cbrc.jp/alignment/server/) (Katoh et al. 2019) and manually adjusted by BioEdit 7.0.9.0 (Hall 1999). The aligned datasets were combined with Mega 5.02 (Tamura et al. 2011). MrModeltest v.2.3 was used to determine the optimal substitution model for each locus with the Akaike Information Criterion (Nylander 2004). Bayesian Inference (BI) analyses, executed in MrBayes v.3.2.7a (Ronquist et al. 2012), were run for 1,235,000 generations using four Metropolis-Coupled Monte Carlo Markov chains to calculate posterior probabilities and the standard deviation of the split frequencies was terminated at 0.009977. Maximum Likelihood (ML) analysis was conducted in W-IQ-TREE Web Service (http://iqtree.cibiv.univie.ac.at/) with 1,000 replicates (Trifinopoulos et al. 2016).
Toxin detection
Ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) was performed for toxin detection. Detailed mushroom sample preparations, analysis of muscarine, amatoxins and phallotoxins (Alta Scientific Co., Ltd., Tianjin, China) referred to our previous works (Xu et al. 2020a, 2020b).
Detailed information for analysis of ibotenic acid and muscimol (Alta Scientific Co., Ltd., Tianjin, China) are as follows: chromatographic separation was conducted on an ACQUITY UPLC C8 column (2.1 × 100 mm, 1.7 μm; Waters, USA). Acetonitrile (A) and 4% formic acid aqueous solution (B) were used as mobile phase solvent flowing at 0.3 ml/min. The column was eluted by 2% A for 1.0 min, followed by 2%–70% A for 1.0 min, then by 70% A for 1.0 min and then by 70%–2% A for 0.5 min, finally by 2% A for 1.5 min. The analytical column was set at 40 °C. The injection volume was 10 μl. The positive MS/MS conditions can refer to muscarine (Xu et al. 2020b). The ion pairs were 115.1 > 68.1 (Cone at 16 V; Collision at 12 V), 159.1 > 113.1 (Cone at 16 V; Collision at 12 V) for ibotenic acid, as well as 115.1 > 98.1 (Cone at 15 V; Collision at 10 V), 115.1 > 68.1 (Cone at 15 V; Collision at 18 V) for muscimol.
For the analysis of psilocybin and psilocin (Alta Scientific Co., Ltd., Tianjin, China), the detailed descriptions are as follows. ACQUITY UPLC T3 column (2.1 × 100 mm, 1.7 μm; Waters, USA) was used as the separation column. The mobile phases were acetonitrile (A) and 10 mmol/l ammonium acetate aqueous solution (B). The flow rate was 0.3 ml/min. The column was eluted by 0% A for 0.5 min, followed by 0%–85% A for 4 min, then by 85% A for 1.5 min and then by 85%–0% A for 1.5 min, finally by 0% A for 2 min. The analytical column was set at 40 °C and the injection volume was 10 μl. The positive MS/MS conditions can refer to muscarine (Xu et al. 2020b). The ion pairs were 285.1 > 85.2 (Cone at 16 V; Collision at 18 V), 285.1 > 240.1 (Cone at 16 V; Collision at 17 V) for psilocybin and 205.1 > 58.2 (Cone at 26 V; Collision at 13 V), 205.1 > 160.1 (Cone at 26 V; Collision at 13 V) for psilocin.
Results
Phylogenetic analyses
Nine sequences (three ITS, three LSU and three rpb2) were newly generated and submitted to GenBank. The best-fit model selected by MrModeltest was GTR+I+G for each gene equally. The three-gene data matrix consisted of 104 taxa and 2890 sites. The final multilocus alignment used for phylogenetic reconstruction was submitted to TreeBase (ID29310). The Bayesian Inference and Maximum Likelihood trees were similar in topology; thus, only the BI tree was presented (Fig. 1). In the BI tree (Fig. 1), all Pseudosperma taxa grouped in a fully supported clade and three Chinese specimens and a European specimen (JV26578) clustered in an independent lineage with full support. The lineage clustered with the lineage composed of I.aureocitrinum (Esteve-Rav.) Matheny & Esteve-Rav. but with limited support.
Figure 1.
Phylogram generated by Bayes Inference (BI) analysis, based on a combined sequences dataset from nuclear genes (rDNA-ITS, nrLSU and rpb2), rooted with Mallocybeterrigena (Pruned). Bayesian Inference posterior probabilities (BI-PP) ≥ 0.95 and ML bootstrap proportions (ML-BP) ≥ 70 are represented as BI-PP/ML-BP.
Taxonomy
. Pseudosperma arenarium
Y.G. Fan, Fei Xu, Hai J. Li & Vauras sp. nov.
2575C825-2C17-5A12-9CB6-ECD00C8718B9
842603
Figure 2.
Pseudospermaarenarium and its habitat a basidiospores b, c cheilocystidia d–h basidiomata. Scale bars: 10 μm (a–c); 10 mm (d–h). Photos by Xu Fei, Li Hai-Jiao & Zhao Li-Na.
Figure 3.
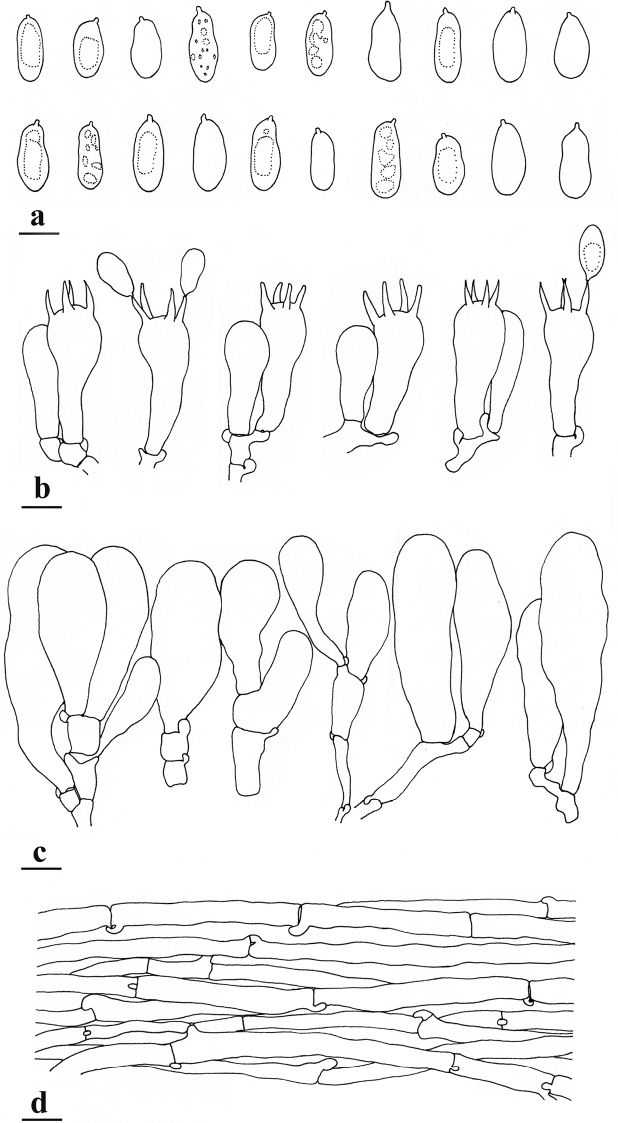
Microfeatures of Pseudospermaarenarium (holotype) a basidiospores b basidia c cheilocystidia d pileipellis. Scale bars: 10 μm (a–d). Line drawings by Li Hai-Jiao.
Etymology.
refers to its habitat of sandy soils.
Holotype.
China, Ningxia Hui Autonomous Region, Wuzhong, Yanchi County, Yanchi Railway Station, on sandy ground under Populusalba × P.berolinensis, 5 Oct 2020, NXYC20201005-01 (FCAS3571, holotype). GenBank accession nos.: ITS-OM304278, LSU-OM304287, rpb2-OM421667.
Diagnosis.
Basidiomata robust, pileus beige, ivory white or yellowish; basidiospores > 13 μm, cylindrical to cylindrical-ellipsoid, cheilocystidia thin-walled. Occurs under artificial plantations of Populusalba × P.berolinensis or open seashore forest of Pinussylvestris Linn. Differs from P.arenicola by longer basidiospores and phylogenetic distance.
Basidiomata.
medium-sized, robust. Pileus 35–65 mm in diameter, spherical to hemispherical when young, convex, dome-shaped to applanate when mature, not umbonate, margin inrolled at first, becoming depressed, straight, to uplifted or recurved in age; surface dry, glabrous to slightly fibrillose, occasionally rimulose to rimose at the margin, with distinct sandy remnants; yellowish (1A2) to ochraceous (1A4), paler outwards, ivory white (1B1) to greyish-white (1B2) when dried. Lamellae crowded, up to 8 mm in width, adnexed to sub-free, not equal, alternately distributed with three tiers of lamellula, initially pure white to creamy white (1A2), becoming yellowish (4A3), brownish (5B6) to cinnamon (5C8) with age, yellowish-brown (4B8) to dark brown (6C7) after drying, edge pinkish-white, fimbricate. Stipe 40–100 × 7–20 mm, solid, equal or slightly tapering downwards, sometimes swollen towards the base, but not marginate, longitudinally fibrillose with scattered squamules, white to ivory white (1B1–1B2) with pinkish tinge (11A3) when fresh, yellowish (5A4) to brownish (5B5) upon drying. Context solid, white and fleshy in pileus, 2–5 mm in thickness, fibrillose in the stipe, striate and shiny, white to somewhat pinkish (7A2). Odour fungoid or slightly spermatic.
Basidiospores.
[170/6/4] (13–)14–20(–21) × (6–)7–9.2(–11) μm, median 16.4 × 7.8 μm, Q = (1.6–)1.75–2.64(–2.95), Qm = 2.12 ± 0.27, yellow-brown, smooth, mostly cylindrical to cylindrical-ellipsoid, less often narrowly ellipsoid to nearly phaseoliform. Basidia 32–42 × 11–14 μm, clavate, usually narrower downwards, four-spored, sterigmata up to 10 μm long, translucent with oily inclusions, occasionally with yellowish pigments. Pleurocystidia absent. Lamellae edge sterile. Cheilocystidia 30–77 × 12–23 μm, thin-walled, colourless, broadly clavate or fusiform, rarely septate, translucent or occasionally with golden yellow inclusions, walls yellowish. Caulocystidia not observed. Hymenophoral trama nearly regularly arranged, composed of translucent and pale yellow, thin-walled hyphae up to 22 μm wide. Pileipellis a cutis, regularly arranged, orange-brown to brownish in 5% KOH, composed of cylindrical hyphae 4–15 μm in diameter; pileal trama made up of compact, parallel, hyaline hyphae, pale yellow in mass. Stipitipellis a cutis frequently disrupted by loose hyphal projections, hyphae thin-walled, colourless, 3–16 μm wide. Stipe trama regularly and densely arranged, yellowish in mass, hyphae thin-walled, colourless, 8–21 μm wide. Oleiferous hyphae 4–15 μm wide, present in pileus and stipe, bright yellow, smooth, often bent, occasionally branched or catenate. Clamp connections are common in all tissues.
Habitat.
individual or scattered on sandy and saline-alkali soil under artificial plantations of Populusalba × P.berolinensis in China and open seashore forest of Pinussylvestris in Estonia. Fruiting in autumn, from late September to early October.
Known distribution.
China (Ningxia and Shaanxi), Estonia.
Additional materials examined.
China. Ningxia Hui Autonomous Region, Wuzhong, Yanchi Country, on sandy ground under Populusalba × P.berolinensis, 22 Sep 2021, NX20210922-57 (FCAS3572), GenBank accession nos.: ITS-OM304279, LSU-OM304288, rpb2-OM421668; Shaanxi Province, Yulin, Dingbian Country, Yanchangbao Country, Xiliangwan Village, on sandy ground under Populusalba × P.berolinensis, 30 Sep 2021, SX20210930-65 (FCAS3573), GenBank accession nos.: ITS-OM304280, LSU-OM304289, rpb2-OM421669. Estonia. Saaremaa, Kaarma Municipality, Mändjala, open seashore forest with Pinussylvestris Linn., on fine calcareous sand, 19 Sep 2008, Jukka Vauras 26578F (TUR-A182630), GenBank no.: ITS and LSU-FJ904154.
Muscarine detection
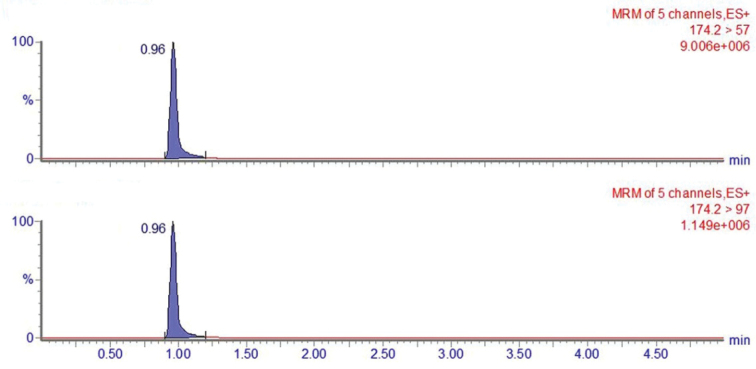
The new species contained only muscarine (Fig. 4), whereas amatoxins, phallotoxins, ibotenic acid, muscimol, psilocybin and psilocin were not detected. The quantitative results are expressed by (X ± U; k = 2, p = 95%; Xu et al. 2020a, 2020b). The muscarine content in the holotype (NXYC20201005-01) between different basidiomata ranged from 3981.4 ± 796.4 to 4074.2 ± 801.3 mg/kg (k = 2, p = 95%) in the pilei and from 811.2 ± 162.3 to 883.3 ± 176.5 mg/kg (k = 2, p = 95%) in the stipes (Table 1). The muscarine content from different specimens ranged from 4012.2 ± 803.1 to 4302.3 ± 863.2 mg/kg (k = 2, p = 95%) in the pilei and from 850.4 ± 171.1 to 929.1 ± 184.2 mg/kg (k = 2, p = 95%) in the stipes (Table 2).
Figure 4.
MRM chromatograms of muscarine from Pseudospermaarenarium (holotype).
Table 1.
Muscarine content in different parts from different basidiomata of the holotype (mg/kg).
| Collection number | Basidiomata 1 | Basidiomata 2 | Basidiomata 3 | Basidiomata 4 | Basidiomata 5 | |||||
| Stipe | Pileus | Stipe | Pileus | Stipe | Pileus | Stipe | Pileus | Stipe | Pileus | |
| NXYC20201005-01 | 816.2 ± 163.1 | 3981.4 ± 796.4 | 816.8 ± 17.1 | 4004.4 ± 801.1 | 811.2 ± 162.3 | 4025.4 ± 805.3 | 834.3 ± 167.1 | 4054.3 ± 801.6 | 883.3 ± 176.5 | 4074.2 ± 801.3 |
Table 2.
Muscarine content in different parts from different specimens collected from different locations.
| Collection numbers | Muscarine (mg/kg) | |
|---|---|---|
| Stipes | Pilei | |
| NXYC20201005-01 | 850.4 ± 171.1 | 4012.2 ± 803.1 |
| NX20210922-57 | 929.1 ± 184.2 | 4302.3 ± 863.2 |
| SX20210930-05 | 863.2 ± 172.5 | 4085.2 ± 816.2 |
Discussion
The new species is known from three localities in Ningxia and Shaanxi of north-western China and is a locally common mushroom that occurs in late autumn under sandy poplar plantations (Fig. 2). As Populusalba × P.berolinensis plantations are widely distributed over north-western China, the new species may have broader distribution in adjacent areas. Moreover, the European material JV26578 collected in Estonia clustered with the new species with full support in the phylogenetic results. This collection also grew on calcareous fine sand, but under Pinussylvestris. According to the file notes, the specimen also has robust basidiomata (pileus up to 38 mm broad, stipes 50–55 × 7–9 mm) with ochraceous pileus and stipes and fungoid or slightly spermatic odour. The microfeatures of JV26578 have cylindrical-ellipsoid basidiospores measuring (13.5–)13.9–16.5(–18.2) × (7.1–)7.2–8.5(–8.8) μm (average, 15.3 × 7.7 μm), Q = 1.8–2.2(–2.25) [average, 1.98 (n = 20)] and clavate cheilocystidia measuring (34–)40–62(–70) × 14–20(–22) μm [average, 50 × 18 μm (n = 20)]. Except for the ochraceous tinge in pileus and stipes, no distinct macroscopical difference was observed between the Chinese materials and the Estonian specimen. The European specimen JV26578 is now considered conspecific with the Chinese materials. Accordingly, the new species has a Eurasian distribution.
Pseudospermaarenarium is characterised by its tricholomoid habit, dirty whitish to ochraceous and glabrous pileus, crowded lamellae with fimbriate edges, large cylindrical basidiospores and thin-walled cheilocystidia. The thick and long persistent velipellis gives its pileus a nearly smooth and whitish appearance. In the field, the pileus, stipe and lamellae surfaces are usually covered with humose sands, showing a dirty yellowish or sometimes brownish colour, especially in older individuals. Its mostly large cylindrical basidiospores are microscopically impressive, but cylindrical ellipsoid to elongated ellipsoid basidiospores also exist in the same individual. With the combination of the characteristics listed above, the new species is distinctive. Without examining its microscopic features or molecular sequence analyses, a mycologist or even an Inocybaceae specialist is unlikely to be able to identify it exactly into the genus Pseudosperma. Unexpectedly, the three-gene phylogeny places P.arenarium in the P.rimosum complex, which clusters with the lineage that unified the type material of P.aureocitrinum and a sample labelled as ‘P.cf.rimosum.’ However, P.aureocitrinum has a typical inocyboid habit, yellowish-tinged basidiomata and broadly ellipsoid to subovoid basidiospores and occurs in Mediterranean evergreen oak forests (Esteve-Raventós 2014).
Pseudospermaarenicola (R. Heim) Matheny & Esteve-Rav., a European species also occurring on coastal sandy soils, is similar in having a whitish appearance, long-persisting thick velipellis and long basidiospores, but it has a less robust habit, relatively short basidiospores measuring 11.5–12–18.5 × 6.0–6.4–7.5 μm (Kuyper 1986), different ecological associations and different phylogenetic positions (Heim 1931; Kuyper 1986; Stangl 1989). Pseudospermapseudo-orbatum (Esteve-Rav. & García Blanco) Matheny & Esteve-Rav. is a whitish species originally described from Spain, resembling the new species in having thick velipellis, non-rimose pileus, large cylindrical basidiospores and clavate cheilocystidia. However, it is distinguished by having pinkish lamellae when young, stockier stipes and an association with Pinuspinaster Ait. and P.pinea L. (Esteve-Raventós et al. 2003). Pseudospermaniveivelatum (D.E. Stuntz ex Kropp, Matheny & L.J. Hutchison) Matheny & Esteve-Rav., a North American species, shares white thick velipellis, elongated large basidiospores and ecological association with Populustremuloides Michx. and conifers. However, it differs by its sericeous pileus, shorter basidiospores measuring 11.5–13.9–18.5 × 6.0–6.4–7.5 μm, slender cheilocystidia (Kropp et al. 2013) and a clinically insignificant amount of muscarine (Kosentka et al. 2013).
Muscarine is a neurotoxin that causes salivation, sweating, delirium and even coma or death (Işiloğlu et al. 2009; Xu et al. 2020b). In recent years, more and more poisoning cases have been caused by eating Inocybaceae mushrooms containing toxic muscarine (Li et al. 2020, 2021a, 2022; Xu et al. 2020b). According to literature, five Pseudosperma species have been assayed; of these, P.rimosum (Bull.) Matheny & Esteve-Rav., P.niveivelatum, P.sororium (Kauffman) Matheny & Esteve-Rav. and P.spurium (Jacobsson & E. Larss.) Matheny & Esteve-Rav. contain muscarine; only P.perlatum (Cooke) Matheny & Esteve-Rav. was reported lacking muscarine (Kosentka et al. 2013). Xu et al. (2020b) reported that the muscarine content of I.serotina Peck in a poisoning incident was 324.0 ± 62.4 mg/kg wet weight. Li et al. (2021b) reported that the muscarine content in I.squarrosolutea (Corner & E. Horak) Garrido and I.squarrosofulva S.N. Li, Y.G. Fan & Z.H. Chen were 136.4 ± 25.4 to 1683.0 ± 313.0 and 31.2 ± 5.8 to 101.8 ± 18.9 mg/kg dry weight, respectively. Deng et al. (2021b, 2022) found that muscarine content in Inospermamuscarium Y.G. Fan, L.S. Deng, W.J. Yu & N.K. Zeng, I.hainanense Y.G. Fan, L.S. Deng, W.J. Yu & N.K. Zeng and I.zonativeliferum Y.G. Fan, H.J. Li, F. Xu, L.S. Deng & W.J. Yu were 16.03 ± 1.23, 11.87 ± 3.02 and [2.08 ± 0.05 (pileus) and 6.53 ± 1.88 (stipes)] g/kg dry weight, respectively. In this study, results showed that P.arenarium is a muscarine-positive species with middle- and upper-level muscarine content and also led to three poisoning incidents with a total of seven patients in northwest China during the past 2 years (Li et al. 2021a, 2022). Interestingly, the muscarine content in caps was approximately five times higher than in stipes. Although some studies showed that the toxin amount in the cap is higher than in the stipes (Hu et al. 2012; Garcia et al. 2015; Sun et al. 2018, 2019), the mechanism of such difference is still not clear. Li et al. (2021b) reported that the muscarine content of Inocybesquarrosolutea varied a lot in different specimens. However, in this study, the muscarine content in the mushroom samples collected from the same or three different places showed no significant difference. Additionally, no amatoxins, phallotoxins, ibotenic acid, muscimol, psilocybin and psilocin were detected in all samples. This study described P.arenarium as a new species, based on morphological, ecological, molecular and toxic evidence. The publicity and education of the new species are needed to control and prevent mushroom poisoning incidents.
Supplementary Material
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 31501814, 31860009 & 31400024), the Natural Science Foundation of Ningxia (No. 2020AAC03437) and the Hainan Basic and Applied Research project for cultivating high level talents (No. 2019RC230). We also thank the anonymous reviewers for their corrections and suggestions to improve our work.
Citation
Yan Y-Y, Zhang Y-Z, Vauras J, Zhao L-N, Fan Y-G, Li H-J, Xu F (2022) Pseudosperma arenarium (Inocybaceae), a new poisonous species from Eurasia, based on morphological, ecological, molecular and biochemical evidence. MycoKeys 92: 79–93. https://doi.org/10.3897/mycokeys.92.86277
Supplementary materials
Table S1
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Ya-Ya Yan ,Yi-Zhe Zhang, Jukka Vauras, Li-Na Zhao, Yu-Guang Fan, Hai-Jiao Li, Fei Xu
Data type
Table (pdf file)
Explanation note
Information of taxa used in phylogenetic analysis. Newly sequenced collections are bold.
References
- Bandini D, Oertel B. (2020) Three new species of the genus Pseudosperma (Inocybaceae). Czech Mycology 72(2): 221–250. 10.33585/cmy.72205 [DOI] [Google Scholar]
- Bandini D, Oertel B, Eberhardt U. (2021) Even more fibre-caps (2): Thirteen new species of the family Inocybaceae. Mycologia Bavarica = Bavarian Journal of Mycology 21: 27–98. [Google Scholar]
- Bau T, Fan YG. (2018) Three new species of Inocybesect.Rimosae from China. Junwu Xuebao 37(6): 693–702. 10.13346/j.mycosystema.180033 [DOI] [Google Scholar]
- Cervini M, Bizio E, Alvarado P. (2020) Quattro nuove specie italiane del Genere Pseudosperma (Inocybaceae) con odore di miele. Rivista di Micologia 63(1): 3–36. [Google Scholar]
- Deng LS, Yu WJ, Zeng NK, Liu LJ, Liu LY, Fan YG. (2021a) Inospermasubsphaeorosporum, a new species (Inocybaceae) from tropical China. Phytotaxa 502(2): 169–178. 10.11646/phytotaxa.502.2.5 [DOI] [Google Scholar]
- Deng LS, Kang R, Zeng NK, Yu WJ, Chang C, Xu F, Deng WQ, Qi LL, Zhou YL, Fan YG. (2021b) Two new Inosperma (Inocybaceae) species with unexpected muscarine contents from tropical China. MycoKeys 85: 87–108. 10.3897/mycokeys.85.71957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng LS, Yu WJ, Zeng NK, Zhang YZ, Wu XP, Li HJ, Xu F, Fan YG. (2022) A new muscarine-containing Inosperma (Inocybaceae, Agaricales) species discovered from one poisoning incident occurring in tropical China. Frontiers in Microbiology 13: 1–12. 10.3389/fmicb.2022.923435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve-Raventós F. (2014) Inocybeaureocitrina (Inocybaceae), a new species of section Rimosae from Mediterranean evergreen oak forests. Plant Biosystems 148(2): 377–383. 10.1080/11263504.2013.877532 [DOI] [Google Scholar]
- Esteve-Raventós F, Blanco AG, Carazo MS, Val JBD. (2003) Inocybeaurantiobrunnea and I.pseudoorbata, two new Mediterranean species found in the Iberian peninsula. Österreichische Zeitschrift für Pilzkunde 12: 89–99. [Google Scholar]
- Fan YG, Bau T. (2013) Two striking Inocybe species from Yunnan Province, China. Mycotaxon 123(1): 169–181. 10.5248/123.169 [DOI] [Google Scholar]
- Garcia J, Oliveira A, Pinho PG, Freitas V, Carvalho A, Baptista P, Pereira E, Bastos ML, Carvalho F. (2015) Determination of amatoxins and phallotoxins in Amanitaphalloides mushrooms from northeastern Portugal by HPLC-DAD-MS. Mycologia 107(4): 679–687. 10.3852/14-253 [DOI] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. (1993) ITS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Molecular Ecology 2(2): 113–118. 10.1111/j.1365-294X.1993.tb00005.x [DOI] [PubMed] [Google Scholar]
- Ge YP, Liu Z, Zeng H, Cheng X, Na Q. (2021) Updated description of Atheniella (Mycenaceae, Agaricales), including three new species with brightly coloured pilei from Yunnan Province, southwest China. MycoKeys 81: 139–164. 10.3897/mycokeys.81.67773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41(41): 95–98. [Google Scholar]
- Heim R. (1931) Le genre Inocybe. Encyclopédi mycologique 1: 1–429. [Google Scholar]
- Hu JS, Zhang P, Zeng J, Chen ZH. (2012) Determination of amatoxins in different tissues and development stages of Amanitaexitialis. Journal of the Science of Food and Agriculture 92(13): 2664–2667. 10.1002/jsfa.5685 [DOI] [PubMed] [Google Scholar]
- Işiloğlu M, Helfer S, Alli H, Yilmaz F. (2009) A fatal Inocybe (Fr.) Fr. poisoning in Mediterranean Turkey. Turkish Journal of Botany 33(1): 71–73. 10.3906/bot-0605-2 [DOI] [Google Scholar]
- Jabeen S, Khalid AN. (2020) Pseudospermaflavorimosum sp. nov. from Pakistan. Mycotaxon-Ithaca Ny 135(1): 183–193. 10.5248/135.183 [DOI] [Google Scholar]
- Jabeen S, Zainab Bashir H, Khalid AN. (2021) Pseudospermaalbobrunneum sp. nov. from coniferous forests of Pakistan. Mycotaxon-Ithaca Ny 136(2): 361–372. 10.5248/136.361 [DOI] [Google Scholar]
- Katoh K, Rozewicki J, Yamada KD. (2019) MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 20(4): 1160–1166. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. (1978) The methuen handbook of colour 3rd edn. Eyre Methuen Ltd. Reprint, London, 252 pp. [Google Scholar]
- Kosentka P, Sprague S, Ryberg M, Gartz J, May A, Campagna S, Matheny PB. (2013) Evolution of the toxins muscarine and psilocybin in a family of mushroom-forming fungi. PLoS ONE 8(5): e64646. 10.1371/journal.pone.0064646 [DOI] [PMC free article] [PubMed]
- Kropp BR, Matheny PB, Hutchison LJ. (2013) InocybesectionRimosae in Utah: Phylogenetic affinities and new species. Mycologia 105(3): 728–747. 10.3852/12-185 [DOI] [PubMed] [Google Scholar]
- Kuyper TW. (1986) A revision of the genus Inocybe in Europe. I. Subgenus Inosperma and the smooth-spored species of subgenus Inocybe. Persoonia (Suppl 3): 1–247.
- Larsson E, Ryberg M, Moreau PA, Mathiesen AD, Jacobsson S. (2009) Taxonomy and evolutionary relationships within species of section Rimosae (Inocybe) based on ITS, LSU and mtSSU sequence data. Persoonia 23(1): 86–98. 10.3767/003158509X475913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Zhang HS, Zhang YZ, Zhang KP, Zhou J, Yin Y, Jiang SF, Ma PB, He Q, Zhang YT, Wen K, Yuan Y, Lang N, Lu JJ, Sun CY. (2020) Mushroom Poisoning Outbreaks – China, 2019. China CDC Weekly 2(2): 19–24. 10.46234/ccdcw2020.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Zhang HS, Zhang YZ, Zhou J, Yin Y, He Q, Jiang SF, Ma PB, Zhang YT, Wen K, Yuan Y, Lang N, Cheng BW, Lu JJ, Sun CY. (2021a) Mushroom poisoning outbreaks – China, 2020. China CDC Weekly 3(3): 41–45. 10.46234/ccdcw2021.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SN, Xu F, Jiang M, Liu F, Wu F, Zhang P, Fan YG, Chen ZH. (2021b) Two new toxic yellow Inocybe species from China: Morphological characteristics, phylogenetic analyses and toxin detection. MycoKeys 81: 185–204. 10.3897/mycokeys.81.68485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Zhang HS, Zhang YZ, Zhou J, Yin Y, He Q, Jiang SF, Ma PB, Zhang YT, Yuan Y, Lang N, Cheng BW, Wang M, Sun CY. (2022) Mushroom Poisoning Outbreaks – China, 2021. China CDC Weekly 4(3): 35–40. 10.46234/ccdcw2022.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny PB. (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Molecular Phylogenetics and Evolution 35(1): 1–20. 10.1016/j.ympev.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Matheny PB. (2009) A phylogenetic classification of the Inocybaceae. McIlvainea 18(1): 11–21. [Google Scholar]
- Matheny PB, Hobbs AM, Esteve-Raventós F. (2020) Genera of Inocybaceae: New skin for the old ceremony. Mycologia 112(1): 83–120. 10.1080/00275514.2019.1668906 [DOI] [PubMed] [Google Scholar]
- Na Q, Hu YP, Zeng H, Song ZZ, Ding H, Cheng XH, Ge YP. (2022) Updated taxonomy on Gerronema (Porotheleaceae, Agaricales) with three new taxa and one new record from China. MycoKeys 89: 87–120. 10.3897/mycokeys.89.79864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander J. (2004) MrModeltest V2. program distributed by the author. Bioinformatics (Oxford, England) 24: 581–583. 10.1093/bioinformatics/btm388 [DOI] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba M, Haelewaters D, Pfister DH, Khalid AN. (2020) New species of Pseudosperma (Agaricales, Inocybaceae) from Pakistan revealed by morphology and multi-locus phylogenetic reconstruction. MycoKeys 69: 1–31. 10.3897/mycokeys.69.33563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl J. (1989) Die Gattung Inocybe in Bayern. Hoppea 46: 1–409. [Google Scholar]
- Sun J, Li HJ, Zhang HS, Zhang YZ, Xie JW, Ma PB, Guo C, Sun CY. (2018) Investigating and analyzing three cohorts of mushroom poisoning caused by Amanitaexitialis in Yunnan, China. Human and Experimental Toxicology 37(7): 665–678. 10.1177/0960327117721960 [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang HS, Li HJ, Zhang YZ, He Q, Lu JJ, Yin Y, Sun CY. (2019) A case study of Lepiotabrunneoincarnata poisoning with endoscopic nasobiliary drainage in Shandong, China. Toxicon 161: 12–16. 10.1016/j.toxicon.2019.02.017 [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. (2011) Mega5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28(10): 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. (2016) W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44(W1): 1–4. 10.1093/nar/gkw256 [DOI] [PMC free article] [PubMed]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172(8): 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SN, Hu YP, Chen JL, Qi LL, Zeng H, Ding H, Huo GH, Zhang LP, Chen FS, Yan JQ. (2021) First record of the rare genus Typhrasa (Psathyrellaceae, Agaricales) from China with description of two new species. MycoKeys 79: 119–128. 10.3897/mycokeys.79.63700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Gong BL, Xu ZX, Wang JJ. (2020a) Reverse-phase/phenylboronic-acid-type magnetic microspheres to eliminate the matrix effects in amatoxin and phallotoxin determination via ultra high performance liquid chromatography-tandem mass spectrometry. Food Chemistry 332: 49–55. 10.1016/j.foodchem.2020.127394 [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang YZ, Zhang YH, Guan GY, Zhang KP, Li HJ, Wang JJ. (2020b) Mushroom poisoning from Inocybeserotina: A case report from Ningxia, northwest China with exact species identification and muscarine detection. Toxicon 179: 72–75. 10.1016/j. toxicon.2020.03.003. [DOI] [PubMed] [Google Scholar]
- Yu WJ, Chang C, Qin LW, Zeng NK, Wang SX, Fan YG. (2020) Pseudospermacitrinostipes (Inocybaceae), a new species associated with Keteleeria from southwestern China. Phytotaxa 450(1): 8–16. 10.11646/phytotaxa.450.1.2 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Ya-Ya Yan ,Yi-Zhe Zhang, Jukka Vauras, Li-Na Zhao, Yu-Guang Fan, Hai-Jiao Li, Fei Xu
Data type
Table (pdf file)
Explanation note
Information of taxa used in phylogenetic analysis. Newly sequenced collections are bold.