Abstract
During the Second Tibetan Plateau Scientific Expedition and Research Program, we discovered that white terricolous lichenized fungal species of Buellia De Not. were widely distributed across the Tibetan Plateau. After examining their morphology, chemistry and phylogeny, we describe Buelliaalpina Xin Y. Wang & Li S. Wang, sp. nov. as new to science. It is present in alpine meadows, and is characterized by its effigurate thallus, distinct linear marginal lobes, cover of thick white pruina and four-spored asci. This is also the first report of Buelliaelegans Poelt and Buelliaepigaea (Pers.) Tuck from China. The Buelliaepigaea-group has previously been characterized by white and often effigurate thalli that occur mainly on soil. However, our results show that species in this group actually belong to two distinct clades. This conclusion is based on analyses of the nuITS region and the combined regions dataset (nuITS-nuLSU-mtSSU-β-tubulin). We discuss differences in morphology, anatomy, chemistry and ecology among the putative Buelliaepigaea-group. Detailed descriptions and figures for the three species from China and a key for species of Buelliaepigaea-group are provided.
Keywords: Lichenized fungi, nuITS-nuLSU-mtSSU-β-tubulin, phylogenetic analysis, terricolous, Tibetan Plateau
Introduction
The lichen genus Buellia De Not. (Caliciales, Caliciaceae) comprises approximately 400 species worldwide (Bungartz et al. 2007). Buellia s.l. is characterized by a crustose thallus, black lecideine apothecia, Bacidia-type asci, brown ascospores with one or more septate and reddish-brown or rarely hyaline hypothecia. Several other genera, which were previously included in Buellia s.l. (such as Amandinea M. Choisy, Diplotomma Flot. and Tetramelas Norman), have since been segregated based on their morphology, anatomy, chemistry and ecological environment (Scheidegger 1993; Marbach 2000; Nordin 2000). However, there remain other Buellia s.l. species with distinct morphological characters currently placed in groups instead of defined genera. Two examples are the species related to Buelliaaethalea (Ach.) Th. Fr. or B.epigaea (Pers.) Tuck, which are currently treated as aethalea-group or epigaea-group, respectively (Poelt and Sulzer 1974; Scheidegger 1993). Their current classification is based solely on external morphology, without the support of molecular data, therefore strict phylogenetic relationships between Buellia s.l. species remain unclear.
More than 64 species of the genus Buellia s.l. were previously reported from China, mostly located in the Tibetan Plateau region (Wei 2020; Wang et al. 2020). During the Second Tibetan Plateau Scientific Expedition and Research Program (STEP), a large number of additional lichen specimens were collected, including Buelliaepigaea-group species.
The Buelliaepigaea-group contains seven species; these are characterized by white and often effigurate thalli that occur mainly on soil. Buelliaepigaea, which was reported from Europe, is the core species of this group. Buelliaasterella Poelt & Sulzer and B.elegans were reported from Europe, and B. zoharyi Galun was reported from Asia and Europe by Poelt and Sulzer (1974). Trinkaus and Mayrhofer (2000) published a revision of the four species above. A further three new species were described from Australia: Buelliadijiana Trinkaus, B.georgei Trinkaus, Mayrhofer & Elix, and B.lobata Trinkaus & Elix (Trinkaus et al. 2001). B.epigaea, B.dijiana and B.georgei formed a monophyletic clade, but data is still lacking for the remaining species (Grube and Arup 2001).
The aim of this study is to determine which Buelliaepigaea-group species are distributed in China and whether they form a monophyletic clade. For this purpose, we carried out a phylogenetic study of the Buelliaepigaea-group based on four loci.
Materials and methods
Morphological and chemical analyses
During STEP, 92 specimens of the Buelliaepigaea-group were collected from the Qinghai-Tibetan Plateau and deposited in the Lichen Herbarium, Kunming Institute of Botany, China (KUN). Morphological characteristics of thalli and apothecia were examined under a dissecting microscope (Nikon SMZ 745T). Anatomical characteristics of apothecia were examined under an optical microscope (Nikon Eclipse Ci-S). Photographs were taken using a digital camera (Nikon DS-Fi2). Descriptions of the range of anatomical characteristics for each species were determined by the smallest and largest single values measured for all specimens. Thin-layer chromatography (TLC) was performed in order to identify secondary metabolites using solvent systems C (toluene: acetic acid = 85:15), according to Orange et al. (2001).
DNA extraction, amplification and sequencing
DNA was extracted from fresh apothecia or thallus pieces with a DNA secure Plant Kit (TIANGEN) according to the manufacturer’s instructions. Amplified gene markers and their corresponding primers are shown in Table 1. PCR amplifications were achieved using 1.1 × T3 Super PCR Mix (TSINGKE) in a 25 µL total volume, containing 1 µL of genomic DNA, 1 µL of a 10 mM solution for each primer and 22 µL of 1.1 × T3 Super PCR Mix. The PCR program was: initial denaturation at 98 °C for 3 min, followed by 35 cycles of 98 °C for 10 s, 54–56 °C for 10 s, 72 °C for 15 s, followed by a final extension at 72 °C for 2 min. The PCR products were sequenced with the same amplification primers using Sanger technology by Tsingke Biotechnology Co., Ltd. (Kunming).
Table 1.
Gene markers and primer pairs used in this study.
| Gene markers | Primers | Sequences of Primers 5’-3’ | References |
|---|---|---|---|
| nuITS | ITS1F | CTTGGTCATTTAGAGGAAGTAA | Gardes and Bruns 1993 |
| ITS4 | TCCTCCGCTTATTGATATGC | White et al. 1990 | |
| nuLSU | LR0R | GTACCCGCTGAACTTAAGC | Rehner and Samuels 1994 |
| LR5 | ATCCTGAGGGAAACTTC | Vilgalys and Hester 1990 | |
| mtSSU | SSU1 | AGCAGTGAGGAATATTGGTC | Zoller et al. 1999 |
| SSU3R | ATGTGGCACGTCTATAGCCC | ||
| β-tubulin | Bt3-LM | GAACGTCTACTTCAACGAG | Myllys et al. 2001 |
| Bt10-LM | TCGGAAGCAGCCATCATGTTCTT |
Phylogenetic analyses
All newly obtained original sequences were edited manually using GENEIOUS v8.0.2. Their taxon name, voucher and GenBank accession number are shown in Table 2. All sequences, including those downloaded from GenBank, were aligned using MAFFT v7 with the option of E-INS-I (Katoh et al. 2005). Ambiguous regions were excluded using GBLOCKS (Talavera and Castresana 2007) with the default settings. Congruence between different gene regions was analyzed before combining. Bayesian inference (BI) and maximum likelihood (ML) were employed to determine the phylogenetic relationships. The best-fit partition substitution models were selected based on the lowest Bayesian information criterion (BIC) using PARTITION FINDER 2 (Guindon et al. 2010; Lanfear et al. 2012, 2017): nuITS dataset (TIM2e+G4) and the combined regions dataset (GTR+G for ITS1, ITS2 and mtSSU; GTR+I+G for 5.8S, nuLSU and β-tubulin), respectively.
Table 2.
Specimens used in this study, with taxon name, voucher and GenBank accession number. Newly obtained sequences are in bold font. “*” indicates that the sample was not included in the combined regions’ dataset analysis. “NA” indicates that there is no sequence available.
| Taxon | Voucher | Accession number | |||
|---|---|---|---|---|---|
| nuITS | mtSSU | nuLSU | β-tubulin | ||
| Acoliuminquinans | Wedin 6352 (UPS) | AY450583 | AY143404 | AY453639 | KX529023 |
| Ac.karelicum | Hermansson 16472 (UPS) | KX512897 | NA | KX512879 | NA |
| Amandineapunctata 1 | 18-60759 (KUN) | OL467351 | NA | NA | NA |
| Am.punctata 2 | AFTOL 1306 | HQ650627.1 | NA | DQ986756.1 | NA |
| Buelliaalpina | 16-53720 (KUN) | OM914626 | NA | NA | NA |
| B.alpina | 16-53737 (KUN) | OM914627 | NA | OP060154 | OM925561 |
| B.dijiana | - | AF250788 | NA | NA | NA |
| B.disciformis 1 | EDNA09-01524 | FR799139 | NA | NA | NA |
| B.disciformis 2 | EDNA09-02095 | FR799136 | NA | NA | NA |
| B.disciformis 3 | EDNA09-02116 | FR799138 | NA | NA | NA |
| B.elegans | 18-60340 (KUN) | OM914622 | NA | OM935566 | OM925559 |
| B.elegans | 20-68266 (KUN) | OM914634 | NA | OM935569 | OM925562 |
| B.elegans | XY19-272 (KUN) | OM914624 | NA | OM935567 | OM925560 |
| B.elegans | 18-59513 (KUN) | OM914623 | NA | NA | NA |
| *B.elegans | 18-62336 (KUN) | OM914630 | / | / | / |
| *B.elegans | XY19-1907 (KUN) | OM914632 | / | / | / |
| *B.elegans | XY19-1372 (KUN) | OM914631 | / | / | / |
| *B.elegans | XY19-2308 (KUN) | OM914633 | / | / | / |
| *B.elegans | 12-34754 (KUN) | OM914625 | / | / | / |
| *B.elegans | 10-0089 (KUN) | OM914636 | / | / | / |
| *B.elegans | 16-0084 (NXAC) | MN103116 | / | / | / |
| *B.elegans | Beck 242 (GZU) | AY143411 | / | / | / |
| *B.elegans | Leavitt 19085 | MZ922074 | / | / | / |
| B.epigaea | XY19-1218 (KUN) | OM914628 | OM913210 | OM935568 | NA |
| B.epigaea | XY19-2294 (KUN) | OM914629 | OM913211 | NA | NA |
| *B.epigaea | - | AF250785 | / | / | / |
| *B.epigaea | 18-59162 (KUN) | OM914635 | / | / | / |
| B.georgei | Trinkaus 356a (GZU) | AJ421416 | NA | NA | NA |
| B.zoharyi 1 | SA2 | MG592314 | MG592321 | MG592328 | MG592346 |
| B.zoharyi 2 | MT30 | MG592315 | MG592322 | MG592329 | MG592347 |
| B.zoharyi 3 | SA6 | MG592316 | MG592323 | MG592330 | MG592348 |
| B.zoharyi 4 | TE13 | MG592317 | MG592324 | MG592331 | MG592349 |
| Caliciumnobile 1 | Tibell 21968 (UPS) | KX512913 | KX512988 | KX529070 | NA |
| C.nobile 2 | Tibell 23396 (UPS) | KX512914 | KX512987 | KX529071 | NA |
| Diplotommaalboatrum 1 | 18-60034 (KUN) | MN615696 | OL467286 | OL444781 | OM925557 |
| Di.alboatrum 2 | 18-60448 (KUN) | MZ224658 | OL467287 | OL444782 | OM925558 |
| Di.venustum 1 | 18-58557 (KUN) | OL467349 | OL467284 | OL444779 | OM925555 |
| Di.venustum 2 | 18-58102 (KUN) | OL467350 | OL467285 | OL444780 | OM925556 |
| * Di.venustum 3 | XY19-252 (KUN) | OL467353 | / | / | / |
| Heterodermiaspeciosa | Wetmore (S) | KX512927 | KX512975 | KX512868 | KX529000 |
| He.vulgaris | Frisch 11/Ug1226 (UPS) | KX512928 | KX512989 | KX512857 | NA |
| Phaeophysciaciliata | Prieto (S) | KX512929 | KX512958 | KX512886 | KX529012 |
| Ph.orbicularis | Prieto 3012 (S) | KX512930 | KX512967 | KX512876 | NA |
| Physciaaipolia | Wedin 6145 (UPS) | KX512931 | AY143406 | AY300857 | KX529021 |
| P.tenella | Odelvik and Hellström 0827 (S) | KX512932 | KX512974 | KX512869 | NA |
| Pyxinecoccoes | Prieto (S) | KX512936 | KX512964 | NA | KX529010 |
| Py.subcinerea | - | HQ650705 | NA | DQ883802 | NA |
| Py.sorediata | Wetmore 91254 (S) | KX512937 | KX512973 | KX512870 | KX529001 |
| Tetramelaschloroleucus | Westberg 10–001 (S) | KX512938 | NA | KX512875 | KX529006 |
| Te.geophilus | 20-67496 (KUN) | OL467354 | OL467291 | OL444785 | OM925563 |
| Te.pulverulentus | Nordin 6368 (UPS) | KX512940 | KX512983 | KX512860 | KX528990 |
| Thelommamammosum 1 | Tibell 23775 (UPS) | KX512942 | KX512954 | KX512888 | KX529016 |
| Th.mammosum 2 | Hernández et al. 2002 (UPS) | KX512943 | KX512953 | KX512851 | KX529017 |
| Th.santessonii 1 | Nordin 4011 (UPS) | KX512944 | KX512951 | KX512889 | NA |
| Th.santessonii 2 | Nash 38262 (UPS) | KX512945 | KX512950 | KX512890 | NA |
ML analyses were performed with RAxML v8.2.12 (Stamatakis 2006). Bootstrap support values (MLBS) were estimated from the 70% majority rule tree of all saved trees obtained from 2000 non-parametric bootstrapping pseudo-replicates. BI analyses were performed with MrBayes v3.2.7 (Ronquist et al. 2012) running for 2 million generations. The trees were sampled every 100 generations and the first 25% of the trees were discarded as burn-in, since the average SD of split frequencies had converged at the step of 20% of the total. Bayesian posterior probabilities (BPP) were obtained from the 95% majority rule consensus tree of all saved trees. The final trees were visualized in FigTree v1.4.0 (Rambaut 2012). The final matrices were submitted to TreeBASE: TB2: 29562 for nuITS and TB2: 29563 for the combined regions dataset.
Results
The nuITS matrix (478bp) comprised 55 sequences including 15 newly generated sequences for new species and new records. The combined regions dataset (478bp for 43 nuITS sequences; 740bp for 27 mtSSU sequences; 899bp for 33 nuLSU sequences; 755bp for 23 β-tubulin sequences) comprised 126 terminals, including 31 newly generated sequences (Table 2). In the two phylogenetic analyses, eight representative monophyletic genera (Acolium (Ach.) Gray, Amandinea, Buellia s.str., Calicium Pers., Diplotomma, Pyxine Fr., Tetramelas, Thelomma A. Massal.) were selected from Caliciaceae Chevall. and six Physciaceae Zahlbr. species were selected as the outgroup. The results of the phylogenetic analyses showed that the species in the Buelliaepigaea-group formed two clades (Figs 1, 2).
Figure 1.
Phylogenetic relationships of Caliciaceae based on a Maximum Likelihood analysis of the nuITS matrix. Species positioned in clade 1 and clade 2 belong to the Buelliaepigaea-group. Maximum Likelihood bootstrap values and posterior probabilities are shown near the nodes. New species and records are shown in bold.
Figure 2.
Phylogenetic relationships within Caliciaceae, based on a Maximum Likelihood analysis of a combined regions dataset (nuITS-nuLSU-mtSSU-β-tubulin). Species positioned in clade 1 and clade 2 belong to the Buelliaepigaea-group. Maximum Likelihood bootstrap values and posterior probabilities are shown near the nodes. New species and records are shown in bold.
In clade 1: B.dijiana, B.georgei and B.epigaea formed an independent clade with strong support (100% BS and 1.00 PP in Figs 1, 2). The specimens designated as B.epigaea (including one sequence from GenBank) clustered as a single lineage with high support (96% BS and 1.00 PP in Fig. 1). These specimens had identical morphological and chemical characters to those described for B.epigaea, and thus have been confirmed as a new record for China.
In clade 2: specimens here described as B.alpina formed a well-supported sister clade to B.zoharyi (100% BS and 1.00 PP in Figs 1, 2). These two species are distinctively different in their anatomical and chemical characteristics. We therefore recognize B.alpina as a new species within Buelliaepigaea-group. The collections designated as B.elegans also formed a highly supported monophyletic lineage, clustering with sequences downloaded from GenBank (96% BS and 1.00 PP in Fig. 1). This constitutes the first record of B.elegans from China. These three species (B.zoharyi, B.alpina and B.elegans) formed a monophyletic clade with strong support (100% BS and 1.00 PP in Figs 1, 2). Clade 2 was sister to the genus Tetramelas (previously included in Buellia s.l.), which also contains alpine terricolous species.
Discussion
Although species in Buelliaepigaea-group share common characters, there are still additional diagnostic traits which could be used to distinguish between species within this group. The monophyletic clade 1 is formed by B.dijiana and B.georgei, together with B.epigaea. These three species share the characters of having no distinct marginal lobes and lacking atranorin. Within clade 1, only B.georgei has effigurate thalli; it also has short marginal lobes which often form rosettes. Both B.georgei and B.dijiana contain arthothelin acid and were described from Australia. However, their habitat differs: B.georgei occurs primarily on soft limestone or calcareous outcrops but never directly on calcareous soil, whereas B.dijiana is present on soil in open mallee vegetation (Trinkaus et al. 2001). In contrast, B.epigaea lacks secondary metabolites and could be reliably recognized by its crusty thallus, which is often uneven to wrinkled.
We propose a new species: Buelliaalpina. It was clustered with B.zoharyi and B.elegans within clade 2. The common features of clade 2 are: having slim effigurate thalli covered with granulose pruina, obvious marginal lobes and always containing atranorin. The most distinctive features of the new species B.alpina are: heavily white pruinose apothecia and four-spored asci. B.elegans is similar to B.zoharyi in its external morphology. However, B.elegans can still be reliably distinguished from B.zoharyi, based on the ornamentation of ascospores. B.elegans has a loosely regulate surface (Fig. 3B and Fig. 4B), whereas the surface of B.zoharyi is microfoveate (Fig. 3G). In addition, the two species differ in their secondary metabolites: B.zoharyi contains atranorin, stictic acid and norstictic acid, while B.elegans has four chemotypes (Trinkaus and Mayrhofer 2000). One of these chemotypes (atranorin and 2’-O-methylperlatolic acid) is widely distributed in Asia, and was detected in most of the specimens from Yunnan, Qinghai and Xizang Provinces, China.
Figure 3.
Ornamentation of ascospores ABuelliaalpinaBBuelliaelegansCBuelliaepigaeaDBuellialobataEBuelliadijianaFBuelliaasterellaGBuelliazoharyiHBuelliageorgei (A–C were drawn by Qiu Yi Zhong D–H are from Trinkaus and Mayrhofer 2000; Trinkaus et al. 2001).
Figure 4.
Ornamentation of ascospores (6000× magnification photograph under scanning electron microscope) ABuelliaalpinaBBuelliaelegansCBuelliaepigaea Scale bars: 2 µm (A–C).
In addition to the species discussed above, Buelliaepigaea-group also contains the species B.asterella and B.lobata. These have not been included in this phylogenetic study due to the lack of available sequences. Morphologically, B.asterella and B.lobata are similar to B.alpina in their possession of four mature ascospores within each ascus (Trinkaus and Mayrhofer 2000; Trinkaus et al. 2001). B.alpina differs from these two species by its heavily white pruinose apothecia, granular pruina on the thallus surface and atranorin content. Furthermore, B.alpina has Callispora-type ascospores (with lateral (subapical) thickening, always with tapering ends). However, B.asterella and B.lobata both have fine pruina on their thallus surface and Buellia-type ascospores (lacking distinct wall thickening). B.asterella contains atranorin, norstictic acid and trace quantities of stictic acid, whereas B.lobata contains atranorin and thuringione.
The Buellia species in this study have all been classified as belonging to the Buelliaepigaea-group, based on their terricolous habitat and distinct morphological characters of white and effigurate thalli. However, the phylogenetic trees in this study suggest that this group is not monophyletic. Thus, the previous definition of Buelliaepigaea-group may be artificial, without support from molecular data. Phylogenetic study of both a single region (nuITS) and combined regions (nuITS-nuLSU-mtSSU-β-tubulin) showed that both clades do not group together. Therefore, the fundamental concept of the Buelliaepigaea-group requires further research, including additional samples from across its global distribution.
In conclusion, species of Buelliaepigaea-group share common characters which can be reliably recognized. There are distinct morphological and chemical differences which could be used to distinguish between different species in this group. Ornamentation of ascospores is a useful character by which to distinguish species in Buelliaepigaea-group (Figs 3, 4; Table 3).
Table 3.
Key characteristics of the Buelliaepigaea-group.
| Species | Thallus | Apothecia | Exciple | Spores | Ornamentation of spores | Major chemistry | Pycnidia | Parasitic fungi |
|---|---|---|---|---|---|---|---|---|
| B.alpina | effigurate, lobes linear, closely aggregate; covered with granulose pruina | flat, margin wavy and irregular | dispersa-type | Callispora-type; four-spored | densely rugulate, resulting in rough surface | atranorin | not seen | not seen |
| B.asterella | effigurate, lobes short and connected; surface with fine pruina | convex | aethalea-type | Buellia-type; often four well developed spores | microfoveate | atranorin, stictic acid, norstictic acid | rare | not seen |
| B.dijiana | not effigurate, crustose to granulose-squamulose; surface with fine pruina; dispersive | soon irregularly convex | aethalea-type | Buellia-type | warty, microrugulate | arthothelin | filiform conidia | rare |
| B.elegans | effigurate, lobes short to slender, multi-forked; covered with granulose pruina | flat to convex | dispersa-type | Buellia-type | Loosely rugulate, resulting in rough surface | a) atranorin; b) atranorin and 2'-O-methylperlatoric (in Asia) | not seen | common |
| B.epigaea | not effigurate, crusty, uneven to wrinkled; surface with fine pruina | flat | aethalea-type | Callispora-type | surface densely areolate and rough | no secondary metabolite | rare | rare |
| B.georgei | effigurate, marginal lobes short and often forming rosettes; surface with white granulose pruina | flat or sometimes slightly convex | aethalea-type | Buellia-type | surface densely areolate and rough | arthothelin | filiform conidia | common |
| B.lobata | effigurate, marginal lobes distinct but short, the tips of lobes dark; surface with lightly fine pruina | apothecia disc below margin | aethalea-type | Buellia-type; often four well developed spores | warty, microrugulate | arthothelin, thuringione | filiform conidia | common |
| B.zoharyi | effigurate, lobes obvious; covered with granulose pruina | flat to convex | dispersa-type | Buellia-type | microfoveate | atranorin, norstictic acid, stictic acid | common | rare |
Taxonomy
. Buellia alpina
Xin Y. Wang & Li S. Wang sp. nov.
DC6E31F7-9A7B-522B-910D-5233C6476804
843376
Figure 5.
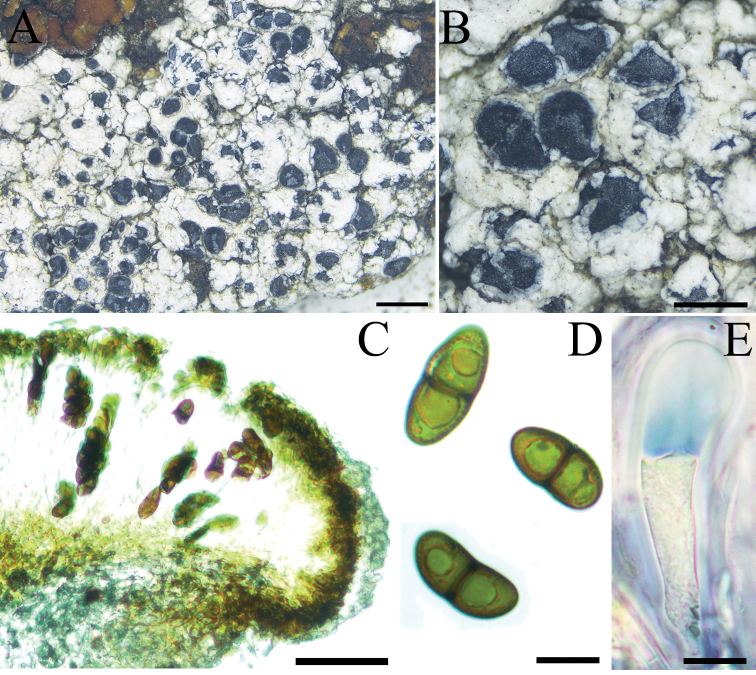
Morphology of Buelliaalpina (16-53720 KUN) A thallus on soil within meadow B black lecideine apothecia covered with white pruina C the section of apothecium, exciple dispersa-type D ascospores with 1-septate, Callispora-type, with tapered ends E mature ascus containing four spores, Bacidia-type F young ascus containing four spores. Scale bars: 2 mm (A); 0.5 mm (B); 50 µm (C); 10 µm (D–F).
Diagnosis.
The species is distinguished from its closest relatives B.elegans and B.zoharyi by its linear lobate thallus, heavily pruinose apothecia and lobes, Callispora-type ascospores and four-spored asci.
Type.
China. Xizang Prov.: Lasa Ci., Namucuo Nature Reserve, on soil beside a lake, 30°46'46"N, 90°52'24"E, alt. 4730 m, 28 Sep. 2016, L.S. Wang et al. 16-53720 (KUN-Holotype; SDNU-Isotype).
Description.
Thallus effigurate, lobate and linear, lobes tightly aggregated, 0.5–1.5 mm wide, prothallus absent; upper surface white to grayish white, dull, covered with granulose pruina; medulla white, non-amyloid (I–). Apothecia sparse to dense, sometimes aggregate, adnate to the thallus, lecideine, margin covered with white pruina which resemble lecanorine apothecia; disc black, roundish, (0.3–)0.5–1.4(–1.6) mm in diam., heavily pruinose, roundish when immature, marginal part becoming wavy and irregular when mature; margin persistent; exciple dispersa-type (Bungartz et al. 2007), dark brown, without aeruginose pigments (HNO3–); epihymenium brown to dark brown; hymenium hyaline, 80–100 µm tall, without oil droplets, paraphyses simple to moderately branched, apically swollen, with a brown pigment cap; hypothecium dark brown; asci oval-clavate, Bacidia-type, four-spored; spores 1-septate, hyaline when young, turning brown when mature, Callispora-type (Bungartz et al. 2007), ellipsoid, with tapering ends, proper septum narrow, not thickening during spore ontogeny, (13–)15–20(–22) × (6–)7–9(–10) µm. Pycnidia not seen.
Chemistry.
Thallus K+ yellow, C–, PD–, UV–, medulla I–; containing atranorin.
Distribution and ecology.
This species is mainly distributed in alpine meadows of the Tibetan Plateau, growing on soil within meadows, between elevations of 4700–5000 m.
Etymology.
The epithet “alpina” refers to the alpine distribution of this species.
Note.
This new species could be distinguished from all other Buellia species by its linear lobate thallus, covered with granulose pruina, black lecideine apothecia with heavy whitish pruina, four-spored asci and its alpine distribution. It might be misidentified as subsquamulose or subfoliose species of Squamarina Poelt, but could be distinguished by the white thickened edges and hyaline simple ascospores.
Specimens examined.
China. Xizang Prov.: Lasa Ci., Namucuo Nature Reserve, on soil beside a lake, 30°46'46"N, 90°52'24"E, alt. 4730 m, 28 Sep. 2016, L.S. Wang et al.16-53737.
. Buellia elegans
Poelt, Nova Hedwigia 25(1–2): 184–186 (1974)
4D597B7C-0C05-5F4C-B0D3-D2660F6D9AFE
Figure 6.
Morphology of Buelliaelegans (16-51770 KUN) A thallus on soil within meadow B lecideine apothecia C the section of apothecium, exciple dispersa-type D ascospores with 1-septate, Buellia-type E mature ascus containing eight spores, Bacidia-type. Scale bars: 2 mm (A); 1 mm (B); 50 µm (C); 10 µm (D, E).
Type.
Italy. Ad terram calcaream supra Clavennam (Madèsimo), Anzi M. (M! -Holotype).
Description.
Thallus effigurate with distinct marginal lobes slim, 0.5–1 mm wide, the edge usually separated from the substrate and clearly foliaceous, thallus radiate, 1–2 cm in diam., prothallus absent; upper surface white, dull, usually covered with granular pruina; the upper cortex about 20 µm thick, with granular crystals, and the lower surface light brown to white, without cortex; medulla white, without calcium oxalate crystals. Apothecia sparse, lecideine; disc and margin black, sometimes lightly pruinose, roundish, 0.3–1.0 mm in diam., immersed and smooth when young but adnate and convex when mature; margin persistent; exciple thick, dispersa-type, without aeruginose pigments (HNO3–); epihymenium brown to dark brown; hymenium hyaline, 70–90 µm tall, without oil droplets, paraphyses simple to moderately branched, apically swollen, with a brown pigment cap; hypothecium dark brown; asci oval-clavate, Bacidia-type, eight-spored; spores 1-septate, hyaline when young, turning brown when mature, Buellia-type, ellipsoid, not thickening during spore ontogeny, 15–22 × 7–10 µm. Pycnidia not seen.
Chemistry.
Thallus K+ yellow, C–, KC–, PD–, UV+ yellow, medulla I–; containing atranorin and norstictic acid (trace) or atranorin and 2’-O-methylperlatolic acid.
Distribution and ecology.
This species is mainly distributed in open and dry soil or soil over rock or within meadows between elevations of 1400–4730 m. This species has been recorded in Asia, Afghanistan, Europe and North America (Thomson, 1997). In China, it is mainly distributed in Gansu, Ningxia, Qinghai, Xizang and Yunnan Provinces.
Note.
This is a new record for China, and is unique among species of Buellia due to its effigurate thallus, marginal lobes linear and slim, branched near the tips. It resembles folicolous species of Physconia Poelt, but could be differentiated by its slim lobes and lack of lower surface. It has a wide distribution across the Tibetan Plateau, especially in arid deserts and meadows. Four chemotypes of the species were previously reported (Trinkaus and Mayrhofer 2000). Only two chemotypes have been detected in Chinese materials: atranorin and 2’-O-methylperlatolic acid account for the majority, atranorin and norstictic acid (trace) constitute only a small proportion.
Selected specimens examined.
China. Gansu Prov.: Jiayuguan Ci., Xigou, mineral, on soil, 39°39'34"N, 97°56'15"E, alt. 2198 m, 28 May 2018, L.S. Wang et al. 18-59611; Yumen Ci., meadow along the route from Yumen to Yuerhong, on soil, 39°57'45"N, 96°39'23"E, alt. 2395 m, 27 May 2018, L.S. Wang et al. 18-59513. Ningxia Prov.: Zhongwei Ci., Shanpotou, Mengjiawan, on soil, 37°36'12"N, 104°55'06"E, alt. 1403 m, 18 Sep. 2010, D.L. Niu et al. 10-0089. Qinghai Prov.: Wulan Co., desert along the route from Wulan to Delingha, on soil, 37°02'08"N, 98°12'29"E, alt. 3072 m, 20 May 2018, L.S. Wang et al. 18-58303; Dulan Co., Xiangjia Vil., on sandy rock, 36°00'53"N, 97°44'36"E, alt. 3056 m, 15 Sep. 2020, L.S. Wang et al. 20-68266. Xizang Prov.: Dazi Dis., Bangdui Vil., on soil, 29°44'06"N, 91°24'55"E, alt. 3709 m, 16 Jul. 2019, L.S. Wang et al. 19-64615; Basu Co., beside Ranwu Lake, on soil over rock, 29°23'34"N, 96°50'20"E, alt. 3901 m, 15 Jul. 2019, X.Y. Wang et al. (XY19-278; XY19-272); Geji Co., beside S301 road, on soil, 32°14'47"N, 82°10'27"E, alt. 4514 m, 21 Jul. 2019, L.S. Wang et al. 19-63808; Bomi Co., along the route to Basu Co., on soil, 29°40'31"N,96°12'38"E, alt. 2920 m, 10 Nov. 2018, L.S. Wang et al. 18-62336; Langkazi Co., Simila Mt., on soil over rock, 28°50'37"N, 89°51'54"E, alt. 4343 m, 24 Jul. 2019, X.Y. Wang et al. XY19-1372; Sangri Co., Sangri Town, on soil over rock, 29°17'29"N, 92°05'30"E, alt. 3595 m, 30 Jul. 2019, X.Y. Wang et al. (XY19-1899; XY19-1907); Jangzi Co., Simila Mt., on soil over rock, 28°50'30"N, 89°51'48"E, alt. 4223 m, 24 Jul. 2019, X.Y. Wang et al. XY19-2308; Jiangda Co., Kakong Vil., on soil over rock, 31°20'22"N, 98°08'01"E, alt. 3785 m, 23 Sep. 2020, L.S. Wang et al. 20-68931. Yunnan Prov.: Deqin Co., Benzilan Vil., on soil over rock, 28°10'27"N, 99°22'53"E, alt. 2007 m, 26 Sep. 2020, L.S. Wang et al. 20-69241; Deqin Co., Benzilan Vil., beside JinSha river, on soil, 28°11'36"N, 99°21'08"E, alt. 2108 m, 19 Aug. 2018, L.S. Wang et al. 18-60340; Deqin Co., Benzilan Vil., on soil, 28°13'38"N, 99°19'20"E, alt. 2110 m, 3 Jul. 2012, L.S. Wang et al. 12-34754.
. Buellia epigaea
(Pers.) Tuck. Gen. lich.: 185 (1872)
E87C9BB5-A2E4-5C9F-B400-C46DD85552A1
Figure 7.
Morphology of Buelliaepigaea (XY19-2294 KUN) A thallus growing on the soil B lecideine apothecia with white pruina, surrounded by a thalline collar (pseudolecanorine) C the section of apothecium, exciple aethalea-type D ascospores with 1-septate, Callispora-type E ascus Bacidia-type. Scale bars: 2 mm (A); 1 mm (B); 50 µm (C); 10 µm (D, E).
Type.
Germany. Hesse, ad terram inter muscos non procul a Monte Meissner, 1794, Persoon (H-Ach-Isotype, not seen).
Description.
Thallus terricolous, tightly attached to the substrate, upper surface white or greyish white, usually with white fine pruina, thallus crusty, uneven to wrinkled, 0.2–1 mm thick, prothallus absent; the upper cortex 60–150 µm thick, with granular crystals, pith completely interspersed with Ca oxalate crystals; medulla white. Apothecia sparse to dense, lecideine, but usually surrounded by a thalline collar (pseudolecanorine); disc black, always with finely white pruina, roundish, 0.5–1.0 mm in diam., mostly flat, rarely slightly convex; young apothecia immersed and margin with finely white pruina breaking out broadly, mature apothecia adnate and margin absent or not obvious; exciple aethalea-type, up to 50 µm thick, without aeruginose pigments (HNO3–); epihymenium brown to dark brown; hymenium hyaline, 60–80 µm tall, without oil droplets, paraphyses simple to moderately branched, apically swollen, with a brown pigment cap; hypothecium hyaline to light brown, up to 100 µm high; asci oval-clavate, Bacidia-type, eight-spored; spores 1-septate, hyaline when young, turning brown when mature, with tapering ends, Callispora-type, often curved, not thickening during spore ontogeny, 12–20 × 6–10 µm. Pycnidia not seen.
Chemistry.
Thallus K–, C–, KC–, PD–, UV–; without secondary metabolites.
Distribution and ecology.
This species mainly occurs on open and dry soil, soil within meadows or on soil over rock, between elevations of 2300–4700 m. This species has been recorded in Asia, Europe and North America (Trinkaus and Mayrhofer 2000). In China, it is mainly distributed in Gansu, Qinghai and Xizang Provinces.
Note.
The species is a new record for China; it could be distinguished from all the other terricolous Buellia species reported in China by the combination of the following characteristics: thallus white crustose, uneven to wrinkled, always covered by finely white pruina, apothecia pseudolecanorine, the ornamentation of the ascospore surface densely areolate and rough, pycnidia rare, Callispora-type ascospores and absence of secondary metabolites. This species is close to Tetramelas species in phylogeny and similar in morphology, but could be distinguished by absence of the secondary metabolites 6-O-methylarthothelin or related xanthones, and pseudolecanorine apothecia covered with white pruina.
Selected specimens examined.
China. Gansu Prov.: Sunan Co., along the route from Linze to Sunan, on soil over rock, 38°52'26"N, 99°44'21"E, alt. 2294 m, 29 May 2018, L.S. Wang et al. 18-58766; Sunan Co., along the route from Linze to Sunan, on soil over rock, 38°52'47"N, 99°43'57"E, alt. 2296 m, 29 May 2018, L.S. Wang et al. 18-59699. Qinghai Prov.: Gonghe Co., meadow beside Qinghai Lake, on soil within meadow, 36°33'26"N, 100°28'45"E, alt. 3431 m, 18 May 2018, L.S. Wang et al. 18-59162. Xizang Prov.: Qushui Co., Niedang Vil., on soil, 29°30'24"N, 90°56'17"E, alt. 3527 m, 22 Jul. 2019, X.Y. Wang et al. XY19-1234; Qushui Co., Niedang Vil., on soil over rock, 29°30'22"N, 90°56'15"E, alt. 3624 m, 22 Jul. 2019, X.Y. Wang et al. XY19-1218; Langkazi Co., entrance to Karuola Glacier, on soil over rock, 28°53'54"N, 90°13'32"E, alt. 4774 m, 24 Jul. 2019, X.Y. Wang et al. XY19-2294.
Key to species of Buelliaepigaea-group
| 1 | Thallus effigurate and marginal lobes long and obvious; containing atranorin | 2 |
| – | Thallus either not effigurate or effigurate but with marginal lobes short and closely aggregate; lacking atranorin | 4 |
| 2 | Ascus four-spored | Buelliaalpina |
| – | Ascus eight-spored | 3 |
| 3 | Spores large, up to 23 µm long, ornamentation of spores rugulate | Buelliaelegans |
| – | Spores smaller, less than 17 µm long, ornamentation of spores microfoveate | Buelliazoharyi |
| 4 | Thallus not effigurate | 5 |
| – | Thallus effigurate, usually with short lobes | 6 |
| 5 | Thallus crustose to granulose-squamulose; containing arthothelin | Buelliadijiana |
| – | Thallus crusty, uneven to wrinkled; lacking secondary metabolites | Buelliaepigaea |
| 6 | On rock; ascus eight-spored; marginal lobes forming rosettes | Buelliageorgei |
| – | On soil; usually four mature spores in each ascus | 7 |
| 7 | Containing atranorin and thuringione; ornamentation of spores warty, microrugulate | Buellialobata |
| – | Containing atranorin, norstictic acid and stictic acid (trace); ornamentation of spores microfoveate | Buelliaasterella |
Supplementary Material
Acknowledgements
We express our sincere thanks to the M herbarium for providing type specimens and digital images. This study was supported by grants from the Flora Lichenum Sinicorum (31750001), the Second Tibetan Plateau Scientific Expedition and Research Program (STEP) (2019QZKK0503), Youth Innovation Promotion Association CAS (2020388), Yunnan Young & Elite Talents Project, National Natural Science Foundation of China (31970022) and State Key Laboratory of Phytochemistry and Plant Resources in West China (P2020-KF08).
Citation
Ai M, Li LJ, Worthy FR, Yin AC, Zhong QY, Wang SQ, Wang LS, Wang XY (2022) Taxonomy of Buellia epigaea-group (Caliciales, Caliciaceae), revealing a new species and two new records from China. MycoKeys 92: 45–62. https://doi.org/10.3897/mycokeys.92.83939
Funding Statement
the Flora Lichenum Sinicorum (31750001) the Second Tibetan Plateau Scientific Expedition and Research Program (STEP) (2019QZKK0503) Youth Innovation Promotion Association CAS (2020388) Yunnan Young & Elite Talents Project, National Natural Science Foundation of China (31970022) State Key Laboratory of Phytochemistry and Plant Resources in West China (P2020-KF08)
Supplementary materials
Ornamentation of ascospores
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Min Ai, Li Juan Li, Fiona Ruth Worthy, An Cheng Yin, Qiu Yi Zhong, Shi Qiong Wang, Li Song Wang, Xin Yu Wang
Data type
Images
Explanation note
Figure S1. Ornamentation of ascospores (6000× magnification photograph under scanning electron microscope). A–D Buelliaalpina. Scale bars: 2 µm. Figure S2. Ornamentation of ascospores (6000× magnification photograph under scanning electron microscope). A–D Buelliaelegans. Scale bars: 2 µm. Figure S3. Ornamentation of ascospores (6000× magnification photograph under scanning electron microscope). A–D Buelliaepigaea. Scale bars: 2 µm.
References
- Bungartz F, Nordin A, Grube M. (2007) Buellia. In: Nash TH III, Gries C, Bungartz F. (Eds) Lichen Flora of the Greater Sonoran Desert Region.Volume 3. Lichens Unlimited, Arizona State University, Tempe, 113–179.
- Gardes M, Bruns TD. (1993) ITS primers with enhanced specificity for basidiomycetes. Application for the identification of mycorrhizae and rusts. Molecular Ecology 2(2): 113–118. 10.1111/j.1365-294X.1993.tb00005.x [DOI] [PubMed] [Google Scholar]
- Grube M, Arup U. (2001) Molecular and morphological evolution in the Physciaceae (Lecanorales, lichenized Ascomycotina), with special emphasis on the genus Rinodina. Lichenologist 33(1): 63–72. 10.1006/lich.2000.0297 [DOI] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Systematic Biology 59(3): 307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- Katoh K, Kuma K, Toh H, Miyata T. (2005) MAFFT version 7, improvement in accuracy of multiple sequence alignment. Nucleic Acids Research 33(2): 511–518. 10.1093/nar/gki198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Ho SY, Guindon S. (2012) PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29(6): 1695–1701. 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. (2017) PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 34: 772–773. 10.1093/molbev/msw260 [DOI] [PubMed] [Google Scholar]
- Marbach B. (2000) Corticole und lignicole Arten der Flechtengattung Buellia sensu lato in den Subtropen und Tropen. Bibliotheca Lichenologica, 74, Cramer J, Berlin, Stuttgart, 384 pp. [Google Scholar]
- Myllys L, Lohtander K, Tehler A. (2001) Beta-tubulin, ITS and group I intron sequences challenge the species pair concept in Physciaaipolia and P.caesia. Mycologia 93: 335–343. [Google Scholar]
- Orange A, James PW, White FJ. (2001) Microchemical methods for the identification of lichens. British Lichen Society, London, 101 pp. [Google Scholar]
- Nordin A. (2000) Taxonomy and phylogeny of Buellia species with pluriseptate spores (Lecanorales, Ascomycotina). Symbolae Botanicae Upsalienses 33(1): 1–117. [Google Scholar]
- Poelt J, Sulzer M. (1974) Die Erdflechte Buelliaepigaea, eine Sammelart. Nova Hedwigia 25: 173–194. [Google Scholar]
- Rambaut A. (2012) FigTree, version 1.4.0, Institute of Evolutionary Biology, University of Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/
- Rehner SA, Samuels GJ. (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98(6): 625–634. 10.1016/S0953-7562(09)80409-7 [DOI] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger C. (1993) A revision of European saxicolous species of the genus Buellia De Not. and formerly included genera. Lichenologist 25(4): 315–364. 10.1006/lich.1993.1001 [DOI] [Google Scholar]
- Stamatakis A. (2006) RAxML-VI-HPC, Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22(21): 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Talavera G, Castresana J. (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology 56(4): 564–577. 10.1080/10635150701472164 [DOI] [PubMed] [Google Scholar]
- Thomson JW. (1997) American Arctic Lichens. 2. The Microlichens. The University of Wisconsin Press, Madison, Wisconsin.
- Trinkaus U, Mayrhofer H. (2000) Revision der Buelliaepigaea-gruppe (lichenisierte Ascomyceten, Physciaceae). I Die Arten der Nordhemisphare. Nova Hedwigia 71(3–4): 271–314. 10.1127/nova/71/2000/271 [DOI] [Google Scholar]
- Trinkaus U, Mayrhofer H, Elix JA. (2001) Revision of the Buelliaepigaea-group (lichenized ascomycetes, Physciaceae) 2. The species in Australia. Lichenologist 33(1): 47–62. 10.1006/lich.2000.0286 [DOI] [Google Scholar]
- Vilgalys R, Hester M. (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172(8): 4238–4246. 10.1128/jb.172.8.4238-4246.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XY, Li LJ, Liu D, Zhang YY, Yin AC, Zhong QY, Wang SQ, Wang LS. (2020) Two new species and six new records of Buellia s.l. (lichenized Ascomycota, Caliciaceae) from China. The Bryologist 123(3): 431–443. 10.1639/0007-2745-123.3.430 [DOI] [Google Scholar]
- Wei JC. (2020) The enumeration of lichenized fungi in China. China Forestry Press, Beijing.
- White TJ, Bruns TD, Lee S, Taylor J. (1990) Amplification and direct sequencing of fungal ribosomal DNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (Eds) PCR protocols: a guide to methods and applications.Academic Press, San Diego, 315–321. 10.1016/B978-0-12-372180-8.50042-1 [DOI]
- Zoller S, Scheidegger C, Sperisen C. (1999) PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31(5): 511–516. 10.1006/lich.1999.0220 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ornamentation of ascospores
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Min Ai, Li Juan Li, Fiona Ruth Worthy, An Cheng Yin, Qiu Yi Zhong, Shi Qiong Wang, Li Song Wang, Xin Yu Wang
Data type
Images
Explanation note
Figure S1. Ornamentation of ascospores (6000× magnification photograph under scanning electron microscope). A–D Buelliaalpina. Scale bars: 2 µm. Figure S2. Ornamentation of ascospores (6000× magnification photograph under scanning electron microscope). A–D Buelliaelegans. Scale bars: 2 µm. Figure S3. Ornamentation of ascospores (6000× magnification photograph under scanning electron microscope). A–D Buelliaepigaea. Scale bars: 2 µm.