Abstract
Infectious disease is hypothesized to be one of the most important causes of morbidity and mortality in wild great apes. Specific socioecological factors have been shown to influence incidences of respiratory illness and disease prevalence in some primate populations. In this study, we evaluated potential predictors (including age, sex, group size, fruit availability, and rainfall) of respiratory illness across three western lowland gorilla groups in the Republic of Congo. A total of 19,319 observational health assessments were conducted during daily follows of habituated gorillas in the Goualougo and Djéké Triangles over a 4-year study period. We detected 1146 incidences of clinical respiratory signs, which indicated the timing of probable disease outbreaks within and between groups. Overall, we found that males were more likely to exhibit signs than females, and increasing age resulted in a higher likelihood of respiratory signs. Silverback males showed the highest average monthly prevalence of coughs and sneezes (Goualougo: silverback Loya, 9.35 signs/month; Djéké: silverback Buka, 2.65 signs/month; silverback Kingo,1.88 signs/month) in each of their groups. Periods of low fruit availability were associated with an increased likelihood of respiratory signs. The global pandemic has increased awareness about the importance of continuous monitoring and preparedness for infectious disease outbreaks, which are also known to threaten wild ape populations. In addition to the strict implementation of disease prevention protocols at field sites focused on great apes, there is a need for heightened vigilance and systematic monitoring across sites to protect both wildlife and human populations.
Keywords: Great apes, Infectious disease, Outbreak, Conservation, Sociality, Ecology, Cough, Sneeze
Introduction
The threat of disease to great ape populations is considerable, particularly considering their slow reproductive rates, longer interbirth intervals, and high rates of infant mortality (Ryan and Walsh 2011; Genton et al. 2012; Masi et al. 2012). Infectious disease, in particular respiratory illness, is hypothesized to be one of the leading causes for mortality in wild great apes habituated to human presence for research or tourism (Köndgen et al. 2008; Patrono et al. 2018; Scully et al. 2018; Negrey et al. 2019; Patrono et al. 2019). The global COVID-19 pandemic has increased awareness of the importance of identifying factors that may predict susceptibility to respiratory illness not only in humans, but also in other species. In this study, we conducted observational health monitoring to examine whether particular social and ecological factors predicted respiratory symptoms and outbreaks across several groups of critically endangered western lowland gorillas. The data collection protocol resulting from this research provides a practical monitoring framework to conduct surveillance for emerging infectious disease outbreaks in wild primate populations.
Sociality, demographics, and disease
While the probability of pathogen transmission within and between groups is contingent on traits of the pathogen (e.g., mode of transmission and infectivity), aspects of social interactions can also influence disease spread (Baudouin et al. 2019; Morrison 2019; Sandel et al. 2020). Increasing our understanding of the relationship between sociality and disease not only has theoretical importance, but also direct implications for the conservation of wild primates.
Social groups
Particular aspects of the structure of great ape societies may be linked to health outcomes. In gorillas, the type of social unit (bachelor group, breeding group, or solitary individual) plays a major role in availability of potential pathways of pathogen transmission (Caillaud et al. 2006). Further, the stability of a group may influence disease transmission and be reliant upon certain positions or individuals within a society. For example, a dominant silverback is typically the focal point of a western lowland gorilla social group, and the death of this individual can result in dispersal of all surviving individuals such that the group ceases to exist. In their research on mountain gorillas, Morrison et al. (2021) found that traits such as age, sex, and adult male dominance could potentially be employed to estimate an individual’s risk of exposure to infectious diseases and their risk of transmitting such diseases. Similar studies have yet to be undertaken for western lowland gorillas.
Sex and age
Sex and age differences in health and overall fitness are found across non-human primates (Hoffman et al. 2008; Kulik et al. 2015; Lonsdorf et al. 2018). Males should theoretically be more susceptible to infections than females due to the male hormone testosterone, which is believed to have a negative effect on immune function (Grossman 1989; Folstad and Karter 1992; Skorping and Jensen 2004). In some primate species, number of males is a key factor associated with increased rates of respiratory infections (Skorping and Jensen 2004; Lonsdorf et al. 2018). In addition to adult males, infants and geriatric individuals are also suspected to be more susceptible to disease. Respiratory signs rise steadily with age among female chimpanzees, with elevated rates also observed in young, low-ranking males and older, high-ranking males (Emery Thompson et al. 2018). Hassell et al. (2017) reported that respiratory infections are more fatal in younger mountain gorillas, particularly those under 1.2 years of age, potentially due to their underdeveloped immune system. As individuals mature, there are considerations regarding the link between sex-biased dispersal and pathogen transmission dynamics that can lead to variable health outcomes depending on the social system (Herrera and Nunn 2019).
Ecological factors and respiratory illness
It is also critical to assess the influence of ecological factors (e.g., variation in rainfall) on respiratory infections, overall health, and mortality. In mountain gorillas, higher monthly rainfall was associated with an increase in fatal respiratory infection (Hassell et al. 2017). Increased susceptibility to respiratory infections and higher mortality rates were also observed in eastern chimpanzees and mountain gorillas in the rainy season, compared with dry seasons (Goodall 1983; Watts 1998). However, fatal respiratory outbreaks in chimpanzees have been observed across both the wet and dry seasons at Gombe and Mahale (Nishida et al. 2003; Kaur et al. 2008; Lonsdorf et al. 2011). Further, Grützmacher et al. (2016) found western lowland gorillas tested positive for the presence of respiratory viruses across seasons and years. For seasonal influenza, higher rates of illness occur during the dry season (Shaman and Kohn 2009; Shaman et al. 2010).
Previous studies have suggested that the consumption of preferred fruits can serve as a proxy for dietary nutritional quality in great apes (Conklin-Brittain et al. 1998; Emery Thompson et al. 2014, 2018). Great apes may lose weight during periods of fruit scarcity (Wallis 1995), and the resulting nutritional stress can impact fitness, making apes increasingly susceptible to pathogens (Nishida et al. 1990; Wallis 1995). However, when fruit is available, apes may travel more and engage in intergroup encounters, which may increase their likelihood of exposure to respiratory pathogens. Compared with the mountain gorillas in East Africa, western gorillas more frequently pursue ripe fruits, the abundance of which varies seasonally and could affect disease transmission dynamics in these apes (Doran et al. 2002).
Respiratory illness
Respiratory infections can range from asymptomatic (Hall et al. 2001) to displaying flu-like signs, as seen in cases of severe bronchiolitis and pneumonia (Falsey and Walsh 2000; van den Hoogen et al. 2001; Harris 2015). Non-invasive approaches to health monitoring include surveillance of syndromic baselines, which involves systematic documentation of clinical respiratory signs (i.e., coughs and sneezes) (Lonsdorf et al. 2011, 2018). The prevalence and timing of these symptoms can be used to detect and monitor disease instances and outbreaks in natural settings (Henning 2004; Morrison et al. 2021).
In the current study, we assessed whether specific social and ecological factors predict clinical respiratory signs in three groups of wild western lowland gorillas. More specifically, we expected that silverback gorillas would display signs associated with respiratory infections more often than any other individual due to the relationship between testosterone and altered immune response, as well as their central position in the group. In addition, we expected infants to have elevated clinical signs of respiratory illness due to their frequent social interactions. We also predicted that disease prevalence would increase with group size. Further, we examined whether respiratory signs increased during periods of high rainfall or times of potential nutritional stress when fruit was scarce. However, it is also possible that respiratory illness would be more frequent when more fruit was available, and individuals gathered to exploit those patchy resources. While within-group transmission has been documented among gorillas, we also attempted to assess if outbreaks may have occurred across groups of western lowland gorillas.
Methods
Study sites and subjects
We conducted this study in two sites in central Africa inhabited by western lowland gorillas: the Goualougo Triangle (2° 05′–2° 13′ N; 16° 24′–16° 34′ E) and the Mondika research site located within the Djéké Triangle (2° 15′–2° 24′ N; 16° 16′–16° 21′ E). The Goualougo Triangle is located within the southernmost portion of the Nouabalé-Ndoki National Park, Republic of Congo. Mondika is a part of the Kabo Forestry Management unit and located inside a region known as the Djéké Triangle. Both sites comprise lowland forest with altitudes ranging between 330 m and 600 m. The climate can be described as transitional between the Congo-equatorial and sub-equatorial climatic zones (Table 1).
Table 1.
Overview of research sites
| Goualougo Triangle | Mondika (Djéké Triangle) | |
|---|---|---|
| Location | Nouabalé Ndoki National Park, Republic of Congo | Kabo Forestry Management Unit, Republic of Congo |
| Ape density estimates | 1.43 apes/km2 | 1.47 apes/km2 |
| Annual rainfall |
1714.3 ± 177.1 mm (2007 to 2016) |
1915.3 ± 107.5 mm (2015 to 2018) |
| Minimum temperature | 21.4 ± 0.2 °C | 21.6 ± 0.3 °C |
| Maximum temperature | 24.8 ± 1.6 °C | 27.3 ± 0.9 °C |
Rainfall and temperature are mean annual values
To characterize respiratory symptoms in the gorilla population of northern Congo, we chose to include neighboring as well as geographically separated Gorilla groups, as the occurrence and prevalence of signs can vary between social units even at small spatial scales. Focal groups are named after their respective silverbacks. Kingo and Buka groups are located in the Djéké Triangle and were habituated two decades ago to daily follows by research teams originating from the Mondika field site (Doran et al. 2007; Ngokaka et al. 2010). Loya group is located in the Goualougo Triangle and has been habituated to human observers since 2013.
Daily health observations were recorded for individual gorillas in the Loya group from March 2015 to October 2019 (N = 55 months), for the Kingo group from January 2016 to November 2019 (N = 44 months), and the Buka group from December 2015 to November 2019 (N = 46 months).
Age classifications
Age classifications were determined by following the age class system provided by Breuer et al. (2009) for Gorilla gorilla. For individuals who were born during the study period, we used known birth dates to determine age classifications. For individuals born before the group was habituated, we used approximate birth dates to assign age classifications.
Group size
Group size was defined as the total number of individuals in the focal group. We incorporated demographic changes including births, migrations, and deaths to accurately determine group size during the study period (Table 2).
Table 2.
Demographic composition of the gorilla groups during the study period. For each group, the first row represents the group demography at the start of the study period. Each subsequent row indicates each time there was a transition in demography. SB Silverback, YSB Young Silverback, AF Adult Female, BB Blackback, SAB Subadult, JUV Juvenile, INF Infant. P + I = # of pregnant females and infants in the group
Behavioral observations
Upon the location of the focal group and identification of all group members present, research assistants for the Goualougo Triangle and Mondika research projects record daily follow information (such as group composition and location) using Cybertracker on Android telephones that can be imported to Spatial Monitoring and Reporting Tool (SMART). They also use a custom configuration of the Animal Observer App (Version 1.4) on iPad mini units to conduct group scan sampling at 20-min intervals to record the activity and location of all members of the focal group.
Health observations (clinical signs)
Clinical signs were recorded on the basis of overt detectable signs associated with a potential respiratory illness (e.g., coughing, sneezing) (Loudon and Brown 1967; Monto et al. 2000; Morton et al. 2013; Morrison et al. 2021). However, it is important to note respiratory symptoms are not necessarily indicators of infection, as this could also be attributed to the presence of parasites or other illness. Outbreaks can be identified by a sudden increase in the incidence of a disease or condition, which we operationalized as at least 25% of group members showing clinical signs (cough, sneeze) within a daily follow. Similar to Morrison et al. (2021) we apply a different criterion for small groups and specify that at least three members in a group of 12 individuals or fewer must show symptoms to be considered an outbreak.
The Animal Observer configuration used at Goualougo and Djéké also includes a focal observational health assessment form, which is completed at the end of each daily follow on all individuals present and adequately viewed. While our data collection protocol did not allow us to specify if one individual was more in view than others, the methods for conducting daily health observations required that observers completing health assessments must have had adequate opportunity to observe the activity patterns of each individual ape, assess body condition, and to allow detection of respiratory signs, observation of lesions, drainage, or eye conditions during that day. The daily follow and scan protocols also have a notation for recording the occurrence of respiratory symptoms as they occur throughout the day. For daily and monthly sampling points, we tallied respiratory signs by individual gorillas across all data collection platforms (health observation surveys in Animal Observer, scans recorded in Animal Observer, and reconnaissance information recorded in SMART) and wrote a customized script to screen for any duplicative recording of clinical signs. Standardized data collection, reliability assessments, and training protocols were used across sites.
We also monitored activity patterns to account for behaviors associated with declining health (e.g., decreased play behavior, a decline in food intake, lethargy, etc.). Social interactions are also described when relevant as they provide a clear pathway for pathogen transmission within and between groups, given that aerosols or droplets are a primary transmission mechanism (Ott-Joslin 1993; Tegner 2013; Gilardi et al. 2015). Respiratory droplets can reach up to 3 m, and when sneezing they can spread up to 10 m (Gilardi et al. 2015).
Prevalence
We first tallied the number of days that health observations were conducted for each individual (Table 3). Then we calculated the average number of symptomatic days per month per individual, within each age and sex category for each group. Then, we averaged across individuals within the same age and sex category to get monthly average prevalence. Not applicable (NA) was listed for age–sex class categories for which there were no individuals represented or too few data points to calculate averages.
Table 3.
Comparison of total sampling effort, respiratory signs, and prevalence of signs across age–sex classes for all health observations
| Age class | Sex | Group | Number of individuals | Total days observed | Days respiratory symptoms observed | Average monthly prevalence |
|---|---|---|---|---|---|---|
| Silverback | Male | Buka | 1 | 904 | 138 | 2.65 |
| Kingo | 1 | 888 | 98 | 1.88 | ||
| Loya | 1 | 1228 | 533 | 9.35 | ||
| Mature | Male | Buka | 3 | 1431 | 33 | 0.27 |
| Kingo | 3 | 1476 | 36 | 0.3 | ||
| Loya | 2 | 1625 | 49 | 0.58 | ||
| Female | Buka | 2 | 1643 | 40 | 0.41 | |
| Kingo | 5 | 1926 | 68 | 0.41 | ||
| Loya | 1 | 966 | 14 | 0.26 | ||
| Immature | Male | Buka | 4 | 1851 | 17 | 0.17 |
| Kingo | 8 | 2592 | 51 | 0.24 | ||
| Loya | 4 | 1417 | 43 | 0.27 | ||
| Female | Buka | 0 | NA | NA | NA | |
| Kingo | 2 | 1370 | 24 | 0.27 | ||
| Loya | 1 | 2 | 2 | NA |
Fruit availability
Monthly phenological monitoring of fruit, leaves, and flowers was conducted for 30 species of trees identified as important food sources for gorillas and chimpanzees (for ape food lists see Morgan and Sanz 2006, 2007). Multiple trees of each species were identified and tagged on a phenology circuit within the study area, and each month the presence of fruit in the canopy or on the ground was scored. We calculated the proportion of phenology trees with fruit each month as an indicator of fruit availability, which provides an indication of the annual fruiting cycles that have been documented in this area (Adamescu et al. 2018).
Statistical analysis
We investigated the effects of age, sex, group membership, group size, fruit availability, and amount of rainfall (referred to here as “test predictors”) on the likelihood of an individual experiencing a symptomatic cough or sneeze in a given month. We ran a generalized linear mixed model with binomial error structure and logit link function using R (version 3.4.3, R Core Team 2017). All test predictors were included in the model as fixed effects (Table 4). The dataset contained 995 data points, each of which corresponded to one month’s observations for a single individual: the response variable was whether or not the individual experienced at least one symptomatic cough or sneeze in that month. All continuous predictors were z-transformed before running the model. To allow for the possibility of a nonlinear effect of age, we also included age2 in the model. Due to the non-independence of the data points, we included individual ID (n = 28) and the ID for each combination of year and month (“year–month”, n = 57) as random effects (Baayen 2008). We also included the number of days per month that the individual was observed (range 8–29, mean 18.25, SD 4.84) as a control predictor. Prior to running the model, we calculated variance inflation factors (VIFs) for all fixed effects. All VIFs were at or below 2.56, suggesting multicollinearity was not an issue (Quinn and Keough 2002; Field 2005). To assess model stability, we removed data points from each individual and each year–month sequentially and reran the model. The model estimates remained similar and the significance of almost all predictors remained the same. The two exceptions were the rain and age2 terms, the stability of which is described in the results section below.
Table 4.
Results of model predicting clinical signs of respiratory health in western lowland gorillas
| Estimate | SE | X2 | df | p | |
|---|---|---|---|---|---|
| Age | 1.06 | 0.14 | 32.72 | 1 | < 0.01 |
| Sex | 0.99 | 0.30 | 8.41 | 1 | < 0.01 |
| Group size | −0.26 | 0.28 | 0.85 | 1 | 0.36 |
| Fruit Availability | −0.46 | 0.16 | 8.31 | 1 | < 0.01 |
| Rainfall | −0.25 | 0.15 | 2.96 | 1 | 0.09 |
| Group (Buka versus Kingo) | 0.68 | 0.50 | 11.91 | 2 | < 0.01 |
| Group (Buka versus Loya) | 1.46 | 0.47 | |||
| Number observation days | 0.07 | 0.11 | (a) | (a) | (a) |
(a) Not calculated because term is control term
We first established the collective significance of the test predictors age, age2, sex, group, group size, fruit availability, and rainfall by comparing the full model, which contained all fixed and random effects, to a reduced model, which omitted the test predictors, using a likelihood ratio test (Dobson 2002; Forstmeier et al. 2011). We then tested individual test predictors using likelihood ratio tests.
Ethical note
Our study adhered to the legal requirements of the Republic of Congo where the research was conducted. The research was approved by the Nouabalé-Ndoki Foundation and the Wildlife Conservation Society’s Congo Program. We also received endorsement to conduct this research from the Institutional Animal Care and Use Committee of Washington University in St. Louis. Finally, the authors declare that they have no conflict of interest.
The combination of emerging infectious diseases and human presence in close proximity to wild apes has made health monitoring and the investigation of measures to protect both ape and human lives an immediate priority for researchers and park managers at the Nouabalé-Ndoki National Park (NNNP) and the Sangha Trinational conservation area. The Goualougo and Mondika research teams use standardized health monitoring protocols and adhere to best practices developed to reduce the possibility of pathogen transmission. By circulating the protocols and results of health monitoring, we aim to raise awareness about such standards among all stakeholders and ensure that information is available to implement precautionary measures across the region.
Results
In 19,319 observational health records, we observed a total of 1146 incidences of respiratory signs (Table 3). Gorilla ages ranged from 0.05 to 41.87 years (mean 16.70, SD 11.82), group size ranged from 3 to 12 individuals (mean 7.92, SD 2.40), percent fruit availability ranged from 0.22 to 0.59 (mean 0.35, SD 0.10), and monthly rainfall ranged from 0 mm to 439.60 mm (mean 150.96, SD 97.85). The number of observation days per month ranged from 8 to 29 (mean 18.25, SD 4.84). In the generalized linear model examining individual respiratory signs in a given month, the predictors taken together were significant (X2 = 58.60, df = 8, p < 0.01).
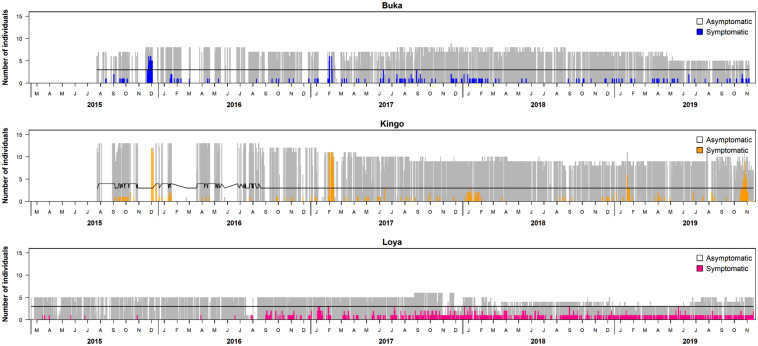
During the course of this study, several punctuated time periods occurred, during which the majority of group members in particular groups showed respiratory clinical signs (Fig. 1).
Fig. 1.
Focal group members with respiratory signs relative to number of members not displaying signs of respiratory illness by month. The black line indicates potential outbreaks within the groups. Note that simultaneous outbreaks across groups were identified in December 2015 and February 2017
Differences in respiratory signs across groups
There were differences in the presence of clinical respiratory signs across the study groups (Fig. 1). Loya group’s average prevalence of coughing per month (0.45 signs/month) was higher than either Buka (0.23 signs/month) or Kingo (0.19 signs/month) group. While the number of individuals in the groups changed during the study period, group size was not a significant predictor of respiratory signs.
Social and demographic influences on clinical respiratory signs
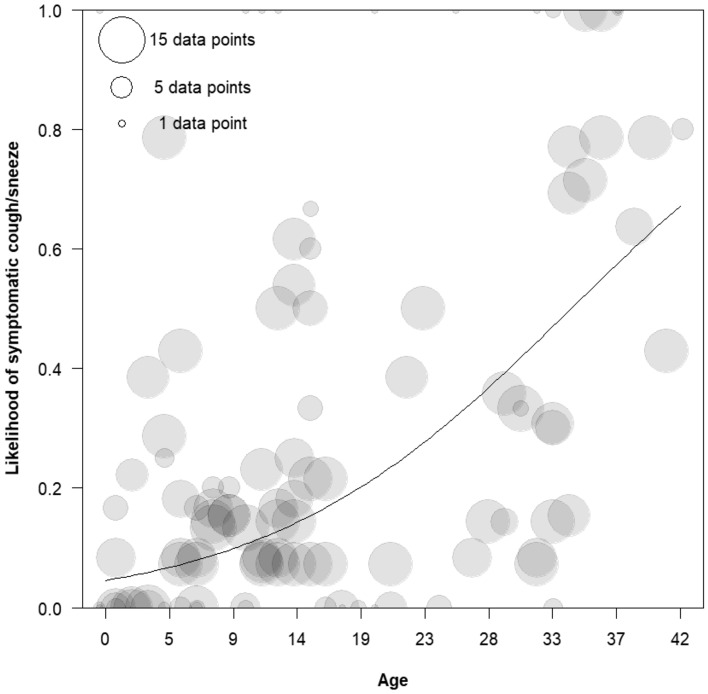
Age2 was found not to be significant (X2 = 2.16, df = 1, p = 0.14) and was subsequently excluded from the model (see the section below on model stability). The remaining effects were tested in comparison to the model lacking age2. There were significant differences among the groups (X2 = 11.91, df = 2, p < 0.01) and males were more likely to show signs than females (estimate 0.99, SE 0.30, X2 = 8.41, df = 1, p < 0.01). The linear effect of age was significant, with increasing age resulting in a higher likelihood of symptomatic coughs or sneezes (estimate 1.06, SE 0.14, X2 = 32.72, df = 1, p < 0.01).
Older individuals showed a higher likelihood of symptomatic coughs or sneezes (Fig. 2), with silverback males showing the highest rates of respiratory signs across the three focal gorilla groups (Table 3). Overall, the prevalence of symptomatic respiratory signs among mature males and females was less than half of that of the silverback. Males showed higher frequencies of respiratory signs than females.
Fig. 2.
Probability of the presence of respiratory signs by age for all groups. Ages have been binned. Each circle represents one individual per age category
As mentioned in the Statistical Analysis section above, there was evidence that the age2 term is not completely stable. Removing data points from either of two individuals (BUK and KIN) resulted in the age2 term becoming significant, whereas removing data points from any of the other individuals did not change its significance. However, the nature of the relationship between age and likelihood of symptomatic coughs or sneezes remained the same regardless of the significance of age2, with increasing age being associated with an increased likelihood.
Ecological influences on clinical respiratory signs
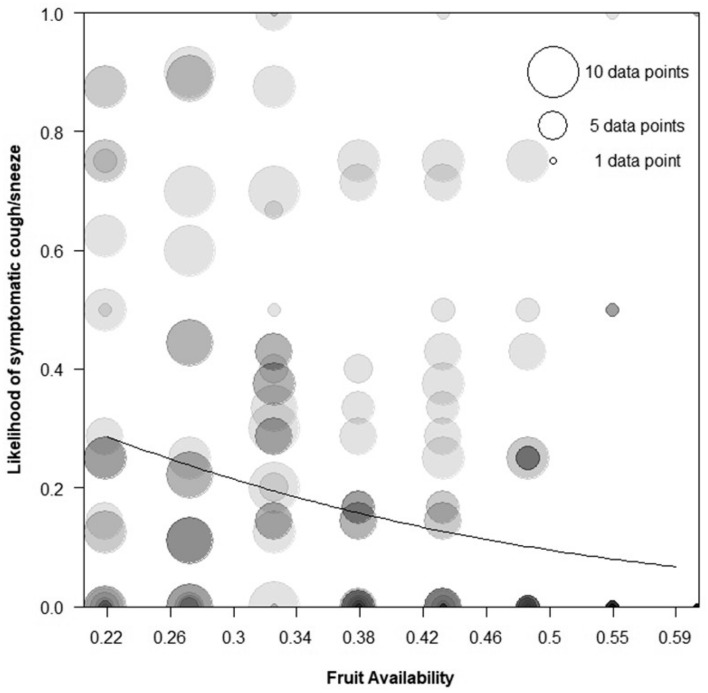
Higher values of fruit availability were associated with a lower likelihood of symptomatic observations (estimate −0.46, SE 0.16, X2 = 8.31, df = 1, p < 0.01) (Fig. 3). Incidences of respiratory signs were most frequent when fruit was scarce. We did not detect a significant effect of rainfall in this study, however, this term was found to be unstable. When one individual (KAO) or any of five year–months (July 2016, June 2017, September 2018, November 2018, and March 2019) were omitted from the data, rainfall became significant. Therefore, the results for rainfall should be treated with caution.
Fig. 3.
Probability of observed respiratory signs (coughing and sneezing) across fruit availability by month for all groups. Fruit availability values have been binned, with each circle representing one individual per fruit availability category
Discussion
The Congo Basin is considered an epicenter of emerging infectious diseases (Leroy et al. 2004; Jones et al. 2008). Further, COVID-19 has clearly demonstrated the risk that emerging infectious diseases pose globally and the importance of disease surveillance in wildlife and human populations. Our study builds on previous research demonstrating how long-term syndromic surveillance can be used to identify illness in wild ape populations (Lonsdorf et al. 2018; Masi et al. 2012; Morrison et al. 2021). While there were differences between groups in their health profiles, we identified several predictors of respiratory symptoms which were consistent within this population of western lowland gorillas. Males were more likely to show symptoms than females, and age was positively related to symptomatic observations. We also found that times of low fruit availability were associated with increased clinical signs of respiratory illness. Further, longitudinal observational health monitoring enabled us to identify potential outbreaks within and across groups, which were characterized by sudden increases in the incidence of disease symptoms. While within-group transmission is expected on the basis of studies in mountain gorillas, the timing of outbreaks in Kingo and Buka’s groups raises the possibility that transmission occurred across groups. We hope that our findings can be used to improve best practices and management protocols relevant to disease prevention in wild ape populations, which may also be relevant to some captive settings.
Social factors and respiratory illness
Similar to previous studies of factors linked to viral infections in primates (Robinson et al. 2011, 2014; Klein and Flanagan 2016; Channappanavar et al. 2017), we found that age and sex were predictors of respiratory symptoms. In this study, older gorillas were more likely to show clinical signs of respiratory illness than younger gorillas. Coughing and sneezing was more prevalent in males than females. Further, the dominant silverbacks of each group showed much higher prevalence of respiratory signs of illness than any other age–sex class. These males may be more at risk to pathogen exposure due to the central position that they play in their social groups. They could also be exposed to infection during intergroup encounters.
On the basis of previous literature, we expected to find a positive relationship between group size and disease prevalence that would be fostered by the increased number of contacts within larger groups (Møller et al. 1993; Loehle 1995). However, group size was not a predictor of respiratory signs. In fact, the smallest group in our study had the highest rates of clinical signs associated with respiratory illness. It has been reported that the potential for within-group transmission of pathogens among gorillas is relatively high (Morrison et al. 2021) and may be even more elevated when there are fewer individuals to associate with, as is the case in Loya’s group. While our study is the largest-scale study of western lowland gorilla health to date, we recognize that our ability to detect an effect of group size may have been hampered by the limited sample of gorilla groups and relatively narrow range of group sizes. However, group sizes fluctuated with demographic events during the study period and were within the range of those reported across the region. It is also arguable that these limitations were compensated for by the information gained from the longitudinal study of the groups in our study. For example, six additional members joined Buka’s group and two individuals joined Kingo’s group after the study period. It should also be noted that a peripheral male was shadowing Loya’s group during our study and eventually took control of the group. Overall, the social and spatial dynamics of gorillas were much more variable and flexible than expected and likely have implications for pathogen transmission within gorilla society.
The sudden increase in the incidence of a disease or condition in several group members can serve as an indicator of a probable outbreak within a group, whereas heightened prevalence of respiratory signs across groups could be indicative of broader scale outbreaks in a population. In Fig. 1, we illustrate how prevalence levels may have exceeded outbreak thresholds, indicating that outbreaks occurred in the focal gorilla groups over the course of the study period (Fig. 1). We also observed simultaneous outbreaks in different groups. On one such occasion, several members of Kingo’s group who were experiencing respiratory signs were observed interacting with Buka group members who were not exhibiting clinical signs. Respiratory symptoms were subsequently detected in the Buka group on days after their interaction with Kingo group, indicating the possibility of between-group transmission of pathogens. However, simultaneous respiratory outbreaks across groups in Dzangha-Ndoki National Park were not attributed to intergroup transmission events because no between-group interactions were observed (Grutzmacher et al. 2016). Also, research on transmission of respiratory disease between mountain gorilla groups did not attribute simultaneous outbreaks to transmission of pathogens between groups (Morrison et al. 2021). Future research incorporating real-time reporting of symptoms and spatial data on overlap in home ranges between western lowland gorilla groups could be informative about potential intergroup disease transmission. In the case that an outbreak is detected in one or several groups, steps could be taken to reduce any external pressures, such as tourism, while the group members recover (see Table 5 for recommendations on how symptom monitoring can be incorporated into site management).
Table 5.
Overview of recommendations for incorporating symptom monitoring in site management
| Recommendations |
|---|
| • Adhere to best practices and field protocols for disease prevention and health monitoring (Gilardi et al. 2015) |
| • Implement standardized ape health monitoring, which includes establishing baselines when in good health and monitoring of clinical signs (Lonsdorf et al. 2011) |
| • Incorporate health indicators in all types of daily monitoring and data collection so as to maximize likelihood of detection of clinical signs |
| • Compile symptomatic observations to assess trends and identify outbreaks at the site in real time |
| • When outbreak detected in a group, halt tourism visits so as to reduce anthropogenic pressure on that group |
| • Conduct diagnostic sampling on the basis of established protocols and within framework of collaborations to identify pathogens and diseases (Gillespie et al. 2008) |
| • Establish communication network to inform local health officials and other sites in the region about health concerns as they emerge |
Ecological factors and respiratory illness
We found that clinical signs of respiratory infection were higher when fruit availability was low. Nutritional stress or associated influences could result in higher susceptibility to respiratory infection during times of fruit scarcity. For example, Masi et al. (2012) posited that the high levels of parasite loads detected in Bai Hokou gorillas during the dry season were the result of “dietary stress,” with individuals affected by the decline in fruit availability and elevated visitation to swamps. When opportunities to forage fruits are scarce, gorillas spend more time feeding on terrestrial herbaceous vegetation. Group members often feed in close proximity to one another within such foraging contexts, which could elevate risks of pathogen transmission. While our results provide preliminary indication that availability of preferred foods such as fruits may be linked to the prevalence of respiratory symptoms, further research is needed to disentangle the influences of food availability, habitat use, and climate on health outcomes.
On the basis of findings of respiratory disease in western lowland gorillas in neighboring Central African Republic, we expected a higher prevalence of symptomatic signs in the months associated with moderate-to-high levels of rainfall (Masi et al. 2012). It is possible that effect lags or variable rates of pathogen transmission across seasons could have reduced our ability to identify rainfall as a key factor. Assessment of rainfall in this study may also have been influenced by other environmental factors that were not accounted for in our model.
Conservation implications
The long-term implementation of an observational health monitoring program that serves as a sentinel system for disease outbreak detection in wild western lowland gorillas is an important advancement for ape conservation in this region. The program also includes daily surveillance of sympatric chimpanzees, continuous monitoring for wildlife mortality events, and a progressive health program for staff at the research sites. On the basis of continued syndromic surveillance across an expanding network of great ape sites and forthcoming results from diagnostic testing, we hope the data from this study will assist in efforts to strengthen best practice guidelines to safeguard wild ape populations as they face increasing anthropogenic pressures. With the future development of international tourism and planned mobility between key tourism destinations in the region, the risk of disease transmission is likely to increase for western lowland gorillas in the Sangha Trinational Conservation Area. There are also increasing anthropogenic pressures at human–ape interfaces associated with degraded, converted, or fragmented habitats. Systematic surveillance of health indicators across sites is urgently needed so that broader patterns of emerging diseases can be detected, pathogens of human origin can be mitigated, and information is made available to inform conservation initiatives to protect great apes (Table 5). Implemented in concert with local conservation initiatives, such research outputs also hold the promise of maximizing chances to identify emerging infectious diseases and implementing preventative steps for the benefit of apes and humans alike (Calvignac-Spencer et al. 2012).
Acknowledgements
We are deeply appreciative for the opportunity to work in the Djeke Triangle. This research would not have been possible without the continued support of the Ministère de l’Economie Forestière du gouvernement de la République du Congo and the Agence Congolaise de la Faune et des Aires Protégées (ACFAP). The Wildlife Conservation Society’s Congo Program and the Nouabalé-Ndoki Foundation are integral partners in this continuing research. Special thanks are due to M. Gately, E. Stokes, T. Breuer, P. Ngouembe, D. Dos Santos, E. Arnhem, M. Ngangoue, M. Mviri, and B. Evans. We would also like to recognize the tireless dedication of J. R. Onononga, C. Eyana-Ayina, S. Ndolo Ebika, W. Mayoukou, S. Ndassoba, J. Wawa, D. Koni, I. Singono, M. Meguessa, and the Goualougo and Mondika tracking team. We are also thankful for the many contributions of J. Ortega and A. Zambarda toward facilitating the ongoing fieldwork at these sites. In addition, we appreciate the constructive feedback provided by the editors (including Guest Editor C. Stephan), anonymous reviewers, and E. Lonsdorf on this manuscript. Grateful acknowledgment of funding is due to the Arcus Foundation; the Conservation, Food and Health Foundation; Indianapolis Zoo; the Cincinnati Zoo and Botanical Garden; the Saint Louis Zoo; and Primate Conservation, Inc.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adamescu GS, Plumptre AJ, Abernethy KA, Polansky L, Bush ER, Chapman CA, Shoo LP, Fayolle A, Janmaat KRL, Robbins MM, Ndangalasi HJ, Cordeiro NJ, Gilby IC, Wittig RM, Breuer T, Hockemba M, Sanz CM, Morgan DB, Pusey AE, Mugerwa B, Gilagiza B, Tutin C, Ewango CEN, Sheil D, Dimoto E, Baya F, Bujo F, Ssali F, Dikangadissi J-T, Jeffery K, Valenta K, White L, Masozera M, Wilson ML, Bitariho R, NdoloEbika ST, Gourlet-Fleury S, Mulindahabi F, Beale CM. Annual cycles are the most common reproductive strategy in African tropical tree communities. Biotropica. 2018;50(3):418–430. doi: 10.1111/btp.12561. [DOI] [Google Scholar]
- Baayen RH. Analyzing linguistic data. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Baudouin A, Gatti S, Levréro F, Genton C, Cristescu RH, Billy V, Motsch P, Pierre J-S, Gouar PL, Ménard N. Disease avoidance, and breeding group age and size condition the dispersal patterns of western lowland gorilla females. Ecology. 2019;100:e02786. doi: 10.1002/ecy.2786. [DOI] [PubMed] [Google Scholar]
- Breuer T, Hockemba MB, Olejniczak C, Parnell RJ, Stokes EJ. Physical maturation, life-history classes and age estimates of free-ranging western gorillas—Insights from Mbeli Bai, Republic of Congo. Am J Primatol. 2009;71:106–119. doi: 10.1002/ajp.20628. [DOI] [PubMed] [Google Scholar]
- Caillaud D, Levrero F, Cristescu R, Gatti S, Dewas M, Douadi M, Gautier-Hion A, Raymond M, Ménard N. Gorilla susceptibility to Ebola virus: the cost of sociality. Curr Biol. 2006;16:R489–R491. doi: 10.1016/j.cub.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Calvignac-Spencer S, Leendertz SAJ, Gillespie TR, Leendertz FH. Wild great apes as sentinels and sources of infectious disease. Clin Microbiol Infect. 2012;18:521–527. doi: 10.1111/j.1469-0691.2012.03816.x. [DOI] [PubMed] [Google Scholar]
- Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin-Brittain NL, Wrangham RW, Hunt KD. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. II Macronutrients. Int J Primatol. 1998;19:971–998. doi: 10.1023/A:1020370119096. [DOI] [Google Scholar]
- Cooksey K, Sanz C, Ebombi TF, Massamba JM, Teberd P, Magema E, Abea G, Ortega Peralejo JSO, Kienast I, Stephens C, Morgan D. Socioecological factors influencing intergroup encounters in western lowland gorillas (Gorilla gorilla gorilla) Int J Primatol. 2020;41:181–202. doi: 10.1007/s10764-020-00147-6. [DOI] [Google Scholar]
- Dobson AJ. An introduction to generalized linear models. Florida: Chapman; 2002. [Google Scholar]
- Doran DM, McNeilage A, Greer D, Bocian C, Mehlman P, Shah N. Western lowland gorilla diet and resource availability: new evidence, cross-site comparisons, and reflections on indirect sampling methods. Am J Primatol. 2002;58:91–116. doi: 10.1002/ajp.10053. [DOI] [PubMed] [Google Scholar]
- Doran-Sheehy DM, Derby AM, Greer D, Mongo P. Habituation of western gorillas: the process and factors that influence it. Am J Primatol. 2007;69(12):1354–1369. doi: 10.1002/ajp.20442. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Wrangham RW. Male chimpanzees compromise the foraging success of their mates in Kibale National Park, Uganda. Behav Ecol Sociobiol. 2014;68:1973–1983. doi: 10.1007/s00265-014-1803-y. [DOI] [Google Scholar]
- Emery Thompson M, Machanda ZP, Scully EJ, Enigk DK, Otali E, Muller MN, Goldberg TL, Chapman CA, Wrangham RW. Risk factors for respiratory illness in a community of wild chimpanzees (Pan troglodytes schweinfurthii) R Soc Open Sci. 2018;5:1–17. doi: 10.1098/rsos.180840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–384. doi: 10.1128/CMR.13.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. London: Sage Publications; 2005. [Google Scholar]
- Forstmeier W, Schielzeth H. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol. 2011;65:47–55. doi: 10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat. 1992;139:603–622. doi: 10.1086/285346. [DOI] [Google Scholar]
- Genton C, Cristescu R, Gatti S, Levrero F, Bigot E, Caillaud D, Pierre JS, Menard N. Recovery potential of a western lowland gorilla population following a major Ebola outbreak: results from a ten-year study. PLoS ONE. 2012;7:1–10. doi: 10.1371/journal.pone.0037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KV, Gillespie TR, Leendertz FH, Macfie EJ, Travis DA, Whittier CA, Williamson EA. Best practice guidelines for health monitoring and disease control in great ape populations. Gland: IUCN SSC Primate Specialist Group; 2015. [Google Scholar]
- Gillespie TR, Nunn CL, Leendertz FH. Integrative approaches to the study of primate infectious disease: implications for biodiversity conservation and global health. Am J Phys Anthropol. 2008;137:53–69. doi: 10.1002/ajpa.20949. [DOI] [PubMed] [Google Scholar]
- Goodall J. Population dynamics during a 15-year period in one community of free-living chimpanzees in the Gombe National Park, Tanzania. Zeitschrift Fuer Tierpsychologie. 1983;61:1–60. doi: 10.1111/j.1439-0310.1983.tb01324.x. [DOI] [Google Scholar]
- Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem. 1989;34:241–251. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- Grützmacher KS, Köndgen S, Keil V, Todd A, Feistner A, Herbinger I, Petrzelkova K, Fuh T, Leendertz SA, Calvignac-Spencer S, Leendertz FH. Codetection of respiratory syncytial virus in habituated wild western lowland gorillas and humans during a respiratory disease outbreak. EcoHealth. 2016;13:499–510. doi: 10.1007/s10393-016-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GL, Hantos Z, Sly PD. Altered respiratory tissue mechanics in asymptomatic wheezy infants. Am J Respir Crit Care Med. 2001;164:1387–1391. doi: 10.1164/ajrccm.164.8.2012148. [DOI] [PubMed] [Google Scholar]
- Harris L (2015) Epidemiology of respiratory illness in endangered mountain gorillas (Gorilla beringei beringei) at a One Health Interface in Africa. Dissertation, University of California, Davis
- Hassell JM, Zimmerman D, Cranfield MR, Gilardi K, Mudakikwa A, Ramer J, Nyirakaragire E, Lowenstine LJ. Morbidity and mortality in infant mountain gorillas (Gorilla beringei beringei): a 46-year retrospective review. Am J Primatol. 2017;79:1–13. doi: 10.1002/ajp.22686. [DOI] [PubMed] [Google Scholar]
- Henning KJ. What is syndromic surveillance. Morb Mortal Wkly Rep. 2004;53:7–11. [Google Scholar]
- Herrera J, Nunn CL. Behavioral ecology and infectious disease: implications for conservation of biodiversity. Philos Trans R Soc B. 2019;374:1–11. doi: 10.1098/rstb.2018.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CL, Ruiz-Lambides AV, Davila E, Maldonado E, Gerald MS, Maestripieri D. Sex differences in survival costs of reproduction in a promiscuous primate. Behav Ecol Sociobiol. 2008;62:1711–1718. doi: 10.1007/s00265-008-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur T, Singh J, Tong S, Humphrey C, Clevenger D, Tan W, Szekely B, Wang Y, Li Y, Alex Muse E, Kiyono M. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. Am J Primatol. 2008;70:755–765. doi: 10.1002/ajp.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- Köndgen S, Kühl H, N’Goran PK, Walsh PD, Schenk S, Ernst N, Biek R, Formenty P, Mätz-Rensing K, Schweiger B, Junglen S. Pandemic human viruses cause decline of endangered great apes. Curr Biol. 2008;18(4):260–264. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Kulik L, Amici F, Langos D, Widdig A. Sex differences in the development of social relationships in rhesus macaques (Macaca mulatta) Int J Primatol. 2015;36:353–376. doi: 10.1007/s10764-015-9826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy EM, Telfer P, Kumulungui B, Yaba P, Rouquet P, Roques P, Gonzalez JP, Ksiazek TG, Rollin PE, Nerrienet EA. A serological survey of Ebola virus infection in central African nonhuman primates. J Infect Dis. 2004;190:1895–1899. doi: 10.1086/425421. [DOI] [PubMed] [Google Scholar]
- Loehle C. Social barriers to pathogen transmission in wild animal populations. Ecology. 1995;76:326–335. doi: 10.2307/1941192. [DOI] [Google Scholar]
- Lonsdorf EV, Murray CM, Travis DA, Gilby IC, Chosy J, Goodall J, Pusey AE. A retrospective analysis of factors correlated to chimpanzee (Pan troglodytes schweinfurthii) respiratory health at Gombe National Park, Tanzania. EcoHealth. 2011;8:26–35. doi: 10.1007/s10393-011-0683-0. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Gillespie TR, Wolf TM, Lipende I, Raphael J, Bakuza J, Murray CM, Wilson ML, Kamenya S, Mjungu D, Collins DA. Socioecological correlates of clinical signs in two communities of wild chimpanzees (Pan troglodytes) at Gombe National Park, Tanzania. Am J Primatol. 2018;80:1–20. doi: 10.1002/ajp.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon RG, Brown LC. Cough frequency in patients with respiratory disease. Am Rev Respir Dis. 1967;96:1137–1143. doi: 10.1164/arrd.1967.96.6.1137. [DOI] [PubMed] [Google Scholar]
- Masi S, Chauffour S, Bain O, Todd A, Guillot J, Krief S. Seasonal effects on great ape health: a case study of wild chimpanzees and western gorillas. PLoS ONE. 2012;7:1–13. doi: 10.1371/journal.pone.0049805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Dufva R, Allander K. Parasites and the evolution of host social behavior. Adv Study Behav. 1993;22:60405. [Google Scholar]
- Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and signs predicting influenza infection. Arch Intern Med. 2000;160:3243–3247. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- Morgan D, Sanz C. Chimpanzee feeding ecology and comparisons with sympatric gorillas in the Goualougo Triangle, Republic of Congo. In: Hohmann G, Robbins M, Boesch C, editors. Primate feeding ecology in apes and other primates: ecological, physiological, and behavioural aspects. Cambridge: Cambridge University Press; 2006. pp. 97–122. [Google Scholar]
- Morgan D, Sanz C. Best practice guidelines for reducing the impact of commercial logging on wild apes in west equatorial Africa. IUCN/SSC Primate Specialist Group (PSG): Gland; 2007. p. 32. [Google Scholar]
- Morrison RE, Mushimiyimana Y, Stoinski TS, Eckardt W. Rapid transmission of respiratory infections within but not between mountain gorilla groups. Sci Rep. 2021;11:19622. doi: 10.1038/s41598-021-98969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RE (2019) Western gorilla social structure and inter-group dynamics. Dissertation, University of Cambridge
- Morton FB, Todd AF, Lee P, Masi S. Observational monitoring of clinical signs during the last stage of habituation in a wild western gorilla group at Bai Hokou, Central African Republic. Folia Primatol. 2013;84:118–133. doi: 10.1159/000350916. [DOI] [PubMed] [Google Scholar]
- Negrey JD, Reddy RB, Scully EJ, Phillips-Garcia S, Owens LA, Langergraber KE, Mitani JC, Emery Thompson M, Wrangham RW, Muller MN, Otali E. Simultaneous outbreaks of respiratory disease in wild chimpanzees caused by distinct viruses of human origin. Emerg Microb & Infect. 2019;8:139–149. doi: 10.1080/22221751.2018.1563456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngokaka C, Akouango F, Mbété P, Nziendolo HGL. Contribution à l'habituation des Gorilles de plaine de l'ouest (Gorilla gorilla gorilla) à la présence humaine, en vue de leur protection, leur conservation et du développement de l'écotourisme. J Animal Plant Sci. 2010;8(2):981–992. [Google Scholar]
- Nishida T, Takasaki H, Takahata Y. Demography and reproductive profiles. In: Nishida T, editor. The Chimpanzees of the Mahale Mountains. Tokyo: University of Tokyo Press; 1990. pp. 63–98. [Google Scholar]
- Nishida T, Corp N, Hamai M, Hasegawa T, Hiraiwa-Hasegawa M, Hosaka K, Hunt KD, Itoh N, Kawanaka K, Matsumoto-Oda A, Mitani JC. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. Am J Primatol. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- Ott-Joslin JE. Zoonotic diseases of nonhuman primates. In: Fowler M, editor. Zoo and wild animal medicine current therapy. Pennsylvania: Saunders Co; 1993. pp. 358–373. [Google Scholar]
- Patrono LV, Leendertz F. Acute infectious diseases occurring in the Taï chimpanzee population: a review. In: Boesch C, Wittig R, editors. The Chimpanzees of the Taï Forest: 40 years of research. Cambridge: Cambridge University Press; 2019. pp. 385–393. [Google Scholar]
- Patrono LV, Samuni L, Corman VM, Nourifar L, Röthemeier C, Wittig RM, Drosten C, Calvignac-Spencer S, Leendertz FH. Human coronavirus OC43 outbreak in wild chimpanzees, Côte d´ Ivoire, 2016. Emerg Microb Infect. 2018;7:1–4. doi: 10.1038/s41426-018-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ. Experimental designs and data analysis for biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011;7:1–9. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. 2014;88:4711–4720. doi: 10.1128/JVI.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Walsh PD. Consequences of non-intervention for infectious disease in African great apes. PLoS ONE. 2011;6:1–9. doi: 10.1371/journal.pone.0029030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandel AA, Rushmore J, Negrey JD, Mitani JC, Lyons DM, Caillaud D. Social network predicts exposure to respiratory infection in a wild chimpanzee group. EcoHealth. 2020;17:437–448. doi: 10.1007/s10393-020-01507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully EJ, Basnet S, Wrangham RW, Muller MN, Otali E, Hyeroba D, Grindle KA, Pappas TE, Thompson ME, Machanda Z, Watters KE, Palmenberg AC, Gern JE, Goldberg TL. Lethal respiratory disease associated with human rhinovirus C in wild chimpanzees, Uganda, 2013. Emerg Infect Dis. 2018;24:267–274. doi: 10.3201/eid2402.170778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 2010;8:1–13. doi: 10.1371/annotation/35686514-b7a9-4f65-9663-7baefc0d63c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorping A, Jensen K. Disease dynamics: all caused by males? Trends Ecol Evol. 2004;19:219–220. doi: 10.1016/j.tree.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Tegner C. Zoonotic respiratory infections and great ape conservation-an emerging challenge. First cycle, G2E. Uppsala: Dept. of Biomedical Sciences and Veterinary Public Health; 2013. [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J. Seasonal influences on reproduction in chimpanzees of Gombe National Park. Int J Primatol. 1995;16:435–452. doi: 10.1007/BF02735796. [DOI] [Google Scholar]
- Watts DP. Seasonality in the ecology and life histories of mountain gorillas (Gorilla gorilla beringei) Int J Primatol. 1998;19:929–948. doi: 10.1023/A:1020366018187. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.