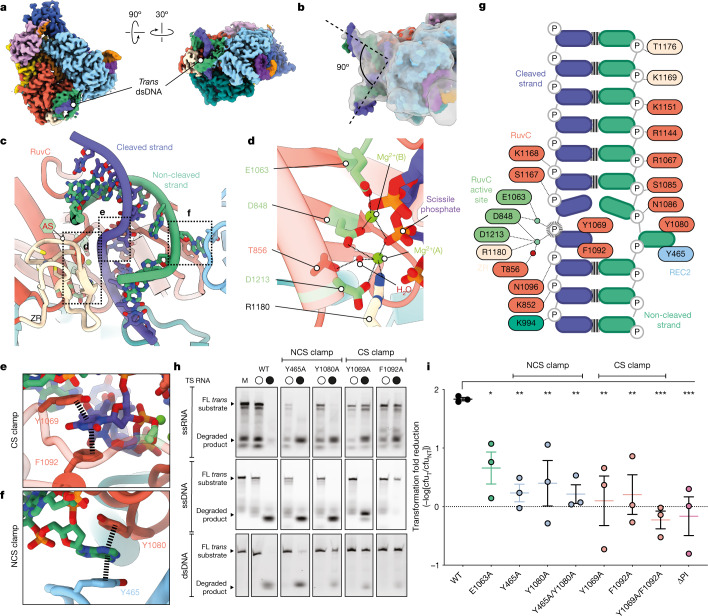
Fig. 3. Cas12a2 binds and clamps duplex DNA.
a, Cryo-EM structure of Cas12a2 quaternary complex. Trans-dsDNA is shown as slate blue and sea green. b, Atomic model of Cas12a2 quaternary complex, with 5 Å low-pass filtered map shown in transparent grey. To highlight the 90º kink in the collateral duplex, two linear ideal B-form dsDNA models have been rigid-body fitted into the map. c, dsDNA situated within active site. d, Close-up view of Cas12a2 active site. e, CS held in place through aromatic clamp. f, NCS held in place through aromatic clamp. g, Schematic of interactions between Cas12a2 and the collateral dsDNA substrate. CS scissile phosphate is denoted by dashed outline. h, Cleavage of ssRNA (top), ssDNA (middle) and dsDNA (bottom) by Cas12a2, and aromatic clamp mutants. M, size marker (intact substrate). Representative of three independent experiments with similar results. i, Alterations to essential residues resulted in loss of the ability of Cas12a2 to clear plasmid (that is, lower transformation fold reduction, calculated as −log10[cfuT/cfuNT], in which cfuT and cfuNT represent the number of colony-forming units for target and non-target plasmids, respectively). Significance between WT and mutant SuCas12a2 was determined by two-sided Student t-test. *P < 0.05, **P < 0.01, ***P < 0.001. Experiments were carried out in triplicate, and error bars correspond to the mean and standard error. For gel source data, see Supplementary Fig. 1.