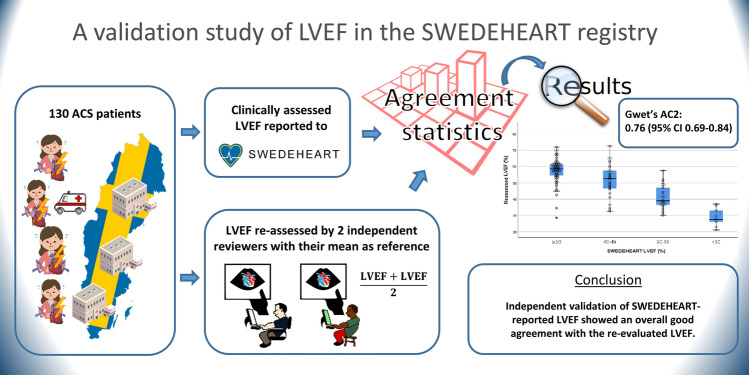
Abstract
Objective
Patients hospitalized with acute coronary syndrome (ACS) in Sweden routinely undergo an echocardiographic examination with assessment of left ventricular ejection fraction (LVEF). LVEF is a measurement widely used for outcome prediction and treatment guidance. The obtained LVEF is categorized as normal (> 50%) or mildly, moderately, or severely impaired (40–49, 30–39, and < 30%, respectively) and reported to the nationwide registry for ACS (SWEDEHEART). The purpose of this study was to determine the reliability of the reported LVEF values by validating them against an independent re-evaluation of LVEF.
Methods
A random sample of 130 patients from three hospitals were included. LVEF re-evaluation was performed by two independent reviewers using the modified biplane Simpson method and their mean LVEF was compared to the LVEF reported to SWEDEHEART. Agreement between reported and re-evaluated LVEF was assessed using Gwet’s AC2 statistics.
Results
Analysis showed good agreement between reported and re-evaluated LVEF (AC2: 0.76 [95% CI 0.69–0.84]). The LVEF re-evaluations were in agreement with the registry reported LVEF categorization in 86 (66.0%) of the cases. In 33 (25.4%) of the cases the SWEDEHEART-reported LVEF was lower than re-evaluated LVEF. The opposite relation was found in 11 (8.5%) of the cases (p < 0.005).
Conclusion
Independent validation of SWEDEHEART-reported LVEF shows an overall good agreement with the re-evaluated LVEF. However, a tendency towards underestimation of LVEF was observed, with the largest discrepancy between re-evaluated LVEF and registry LVEF in subjects with subnormal LV-function in whom the reported assessment of LVEF should be interpreted more cautiously.
Graphical abstract
Keywords: LVEF, Registry, Validation, SWEDEHEART, Echocardiography
Background
Left ventricular ejection fraction (LVEF) is one of the most robust predictive parameters post-myocardial infarction offering guidance in both treatment and follow-up strategies [1]. However, despite its recognized value, LVEF by biplane Simpson is subject to a non-negligible inter-observer variability that increases with declining image quality [2–4]. Poor image quality may prevent reliable delineation of the endocardial border and hinder a quantitative assessment of LVEF [5]. In such cases, LVEF may be assessed using visual “eye-balling”. This technique implicates an even greater uncertainty with a reported inter-observer variability of up to ± 14% LVEF [6].
The SWEDEHEART registry contains patient data from subjects admitted to Swedish hospitals nationwide due to acute coronary syndrome (ACS). The registry was established in 2009 by merging of four preexisting registries on ischemic heart disease into a larger, more comprehensive registry. The registry encompasses more than 450 variables including left ventricular ejection fraction (LVEF) by echocardiography [7]. SWEDEHEART has been a valuable source of insight in different aspects of ischemic heart disease and data from SWEDEHEART, including LVEF, has been used in a large number of studies [8].
Given the importance of LVEF and that the LVEF assessments in SWEDEHEART, and in several other registries, have not previously been validated we aimed to validate the LVEF assessments reported to SWEDEHEART.
Methods
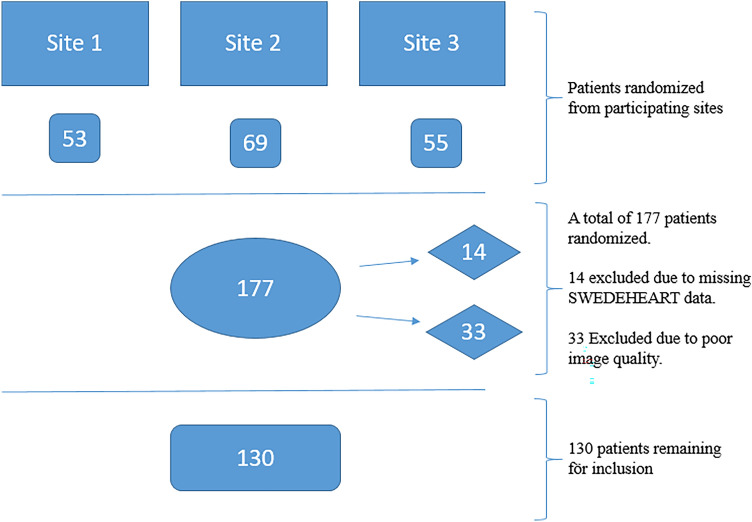
The study cohort consisted of a random sample of 177 patients with ACS from three different Swedish hospitals [Uppsala University Hospital, Uppsala (site 1), Lund University Hospital, Lund (site 2) and Danderyd University Hospital, Stockholm (site 3)], in whom LVEF had been assessed according to local routine by 2D echocardiography during the index hospital stay. LVEF was then reported to SWEDEHEART in four categories: < 30%, 30–39%, 40–49%, and ≥ 50%. Missing data on LVEF in the registry prompted exclusion from the study (Fig. 1).
Fig. 1.
Flow chart illustrating patient selection
The echocardiographic raw data was collected from the imaging databases at the participating hospitals and LVEF was re-evaluated at the core lab in Uppsala by two independent reviewers according to the modified biplane Simpson method using TOMTEC-ARENA TTA2 software (TOMTEC IMAGING SYSTEMS, GMBH EDISONSTRASSE, Unterschleissheim DE) with manual delineation of the endocardial border [9]. Subjects with more than two untraceable myocardial segments due to insufficient image quality were excluded. The reviewers were experienced echocardiographers blinded to all patient related clinical data. The mean LVEF value of the two reassessments was used as reference when compared to SWEDEHEART data. Before comparison, the reassessed reference values were categorized from continuous into ordinal scale variables in accordance with the LVEF ranges found in SWEDEHEART.
Statistical analysis
Spearman’s rank correlation was used to test correlation and Wilcoxon signed-rank test was used to assess bias. Gwet’s AC1 and AC2 statistics were used to test agreement between SWEDEHEART data and the reassessments. The Gwet’s AC2 analysis was performed with pre-specified linear weights. The Intraclass correlation coefficient (ICC) was used for inter-observer variability assessment between the two reference reviewers by a two-way mixed-effects model examining consistency in “single rater” type [10, 11]. The analysis was performed using SPSS software 26.0, SPSS Inc., Chicago, IL, USA and R (4.0.2) with statistical significance defined by p < 0.05. The results were presented in a tabular format with 95% confidence intervals. The study was approved by the Regional Committee for Medical Research Ethics (DNR 2017/759-31).
Results
The median age in the study population was 65 years, and 76% were men. See Table 1 for further baseline characteristics.
Table 1.
Patient demographics and medical history
| Characteristics | n = 130 |
|---|---|
| Age, years median (IQR) | 65 (58–72) |
| Male sex (%) | 99 (76) |
| Active smoker, (%) | 36 (28) |
| Diagnosis of ACS | |
| STEMI (%) | 65 (50) |
| NSTEMI (%) | 62 (48) |
| Other (UA, type 2 MI, Takotsubo) (%) | 3 (2) |
| Medical history | |
| Hypertension (%) | 72 (55) |
| Diabetes mellitus (%) | 27 (21) |
| Atrial fibrillation (%) | 6 (5) |
| Heart failure (%) | 22 (17) |
| History of stroke (%) | 10 (8) |
| History of MI (%) | 29 (22) |
IQR Inter quartile range, STEMI ST-elevation myocardial infarction, NSTEMI non-ST-elevation myocardial infarction, BMI Body mass index, UA Unstable angina pectoris
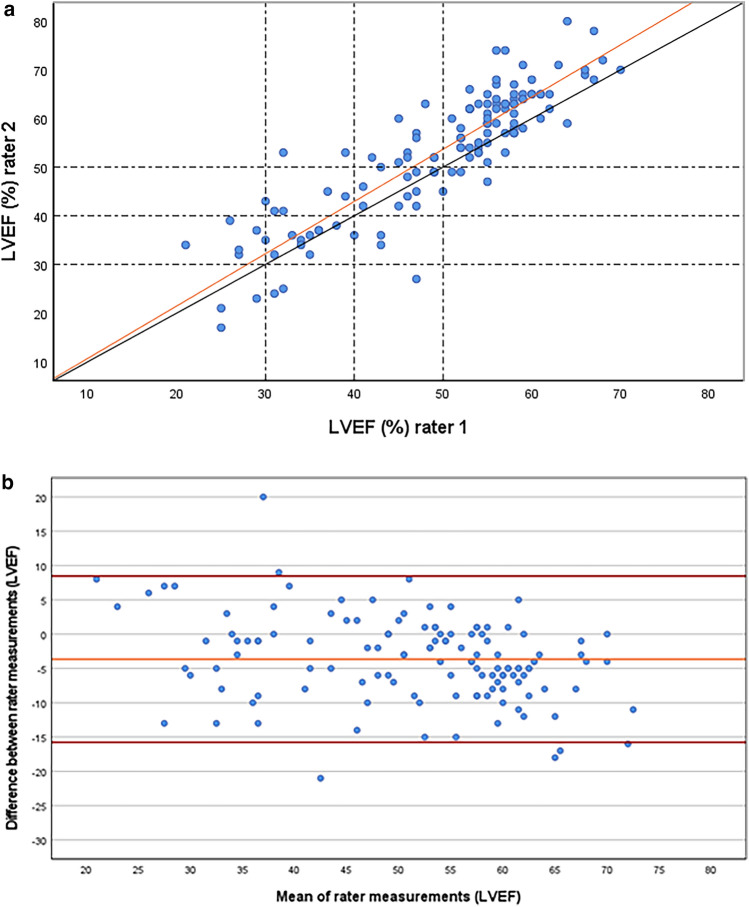
After LVEF was re-evaluated by the two reviewers with the modified biplane Simpson method, the mean LVEF was calculated for comparison with the SWEDEHEART data. The inter-observer variability between the two reference reviewers re-evaluating LVEF by biplane Simpson was close to excellent [ICC 0.87 (95% CI 0.82–0.92)]. Likewise, there was an excellent correlation with Spearman’s R of 0.88 (p < 0.001). Figure 2a displays a scatterplot of the reassessments by the two reviewers and Fig. 2b illustrates their agreement in a Bland–Altman plot.
Fig. 2.
a A scatter plot illustrating inter-observer variability between the two reviewers. The dotted lines mark the cut-off values defining LVEF categories in SWEDEHEART. b Bland–Altman plot illustrating the difference between the reviewers’ measurements to their mean. Orange line = mean difference, Red lines = mean difference ± 1.96* standard deviation of the difference
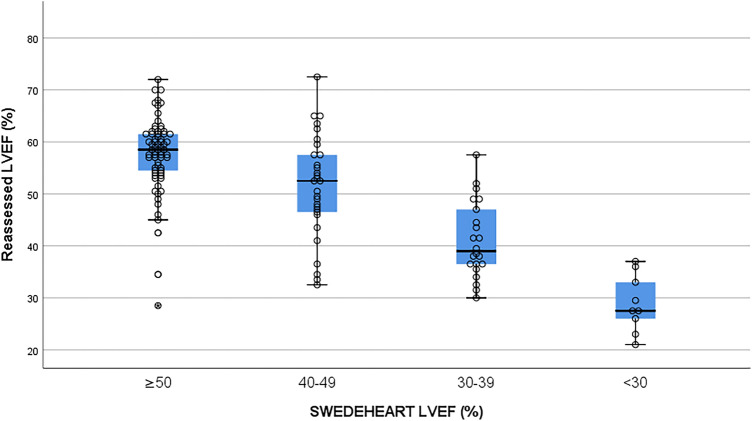
As displayed in Table 2 there is an overall good correlation between reassessed LVEF and SWEDEHEART LVEF (Spearman’s r = 0.69, p < 0.01). Agreement as assessed by Gwet’s AC1 was moderate [0.58 (95% CI 0.47–0.69)], however, when adjusted for misclassification errors (AC2), the agreement between SWEDEHEART LVEF and the gold standard was good [0.76 (95% CI 0.69–0.84)]. There was a trend towards lower LVEF values in the registry when compared to the reassessments. This bias was further explored in Table 3 by the Wilcoxon signed-rank test. The analysis showed a lower category LVEF in 25.4% (p < 0.001) of cases in SWEDEHEART and an absolute agreement in 66% of cases. Figure 3 displays a particularly wide distribution of reassessed LVEF among patients registered in the LVEF range between 40 and 49%, with a median above the 50% cut-off limit. There appears to be a slight bias towards registering a lower category LVEF in subjects with borderline function.
Table 2.
Crosstab displaying the overlap and distribution of the categorized re-evaluated LVEF (gold standard) and the LVEF registered in SWEDEHEART
| SWEDEHEART | Reassessed LVEF | ||||
|---|---|---|---|---|---|
| LVEF ≥ 50% | LVEF 40–49% | LVEF 30–39% | LVEF < 30% | Total | |
| LVEF ≥ 50% | 62 | 5 | 1 | 1 | 69 |
| LVEF 40–49% | 18 | 8 | 4 | 0 | 30 |
| LVEF 30–39% | 3 | 8 | 11 | 0 | 22 |
| LVEF < 30% | 0 | 0 | 4 | 5 | 9 |
| Total | 83 | 21 | 20 | 6 | 130 |
Table 3.
A Wilcoxon signed-rank test of bias between re-evaluated LVEF and LVEF estimates in the SWEDEHEART registry
| Wilcoxon signed-rank test | n (%) |
|---|---|
| Reassessed LVEF > SWEDEHEART | 33 (25.4) |
| Reassessed LVEF < SWEDEHEART | 11 (8.6%) |
| Ties | 86 (66.0) |
| Total | 130 |
Fig. 3.
Combined boxplot and dotplot with distribution of the reassessed LVEF in LVEF categories according to SWEDEHEART. Middle line = median; Box = interquartile range (IQR); Whiskers = lowest and maximum LVEF excluding outliers; Circle outside whiskers = mild outliers; asterisk in circle = severe outliers
Discussion
Independent validation of LVEF in the SWEDEHEART registry shows an overall good agreement when re-evaluated by two independent reviewers using the modified biplane Simpson method. However, there was a tendency towards underestimation of LVEF in SWEDEHEART, with the largest discrepancy between re-evaluated LVEF and SWEDEHEART LVEF in subjects with LVEF < 50%. Only five patients had a greater than one category difference.
To our knowledge this is the first validation study of LVEF assessed by echocardiography in a quality registry by reassessment of the raw data. Consequently, it may be difficult to put these results in relation to previous registry validations. In 2016, Govatsmark et al. published a validation of the Norwegian myocardial infarction register with medical records as reference, without reinterpretation of the echocardiography raw data [12]. This approach, with medical records as gold standard, is common in registry validation studies, however, it fails to address potential flaws in index data acquisition [13, 14]. Publications on inter-observer variability in LVEF may be more feasible as a benchmark since they more closely reflect this SWEDEHEART validation study design. Such studies, covering different clinical settings, have presented good to excellent inter-observer agreement of LVEF in populations with preserved ejection fraction, yet only moderate agreement in subjects with subnormal ejection fraction [15–17]. The findings from the SWEDEHEART registry are thus in accordance with previous results.
There were 47 subjects (36%) with LVEF < 50% in this study sample collected between 2008 and 2014. As of 2014, 35% of all the subjects registered to SWEDEHEART had an LVEF < 50%, indicating that the study sample was representative of the overall population [18].
Assessment of LVEF by visual eye-balling was, at the time of enrollment, the most common method of LVEF assessment at all three participating centers. This approach has repeatedly shown to underestimate LVEF compared to quantitative assessment by the modified Simpson method [19–21]. Visually assessed LVEF has substantial inter-observer variability of up to ± 14% LVEF [6]. In such settings, grouping the values into larger categories might be reasonable. However, current guidelines on cardiac chamber quantification favor the more robust modified biplane Simpson approach that generates a continuous variable [22]. In such quantitative setting, it may be more reasonable to report the obtained LVEF value to avoid the obvious loss of granularity in categorized data. Categorization may also increase the perceived difference between two assessments that end up on different sides of a cut-off limit, despite being close in numeric value. The current categorization also fails to harmonize with important clinical cut-off limits such as the 35% LVEF limit in deciding on ICD and CRT implantation [23]. Algorithms enabling an automatic tracing of the endocardial border, generating an instant assessment of LVEF, are now common and validated tools in the echocardiography lab [24]. These techniques further lower the barriers to a quantitative assessment of a continuous LVEF variable. However, should quantitatively assessed LVEF come to be reported as a continuous variable it must still be interpreted with respect to a smaller, yet significant, inter-observer variation and a smallest detectable change of almost 7 % points [3, 25].
Kappa statistics has repeatedly been used in inter-observer variability studies on LVEF due to its property of adjusting for chance agreement [26]. However, Cohen’s kappa is prone to underestimating agreement in populations with symmetrical imbalance [26] [27]. As the majority (63%) of the studied SWEDEHEART subjects presented with a preserved ejection fraction (LVEF > 50%), Cohen’s kappa was rendered unfeasible in this setting. Instead, the weighed Gwet’s AC2 method offered a more robust analysis, yet despite its advantages, it appears to be rarely performed in publications on assessments of LVEF agreement [12, 28, 29].
There was an agreement on categorized LVEF in roughly 50% of the cases with reduced LVEF (as determined by the reference method). This discrepancy is largest in the mid-range categories of 30–49% where important clinical cut-off limits are present. An improved precision of LVEF assessments in this subgroup would be most favorable. A Japanese study examined two different teaching interventions with regard to improved inter-rater variability in visually assessed LVEF [30]. The interventions significantly reduced the misclassification rates of LVEF regardless of operator experience and image quality. There are several other studies in support of similar teaching interventions [6, 31]. Such initiatives could possibly further improve the quality of LVEF assessments entered in SWEDEHEART.
Limitations
There is a theoretical risk that some LVEF assessments have been misclassified into the wrong category in SWEDEHEART since this process is performed manually. As we have not validated the registry LVEF in relation to patient records such faults cannot be accounted for in this study which may be considered a limitation. The extreme outlier in Fig. 3 may be an example of such accidental misclassification. LVEF lacks a gold standard and, as a consequence, the proper reference method in LVEF validation studies is a subject open for debate. Given that alternative imaging modalities, such as cardiac magnetic resonance imaging (CMR) or cardiac computed tomography (CCT), have not been routinely performed in Swedish ACS subjects we deemed it appropriate that the reference in this study should be based on the 2D echocardiographic raw data. The use of a mean from two independent reassessments by the modified Simpson rule was proposed to strengthen the robustness in the reference method, however, there is scarce literature in support of this notion.
The patients were included at three university hospitals which may give rise to questions of sample representation since SWEDEHEART contains data from all Swedish hospitals treating ACS. A larger nationwide follow-up with a greater variation in hospital size may provide additional information on the data validity in SWEDEHEART. However, as previously mentioned, the representability of the material is supported by the concurring proportion of subjects categorized with reduced ejection fraction in this study and in the SWEDEHEART registry [18].
Conclusion
Independent validation of SWEDEHEART-reported LVEF shows an overall good agreement with the re-evaluated LVEF. However, a tendency towards underestimation of LVEF was observed, with the largest discrepancy between re-evaluated LVEF and registry LVEF found in subjects with subnormal LV-function in whom the reported assessment of LVEF should be interpreted more cautiously.
Funding
Open access funding provided by Uppsala University. No funding was received for conducting this study.
Availability of data and material
The data may be provided upon request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical standards
This study has been approved by the regional ethics committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
All study subjects gave their informed consent prior to their inclusion in the study.
References
- 1.Collet J-P, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2021;42(14):1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 2.Thavendiranathan P, et al. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes. J Am Coll Cardiol. 2013;61(1):77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 3.Baron T, et al. Test–retest reliability of new and conventional echocardiographic parameters of left ventricular systolic function. Clin Res Cardiol. 2019;108(4):355–365. doi: 10.1007/s00392-018-1363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole GD, et al. Defining the real-world reproducibility of visual grading of left ventricular function and visual estimation of left ventricular ejection fraction: impact of image quality, experience and accreditation. Int J Cardiovasc Imaging. 2015;31(7):1303–1314. doi: 10.1007/s10554-015-0659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGowan JH, Cleland JGF. Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of 3 methods. Am Heart J. 2003;146(3):388–397. doi: 10.1016/S0002-8703(03)00248-5. [DOI] [PubMed] [Google Scholar]
- 6.Johri AM, et al. Can a teaching intervention reduce interobserver variability in LVEF assessment: a quality control exercise in the echocardiography lab. JACC: Cardiovasc Imaging. 2011;4(8):821–829. doi: 10.1016/j.jcmg.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Desta L, et al. Heart failure with normal ejection fraction is uncommon in acute myocardial infarction settings but associated with poor outcomes: a study of 91,360 patients admitted with index myocardial infarction between 1998 and 2010. Eur J Heart Fail. 2016;18(1):46–53. doi: 10.1002/ejhf.416. [DOI] [PubMed] [Google Scholar]
- 8.Hambraeus K, et al. SWEDEHEART annual report 2012. Scand Cardiovasc J. 2014;48:1. doi: 10.3109/14017431.2014.931551. [DOI] [PubMed] [Google Scholar]
- 9.Foley T, et al. Measuring left ventricular ejection fraction—techniques and potential pitfalls. Eur Cardiol Rev. 2012;8:108. doi: 10.15420/ecr.2012.8.2.108. [DOI] [Google Scholar]
- 10.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunting KV, et al. A practical guide to assess the reproducibility of echocardiographic measurements. J Am Soc Echocardiogr. 2019;32(12):1505–1515. doi: 10.1016/j.echo.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Govatsmark RE, et al. Interrater reliability of a national acute myocardial infarction register. Clin Epidemiol. 2016;8:305–312. doi: 10.2147/CLEP.S105933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vikholm P, et al. Validity of the Swedish cardiac surgery registry. Interact Cardiovasc Thorac Surg. 2018;27(1):67–74. doi: 10.1093/icvts/ivy030. [DOI] [PubMed] [Google Scholar]
- 14.Ljung R, et al. The Swedish dental health register—validation study of remaining and intact teeth. BMC Oral Health. 2019;19(1):116. doi: 10.1186/s12903-019-0804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gadsbøll N, et al. Interobserver agreement and accuracy of bedside estimation of right and left ventricular ejection fraction in acute myocardial infarction. Am J Cardiol. 1989;63(18):1301–1307. doi: 10.1016/0002-9149(89)91039-4. [DOI] [PubMed] [Google Scholar]
- 16.De Geer L, Oscarsson A, Engvall J. Variability in echocardiographic measurements of left ventricular function in septic shock patients. Cardiovasc Ultrasound. 2015;13(1):19. doi: 10.1186/s12947-015-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frikha Z, et al. Reproducibility in echocardiographic assessment of diastolic function in a population based study (The STANISLAS Cohort Study) PLoS ONE. 2015;10:e0122336. doi: 10.1371/journal.pone.0122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szummer K, et al. Relations between implementation of new treatments and improved outcomes in patients with non-ST-elevation myocardial infarction during the last 20 years: experiences from SWEDEHEART registry 1995 to 2014. Eur Heart J. 2018;39(42):3766–3776. doi: 10.1093/eurheartj/ehy554. [DOI] [PubMed] [Google Scholar]
- 19.Sievers B, et al. Visual estimation versus quantitative assessment of left ventricular ejection fraction: a comparison by cardiovascular magnetic resonance imaging. Am Heart J. 2005;150(4):737–742. doi: 10.1016/j.ahj.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Rana S, et al. Left ventricle ejection fraction estimation by point of care echocardiography in patients admitted in intensive care unit. J Chitwan Med Coll. 2020;10:54–57. doi: 10.54530/jcmc.120. [DOI] [Google Scholar]
- 21.Jakobsen CJ, Torp P, Sloth E. Assessment of left ventricular ejection fraction may invalidate the reliability of EuroSCORE. Eur J Cardiothorac Surg. 2006;29(6):978–982. doi: 10.1016/j.ejcts.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 23.Priori SG, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36(41):2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 24.Knackstedt C, et al. Fully automated versus standard tracking of left ventricular ejection fraction and longitudinal strain: The FAST-EFs Multicenter Study. J Am Coll Cardiol. 2015;66(13):1456–1466. doi: 10.1016/j.jacc.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 25.Thorstensen A, et al. Reproducibility in echocardiographic assessment of the left ventricular global and regional function, the HUNT study. Eur J Echocardiogr. 2010;11(2):149–156. doi: 10.1093/ejechocard/jep188. [DOI] [PubMed] [Google Scholar]
- 26.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 27.Feinstein AR, Cicchetti DV. High agreement but low Kappa: i the problems of two paradoxes. J Clin Epidemiol. 1990;43(6):543–549. doi: 10.1016/0895-4356(90)90158-L. [DOI] [PubMed] [Google Scholar]
- 28.Wongpakaran N, et al. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: a study conducted with personality disorder samples. BMC Med Res Methodol. 2013;13(1):61. doi: 10.1186/1471-2288-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh P, et al. Approaches to describing inter-rater reliability of the overall clinical appearance of febrile infants and toddlers in the emergency department. PeerJ. 2014;2:e651. doi: 10.7717/peerj.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusunose K, et al. Reduced variability of visual left ventricular ejection fraction assessment with reference images: The Japanese Association of Young Echocardiography Fellows multicenter study. J Cardiol. 2018;72(1):74–80. doi: 10.1016/j.jjcc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Anilkumar S, et al. A teaching intervention increases the performance of handheld ultrasound devices for assessment of left ventricular ejection fraction. Heart Views. 2019;20(4):133–138. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_91_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data may be provided upon request.
Not applicable.