Abstract
We aimed to assess the systemic and hepatic renin-angiotensin-system (RAS) fingerprint in advanced chronic liver disease (ACLD). This prospective study included 13 compensated (cACLD) and 12 decompensated ACLD (dACLD) patients undergoing hepatic venous pressure gradient (HVPG) measurement. Plasma components (all patients) and liver-local enzymes (n = 5) of the RAS were analyzed using liquid chromatography–tandem mass spectrometry. Patients with dACLD had significantly higher angiotensin (Ang) I, Ang II and aldosterone plasma levels. Ang 1–7, a major mediator of the alternative RAS, was almost exclusively detectable in dACLD (n = 12/13; vs. n = 1/13 in cACLD). Also, dACLD patients had higher Ang 1–5 (33.5 pmol/L versus cACLD: 6.6 pmol/L, p < 0.001) and numerically higher Ang III and Ang IV levels. Ang 1–7 correlated with HVPG (ρ = 0.655; p < 0.001), von Willebrand Factor (ρ = 0.681; p < 0.001), MELD (ρ = 0.593; p = 0.002) and interleukin-6 (ρ = 0.418; p = 0.047). Considerable activity of ACE, chymase, ACE2, and neprilysin was detectable in all liver biopsies, with highest chymase and ACE2 activity in cACLD patients. While liver-local classical and alternative RAS activity was already observed in cACLD, systemic activation of alternative RAS components occurred only in dACLD. Increased Ang 1–7 was linked to severe liver disease, portal hypertension, endothelial dysfunction and inflammation.
Subject terms: Portal hypertension, Ascites, Liver
Introduction
While advanced chronic liver disease (ACLD) is initially asymptomatic and therefore considered compensated (cACLD), it eventually progresses into decompensated disease (dACLD), displaying characteristic symptoms which arise from portal hypertension such as ascites and bleeding of oesophageal varices1,2. Aside from symptom-dependent treatment, the current therapies of dACLD focus on lowering portal pressure and include pharmacological treatment using non-selective betablockers3,4 as well as invasive procedures such as the implantation of a transjugular intrahepatic portosystemic shunt (TIPS)5.
A better understanding of the role of the renin-angiotensin-system (RAS) including its classical and alternative components in ACLD may reveal novel therapeutic targets. Through renin cleaving hepatic angiotensinogen, angiotensin I (Ang I) is created and further converted to angiotensin (Ang) II by angiotensin-converting enzyme (ACE). Ang II is also frequently converted by the locally active chymase6 and mediates its effects through binding to members of the angiotensin-receptor family, which lead to vasoconstriction and aldosterone secretion facilitating sodium and water retention in the kidneys as well as mediating inflammation7. By acting not only systemically but also locally, the RAS is involved in growth and remodeling processes in the heart and blood vessels8. A permanently upregulated RAS is strongly associated with various health complications such as hypertension, diabetes and aging processes9. In liver disease, elevated Ang II is associated with both hepatic resistance as well as portal pressure10. Additionally, an increased expression of RAS components has even been observed in activated hepatic stellate cells (HSC) following liver injury, which enables local synthesis of Ang II mediating fibrosis of the liver11,12.
As opposed to the classical RAS-axis, the metalloprotease angiotensin-converting enzyme 2 (ACE2) has been identified as the key enzyme of the alternative RAS. It is able to use Ang II as substrate, converting it into Angiotensin 1–7 (Ang 1–7)7, which binds to the mas-receptor (masR) and has vasodilative and anti-inflammatory qualities13.
Ang 1–7 can also be formed by way of conversion through neprilysin (NEP) from Ang I. Upregulated Ang 1–7 is associated with lower incidence of steatosis14 and even plays a role in the inhibition of Ang II-mediated pathways facilitating fibrosis in cultured HSC15. Hence it can be argued, that ACE2 and Ang 1–7 as part of the alternate RAS axis act as a compensating measure to the classical RAS in the liver.
Considering the growing body of evidence on the involvement of RAS in liver disease, it seems essential to systematically investigate the classical and non-classical RAS fingerprint in patients across the full spectrum of liver disease. Thus, we performed a study to assess the blood and hepatic fingerprint of the classical and non-classical RAS components in the systemic (blood) and local (liver) compartments in patients with both compensated and decompensated ACLD.
Results
Patient characteristics (Table 1)
Table 1.
Patient characteristics of patients with compensated (cACLD) and decompensated cirrhosis (dACLD).
| Patient characteristics | cACLD (n = 13) | dACLD (n = 12) | p-value |
|---|---|---|---|
| Sex, male/female (% male) | 11/2 (84.6%) | 7/5 (58.3%) | 0.144 |
| Age, years (IQR) | 59.3 (13.5) | 62.5 (19.8) | 0.999 |
| Body mass index, kg m−2 (IQR) | 24.6 (5.7) | 28.3 (9.4) | 0.017 |
| Etiology | 0.227 | ||
| ALD, n (%) | 3 (23.1%) | 7 (58.3%) | |
| Viral hepatitis, n (%) | 7 (53.8%) | 2 (16.7%) | |
| NASH, n (%) | 2 (15.4%) | 2 (16.7%) | |
| Cholestatic, n (%) | 1 (7.7%) | 1 (8.3%) | |
| Varices, n (%) | 3 (23.1%) | 11 (91.7%) | < 0.001 |
| History of variceal bleeding, n (%) | 0 (0.0%) | 4 (33.3%) | 0.023 |
| Ascites, n (%) | 0 (0.0%) | 12 (100.0%) | < 0.001 |
| Refractory ascites, n (%) | 0 (0.0%) | 6 (50.0%) | 0.003 |
| History of spontaneous bacterial peritonitis | 0 (0.0%) | 1 (8.3%) | 0.288 |
| Hepatic encephalopathy, n (%) | 0 (0.0%) | 8 (66.7%) | 0.003 |
| Child–Turcotte–Pugh Score, points (IQR) | 5.0 (0.0) | 8.0 (3.0) | < 0.001 |
| Child-stage | < 0.001 | ||
| A, n (%) | 13 (100.0%) | 0 (0.0%) | |
| B, n (%) | 0 (0.0%) | 9 (75.0%) | |
| C, n (%) | 0 (0.0%) | 3 (25.0%) | |
| MELD, points (IQR) | 9.0 (2.5) | 15.5 (10.3) | < 0.001 |
| Hepatic venous pressure gradient, mmHg (IQR) | 8.0 (3.0) | 19.0 (10.0) | < 0.001 |
| Liver stiffness measurement, kPa (IQR) | 13.8 (6.2) | 62.4 (44.6) | < 0.001 |
| Mean arterial pressure, mmHg (IQR) | 98.0 (17.8) | 96.0 (29.1) | 0.999 |
| Sodium, mmol L−1 (IQR) | 140.0 (5.0) | 134.0 (5.0) | 0.015 |
| Bilirubin, mg dL−1 (IQR) | 0.8 (0.8) | 1.2 (1.2) | 0.115 |
| International normalized ratio, units (IQR) | 1.2 (0.2) | 1.4 (0.6) | 0.036 |
| Albumin, g L−1 (IQR) | 40.4 (4.1) | 31.6 (5.8) | 0.005 |
| Creatinine, mg dL−1 (IQR) | 0.8 (0.3) | 1.0 (1.0) | 0.434 |
| VWF antigen, % (IQR) | 242.0 (155.0) | 413.0 (24.0) | < 0.001 |
| C-reactive protein, mg dL−1 (IQR) | 0.1 (0.3) | 0.5 (1.9) | 0.434 |
| Interleukin-6, pg mL−1 (IQR) | 4.0 (18.2) | 22.2 (19.0) | 0.100 |
cACLD compensated advanced chronic liver disease, ALD alcoholic liver disease, dACLD decompensated advanced chronic liver disease, IQR interquartile range, MELD model for end-stage liver disease, NASH non-alcoholic steatohepatitis, VWF von Willebrand factor.
Overall, 25 patients (cACLD: n = 13, dACLD: n = 12) with a median age of 61.1 years and male predominance (n = 18/25; 72.0%) were included. Etiologies of liver disease included alcoholic liver disease (ALD; n = 10/25; 40.0%), viral hepatitis (n = 9/25; 36.0%), NASH (n = 4/25; 16.0%) and cholestatic liver disease (n = 2; 8.0%).
A detailed comparison of the characteristics of patients with cACLD and dACLD is given in Table 1. In short, there was no difference in sex, median age or liver disease etiology between patients with cACLD and dACLD. Patients with cACLD had lower prevalence of varices, lower MELD score, HVPG, LSM, as well as higher plasma sodium and plasma albumin levels as compared to patients with dACLD.
Interestingly, median MAP did not differ between the cACLD and the dACLD cohort (cACLD: 98.0 mmHg vs. dACLD: 96.0 mmHg; p = 0.999).
VWF antigen (cACLD: 242.0% vs. dACLD: 413.0%; p < 0.001) and in tendency IL-6 levels (cACLD: 4.0 pg/mL vs. dACLD: 22.2 pg/mL; p = 0.100) were lower in patients with cACLD than in patients with dACLD, while CRP was not significantly different(cACLD: 0.1 mg/dL vs. dACLD: 0.5 mg/dL; p = 0.434).
Plasma equilibrium levels of classical and alternative RAS components in patients with cACLD and dACLD (Table 2, Fig. 1)
Table 2.
Plasma levels of classical and alternative renin-angiotensin system (RAS) components in patients with compensated (cACLD) and decompensated cirrhosis (dACLD).
| Parameter | cACLD (n = 13) | dACLD (n = 12) | p-value |
|---|---|---|---|
| PRA-S, pmol L−1 (IQR) | 51.5 (150.0) | 489.7 (710.1) | 0.001 |
| ACE-S, (pmol L−1) × (pmol L−1) (IQR) | 6.6 (4.4) | 3.4 (2.5) | 0.022 |
| Ang I, pmol L−1 (IQR) | 12.4 (14.4) | 93.1 (317.8) | < 0.001 |
| Ang II, pmol L−1 (IQR) | 46.2 (136.4) | 343.7 (666.4) | 0.016 |
| Ang 1–7, pmo L−1 (IQR) | 3.0 (0.0) | 16.1 (68.3) | < 0.001 |
| Undetectable Ang 1–7, n (%) | 12 (92.3%) | 2 (16.7%) | < 0.001 |
| Ang 1–5, pmol L−1 (IQR) | 6.6 (12.8) | 33.5 (114.5) | < 0.001 |
| Ang III, pmol L−1 (IQR) | 2.5 (0.9) | 3.1 (10.5) | 0.309 |
| Ang IV, pmol L−1 (IQR) | 2.0 (2.8) | 5.0 (16.6) | 0.141 |
| Aldosterone, pg mL−1 (IQR)a | 84.0 (100.7) | 383.6 (499.0) | 0.007 |
ACE-S angiotensin converting enzyme surrogate, Ang angiotensin, cACLD compensated advanced chronic liver disease, dACLD decompensated advanced chronic liver disease, IQR interquartile range, PRA-S plasma renin activity surrogate, RAS renin–angiotensin system.
aAvailable in n = 14 patients (cACLD: n = 7; dACLD: n = 7).
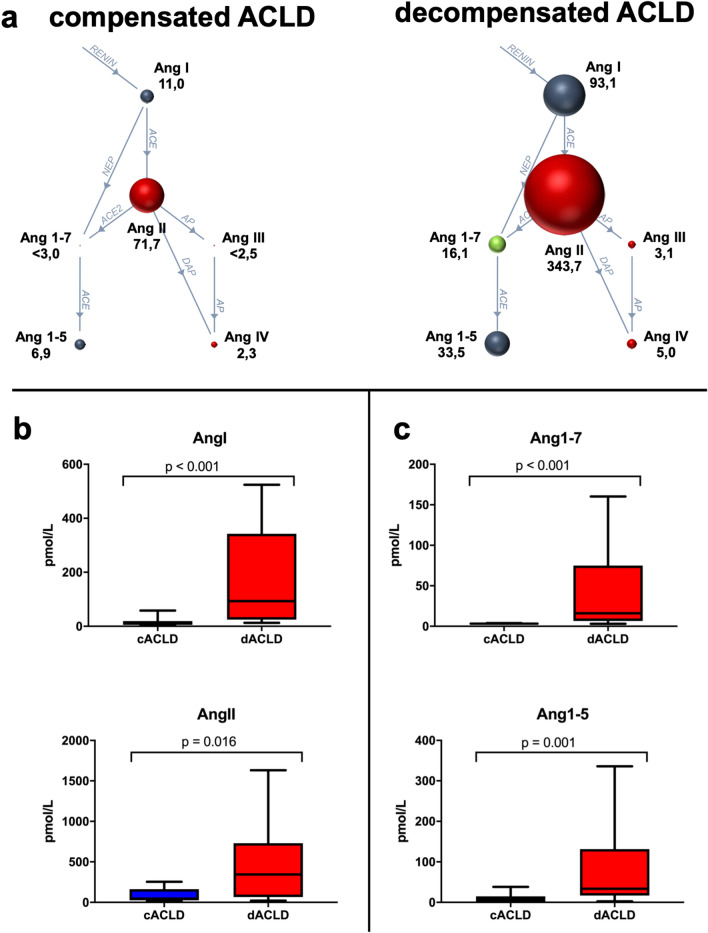
Figure 1.
Components of the classical and alternative renin–angiotensin system (RAS) in patients with compensated (cACLD, n = 13) and decompensated cirrhosis (dACLD, n = 12). (a) Depiction of the RAS fingerprint in patients with cACLD and dACLD. Comparison of quantitative levels of (b) classical RAS components (Ang I and Ang II) and (c) alternative RAS components (Ang 1–7, Ang 1–5) between patients with cACLD and dACLD. For RAS fingerprint analysis, the size of the spheres and numbers beside them represent the median absolute concentrations of angiotensins (pmol/L) analyzed by mass spectrometry. Ang angiotensin, ACE angiotensin converting enzyme, AP aminopeptidase, cACLD compensated advanced chronic liver disease, dACLD decompensated advanced chronic liver disease, DAP dipeptidyl aminopeptidase, NEP neprilysin, RAS renin–angiotensin system.
Figure 1A shows the systemic RAS fingerprint in patients with cACLD and dACLD. The RAS was generally upregulated in dACLD with high levels of Ang I, Ang II, but also Ang 1–7 and Ang 1–5. RAS activity in the plasma of patients with cACLD was less pronounced. Specifically, PRA-S (cACLD: 51.5 pmol/L vs. dACLD: 489.7 pmol/L; p = 0.001), Ang I (cACLD: 12.4 pmol/L vs. dACLD: 93.1 pmol/L; p < 0.001), Ang II (cACLD: 46.2 pmol/L vs. dACLD: 343.7 pmol/L; p = 0.016) and aldosterone (cACLD: 84.0 pg/mL vs. dACLD: 383.6 pg/mL; p = 0.007) were lower in patients with cACLD, in comparison to patients with dACLD.
Ang 1–7, a key component of the alternative RAS, was below the lower limit of quantification (i.e. < 3 pmol/L) in 92.3% (n = 12/13) of patients with cACLD, while in 83.3% (n = 10/12) of patients with dACLD patients, Ang 1–7 was detectable at a median level of 16.1 pmol/L (vs. 3.0 pmol/L in the one patient with cACLD; p < 0.001). Similarly, Ang 1–5 levels were lower in patients with cACLD than patients with dACLD (cACLD: 6.6 pmol/L vs. dACLD: 33.5 pmol/L; p < 0.001).
There was no significant difference in plasma levels of Ang III and Ang IV between patients with cACLD and patients with dACLD (Table 2).
Correlations of RAS components and severity of liver disease, portal hypertension, liver stiffness, endothelial dysfunction and inflammation (Table S1, Fig. 2)
Figure 2.
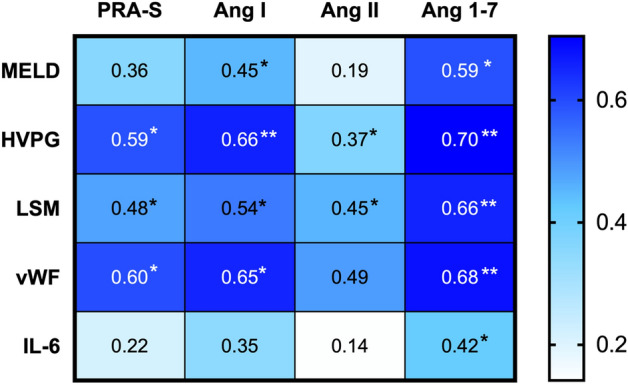
Correlations between components of the classical and alternative RAS (PRA-S, Ang I, Ang II and Ang 1–7) and parameters of liver disease severity (MELD), severity of portal hypertension (HVPG), liver stiffness (LSM), endothelial dysfunction (vWF) and inflammation (IL-6). Correlations were assessed using Spearman’s Rho. ∗ denotes p values < 0.05, whereas ∗∗ indicates p values < 0.001. Ang angiotensin, HVPG hepatic venous pressure gradient, IL-6 interleukin-6, LSM liver stiffness measurement, MELD model for end-stage liver disease, RAS renin–angiotensin system, PRA-S plasma renin activity surrogate, vWF von Willebrand factor antigen.
We determined associations between components of the classical (PRA-S, Ang I, Ang II) and alternative RAS (Ang 1–7) and parameters of liver disease severity (i.e. MELD), LSM, portal hypertension (i.e. HVPG), endothelial dysfunction (i.e. VWF antigen) and inflammation (i.e. IL-6).
Importantly, Ang 1–7 showed the strongest correlations of all RAS components with all assessed parameters. Ang 1–7 levels correlated with LSM (ρ = 0.704; p < 0.001), HVPG (ρ = 0.655; p < 0.001), VWF antigen (ρ = 0.681; p < 0.001), MELD (ρ = 0.593; p = 0.002) and IL-6 (ρ = 0.418; p = 0.047).
PRA-S exhibited correlations with LSM (ρ = 0.479; p = 0.028), HVPG (ρ = 0.592; p = 0.002), VWF antigen (ρ = 0.598; p = 0.002) and in tendency with MELD score (ρ = 0.360; p = 0.078). Ang I correlated with MELD score (ρ = 0.449; p = 0.024), LSM (ρ = 0.536; p = 0.012), HVPG (ρ = 0.658; p < 0.001) and VWF antigen (ρ = 0.654; p = 0.001), while Ang II correlated with LSM (ρ = 0.455; p = 0.038), VWF antigen (ρ = 0.489; p = 0.015) and tendentially with HVPG (ρ = 0.371; p = 0.068).
Assessed by linear regression analysis, HVPG (HR 2.61; 95% confidence interval [95% CI]: 0.69–4.52; p = 0.010; Table 3), VWF (HR 0.15; 95% CI: 0.01–0.30; p = 0.049) and dACLD (HR 7.67; 95% CI: 1.65–13.70; p = 0.015) were associated with Ang 1–7. In multivariate analysis, HVPG remained as the only parameter independently linked to Ang 1–7 plasma levels (aHR 2.57; 95% CI: 0.60–4.54; p = 0.013).
Table 3.
Factors associated with Ang 1–7 assessed by linear regression analysis. Both univariate and multivariate regression analyses are shown.
| Parameters | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | aHR | 95% CI | p-value | |
| Age, years | 1.10 | −0.61 to 2.81 | 0.195 | – | – | – |
| Sex, male | 14.7 | −23.01 to 52.42 | 0.428 | – | – | – |
| MELD, points | 2.10 | −0.78 to 5.00 | 0.145 | – | – | – |
| HVPG, mmHg | 2.61 | 0.69 to 4.52 | 0.010 | 2.57 | 0.60 to 4.54 | 0.013 |
| VWF, % | 0.15 | 0.01 to 0.30 | 0.049 | 0.01 | −0.23 to 0.26 | 0.905 |
| IL-6, pg mL−1 | 0.21 | −0.89 to 1.31 | 0.697 | – | – | – |
| dACLD, yes | 7.67 | 1.65 to 13.70 | 0.015 | 2.96 | −8.15 to 14.05 | 0.586 |
Ang angiotensin, HVPG hepatic venous pressure gradient, IL-6 interleukin-6, LSM liver stiffness measurement, MELD model for end-stage liver disease, vWF von Willebrand factor antigen.
Differences of RAS activity in the plasma and liver tissue of ACLD patients (Table 4, Fig. 3, Fig. S1)
Table 4.
Hepatic and systemic RAS components in liver tissue samples and plasma samples of 5 ACLD patients (#1–5).
| #1 | #2 | #3 | #4 | #5 | |
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Age, years | 69 | 67 | 62 | 64 | 52 |
| Body mass index, kg m−2 | 26.1 | 24.6 | 23.1 | 33.9 | 19.3 |
| Etiology | NASH | VIRAL | ALD | NASH | ALD |
| cACLD/dACLD | cACLD | cACLD | cACLD | dACLD | dACLD |
| HVPG, mmHg | 15 | 3 | 8 | 10 | 18 |
| MAP, mmHg | 112 | 94 | 106 | 119 | 123 |
| CTP score | 5 | 5 | 5 | 6 | 8 |
| MELD | 11 | 12 | 9 | 8 | 19 |
| Liver tissue | |||||
| ACE activity | 286.0 | 292.0 | 119.0 | 116.0 | 185.0 |
| Chymase activity | 6971.0 | 302.0 | 359.0 | 731.0 | 2237.0 |
| NEP activity | 59.0 | 669.0 | 304.0 | 403.0 | 86.0 |
| ACE2 activity | 186.0 | 80.0 | 54.0 | 130.0 | 110.0 |
| Plasma | |||||
| PRA-S | 28.6 | 137.4 | 37.2 | 147.2 | 446.7 |
| Ang I (1–10) | 12.4 | 18.2 | 7.9 | 49.2 | 151.8 |
| Ang II (1–8) | 16.1 | 119.2 | 29.3 | 98.0 | 295.0 |
| Ang 1–7 | 3.0 | 3.0 | 3.0 | 6.0 | 23.9 |
| Ang 1–5 | 2.0 | 7.4 | 6.6 | 27.9 | 114.0 |
| Ang III (2–8) | 7.6 | 22.1 | 3.0 | 5.1 | 23.3 |
| Ang IV (3–8) | 2.0 | 5.7 | 2.0 | 2.9 | 12.0 |
| Aldosterone | 43.3 | 158.7 | 66.9 | 383.6 | 1339.9 |
In the liver tissue, activity of ACE and chymase as enzymes of the classical RAS and ACE2 and NEP as enzymes of the non-classical RAS were assessed. In plasma, levels of classical (PRA-S, Ang I, Ang II, aldosterone) and non-classical RAS (Ang 1–7, Ang III, Ang IV, Ang 1–5) were quantified.
ACE angiotensin converting enzyme, Ang angiotensin, cACLD compensated advanced chronic liver disease, CTP Child–Turcotte–Pugh, dACLD decompensated advanced chronic liver disease, HVPG hepatic venous pressure gradient, MAP mean arterial pressure, MELD model for end-stage liver disease, NEP neprilysin, PRA-S plasma renin activity surrogate, RAS renin–angiotensin system.
Figure 3.
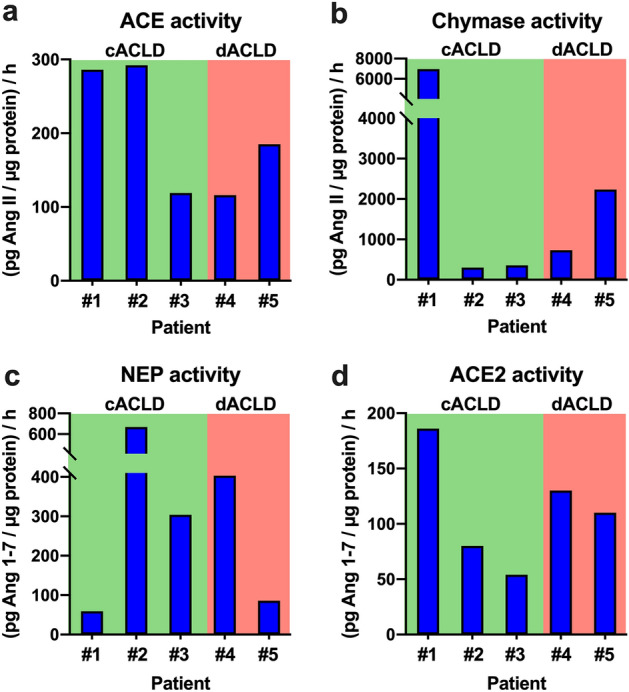
Tissue activity of components of the (a,b) classical (ACE, chymase) and (c,d) alternative RAS (neprilysin [NEP], ACE2) in 5 patients with compensated (cACLD; n = 3) and decompensated advanced chronic liver disease (dACLD; n = 2). ACE angiotensin converting enzyme, cACLD compensated advanced chronic liver disease, dACLD decompensated advanced chronic liver disease, NEP neprilysin, RAS renin–angiotensin system.
In five patients (cACLD: n = 3; dACLD: n = 2), components of the classical and alternative RAS were assessed both in plasma and liver tissue, as shown in Table 3. Again, plasma levels of classical (PRA-S, Ang I, Ang II, aldosterone) and alternative RAS components (Ang 1–7, Ang 1–5) were particularly high in dACLD patients (Fig. S1).
However, interestingly, the enzymatic activity of the RAS was more complex in the liver tissue, as outlined in Fig. 3.
Of note, patient #1 had compensated NASH and only moderate systemic classical or alternative RAS activity, and exhibited the highest hepatic activity of the classical RAS (ACE activity: 286.0 (pg Ang II/µg protein)/h; chymase activity: 6971.0 (pg Ang II/µg protein)/h) and ACE2 (186.0 (pg Ang 1–7/µg protein)/h), while NEP activity was the lowest among all patients (59.0 (pg Ang 1–7/µg protein)/h).
Patient #2 had viral hepatitis without portal hypertension (HVPG: 3 mmHg) and systemic levels moderate systemic levels of Ang II and Ang IV, while Ang I was low and Ang 1–7 was below the lower limit of quantification. This patient exhibited the highest hepatic activity of ACE (292.0 (pg Ang II/µg protein)/h) and NEP (669.0 (pg Ang II/µg protein)/h).
Patient #3 had compensated ALD and subclinical portal hypertension and had been abstinent for one month before study inclusion. This patients had virtually no systemic activation of the classical or alternative RAS and exhibited low hepatic activity of both the classical (ACE activity: 119.0 (pg Ang II/µg protein)/h; chymase activity: 359.0 (pg Ang II/µg protein)/h) and alternative RAS (ACE2 activity: 54.0 (pg Ang 1–7/µg protein)/h; NEP activity: 304.0 (pg Ang 1–7/µg protein)/h).
Patient #4 had decompensated NASH and relatively low systemic RAS activity, and exhibited moderate and high hepatic activity of the classical (ACE activity: 116.0 (pg Ang II/µg protein)/h; chymase activity: 731.0 (pg Ang II/µg protein)/h) and alternative RAS (ACE2 activity: 130.0 (pg Ang 1–7/µg protein)/h; NEP activity: 403.0 (pg Ang 1–7/µg protein)/h), respectively.
Patient #5, had decompensated ALD, high systemic classical and alternative RAS activation and exhibited pronounced hepatic activity of the classical RAS (ACE activity: 185.0 (pg Ang II/µg protein)/h; chymase activity: 2237.0 (pg Ang II/µg protein)/h). However, although systemic alternative RAS activity was high, hepatic activity of ACE2 (110.0 (pg Ang 1–7/µg protein)/h) and NEP (86.0 (pg Ang 1–7/µg protein)/h) were relatively low.
Discussion
In this study, we assessed the classical and alternative RAS fingerprint in well-characterized patients with ACLD, divided into two cohorts: cACLD versus dACLD. We found that patients with dACLD had pronounced systemic activation of the classical RAS, and activation of the alternative RAS, Ang 1–7 being detectable in 83.3% of patients with dACLD (and in only one patient with cACLD [7.7%]). Importantly, we also demonstrated that, compared to other RAS components, Ang 1–7 showed the strongest correlations with parameters of liver disease severity, portal hypertension, LSM and endothelial dysfunction. Moreover, Ang 1–7 was the only angiotensin that correlated with IL-6 as a marker for systemic inflammation. Finally, suggest that the local activity of the classical and non-classical RAS in the liver is different from systemic RAS activity. Of note, patients with cACLD and patients with clinically significant portal hypertension exhibited the highest hepatic activities of classical and alternative RAS enzymes.
A systemic activation of the classical RAS in patients with dACLD, particularly in patients with ascites is well-documented16–19. In line with this, patients with dACLD in our cohort showed pronounced systemic elevation of classical RAS components including PRA-S, Ang I, Ang II and aldosterone. Higher classical RAS activity in dACLD aggravates hyperdynamic circulation, as it promotes splanchnic vasodilation and higher intrahepatic vascular resistance19. Moreover, ACE inhibition lowers portal pressure in cirrhotic patients20–22.
To date, data on alternative RAS activity in patients with ACLD are still scarce and evidence of alternative RAS activation in liver cirrhosis is mostly based on animal studies19. Our study shows that in the plasma of patients with dACLD, alternative RAS components including Ang 1–7 and Ang 1–5 were higher than in patients with cACLD. Similarly, activation of the alternative RAS was observed in diabetic patients with vascular disease and hypertension, in which ACE2 upregulation was interpreted as an attempt to counteract disease progression23. Whether this is the case in patients with dACLD or whether increased Ang 1–7 and Ang 1–5 are simply byproducts of overall RAS activation cannot be answered definitively with our data and requires further research.
At the same time, Ang III and Ang IV levels did not differ significantly between patients with cACLD and dACLD, but were numerically higher in patients who were decompensated. This result is in line with previous findings of a study displaying activation of the Ang 1–7/Mas receptor axis in 7 patients with liver cirrhosis undergoing liver transplantation24 and another study that showed increased levels of Ang 1–7 in the plasma of 9 cirrhotic patients and 23 non-cirrhotic patients with hepatitis C compared to healthy controls25. Moreover, several animal studies demonstrated systemic elevation of alternative RAS components including Ang 1–7 and ACE2 in chronic liver disease26,27.
Interestingly, previous studies suggest an activation of the systemic alternative RAS already in pre-cirrhotic stages of chronic liver disease, particularly in viral hepatitis25. In contrast, our cohort of patients who had strictly compensated ACLD of majorly viral etiology with mostly subclinical portal hypertension showed virtually no alternative RAS activity in their plasma, with Ang 1–7 being undetectable in 92.3% of patients in this cohort. This may be due to the fact that these were primarily patients after cure of chronic hepatitis C with correspondingly lower levels of (hepatic) inflammation.
Importantly, there were statistical correlations between Ang 1–7 and parameters of liver disease severity (MELD), LSM, portal hypertension (assessed by the gold standard HVPG) and endothelial dysfunction (assessed by its well-established surrogate biomarker VWF antigen28–31), suggesting a link between activation of the alternative RAS and worse outcomes of patients with ACLD32,33. Moreover, unlike other components of the RAS, Ang 1–7 correlated with IL-6, linking alternative RAS activation in ACLD to systemic inflammation1. Of note, systemic Ang II did not correlate with MELD or HVPG, indicating a special status of particularly the systemic alternative RAS in ACLD and portal hypertension. Interestingly, HVPG was the only parameter independently associated with Ang 1–7 in linear regression analysis, linking portal hypertension to alternative RAS activation.
An important role of the local intrahepatic classical RAS has been reported to drive an increase in portal hypertension19,34. Moreover, ACE inhibitors were associated with a decreased risk of liver-related events in non-alcoholic fatty liver disease35. There has been evidence that ACE2 is upregulated in livers of humans and rats24,26,36 with chronic liver disease. Furthermore, NEP was higher in livers of cirrhotic animals37. Our study, examining liver biopsies of 2 individuals with cACLD and 2 with dACLD, demonstrated that hepatic expression of enzymes of the classical and alternative RAS is complex and not limited to patients with dACLD. Despite exhibiting little classical and alternative RAS activity in their plasma, patients with cACLD had the highest hepatic activity of ACE, chymase, NEP and ACE2, suggesting a pronounced activation of intrahepatic classical and alternative RAS already in early stages of ACLD. This finding is of high relevance, as Ang II is known to induce and promote liver fibrosis via contraction, proliferation and activation of HSCs11,38,39 and hepatic chymase has also been linked to liver fibrosis in animals and humans40,41. Moreover, induction of intrahepatic ACE2 activity has been suggested to exert antifibrotic effects42 and NEP seems to be critically involved in the development of portal hypertension37 and kidney function in cirrhotic animal models43. Modifications such as ACE2 activation or Ang 1–7 supplementation seemed to have beneficial effects in murine models44,45.Thus, targeted pharmacological interventions targeting RAS components in cACLD patients may attenuate disease progression.
Our study also has limitations. Firstly, we recruited strictly compensated patients with cACLD, as well as patients with dACLD with a history of at least two decompensation events. Accordingly, this study does not investigate RAS activation patterns in other stages of ACLD. Secondly, this is a pilot study, including only a small number of patients with cACLD and dACLD. This limitation applies for the systemic (plasma) RAS fingerprint results and even more so for the intrahepatic RAS characterization (assessed in only 5 patients). Thus, the limited sample size represents an important limitation of our study and further studies are required to examine the systemic RAS fingerprint throughout all stages of ACLD, as well as the intrahepatic RAS in cACLD and dACLD. Still we want to emphasize that these data are novel and of important value for the liver community. Thirdly, collinearity between dACLD and other parameters, which were correlated with Ang 1–7, cannot be excluded, since these parameters increase with ACLD severity1,46. To account for this, linear regression analysis considering dACLD as a potential confounding factor was conducted. Also, patients with dACLD in our study still had relatively high median MAP, which indicates that patients with hyperdynamic circulation may be underrepresented in our study. Moreover, the majority of patients with dACLD had intake of aldosterone antagonists. This represents a confounding factor regarding the aldosterone levels reported in this study. Finally, plasma aldosterone levels were not available for all patients, but our results are well in line with previous studies18,47.
In conclusion, in this comprehensive characterization of the RAS fingerprint in patients with cACLD and dACLD, we were able to show that in addition to the classical RAS, the alternative RAS is activated in the plasma of those with dACLD. Importantly, Ang 1–7 correlated with parameters of liver disease severity, portal hypertension, endothelial dysfunction and inflammation. Hepatic activation of classical and alternative RAS components is complex and ACE, chymase, ACE2 and NEP are already upregulated in liver tissue of patients with cACLD.
Methods
Study population and sampling
This prospective single center study included patients who underwent hepatic venous pressure gradient (HVPG) measurement at the Vienna Hepatic Hemodynamic Lab (Division of Gastroenterology and Hepatology, Medical University of Vienna) between 06/2017 and 12/2021. ACLD was defined as liver stiffness measurement (LSM) ≥ 10 kPa and/or hepatic venous pressure gradient > 5 mmHg and/or histological fibrosis stage F3/F4. While exclusively strictly compensated ACLD patients in Child-stage A with without intake of diuretics or ACE inhibitors were evaluated for inclusion in the cACLD group, only clinically stable decompensated patients with ascites and another hepatic decompensation event (hepatic encephalopathy, refractory ascites, history of variceal bleeding, history of spontaneous bacterial peritonitis) were considered for the dACLD group. Patients with evidence of infection at the time of HVPG measurement, as well as patients with portal vein thrombosis, TIPS, hepatocellular carcinoma, or liver transplantation were excluded.
We aimed to measure components of both the classical and the alternate RAS from local and systemic sources. Liquid biopsies were collected as EDTA plasma through peripherally drawn venous blood samples. Liver tissue was collected by way of transjugular liver biopsy as previously described48. Both plasma and liver tissue samples were immediately stored at −80 °C.
HVPG measurement
HVPG was measured according to a standardized procedure48. After local anesthesia, a catheter introducer sheath was placed in the right internal jugular vein. Using fluoroscopic guidance, a specifically designed angled-tip balloon catheter49 and was advanced into a large hepatic vein via the inferior vena cava. HVPG was calculated by subtracting free from wedged hepatic venous pressure. For further analyses, the mean of 3 measurements was used. Hemodynamic parameters including mean arterial pressure (MAP) were measured at the beginning of HVPG measurement.
Assessment of liver stiffness measurement, liver function and endothelial dysfunction
Liver stiffness measurement (LSM) was measured via vibration-controlled transient elastography (VCTE) using Fibroscan© (Echosens, Paris, France). Severity of portal hypertension was evaluated by calculating the HVPG during liver vein catheterization. Serum von-Willebrand-factor was measured for evaluation of endothelial dysfunction and as an additional surrogate marker for portal pressure33. Child–Turcotte–Pugh (CTP) and Model for End stage Liver Disease score (MELD) of 201632 were used to assess severity of ACLD.
Laboratory analysis
Measurement of routine laboratory parameters such as interleukin-6 (IL-6) were performed by the facility’s department of laboratory medicine.
For assaying enzyme activities in tissue samples, liver tissue samples were homogenized under liquid nitrogen to enable stabilized peptide extraction and assayed as described previously50. Quantification of equilibrium RAS metabolites Ang I, Ang II, Ang III Ang IV as well as Ang 1–5 and Ang 1–7 in plasma was performed by liquid chromatography-tandem mass spectrometry (Attoquant Diagnostics, Vienna, Austria) using previously established protocols51. Plasma renin activity surrogate (PRA-S), a well-established biomarker for plasma renin activity was calculated as the sum of Ang I and Ang II52,53 and systemic ACE surrogate (ACE-S) was calculated as Ang II divided by Ang I53,54. Plasma aldosterone was assessed by chemiluminsescence immunoassay (DiaSorin, Liaision XL, Saluggia, Italy). Of note, plasma aldosterone levels were not available in all patients, as they were only measured starting 04/2018.
Statistical analysis
Categorical variables were reported as number (n) of patients and % of patients with the characteristic of interest. Continuous data was presented as median and interquartile range (IQR). The data sets were tested for normal distribution with D’Agostino & Pearson and Shapiro–Wilk normality test. Mann–Whitney U test was used for comparing continuous variables without normal distribution between two groups and group comparisons of categorical variables were conducted using Pearson’s Chi-squared test. Correlations between two metric non-normally distributed variables were assessed with Spearman’s Rho. Linear regression analysis was conducted to investigate factors associated with Ang 1–7. Parameters that were at least tendentially associated with Ang 1–7 in univariate analysis (p < 0.100) were included in the multivariate model. GraphPad Prism 8 (Graphpad Software, La Jolla, CA, USA) and IBM SPSS 25.0 statistic software (IBM, Armonk, NY, USA) were used for statistical analysis. Two-sided p-values equal to or below 0.05 were considered as statistically significant.
Ethics
All included patients were part of the Vienna Cirrhosis Study (VICIS; NCT: NCT03267615), a prospective observational trial. This study was performed in accordance with the current version of the Helsinki declaration and approved by the local Ethics Committee of the Medical University of Vienna (No. 1262/2017). All patients gave their informed written consent before study inclusion.
Supplementary Information
Abbreviations
- 95%CI
95% Confidence interval
- Ang
Angiotensin
- ACE
Angiotensin converting enzyme
- ACLD
Advanced chronic liver disease
- ALD
Alcoholic liver disease
- BMI
Body mass index
- cACLD
Compensated advanced chronic liver disease
- CSPH
Clinically significant portal hypertension
- CTP
Child–Turcotte–Pugh
- EC
Ethics committee
- HVPG
Hepatic venous pressure gradient
- HSC
Hepatic stellate cell
- IQR
Interquartile range
- IL-6
Interleukin-6
- INR
International normalized ratio
- LSM
Liver stiffness measurement
- MAP
Mean arterial pressure
- MELD
Model for end-stage liver disease
- n
Number
- NASH
Non-alcoholic steatohepatitis
- NEP
Neprilysin
- PRA-S
Plasma renin activity
- RAS
Renin–aldosterone system
- TIPS
Transjugular intrahepatic portosystemic shunt
- VCTE
Vibration-controlled transient elastography
- VWF
Von Willebrand factor
Author contributions
All authors contributed either to research design (L.H., B.R. R.R.S., M.H and T.R.) and/or the acquisition (L.H., B.R., B.S., D.B., R.P., M.M. and T.R.), analysis (L.H.) or interpretation (all authors) of data. L.H., B.R. and T.R. drafted the manuscript, which was critically revised by all other authors.
Funding
This study was supported by the PhD Martina Hamböck Grant Programme of the Austrian Medical Association (ÖÄK).
Data availability
The data is available upon reasonable request to the corresponding author.
Competing interests
The authors have nothing to disclose regarding the work under consideration for publication. Conflicts of interests outside the submitted work: L.H., B.R. M.J., R.P. have nothing to disclose. O.D. and M.P: are employed at Attoquant Diagnostics. B.Sim. received travel support from AbbVie and Gilead. D.B. received speaker fees from AbbVie and Siemens, as well as grant support form Gilead and Siemens, as well as travel support from AbbVie and Gilead. M.T. served as a speaker and/or consultant and/or advisory board member for Albireo, BiomX, Falk, Boehringer Ingelheim, Bristol-Myers Squibb, Falk, Genfit, Gilead, Intercept, Janssen, MSD, Novartis, Phenex, Pliant, Regulus, and Shire, and received travel support from AbbVie, Falk, Gilead, and Intercept, as well as grants/research support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, and UltraGenyx. He is also co-inventor of patents on the medical use of 24-norursodeoxycholic acid. M.M. served as a speaker and/or consultant and/or advisory board member for AbbVie, Gilead, and W. L. Gore & Associates and received travel support from AbbVie and Gilead. M.H. served as a speaker and/or consultant for Astellas Pharma, AstraZeneca, Eli Lilly, Fresenius Medical Care, Janssen-Cilag, Siemens Healthcare and Vifor and received academic study support from Astellas Pharma, Boehringer Ingelheim, Eli Lilly, Nikkiso and Siemens Healthcare. T.R. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bayer, Boehringer Ingelheim, Gilead, Intercept, MSD, Siemens, and W. L. Gore & Associates and received grants/research support from AbbVie, Boehringer Ingelheim, Gilead, Intercept, MSD, Myr Pharmaceuticals, Pliant, Philips, Siemens, and W. L. Gore & Associates as well as travel support from Abbvie, Boehringer Ingelheim, Gilead and Roche.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lukas Hartl and Benedikt Rumpf.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-28239-2.
References
- 1.Costa D, et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J. Hepatol. 2021;74:819–828. doi: 10.1016/j.jhep.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Königshofer P, et al. Distinct structural and dynamic components of portal hypertension in different animal models and human liver disease etiologies. Hepatology. 2022;75:610–622. doi: 10.1002/hep.32220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J. Hepatol. 2017;66:849–859. doi: 10.1016/j.jhep.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Kalambokis GN, Baltayannis G, Christodoulou D, Christou L. Beta adrenergic blockade and advanced cirrhosis: Does it really improve survival in patients with acute-on-chronic liver failure? J. Hepatol. 2017;67:878–880. doi: 10.1016/j.jhep.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Fidelman N, et al. The transjugular intrahepatic portosystemic shunt: An update. Am. J. Roentgenol. 2012;199:746–755. doi: 10.2214/AJR.12.9101. [DOI] [PubMed] [Google Scholar]
- 6.Dell’Italia LJ, Collawn JF, Ferrario CM. Multifunctional role of chymase in acute and chronic tissue injury and remodeling. Circ. Res. 2018;122:319–336. doi: 10.1161/CIRCRESAHA.117.310978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BMW. Renin–angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 8.Paul, M., Poyan Mehr, A. & Kreutz, R. Physiology of local renin–angiotensin systems. Physiol. Rev.86, 747–803. 10.1152/physrev.00036.2005 (2006). [DOI] [PubMed]
- 9.Kamo T, Akazawa H, Komuro I. Pleiotropic effects of angiotensin II receptor signaling in cardiovascular homeostasis and aging. Int. Heart J. 2015;56:249–254. doi: 10.1536/ihj.14-429. [DOI] [PubMed] [Google Scholar]
- 10.Grace JA, Herath CB, Mak KY, Burrell LM, Angus PW. Update on new aspects of the renin-angiotensin system in liver disease: Clinical implications and new therapeutic options. Clin. Sci. (Lond.) 2012;123:225–239. doi: 10.1042/cs20120030. [DOI] [PubMed] [Google Scholar]
- 11.Bataller R, et al. Activated human hepatic stellate cells express the renin–angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117–125. doi: 10.1016/S0016-5085(03)00695-4. [DOI] [PubMed] [Google Scholar]
- 12.Herath, C. B. et al. Role of the alternate RAS in liver disease and the GI tract. in The Protective Arm of the Renin Angiotensin System (RAS). 239–247 (Elsevier, 2015).
- 13.Warner, F.J., Lubel, J.S., McCaughan, G.W. & Angus, P.W. Liver fibrosis: A balance of ACEs? Clin. Sci.113, 109–118. 10.1042/cs20070026 (2007). [DOI] [PubMed]
- 14.Cao X, et al. Angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis. Sci. Rep. 2016;6:21592. doi: 10.1038/srep21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osterreicher CH, et al. Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology. 2009;50:929–938. doi: 10.1002/hep.23104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartl L, et al. The differential activation of cardiovascular hormones across distinct stages of portal hypertension predicts clinical outcomes. Hepatol. Int. 2021 doi: 10.1007/s12072-021-10203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paternostro R, et al. Plasma renin concentration represents an independent risk factor for mortality and is associated with liver dysfunction in patients with cirrhosis. J. Gastroenterol. Hepatol. 2017;32:184–190. doi: 10.1111/jgh.13439. [DOI] [PubMed] [Google Scholar]
- 18.Bosch J, et al. Hepatic hemodynamics and the renin–angiotensin–aldosterone system in cirrhosis. Gastroenterology. 1980;78:92–99. doi: 10.1016/0016-5085(80)90197-3. [DOI] [PubMed] [Google Scholar]
- 19.Sansoè G, Aragno M, Wong F. Pathways of hepatic and renal damage through non-classical activation of the renin-angiotensin system in chronic liver disease. Liver Int. 2020;40:18–31. doi: 10.1111/liv.14272. [DOI] [PubMed] [Google Scholar]
- 20.Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J. Renin–angiotensin–aldosterone inhibitors in the reduction of portal pressure: A systematic review and meta-analysis. J. Hepatol. 2010;53:273–282. doi: 10.1016/j.jhep.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Schepke M, et al. Hemodynamic effects of the angiotensin II receptor antagonist irbesartan in patients with cirrhosis and portal hypertension. Gastroenterology. 2001;121:389–395. doi: 10.1053/gast.2001.26295. [DOI] [PubMed] [Google Scholar]
- 22.Schepke M, et al. Irbesartan plus low-dose propranolol versus low-dose propranolol alone in cirrhosis: a placebo-controlled, double-blind study. Am. J. Gastroenterol. 2008;103:1152–1158. doi: 10.1111/j.1572-0241.2007.01759.x. [DOI] [PubMed] [Google Scholar]
- 23.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/angiotensin 1–7 axis of the renin–angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/circresaha.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grace JA, et al. Activation of the MAS receptor by angiotensin-(1–7) in the renin–angiotensin system mediates mesenteric vasodilatation in cirrhosis. Gastroenterology. 2013;145:874–884.e875. doi: 10.1053/j.gastro.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 25.Lubel JS, et al. Angiotensin-(1–7), an alternative metabolite of the renin–angiotensin system, is up-regulated in human liver disease and has antifibrotic activity in the bile-duct-ligated rat. Clin. Sci. (Lond.) 2009;117:375–386. doi: 10.1042/cs20080647. [DOI] [PubMed] [Google Scholar]
- 26.Paizis G, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herath CB, et al. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1–7) levels in experimental biliary fibrosis. J. Hepatol. 2007;47:387–395. doi: 10.1016/j.jhep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandorfer M, et al. Von Willebrand factor indicates bacterial translocation, inflammation, and procoagulant imbalance and predicts complications independently of portal hypertension severity. Aliment Pharmacol. Ther. 2018;47:980–988. doi: 10.1111/apt.14522. [DOI] [PubMed] [Google Scholar]
- 29.Jachs M, et al. Decreasing von Willebrand factor levels upon nonselective beta blocker therapy indicate a decreased risk of further decompensation, acute-on-chronic liver failure, and death. Clin. Gastroenterol. Hepatol. 2021 doi: 10.1016/j.cgh.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Ferlitsch M, et al. von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology. 2012;56:1439–1447. doi: 10.1002/hep.25806. [DOI] [PubMed] [Google Scholar]
- 31.Hartl, L. et al. Cirrhosis-associated RAS-inflammation–coagulation axis anomalies: Parallels to severe COVID-19. J. Pers. Med. 10.3390/jpm11121264 (2021). [DOI] [PMC free article] [PubMed]
- 32.Alcorn, J. B. United Organ Sharing Network. Important Policy Note (2015).
- 33.Györi GP, et al. The von Willebrand factor facilitates model for end-stage liver disease-independent risk stratification on the waiting list for liver transplantation. Hepatology. 2020;72:584–594. doi: 10.1002/hep.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bataller R, Sancho-Bru P, Gines P, Brenner DA. Liver fibrogenesis: A new role for the renin-angiotensin system. Antioxid. Redox Signal. 2005;7:1346–1355. doi: 10.1089/ars.2005.7.1346. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology. 2022;76:469–482. doi: 10.1002/hep.32294. [DOI] [PubMed] [Google Scholar]
- 36.Paizis G, et al. Up-regulation of components of the renin–angiotensin system in the bile duct-ligated rat liver. Gastroenterology. 2002;123:1667–1676. doi: 10.1053/gast.2002.36561. [DOI] [PubMed] [Google Scholar]
- 37.Sansoè, G. et al. Neutral endopeptidase (EC 3.4.24.11) in cirrhotic liver: A new target to treat portal hypertension? J. Hepatol.43, 791–798. 10.1016/j.jhep.2005.04.017 (2005). [DOI] [PubMed]
- 38.Bataller R, Brenner DA. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/jci24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bataller R, et al. Angiotensin II induces contraction and proliferation of human hepatic stellate cells. Gastroenterology. 2000;118:1149–1156. doi: 10.1016/s0016-5085(00)70368-4. [DOI] [PubMed] [Google Scholar]
- 40.Komeda K, et al. Chymase inhibition attenuates tetrachloride-induced liver fibrosis in hamsters. Hepatol. Res. 2010;40:832–840. doi: 10.1111/j.1872-034X.2010.00672.x. [DOI] [PubMed] [Google Scholar]
- 41.Sansoè, G. et al. Role of chymase in the development of liver cirrhosis and its complications: Experimental and human data. PLoS One11, e0162644. 10.1371/journal.pone.0162644 (2016). [DOI] [PMC free article] [PubMed]
- 42.Rajapaksha IG, et al. The small molecule drug diminazene aceturate inhibits liver injury and biliary fibrosis in mice. Sci. Rep. 2018;8:10175. doi: 10.1038/s41598-018-28490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sansoè, G. et al. Overexpression of kidney neutral endopeptidase (EC 3.4.24.11) and renal function in experimental cirrhosis. Am. J. Physiol. Renal Physiol.290, F1337–F1343. 10.1152/ajprenal.00435.2005 (2006). [DOI] [PubMed]
- 44.Mori J, et al. Angiotensin 1–7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ. Heart Fail. 2014;7:327–339. doi: 10.1161/circheartfailure.113.000672. [DOI] [PubMed] [Google Scholar]
- 45.Murça TM, et al. Oral administration of an angiotensin-converting enzyme 2 activator ameliorates diabetes-induced cardiac dysfunction. Regul. Pept. 2012;177:107–115. doi: 10.1016/j.regpep.2012.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pomej K, et al. Clinical significance of substantially elevated von Willebrand factor antigen levels in patients with advanced chronic liver disease. Dig. Liver Dis. 2022 doi: 10.1016/j.dld.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Bernardi M, Trevisani F, Santini C, De Palma R, Gasbarrini G. Aldosterone related blood volume expansion in cirrhosis before and during the early phase of ascites formation. Gut. 1983;24:761–766. doi: 10.1136/gut.24.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiberger T, Schwabl P, Trauner M, Peck-Radosavljevic M, Mandorfer M. Measurement of the hepatic venous pressure gradient and transjugular liver biopsy. J. Vis. Exp. 2020 doi: 10.3791/58819. [DOI] [PubMed] [Google Scholar]
- 49.Ferlitsch A, et al. Evaluation of a new balloon occlusion catheter specifically designed for measurement of hepatic venous pressure gradient. Liver Int. 2015;35:2115–2120. doi: 10.1111/liv.12783. [DOI] [PubMed] [Google Scholar]
- 50.Kaltenecker CC, et al. Critical role of neprilysin in kidney angiotensin metabolism. Circ. Res. 2020;127:593–606. doi: 10.1161/circresaha.119.316151. [DOI] [PubMed] [Google Scholar]
- 51.Urwyler SA, et al. IL (interleukin)-1 receptor antagonist increases ang (angiotensin [1–7]) and decreases blood pressure in obese individuals. Hypertension. 2020;75:1455–1463. doi: 10.1161/HYPERTENSIONAHA.119.13982. [DOI] [PubMed] [Google Scholar]
- 52.Zoufaly A, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burrello J, et al. Renin–angiotensin–aldosterone system triple-A analysis for the screening of primary aldosteronism. Hypertension. 2020;75:163–172. doi: 10.1161/hypertensionaha.119.13772. [DOI] [PubMed] [Google Scholar]
- 54.Pavo N, et al. Low- and high-renin heart failure phenotypes with clinical implications. Clin. Chem. 2018;64:597–608. doi: 10.1373/clinchem.2017.278705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available upon reasonable request to the corresponding author.