Abstract
Metal oxide glasses are important in various industries because their properties can be tailored to meet application-specific requirements. However, there are few rigorous modeling tools for predicting thermomechanical properties of these materials with acceptable accuracy and speed, yet these properties can play a critical role in material design. In this article, a general multi-scale modeling framework based on Monte Carlo simulation and a cubic equation of state for predicting thermomechanical properties is presented. There are two novel and fundamental aspects of this work: (1) characterization of glass transition and softening temperatures as adjacent saddle points on the heat capacity versus temperature curve, and (2) a new moving boundary equation of state that accounts for structure and ‘soft’ repulsion. In addition, modeling capabilities are demonstrated by comparing thermomechanical properties of a pure B2O3 glass and PbO–B2O3 glass predicted by the equation of state to experimental data. Finally, this work provides a rigorous approach to estimating thermophysical properties for the purpose of guiding experimental work directed at tailoring thermomechanical properties of glasses to fit applications.
Subject terms: Engineering, Materials science, Mathematics and computing
Introduction
Metal oxide glasses (or glass–ceramics) are important industrial materials that exhibit a unique set of thermal, mechanical, and optical properties. They can also provide excellent chemical resistance and are used in a wide range of applications that include adding color, iridescence, and luster to glass, in semi-conductor materials processing, for thermal imaging as thermal paints, optical communication, chemical sensors, hermetic glass-metal seals in biomedical devices, in thermal barrier coatings for ceramic matrix composites and C–C composites for hypersonics, and for immobilizing nuclear waste to name a few. For example, boron trioxide, B2O3, has low glass transition and melting temperatures, a small coefficient of thermal expansion, and is used in many glass formulations (e.g., in borosilicate glasses) to improve hardness and lower water absorption. Also, lead monoxide, PbO, is a common additive to glass that has two stable crystal polymorphs—litharge, which is yellow, is stable at low temperatures, and has a tetragonal structure, and massicot, which is red in color, has an orthorhombic structure, and is stable at higher temperatures (i.e., at and above the transition temperature of 762 K). Metal oxide glasses, like PbO–B2O3, are often used to immobilize nuclear waste and while PbO does not form a glass by itself, the utility of lead monoxide is that when added to glass formers like boron trioxide, the properties of the resulting metal oxide glass can be tailored by adjusting composition to meet application requirements. For example, the relative compositions of PbO and B2O3 can be tailored to provide maximum loading of nuclear waste. However, significant structural changes from planar BO3 triangles to BO4 tetrahedra occur as a function of PbO content above 20 mol% and these structural changes must be considered in order to determine accurate thermophysical properties.
While detailed understanding of the impact of adding metal oxides to glass formers is still an open debate (i.e., whether they function as modifiers or network formers; see1, nonetheless they are very useful in tailoring the properties of the glass by varying the composition of metal oxide/glass mixture to meet specific application requirements.
Creating glass formulations experimentally is a time consuming and expensive task. On the other hand, a robust, general-purpose modeling framework for predicting thermomechanical properties of these materials would be valuable in guiding the experimental design of metal oxide/glass formulations. However, glasses like silicon dioxide (SiO2), boron trioxide (B2O3), germanium oxide (GeO2) as well as mixture of these glasses with metal oxide additives such as PbO, BaO, Al2O3, CaO, etc. are among the most difficult amorphous materials to model. There are several reasons for this:
Pure glasses and metal oxide glasses frequently exhibit unusual pVT behavior such as maxima in density and coefficient of thermal expansion2, negative thermal expansion3,4, and minima in Young’s and shear moduli2.
In certain applications, thermal processing of glass over extended temperature ranges and large temperature gradients is necessary, and this places additional demands on modeling glassy materials (e.g., in hypersonic applications where glassy coatings are used as a thermal barrier to protect SiC and C–C composites. Here temperatures can range from ambient 2000 °C and temperature gradients can exist within the aeroshell). These large temperature ranges and gradients can result in changes in glass–ceramic structure and large changes in properties, which present challenges at all modeling length scales.
The choice of force fields used in modeling glass and metal oxide glass at the molecular length scale can give poor matches to experimental data because the force field terms do not capture the correct physics and/or the force field parameters are inadequate over the temperature range of interest.
There are very few rigorous computational modeling tools capable of predicting important thermomechanical properties of these materials (e.g., density, glass transition temperature, , softening temperature, , coefficient of thermal expansion, , isothermal compressibility, , bulk modulus, , elastic modulus, , enthalpy, , and heat capacity, ) with acceptable accuracy and speed. Quantitative values of these properties can play a critical role in material design. For example, matching the coefficients of thermal expansion of substrates and laminates is important in order to avoid delamination on heating. Also, softening temperature is a key design parameter in formulating thermal paints and thermal barrier coatings, which are frequently used in severe, high- temperature environments.
There are several different types of models for glasses and metal oxide glasses: empirical methods, molecular models, and equations of state. While there are several empirical equations of state (EOS) for polymers [e.g., the Hartmann and Hague equation5, the double domain Tait (DDT) equation6, and the two-domain Schmidt equation7, there are far fewer EOS for glasses8–11. With the exception of the two-state model of Macedo et al.10, most of these EOS are adapted from solid models, relate pressure to volume at constant temperature to quantify shock compression, require many parameters that must be fit to experimental data, and generally provide poor fits to specific volume as a function of temperature. There are also other empirical equations like the method of Gordon and Taylor12 and the Fox equation13 that can be used to determine glass transition temperature, but these methods do not provide estimates for other thermomechanical properties.
Relevant potential energy (PE) models for glasses and glassy metal oxides generally include electrostatic forces (e.g., Ewald summations) but differ in the way in which dispersion terms are included. Bowron14, Bent et al.15, Hannon et al.16, Hannon et al.17, Hannon and Parker18, and Hannon et al.19 have used the Lennard–Jones 6–12 potential for dispersion terms to model amorphous glass materials such as SiO2, GeO2, (Cs2O)0.25(GeO2)0.75, (Cao)0.70(Al2O3)0.30, and (PbO)0.67(Ga2O3)0.33. Kuzuu et al.20 have used four PE models that include 2- and 3-body terms by combining Morse and Born–Mayer–Huggins (BMH) potentials as well as screened/full electrostatic terms to study anomalous behavior of vitreous SiO2. Huang and Kieffer21 have developed and used their charge transfer 3-body potential to study thermomechanical properties and densification of vitreous silica and boron trioxide4. Soper22 developed a method called empirical potential for structure refinement (EPSR) for glassy material and applied it to vitreous boron trioxide23. EPSR consists of dispersion forces modeled using the Lennard–Jones 6–12 potential, Coulomb forces computed using Ewald summation, and an additional repulsive exponential potential to prevent atomic overlap. Finally, there is the family of Betrani–Menziani–Pedone24 empirical PE models for multicomponent oxide glasses and crystals, which use a Morse potential, a repulsive term, electrostatic forces, a Buckingham repulsive term for network former-network former interactions, and a simple/screened harmonic approximation to 3-body interactions.
While EOS such as the Flory–Orwoll–Vrije (FOV) equation25,26, the Sanchez–Lacombe (SL) equation27,28, variants of the statistical associating fluid theory (SAFT) of Chapman et al.29, the cubic plus association (CPA) equation30, and the multiscale Gibbs–Helmholtz Constrained (GHC) equation31–33 could, in principle, be used, these rigorous equations of state have not been applied directly to glasses and metal oxide glasses.
Lucia et al.31 used the Soave modification of the Redlich–Kwong equation
| 1 |
and the Gibbs–Helmholtz equation to constrain the energy (or attraction) parameter, , given by
| 2 |
to develop the multi-scale Gibbs–Helmholtz Constrained (GHC) equation of state, where is pressure, is temperature in Kelvins, is the molar volume, is the universal gas constant, is the internal energy of departure, is the molecular co-volume parameter, and and are critical temperature and critical pressure, respectively. Given Eqs. (1) and (2) the molar volume and all pVT-related properties (e.g., coefficient of thermal expansion, isothermal compressibility, bulk modulus, etc.) can be computed.
The key attributes of the multi-scale GHC equation are as follows:
It uses the Gibbs–Helmholtz equation to constrain the energy parameter.
The boundary condition for the energy parameter used to derive Eq. (1) is defined in the conventional way—by setting the first and second derivatives, ( and , on the critical isotherm equal to zero. This results in a fixed boundary condition for the energy parameter given by , which is not applicable to glasses and metal oxide glasses because many of these materials decompose before ever reaching a critical point.
Monte Carlo simulations are used to gather only pure component molecular length scale information (i.e., internal energies of departure, ) over ranges of temperature and pressure and are placed in lookup tables for use in bulk phase modeling and simulation.
Monte Carlo simulations need only be run once because lookup tables can be used over and over again. Thus, the GHC framework is computationally efficient.
Mixing rules are used to predict properties of mixtures.
The parameters, and , are never regressed to experimental data. Experimental data is only used to validate predictions by the GHC equation.
Equation (1) has also been used for vapors, hexagonal ice, aqueous electrolytes, and gas hydrates.
The objective of this article is to develop a thermodynamically rigorous modeling framework that addresses the open modeling challenges associated with the design of glassy materials. A general multi-scale equation of state framework for modeling and simulating glassy materials is presented that includes the use of Monte Carlo simulation to gather molecular length scale information and a novel moving boundary equation of state for calculating bulk thermomechanical properties of pure glasses and glassy mixtures. The numerical methodology and simulation results for a pure glass and binary glass mixture are compared to experimental data taken from the open literature where available. This is followed by a discussion of results and the conclusions of this work.
Materials and methods
Modeling glasses and their properties with an equation of state
Unfortunately, Eq. (1) breaks down for glassy materials (e.g., asphaltenes, ionic liquids, polymers, pure glass, metal oxide glasses, etc.) because these materials decompose prior to reaching a critical point. This, in turn, makes the boundary condition for the energy parameter in Eq. (1) undefined. To circumvent this Lucia and Gow32,33 modified the expression for the energy parameter for pure glasses as follows:
| 3 |
where the quantity in Eq. (3) denotes the boundary condition for the energy parameter for pure glassy materials. Lucia and Gow32 proposed and used an iterative numerical procedure to estimate that minimizes the error between from Monte Carlo simulation and calculated from fundamental thermodynamics. However, this numerical procedure is computationally expensive and does not consider ‘soft’ repulsive forces34. Therefore, in this work, we propose the following expression for the boundary condition for the energy parameter for glassy materials
| 4 |
Note that Eq. (4) has the following properties:
It is a closed form, temperature-dependent expression for the attraction parameter boundary condition, making it a moving boundary condition.
The first term on the right, , is fixed and replaces the computationally expensive numerical procedure described in Lucia and Gow32.
The second term on the right, , is an ideal gas-like contribution that increases the boundary condition as temperature increases.
The last term, is an entropic contribution to attraction due to soft repulsion, where and decreases the boundary condition as temperature increases.
Regardless of the way in which the boundary condition, , is computed, the energy parameter, , satisfies the Gibbs–Helmholtz equation.
Substitution of Eq. (4a) in Eq. (3) gives the following new expression for the energy parameter
| 5 |
Equation (5) is a closed form and computationally efficient expression for the attraction parameter that accounts for soft repulsive forces through the inclusion of internal energy and entropy of departure terms.
For mixtures, the molecular co-volume parameter, , is computed using
| 6 |
where denotes the mixture property, denotes a mole fraction, the subscript denotes pure component , and is the number of components in the mixture. Internal energies of departure for mixtures are determined using the linear mixing rule
| 7 |
The moving boundary condition for mixtures, , and the temperature dependent attraction parameter, are computed using one fluid theory (i.e., by replacing with , with , and with in Eqs. 4 and 5), which gives the following moving boundary condition for :
| 8 |
and the following expression for :
| 9 |
Molecular length scale information from Monte Carlo simulation
Accurate molecular length scale information is extremely important in building a robust bulk phase model for predicting thermomechanical properties. For the multiscale GHC equation, this means accurate pure component internal energies of departure, , are required. As noted earlier, this information is gathered a priori using Monte Carlo simulations that are run over ranges of temperature and pressure relevant to the application. Results of the Monte Carlo simulations are then placed in pure component lookup tables and used in bulk phase property determinations. In the multiscale GHC framework, pure glass and pure metal oxide properties tend to be very sensitive to values of internal energies of departure. This suggests that large numbers of Monte Carlo cycles and many sets are required to estimate values of .
Force field models
Force field models play a critical role in computing accurate molecular length scale information and many force fields (potentials) have been used for glasses. Table 1 gives a summary of some of the force field models that have been used to model thermomechanical properties for pure glasses and metal oxide glass mixtures using molecular dynamics and Monte Carlo simulation. As can be seen from Table 1, much of the modeling work in the open literature has focused on silica. However, there is no general agreement on the force field used to model glasses or metal oxide glasses.
Table 1.
Force fields/potentials that have been used to model various pure glasses and metal oxides.
| Glass | Phase | Force field or potential | References |
|---|---|---|---|
| SiO2 | Liquid | van Best–Kramer–van Santan (BKS) | Shell et al.3 |
| Vitreous | Bohr–Mayer–Huggins (BMH) + Morse potential | Kuuzu et al.20 | |
| Vitreous | Charge-transfer three-body potential | Huang and Kieffer4 | |
| Vitreous | Lennard–Jones + electrostatic forces | Chu-Cruz et al.35 | |
| Vitreous | Lennard–Jones + electrostatic forces | Bowron14 | |
| Vitreous | Ab initio-derived Lennard–Jones + electrostatic | Butenuth et al.36 | |
| Vitreous | Lennard–Jones + electrostatic forces | Anagnostopoulos et al.37 | |
| B2O3 | Vitreous | Coordination-dependent charge transfer potential | Huang et al.21 |
| Vitreous | Lennard–Jones + electrostatic forces + repulsion | Soper23 | |
| Crystal | Many-body quantum Monte Carlo | Ferlat et al.38 | |
| GeO2 | Vitreous | Lennard–Jones + electrostatic forces | Hannon et al.16 |
| MO | |||
| PbO | In glass | Lennard–Jones + electrostatic forces | Hannon et al.17 |
| Liquid | Lennard–Jones | Arkundato et al.39 | |
| BaO | Solid | Lennard–Jones | Xiaoping40 |
| Al2O3 | Liquid | Lennard–Jones + electrostatic forces | Soper41 |
| In glass | Lennard–Jones + electrostatic forces | Hannon et al.19 | |
| CaO | In glass | Lennard–Jones + electrostatic forces | Hannon and Parker18 |
MO Metal oxide.
In this work, we have chosen to use Lennard–Jones plus electrostatic forces to model the internal energy of departure
| 10 |
where and are the well depth of the potential function and zero potential distance parameters respectively, denotes a partial charge, is the permittivity, is the distance between particles (i.e., atoms), and are atom indices, and is the total number of atoms in a molecule.
Force field parameters
Finding or determining reliable values of the parameters, , , and , for glasses and metal oxides can, at times, be quite challenging. Many of the parameters published in the literature [e.g., Si and O parameters in Cruz-Chu et al.35, Butenuth et al.36, Emani et al.42, Anagnostopolous et al.37] give values of and bulk phase properties that vary all over the map.
Numerical methodology
Before presenting simulation results it is instructive to describe the numerical methodology used in determining thermophysical properties of glass and metal oxide glass, which determines the glass transition and softening temperatures prior to estimating all other desired thermophysical properties. Motivation for the methodology to follow is illustrated using boron trioxide as an example. The zero potential Lennard–Jones parameters, , were taken from Tabrizi et al.43 and the well depth parameters, , were taken from Soper23 and adjusted to remove the EPSR repulsive term. Partial charges were selected to enforce electroneutrality. The B2O3 force field parameters are shown in Table 2.
Table 2.
Force field parameters for boron trioxide.
| Atom | (K) | ( | q (eV) |
|---|---|---|---|
| B | 47.3029 | 3.45 | + 0.6 |
| O | 50.32 | 3.47 | − 0.4 |
NVT Monte Carlo simulations with N = 320 atoms at 1 bar pressure were run to gather molecular information. Smaller ensembles will give inaccurate molecular information at higher temperatures.
Heat capacity
Figure 1 shows a comparison of the Monte Carlo simulation predictions of specific heat capacity, , vs. temperature for boron trioxide with experimental data from D’Angelo and Carini44, where the predicted heat capacity at constant volume was computed using the fundamental expression . For B2O3 glass, , the number of atoms/molecule, is five and the degrees of freedom, , for a polyatomic, nonlinear ideal gas varies from 6 at low temperature to at high temperature. Therefore, and comes from the Monte Carlo simulations. However, the results of the Monte Carlo simulations over-predict the maximum heat capacity (i.e., 2.3 J/g K vs. 2 J/g K) and slightly under-predict the heat capacity (i.e., 1.59 vs. 1.75 J/g K) over the range [600–850 K].
Figure 1.

Heat capacity, , vs. temperature for boron trioxide at 1 bar (a) Predicted by Monte Carlo simulation. (b) Inset: Experimental Cp Data Taken from D’Angelo and Carini44.
Glass transition and softening temperatures
One of the novel aspects of this work is the conjecture that glass transition and softening temperatures can be estimated using adjacent inflection points on each side of the maximum in . Using the numerical procedure described in the Supplemental Material, adjacent saddle points on each side of the maximum in give values of and .
Volume (or density)
Molar volume is computed directly by solving Eq. (1) with the energy parameter determined by Eq. (5).
Volumetric coefficient of thermal expansion
Volume versus temperature results from the equation of state computations can be fit and then differentiated to give approximations of so that a prediction of can be computed from the equation
| 11 |
Fitting of vs. is recommended is because involves the attraction parameter derivative, (. This coupled with the fact that derivatives like from the Monte Carlo simulation can be inaccurate and sometimes lead to errors in the derivatives and needed to determine .
Pseudo-algorithm
A general pseudo-algorithm for computing glass transition and softening temperatures along with other thermophysical properties is as follows:
Compute all pure component molecular properties (i.e., , etc.) for the glass under consideration using Monte Carlo simulations over ranges of temperatures and pressures of interest, considering any structural changes that can occur. Typically, four sets of all-atom Monte Carlo simulations with periodic boundary conditions and 100,000 equilibration and 100,000 production cycles are run for each temperature and pressure and averaged. Store the molecular information in pure component lookup tables. Compute the mixture internal energies of departure using Eq. (7).
Using internal energies of departure and degrees of freedom compute the total internal energy, , and heat capacity at constant volume, , over the desired range of temperature where , , and .
Compute the global maximum of and saddle points on each side of the global maximum using the procedure described in the Supplementary Material. The saddle points on each side of the maximum give predicted values of and , where .
Given , compute the boundary condition for the glass using Eq. (4) (or Eq. 8 for mixtures).
Using the multiscale GHC equation of state, calculate the molar volume and all desired volume-dependent properties such as volumetric thermal expansion coefficient, isothermal compressibility, bulk modulus, elastic modulus, etc. over the temperature and pressure ranges of interest.
Steps 1 is a critical step of the modeling since it is well known that glasses can exhibit temperature-dependent changes in structure. For example, it is well known that boron trioxide is comprised of two types of elementary building blocks—BO3 units and boroxol rings (B3O6) and that the fraction of boroxol rings decreases with increasing temperature (i.e., from 0.6 at 500 K in the glassy region to 0.2 at 1900 K in the liquid state). See Fig. 2 and Ferlat38.
Figure 2.
Different structural units of boron trioxide. (a) BO3 triangles, (b) Boroxol rings.
However, it is possible to incorporate structure in the Monte Carlo simulations by using an initial structure in the simulation box that reflects structure. In the case of B2O3 that would mean initializing the atoms in the simulation box to reflect the ratios of the elementary building blocks BO3 to B3O6.
Step 2 and 3 provide a way of directly calculating glass transition and softening temperatures for pure glasses or metal oxide glasses and set the stage for subsequently computing volume and all volume-dependent thermophysical properties in steps 4 and 5. However, it is important to understand that accurate Monte Carlo simulations must be performed in step 1 to get reliable estimates of and the functionality, particularly at high temperature. This means that several sets of Monte Carlo simulations with a large number of equilibration and production cycles and large ensemble are required.
Results
To demonstrate the capabilities of the proposed modeling framework, multiscale simulation results for thermomechanical properties of a pure glass and a metal oxide glass mixture are presented and compared to the experimental data in the open literature. All computations were performed on a Dell Vostro 5500 laptop with the Lahey–Fijitsu (LF) 95 compiler using double precision arithmetic.
Pure glass formers
All-atom boron trioxide NVT Monte Carlo simulations were conducted with N = 320 atoms. Four sets of 100,000 equilibration cycles and 100,000 production cycles each were run for each temperature in the range [200, 1600 K] in 50 K intervals. Results for four sets for each temperature were averaged to provide molecular length scale information.
Heat capacity
The heat capacity versus temperature curve shown in Fig. 1a shows that the general trend and magnitude of calculated in this work match the experimental from D’Angelo and Carini44 quite well.However, the results of the Monte Carlo simulations over-predict the maximum heat capacity (i.e., 2.3 J/g K vs. 2 J/g K), slightly under-predict the heat capacity (i.e., 1.59 vs. 1.75 J/g K) over the range [600–850 K] and show a peak slightly to the right of the peak given in the experimental data. Remember, the computed results are truly predictions with no regression to any experimental data. Also, note that there is no clear melting point in Fig. 1a or b because the material is glass. This suggests that the force field model for B2O3 consisting of Lennard–Jones + electrostatic forces together with Monte Carlo simulation is capable of predicting the heat capacity of boron trioxide with reasonably good accuracy. In addition, it is important to note that the comparison of calculated values of in Fig. 1a with experimental values of shown in the Fig. 1b is valid because (a) , (b) the term is small, (c) the pressure is low (i.e., 1 bar), and (d) the glass phase is a condensed phase. Thus, is a good approximation, which implies .
Glass transition temperature
The saddle point (inflection point) to the left of the maximum in shown in Fig. 1a gives a predicted glass transition temperature, Tg, of 535.26 K and falls within the range of reported experimental values in the literature [510, 580 K]. More specifically, the experimental value reported by D’Angelo and Carini44 is 560 K while other literature values for the glass transition temperature for pure B2O3 range from 510 K in Ref.45 to 571 K in Ref.46 to as high as 580 K in Ref.47.
Softening temperature
Softening temperature is generally described as a transport property and characterized as the temperature at which the viscosity, η, of the glass is poise (log10 (η) = 7.6). Experimental viscosity data given in Ref.48 in Table II, p. 616 shows that log10 (η) of B2O3 has a value of 7.6 at 375 °C (648 K). Like many properties of boron trioxide, there is limited experimental information for softening temperature and in the few articles containing data48,53, the reported results vary widely. Nevertheless, the moving boundary equation of state prediction of softening temperature in Fig. 1a is , which is in good agreement with the viscosity-based results reported in Table II on p. 616 in Ref.48 of 375 °C (648 K)—less than 2% error. However, the estimated softening temperature of B2O3 reported on p. 379 in Ref.53 is much lower—315 °C (588 K) and, in our opinion, is much closer to the glass transition temperature.
Volume predictions
Table 3 illustrates the volume predictions of the multiscale GHC equation in the glassy region with and without soft repulsive effects.
Table 3.
Volumes of B2O3 glass calculated with and without soft repulsion effects in .
| T (K) | Volumea (g/cc) | ||
|---|---|---|---|
| Exp datab | |||
| 301 | 0.5297 | 0.5367 | 0.5474 |
| 360 | 0.5347 | 0.5411 | 0.5495 |
| 390 | 0.5376 | 0.5435 | 0.5580 |
| 505 | 0.5518 | 0.5535 | 0.5598 |
| 510 | 0.5526 | 0.5540 | 0.5557 |
| 530 | 0.5557 | 0.5560 | 0.5570 |
| 561 | 0.5603 | 0.5621 | 0.5597 |
| 566 | 0.5611 | 0.5632 | 0.5626 |
| 581 | 0.5634 | 0.5667 | 0.5657 |
| 593 | 0.5652 | 0.5697 | 0.5702 |
| 594 | 0.5654 | 0.5699 | 0.5710 |
| 623 | 0.5699 | 0.5771 | 0.5777 |
| 628 | 0.5706 | 0.5783 | 0.5834 |
| 673 | 0.5777 | 0.5900 | 0.5887 |
| 723 | 0.5861 | 0.5940 | 0.5984 |
| AAD%c | 1.53 | 0.78 | |
a K; cm3/mol; -1.1975× cm6bar/mol2; K.
bExperimental data from Macedo et al.49.
c, where denotes volume.
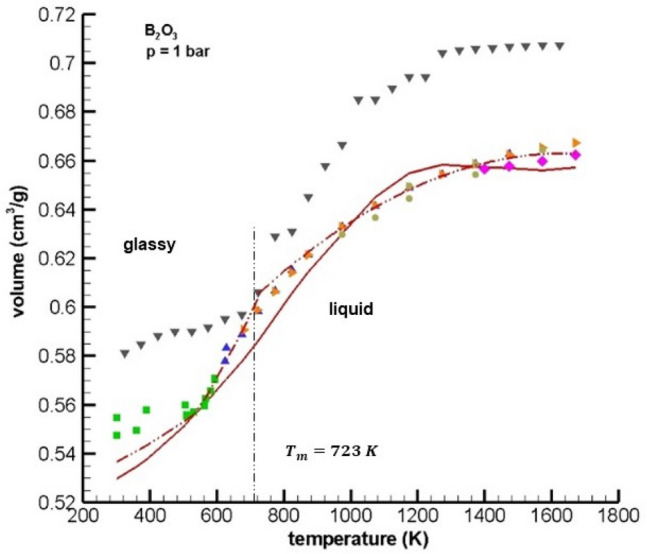
Figure 3 shows a comparison of multiscale GHC equation predictions of B2O3 specific volume with and without soft repulsive forces with experimental volume data for the glassy and melt regions.
Figure 3.
Specific volume vs. temperature for glassy B2O3 and B2O3 melt at 1 bar. experimental data: Macedo and Litovitz10 ( ), Napolitano et al.48 (
), Napolitano et al.48 ( ), Macedo et al.49 (
), Macedo et al.49 ( ), Roebling50 (
), Roebling50 ( ), Chebykin et al.51 (
), Chebykin et al.51 ( ). Simulations: MD Huang and Kieffer4 (
). Simulations: MD Huang and Kieffer4 ( ), multiscale GHC equation without soft repulsion (
), multiscale GHC equation without soft repulsion ( ), moving boundary equation with soft repulsion/temperature-dependent boroxol ring structure (
), moving boundary equation with soft repulsion/temperature-dependent boroxol ring structure ( ).
).
The comparison of predicted specific volume with experimental data shown in Table 3 and Fig. 3 are in good agreement with the five experimental data sets from the literature, which contain a total of 51 experimental data points and were taken from10,48–51. The values of the AAD % error for the multiscale GHC equation of state without (i.e., ) and with soft repulsion and temperature-dependent boroxol ring structure (i.e.,
) and with soft repulsion and temperature-dependent boroxol ring structure (i.e., ) were 1.29 and 0.64% respectively for the temperature range [300, 1600 K] and shows that the inclusion of ‘soft’ repulsion and structure in the moving boundary condition and in the Monte Carlo simulations lead to improvements in model predictions of specific volume.Also shown in Fig. 3 are the molecular dynamics (MD) simulation results from4 (i.e.,
) were 1.29 and 0.64% respectively for the temperature range [300, 1600 K] and shows that the inclusion of ‘soft’ repulsion and structure in the moving boundary condition and in the Monte Carlo simulations lead to improvements in model predictions of specific volume.Also shown in Fig. 3 are the molecular dynamics (MD) simulation results from4 (i.e., ). While the shape of the specific volume versus temperature curve for the MD simulations using the charge-transfer, three-body potential of Huang and Kieffer4 shown in Fig. 3 matches the overall shape of the experimental data quite well, the MD simulation predictions over-estimate the specific volume at all temperatures with an AAD% error of 5.92%.
). While the shape of the specific volume versus temperature curve for the MD simulations using the charge-transfer, three-body potential of Huang and Kieffer4 shown in Fig. 3 matches the overall shape of the experimental data quite well, the MD simulation predictions over-estimate the specific volume at all temperatures with an AAD% error of 5.92%.
Volumetric coefficient of thermal expansion predictions
Accurate model predictions of volume versus temperature are critical to obtain accurate estimates of the coefficients of thermal expansion. The fits of for the moving boundary equation of state shown in Fig. 3 and ( used to predict in this work are given in the Supplementary Material. Figure 4 shows plots of the volumetric coefficient of thermal expansion for the moving boundary equation of state, along with the experimental data reported in Ref.52 and the coefficient of expansion data reported in Ref.48, which appears to have been computed from fits of density versus temperature.
Figure 4.

Volumetric coefficient of expansion vs temperature for boron trioxide at 1 bar. Experimental data: Napolitano et al.48 ( ), Spaght and Parks52 (
), Spaght and Parks52 ( )—partially and carefully annealed (sample 1), Spaght and Parks52 (
)—partially and carefully annealed (sample 1), Spaght and Parks52 ( ) —partially and carefully annealed (sample 2), Fajan and Barber54 (
) —partially and carefully annealed (sample 2), Fajan and Barber54 ( ). Simulation data: moving boundary equation with soft repulsion/temperature-dependent boroxol ring structure (
). Simulation data: moving boundary equation with soft repulsion/temperature-dependent boroxol ring structure ( ).
).
There are only a few data sets in the open literature containing experimental coefficient of thermal expansion data for boron trioxide. While there is general agreement with respect to the order of magnitude of in the range [10–4, 10–5 °C−1], the values of the experimental data vary widely. In Table I, p. 106 Spaght and Parks52 provide different sets of results for for two separate samples of B2O3 glass for partially and carefully annealed samples in which volume was measured using dilatometry and was approximated using the finite difference expression . However, Spaght and Parks present no volume data or experimental error statistics for the coefficients of expansion—so it is difficult to judge the accuracy of their data. However, for partially annealed and carefully annealed boron trioxide, Spaght and Parks show that increases with increasing temperature over the range [122, 275 °C] corresponding to temperatures up to the glass transition temperature. Again, it is important to note that these authors present no raw volume data in their paper. Donoghue and Hubbard53 used interferometry and report a melting point of 450.8 °C (see p. 375) and a softening temperature of 315 °C (see p. 379). However, they only describe the qualitative behavior of for B2O3. Fajans and Barber54 use electronic structure arguments and the experimental data in Ref.53 and in Ref.55 respectively to sketch plots of similar to those given in Ref.52 for glassy B2O3 and in Ref.48 for liquid B2O3. Finally, in Fig. 3, p. 616 in Ref.48 it is shown that for boron trioxide decreases monotonically with increasing temperature over the range [400, 1400 °C], which corresponds to temperatures in the melt regime.
The moving boundary equation of state predictions of volumetric coefficient of thermal expansion are in good agreement with experimental data reported in Refs.48,52,54 both in temperature trends and in order of magnitude [10–4, 10–5 °C−1]. Like the experimental data, for which there is considerable scatter, the moving boundary equation predictions show a rapid rise in with increasing temperature in the glassy region and a decline in with increasing temperature in the melt region.
Metal oxide glass: lead monoxide-boron trioxide
Mixtures of lead monoxide and boron trioxide have been studied by Refs.56–61. To begin, to calculate glass transition and softening temperatures for mixtures of PbO and B2O3 and generate vs. temperature curves similar to Fig. 1a, a force field and force field parameters for PbO are needed. Table 4 gives the Lennard–Jones 6–12 parameters and partial charges for PbO, which were taken from Refs.62,63. We first tested the PbO force field parameters by determining internal energies of departure as a function of temperature. We then used those internal energies of departure to calculate the densities of litharge and massicot using the moving boundary equation of state. In each case the calculated predictions were close to values reported in the literature. More specifically, literature values of densities for litharge and massicot range from 9.14–9.53 and 9.56–10 g/cm3 respectively (e.g., mindat.org) while the moving boundary equation of state predictions were 9.13 g/cm3 at 300 K for litharge and 9.58 g/cm3 at 800 K for massicot.
Table 4.
Force field parameters for lead monoxide.
| Atom | (K) | ( | q (eV) |
|---|---|---|---|
| Pb | 430.29814 | 4.404 | + 1.218 |
| O | 39.4556 | 3.627 | − 1.218 |
Heat capacity vs. temperature
Figure 5 shows a plot of vs. temperature at 1 bar for a mixture of 50 mol% PbO and 50 mol% B2O3 over the temperature range [300, 1500 K], where was calculated using the finite difference formula
| 13 |
where , and was calculated using NVT Monte Carlo simulations with 25,000 equilibration cycles and 75,000 production cycles. Also, the degrees of freedom, , for the ideal gas contribution to the total internal energy were because PbO is a triatomic linear molecule and since B2O3 is a polyatomic nonlinear molecule.
Figure 5.

Heat capacity, , vs. temperature for 50 mol% lead monoxide-50 mol% boron trioxide glass at 1 bar predicted by Monte Carlo Simulation using the linear mixing rule for .
The shape of vs. temperature for the equimolar mixture of PbO and B2O3 is qualitatively the same as that for pure boron trioxide but is shifted to higher temperatures due to the presence of the network former/modifier PbO. Also, the peak in the heat capacity of the mixture is less than that of pure B2O3 by about half because the heat capacity of lead monoxide is small (0.2 J/g K) compared to that of pure boron trioxide (2 J/g K).
Glass transition and softening temperatures of PbO–B2O3
Glass transition and softening temperatures for mixtures are a function of composition and temperature. For mixtures of PbO and B2O3, the addition of the network former/modifier PbO increases the glass transition and softening temperatures. Calculation of the inflection point to the left of the maximum in at using the procedure described in the Supplemental Material gives an estimate of the glass transition temperature of .Calculation of the inflection point to the right of the maximum at yields a softening temperature of .
Volume and volumetric coefficient of thermal expansion
While there is some linear thermal expansion coefficient data reported in Table 3, p. 591 in Ref.66, there is no volume or density data. There is also limited experimental values of density and linear thermal expansion coefficients measured by gamma densitometry in Table 2, p. 11 in Ref.67 for three different molar compositions of PbO and B2O3. Moreover, the data in Ref.66 and in Ref.67 are easily reconciled and provide consistent values of linear thermal expansion coefficients. Here the focus for comparisons of modeling results with experimental data will be an equimolar mixture of PbO and B2O3.
From the calculated glass transition temperature, , and the linear mixing rules for molecular co-volume and internal energy of departure given by Eqs. (6) and (7) respectively, molar volumes and volumetric coefficients of thermal expansion for 50–50 mol% (76.2–23.8 wt%) PbO–B2O3 were calculated using the moving boundary equation of state. The predictions and experimental data are shown in Table 5, in which the volumetric coefficients of thermal expansion were computed analytically from 25 to 300 °C and averaged.
Table 5.
Comparisons of model predictions with experimental data for 50–50 mol% PbO–B2O3.
| Thermophysical property | Geller and Bunting66 | Drotning67 | Model predictions |
|---|---|---|---|
| Density @ 800 °C (g/cm3) | 5.03a | 5.27 | |
| (K−1) | 2.70 × 10–5b | 2.85 × 10–5a,c | 3.79 × 10–5 |
aPolynomial extrapolation of experimental data.
bInterpolated average from room temperature to 250 °C.
cMean value from 50 to 300 °C.
The results in Table 5 show that the moving boundary equation of state gives good estimates of specific volume and average volumetric coefficients of expansion for a 50–50 mol% mixture of PbO and B2O3. The error in the volume prediction at 800 °C is 4.77% while the average value of the volumetric coefficient of expansion, while higher than the experimental values, is certainly the right order of magnitude.
Glass transition temperature
The calculated glass transition temperature for a 50–50 mol% mixture of PbO and B2O3 from Fig. 5 is within 3.67% of the experimental glass transition temperatures of 659 K reported in Table 1, p. 369 in Ref.64 and 658 K reported in Fig. 1, p. 749 in Ref.65. In contrast, a glass transition temperature of for an equimolar mixture of PbO and B2O3 is reported in Ref.58.
Softening temperature
Experimental data for softening temperature for an equimolar mixture of PbO and B2O3 available in the open literature is in Table 3, p. 591 in Ref.65 where the authors report ‘beginning of softening’ temperatures for a range of composition of PbO–B2O3—but not specifically for a 50–50 molar mixture. Interpolation of the values in Ref.65 for an equimolar mixture (76.22 wt% PbO) yields an estimated experimental softening temperature of 685.85 K (412.7 °C). In Fig. 1 in Schwarz and Ticha68 the softening temperature for a molar ratio of PbO/B2O3 equal to 1 is 718 K (450 °C) so there is some disagreement in the experimental data in references65,68. The softening temperature predicted by the moving boundary equation of state is 739.16 K is quite a bit higher than the experimental value. Nonetheless, the error in the modeling prediction is 12.92% when compared to the data in Ref.66, which is reported as the ‘beginning of softening’, and 3.55% when compared to the data in Ref.68. Moreover, it is unclear what Geller and Bunting65 mean by ‘beginning of softening’.
Discussion and conclusions
A moving boundary condition for the multiscale Gibbs–Helmholtz equation of state that accounts for soft repulsion was proposed. This new boundary condition gave rise to a moving boundary equation of state, which was used to determine thermophysical properties of pure boron trioxide and an equimolar mixture of PbO–B2O3. The predicted glass transition temperature for B2O3, 535.27 K, was within the range of experimental values of [510, 580 K] reported in the literature, while the predicted softening temperature of 660.78 K was within 1.97% of the experimental value reported in Napolitano et al.48. The moving boundary equation of state prediction of glass transition temperature for a mixture of 50 mol% PbO and 50 mol% B2O3 was 672.10 K, which is within the range reported in the literature [658, 678 K]. For pure boron trioxide, the equation of state predictions of volume was within 0.64% and volumetric coefficients of thermal expansion were in the same range as those reported in the literature. Equation of state predictions of the same thermophysical properties for an equimolar mixture of lead monoxide and boron trioxide were also good but comparisons were limited to very few available experimental data.
It is also important for the reader to understand that there is limited experimental data for metal oxide glasses in the open literature and, in many cases, there is considerable disagreement among the experimental data. Moreover, because none of the papers used for comparison in our manuscript presents error bars for the experimental data or gives any statistical information regarding experimental errors and with such wide variation in the experimental data for various properties, it is difficult to identify the source of the disagreement between experiments and modeling. In our opinion, what is important from a modeling and simulation perspective is for the modeling framework to give results that (1) fall within the range of published experimental data (i.e., have the right order of magnitude) and (2) show the correct temperature trends. However, this is not to imply that there are not errors introduced in modeling. While computations should not be expected to match experimental data for different properties to the same level of accuracy, the proposed computational framework can introduce errors due to errors in the (1) Monte Carlo simulations of internal energies of departure, and (2) the mixing rules for mixture internal energies of departure and molecular co-volume.
In conclusion, it was shown that glass transition and softening temperatures can be estimated by computing adjacent saddle points on vs. temperature, where is computed from finite difference derivatives of the total internal energy. Internal energies of departure from Monte Carlo simulations needed to compute were used within the multiscale GHC equation of state framework and provided good estimates of volume and volumetric coefficients of thermal expansion.
Finally, the modeling and simulation framework presented in this work can be readily extended to other metal oxide glasses and has the potential to guide the experimental design of these materials.
Supplementary Information
Author contributions
The corresponding author, A.L., developed the modeling and simulation software and the mathematical analysis contained in this work, and prepared the numerical results and figures in the manuscript. Co-author O.G. prepared and edited the materials science aspects of the manuscript. All authors reviewed the manuscript.
Data availability
Input and output data from all numerical simulations and plots presented in this paper are available by contacting the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-27747-5.
References
- 1.Kacem IB, Gautron L, Coillot D, Neuville D. Structure and properties of lead silicate glasses and melts. J. Chem. Geol. 2017;461:104–114. doi: 10.1016/j.chemgeo.2017.03.030. [DOI] [Google Scholar]
- 2.Bruckner R. Properties and structure of vitreous silica. J. Non-Cryst. Solids. 1970;5:123–175. doi: 10.1016/0022-3093(70)90190-0. [DOI] [Google Scholar]
- 3.Shell MS, Debenedetti PG, Panagiotopoulos AZ. Molecular structure order and anomalies in liquid silica. Phys. Rev. E. 2002;66:011202. doi: 10.1103/PhysRevE.66.011202. [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Kieffer J. Thermomechanical anomalies and poly-amorphism in B2O3 glass: A molecular dynamics simulation study. Phys. Rev. B. 2006;74:224107. doi: 10.1103/PhysRevB.74.224107. [DOI] [Google Scholar]
- 5.Hartmann B, Haque MA. Equation of state for polymer solids. J. Appl. Phys. 1985;5:2831–2836. doi: 10.1063/1.335881. [DOI] [Google Scholar]
- 6.Rodgers PA. Pressure–volume–temperature relationships for polymeric liquids: A review of equations of state and their characteristic parameters for 56 polymers. J. Appl. Pol. Sci. 1993;48(6):1061–1080. doi: 10.1002/app.1993.070480613. [DOI] [Google Scholar]
- 7.Schmidt T. W., Menges G. Calculation of the packing phase in injection molding with a two-layer segment model. In Proceedings of the 44th Annual Technical Conference (ANTEC) of the Society of Plastics Engineer (SPE). Boston (1986).
- 8.Murnaghan FD. The compressibility of media under extreme pressures. Proc. Nat. Acad. Sci. 1944;30(9):244–247. doi: 10.1073/pnas.30.9.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birch F. Finite elastic strain of cubic crystals. Phys. Rev. 1947;71(11):809–824. doi: 10.1103/PhysRev.71.809. [DOI] [Google Scholar]
- 10.Macedo PB, Litovitz TA. Ultrasonic viscous relaxation in molten boron trioxide. Phys. Chem. Glasses. 1965;6(3):69–80. [Google Scholar]
- 11.Lemons DS, Lund CM. Thermodynamics of high temperature Mie-Gruneisen solids. Am. J. Phys. 1999;67:1105–1108. doi: 10.1119/1.19091. [DOI] [Google Scholar]
- 12.Gordon M, Taylor JS. Ideal copolymers and the second-order transitions of synthetic rubbers. I. Non-crystalline copolymers. J. Appl. Chem. 1952;2(9):493–500. doi: 10.1002/jctb.5010020901. [DOI] [Google Scholar]
- 13.Fox TG. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956;1:123. [Google Scholar]
- 14.Bowron DT. Building Monte Carlo models of glasses using neutron and/or X-ray diffraction data. Proc. Mater. Sci. 2014;7:38–52. doi: 10.1016/j.mspro.2014.10.007. [DOI] [Google Scholar]
- 15.Bent JF, Hannon AC, Holland D, Karim MMA. The structure of tin silicate glass. J. Non-Cryst. Solids. 1998;232–234:300–308. doi: 10.1016/S0022-3093(98)00388-3. [DOI] [Google Scholar]
- 16.Hannon AC, Howells WS, Soper AK. ATLAS—A suite of programs for the analysis of time-of-flight neutron diffraction data from liquid and amorphous samples. IOP Conf. Ser. 1990;107:193–211. [Google Scholar]
- 17.Hannon AC, Parker JM, Vessal B. Neutron diffraction analysis of atomic short-range order in lead gallate glasses. J. Non-Cryst. Solids. 1998;232:51–58. doi: 10.1016/S0022-3093(98)00372-X. [DOI] [Google Scholar]
- 18.Hannon AC, Parker JM. The structure of aluminate glasses by neutron diffraction. J. Non-Cryst. Solids. 2000;274:102–109. doi: 10.1016/S0022-3093(00)00208-8. [DOI] [Google Scholar]
- 19.Hannon AC, DiMartino D, Santos LF, Almeida RM. Ge–O coordination in cesium germanate glasses. J. Phys. Chem. B. 2007;111:3342–3354. doi: 10.1021/jp066714z. [DOI] [PubMed] [Google Scholar]
- 20.Kuzuu N, Yoshie H, Tamai Y, Wang C. Molecular dynamics study of temperature dependence of volume of amorphous silica. J. Non-Cryst. Solids. 2004;349:319–330. doi: 10.1016/j.jnoncrysol.2004.08.207. [DOI] [Google Scholar]
- 21.Huang L, Kieffer J. Molecular dynamics study of cristobalite silica using a charge-transfer three-body potential: Phase transformation and structural disorder. J Chem. Phys. 2003;118(3):1487–1498. doi: 10.1063/1.1529684. [DOI] [Google Scholar]
- 22.Soper AK. Partial structure factors from disordered materials diffraction data: An approach using empirical potential structure refinement. Phys. Rev. B. 2005;72:104204. doi: 10.1103/PhysRevB.72.104204. [DOI] [Google Scholar]
- 23.Soper AK. Boroxol rings from diffraction data on vitreous boron trioxide. J. Phys. Condens. Matter. 2011;23(36):365402. doi: 10.1088/0953-8984/23/36/365402. [DOI] [PubMed] [Google Scholar]
- 24.Betrani M, Menziani MC, Pedone A. Improved empirical force field for multicomponent oxide glasses and crystals. Phys. Rev. Mater. 2021;5:045602. doi: 10.1103/PhysRevMaterials.5.045602. [DOI] [Google Scholar]
- 25.Flory PJ, Orwoll RA, Vrij A. Statistical thermodynamics of chain molecule liquids. I. An equation of state for normal paraffin hydrocarbons. J. Am. Chem. Soc. 1964;86(17):3507–3514. doi: 10.1021/ja01071a023. [DOI] [Google Scholar]
- 26.Flory PJ, Orwoll RA, Vrij A. Statistical thermodynamics of chain molecule liquids. II. Liquid mixtures of normal paraffin hydrocarbons. J. Am. Chem. Soc. 1964;86(17):3515–3520. doi: 10.1021/ja01071a024. [DOI] [Google Scholar]
- 27.Sanchez IC, Lacombe RH. An elementary molecular theory of classical fluids. Pure Fluids. J. Phys. Chem. 1976;80(21):2352–2362. doi: 10.1021/j100562a008. [DOI] [Google Scholar]
- 28.Sanchez IC, Lacombe RH. Statistical thermodynamics of polymer solutions. Macromolecules. 1978;11(6):1145–1156. doi: 10.1021/ma60066a017. [DOI] [Google Scholar]
- 29.Chapman WG, Gubbins K, Jackson G, Radosz M. SAFT: Equation-of-state solution model for associating fluids. Fluid Phase Equil. 1989;52:31–38. doi: 10.1016/0378-3812(89)80308-5. [DOI] [Google Scholar]
- 30.Kontogeorgis GM, Liang X, Arya A, Tsivintzelis I. Equations of state in three centuries. Are we closer to arriving to a single model of all applications? Chem. Eng. Sci. X. 2020;7:10060. [Google Scholar]
- 31.Lucia A, Bonk BM, Roy A, Waterman RR. A multi-scale framework for multi-phase equilibrium flash. Comput. Chem. Eng. 2012;36:79–98. doi: 10.1016/j.compchemeng.2011.07.011. [DOI] [Google Scholar]
- 32.Lucia A, Gow AS. A cubic equation of state for compounds with no critical point: Application to asphaltenes. Chem. Eng. Res. Des. 2019;151:252–260. doi: 10.1016/j.cherd.2019.09.017. [DOI] [Google Scholar]
- 33.Lucia A, Gow AS. Phase behavior of mixtures involving glassy materials. Comput. Chem. Eng. 2020;135:106742. doi: 10.1016/j.compchemeng.2020.106742. [DOI] [Google Scholar]
- 34.Abbott MM, Prausnitz JM. Generalized van der Waals theory: A classical perspective. Fluid Phase Equil. 1987;37:29–62. doi: 10.1016/0378-3812(87)80042-0. [DOI] [Google Scholar]
- 35.Cruz-Chu ER, Aksimentiev A, Schulten K. Water-silica force field for simulating nanodevices. J. Phys. Chem. 2006;110(43):21497–21508. doi: 10.1021/jp063896o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butenuth A, Moras G, Schneider J, Koleini M, Köppen S, Meißner R, Wright LB, Walsh TR, Ciacchi LC. Ab Initio derived force-field parameters for molecular dynamics simulations of deprotonated amorphous-SiO2/water interfaces. Phys. Status Solidi B. 2012;249:292–305. doi: 10.1002/pssb.201100786. [DOI] [Google Scholar]
- 37.Anagnostopolous A, Alexiadis A, Ding Y. Simplified force field for molecular dynamics simulations of amorphous SiO2 for solar applications. Int. J. Therm. Sci. 2021;160:106647. doi: 10.1016/j.ijthermalsci.2020.106647. [DOI] [Google Scholar]
- 38.Ferlat G, Charpentier T, Seitsonen AP, Takada A, Lazzeri M, Cormier L, Calas G, Mauri F. Boroxol rings in liquid and vitreous B2O3 from first principles. Phys. Rev. Lett. 2008;101:065504. doi: 10.1103/PhysRevLett.101.065504. [DOI] [PubMed] [Google Scholar]
- 39.Arkundato A, Su’ud Z, Abdullah M, Sutrisno W. Molecular dynamic simulation on iron corrosion-reduction in high temperature molten lead bismuth eutectic. Turk. J. Phys. 2013;37:132–144. [Google Scholar]
- 40.Xiaoping, D. Molecular dynamics study of hydrogen on alkali-earth metal cations exchanged zeolites. Int. J. Chem. Eng. 701057 (2014).
- 41.Soper AK. Empirical potential Monte Carlo simulation of fluid structure. Chem. Phys. 1996;202:295–306. doi: 10.1016/0301-0104(95)00357-6. [DOI] [Google Scholar]
- 42.Emami FS, Puddu V, Berry RJ, Varshney V, Patwardhan SV, Perry CC, Heinz H. Force field and a surface model database for silica to simulate interfacial properties in atomic resolution. Chem. Mater. 2014;26(8):2647–2658. doi: 10.1021/cm500365c. [DOI] [Google Scholar]
- 43.Tabrizi NS, Vahid B, Amazat J. Functionalized single-atom thickness boron nitride membrane for separation of arsenite ion from water: A molecular dynamics simulation study. Phys. Chem. Res. 2020;8(3):843–856. [Google Scholar]
- 44.D’Angelo G, Carini G. Effects of topological disorder on thermodynamic and vibrational properties of amorphous solids. AAPP Classed di Scienze Fisiche, Mathematise e Naturali. 2020;98(S1):A1–8. [Google Scholar]
- 45.Abramov I, Vassilis T, Panko I. The glass transition temperature of silicate and borate glasses. J. Non-Cryst. Solids. 2005;351:472–476. doi: 10.1016/j.jnoncrysol.2005.01.044. [DOI] [Google Scholar]
- 46.Soppe W, van der Marel C, van Gunsteren WF, den Hartog HW. New insights into the structure of B2O3 glass. J. Non-Cryst. Solids. 1988;103(2–3):201–209. doi: 10.1016/0022-3093(88)90199-8. [DOI] [Google Scholar]
- 47.Bota WJ, Ota K, Haloumi K, Vaughan G, Yavari AR. Glass transition, thermal expansion, and relaxation in B2O3 glass measured by time-resolved X-ray diffraction. J. Non-Cryst. Solids. 2008;354:325–327. doi: 10.1016/j.jnoncrysol.2007.07.043. [DOI] [Google Scholar]
- 48.Napolitano A, Macedo PB, Hawkins EG. Viscosity and density of boron trioxide. J. Am. Ceram. Soc. 1965;48(12):613–616. doi: 10.1111/j.1151-2916.1965.tb14690.x. [DOI] [Google Scholar]
- 49.Macedo PB, Capps W, Litovitz TA. Two-state model for the free volume of vitreous B2O3. J. Chem. Phys. 1966;44(9):3357–3364. doi: 10.1063/1.1727238. [DOI] [Google Scholar]
- 50.Riebling EF. Structure of B2O3 and binary aluminoborate melts at 1600 °C. J. Am. Ceram. Soc. 1966;49(1):19–23. doi: 10.1111/j.1151-2916.1966.tb13139.x. [DOI] [Google Scholar]
- 51.Chebykin D, Heller H-P, Sanko I, Bartsch G, Endo R, Volkova O. Effect of glass transition: Density and thermal conductivity measurements of B2O3. High Temp. High Press. 2020;49:125–142. doi: 10.32908/hthp.v49.801. [DOI] [Google Scholar]
- 52.Spaght, M. E. & Parks, G. S. The coefficient of thermal expansion of boron trioxide. Studies in Glass. VIII. J. Phys. Chem. 1, 103–110 (1934).
- 53.Donoghue JJ, Hubbard D. Thermal expansion studies of boric oxide glass and of crystalline boric oxide. J. Res. Nat. Bur. Stand. 1941;27:371–379. doi: 10.6028/jres.027.023. [DOI] [Google Scholar]
- 54.Fajans K, Barber SW. Properties and structure of vitreous and crystalline boron oxide. J. Am. Chem. Soc. 1952;74:2761–2768. doi: 10.1021/ja01131a019. [DOI] [Google Scholar]
- 55.Volarovich MP. Acta Physicochim. 1935;2(6):695. [Google Scholar]
- 56.Manaktala, H. K. An assessment of borosilicate glass as a high-level waste form. NRC Center for Nuclear Waste Regulatory Analysis Report Rep. No.: CNWRA 92-017 (1992).
- 57.Forsberg, C. W., Parker, G. W., Rudolph, J. C. Osborne-Lee, I. W., Kenton, M. A. Termination of light-water reactor core-melt accidents with a chemical core catcher: The core-melt source reduction system (COMSORS). Oak Ridge Nat. Lab Rep. ORNL-6899 (1996).
- 58.Erdogan C, Bengisu M, Erenturk SA. Chemical durability and structural analysis of PbO–B2O3 Glasses and testing for simulated radioactive wastes. J. Nucl. Mater. 2014;445:154–164. doi: 10.1016/j.jnucmat.2013.10.025. [DOI] [Google Scholar]
- 59.Deng L, Du J. Development of effective empirical potentials for molecular dynamics simulations of the structures and properties of boroaluminosilicate glasses. J. Non-Cryst. Solids. 2016;453:177–194. doi: 10.1016/j.jnoncrysol.2016.09.021. [DOI] [Google Scholar]
- 60.Risold D, Nagata J, Suzuki RO. Thermodynamic description of the Pb–O system. J. Phase Equil. 1998;19(3):213–233. doi: 10.1361/105497198770342238. [DOI] [Google Scholar]
- 61.Cheng Y, Xiao H, Guo W. Structural and crystallization kinetics of PbO–B2O3 glasses. Ceram. Int. 2007;33(7):1341–1347. doi: 10.1016/j.ceramint.2006.04.025. [DOI] [Google Scholar]
- 62.Zhao L, Liu L, Sun H. Semi-ionic model for metal oxides and their interfaces with organic molecules. J. Phys. Chem. C. 2007;111(28):10610–10617. doi: 10.1021/jp071775y. [DOI] [Google Scholar]
- 63.Maghfiroh CY, Arkundato A, Maulina W. Parameters of Lennard–Jones for Fe, Ni, Pb for potential and Cr based on melting point values using the molecular dynamics method of the LAMMPS program. IOP Conf. Ser. J. Phys. Conf. Ser. 2020;149:012022. doi: 10.1088/1742-6596/1491/1/012022. [DOI] [Google Scholar]
- 64.Xi Y, Xu Z, Hou Z, Liu L, Xu L, Wang W, Affatigato M, Feller S. Second-order optical nonlinearity in bulk PbO/B2O3 glass. Opt. Commun. 2002;210:367–373. doi: 10.1016/S0030-4018(02)01565-1. [DOI] [Google Scholar]
- 65.Klyuev VP. Dependence of the dilatometric properties of glasses on their structure: I. Borate, aluminoborate, and lead-containing glasses. Glass Phys. Chem. 2005;31(6):749–759. doi: 10.1007/s10720-005-0123-8. [DOI] [Google Scholar]
- 66.Geller RF, Bunting EN. The system: PbO–B2O3. J. Res. Natl. Bur. Stand. 1937;18:585–593. doi: 10.6028/jres.018.035. [DOI] [Google Scholar]
- 67.Drotning, W. D. Density and thermal expansion measurements of several mixed oxide glasses in the solid and liquid regions. Sandia Rep. SAND84-2006 (1984).
- 68.Schwarz J, Ticha H. Some optical properties of PbO–BaO–B2O3 glasses. J. Optoelectron. Adv. Mater. 2003;5(1):69–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Input and output data from all numerical simulations and plots presented in this paper are available by contacting the corresponding author.


