Abstract
Purpose
To estimate the prevalence of the sphenoidal emissary foramina (SEF), and the effect of possible moderators on it.
Methods
A systematic online literature search was conducted. The pooled prevalence with 95% confidence intervals was estimated. Outlier and influential analyses were performed. The presence of small-study effect and publication bias were evaluated. Moderator analyses were executed to investigate the effect of the specimens’ continent of origin, type of study (dried skull or imaging), probing for the evaluation of SEF patency (conduction and instruments used), side dominance (bilateral or unilateral), morphometric data [SEF diameter, distances SEF–Foramen ovale (FO) and SEF–Foramen spinosum (FS)], and the methodology used for the morphometric measurements (caliper, DICOM Viewer, and image analysis software) on the estimated prevalence.
Results
In total, 6,460 subjects from 26 studies were included in the meta-analysis. The overall SEF prevalence was estimated as 38.1%. The heterogeneity was high and statistically significant. No indications of publication bias and small-study effect were identified. The conducted subgroup analyses did not yield statistically significant differences in the SEF prevalence between groups, except of the type of side dominance. Both results of the univariable and multivariable regression analyses showed the association of the unilateral dominance with a decrease in the reported SEF prevalence.
Conclusion
The identification of more unilateral than bilateral foramina in a given cohort is associated with a decrease in the reported crude SEF prevalence. Laterality-specific estimates should be established for a precisive estimation of the emissary foramina prevalence.
Keywords: Foramen Vesalius, Sphenoidal emissary foramen, Prevalence, Meta-analysis, Laterality-specific prevalence
Introduction
The constant foramina (foramina ovale and spinosum, FO and FS) are located in the posterior part of the greater wings of the sphenoid bone. FO transmits the mandibular nerve and occasionally the accessory meningeal artery and the lesser superficial petrosal nerve. FS is perforated by the middle meningeal artery and the meningeal branch of the trigeminal nerve [12]. Occasionally, anteromedially or anteriorly to the FO [19, 31], a small sized foramen—the so-called sphenoidal emissary foramen (SEF) or foramen of Vesalius (FV) can be unilaterally or bilaterally identified. SEF has not been identified in any other primates than human [40]. As per its content, SEF transmits a sphenoidal emissary vein (SEV) connecting the pterygoid venous plexus to the cavernous sinus [24]. Therefore, it is important in neurosurgical procedures, such as in FO cannulation for trigeminal nerve rhizotomy, as well as pathway of spreading of extracranial infections into the cavernous sinus [22, 25, 35]. The SEF occurrence varies widely among different studies’ samples [13].
The current meta-analysis provides a more precise estimation of the SEF prevalence and pinpoints the variables associated with the SEF presence.
Materials and methods
Search strategy
A systematic literature search was conducted by two independent assessors in August 2022 using Publish or Perish software [15]. Through this application, all available databases except for the Web of Science (Crossref, GoogleScholar, OpenAlex, PubMed, Scopus, and Semantic Scholar) were scanned using combinations of the following keywords: [“foramen Vesalius”, “sphenoidal emissary foramen”, “presence”, “occurrence”, “prevalence”, “incidence”)]. Notably, in Semantic Scholar and OpenAlex, only single keywords were used since both databases’ application programming interfaces did not support the use of Boolean operators. After duplicates’ removal, each publication’s reference list was manually scanned for potentially non-identified studies. The systematic literature search flowchart (Fig. 1) is based on the PRISMA 2020 Statement [29].
Fig. 1.
Flow chart depicting the systematic search results from the relevant studies' identification and selection
Criteria for study selection and data inclusion and extraction
All original studies reporting data regarding SEF prevalence were included with no restriction on language or publication date. Case reports, review articles, letters to the editor, conference abstracts, doctoral thesis, studies with no full-text or detailed abstracts available, and articles that could not be cross-verified by multiple secondary sources were excluded. Out of each publication, the extracted data included the authors, year of publication, continent of origin (Europe, Asia, and America), type of data (dried skulls and imaging), probing for evaluating SEF patency (yes or no), instrument used for probing (bristle, wire, and other), total sample, reported SEF frequency (total, bilateral, and unilateral), type of dominance (bilateral: when the bilateral to unilateral foramina ratio was greater than 1, otherwise, unilateral), morphometric data (SEF diameter, SEF–FO, and SEF–FS distances), and the methodology used for the morphometric measurements (caliper, DICOM Viewer, image analysis software). In publications not mentioning the continent of origin, the country where the study originated from, was eventually recorded and in case of an article written in a non-Latin language (e.g., Russian), the full paper was downloaded and translated using the Google Translate website (https://translate.google.com). Additionally, in manuscripts where only the bilateral or unilateral percentages were reported, the respective frequencies (bilateral and unilateral frequency) were calculated by converting each percentage to integers with no decimal approximation.
Quality assessment
The quality assessment was performed according to the Anatomical Quality Assessment (AQUA) tool [16], a tool consisting of 25 questions and dividing into 5 areas: 1. Objectives and Subject Characteristics, 2. Study Design, 3. Methodology Description, 4. Descriptive Anatomy, and 5. Results Reporting. For each domain, where all questions were replied affirmatively, the risk of bias was rated as 'low', otherwise as 'high'. Study quality was defined as ‘high’ if at low risk of bias in all five domains, ‘moderate’ if at low risk of bias at least in three domains, and otherwise as ‘low’.
Statistical analysis
Statistical analysis was carried out using RStudio (version: 2022.7.1.554) software (RStudio Team (2022)). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA for MacOS. The DerSimonian and Laird random-effects model was used to estimate the pooled prevalence and its respective 95% confidence intervals (CI). No logit or double arcsine transformation were made since the observed proportions identified across studies were between 0.2 and 0.8 [21, 37]. Heterogeneity presence across studies was estimated by constructing a forest plot and tested using the Cochran’s Q statistic and its respective p value. The Higgins I2 statistic and its respective 95% CI were used for quantifying the magnitude of true heterogeneity in effect sizes. An I2 value of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity. To detect studies that overly contributed to the heterogeneity, a Baujat plot [2] was created. To determine if the potential outlying studies, as evaluated in this plot, were also influential, screening for externally studentized residuals with z-values larger than two in absolute value and leave-one-out diagnostics were performed [38]. With the outlying and influential studies removed, the pooled prevalence, its’ respective 95% CI, and the substantial heterogeneity were re-evaluated through moderator analyses. In the conducted subgroup analyses, the following covariates were evaluated: continent of origin, type of data, probing, sample size, dominance, study quality, and measurements. As per the sample size, all manuscripts were divided into two categories (small and large studies) based on the median sample size (n = 239 subjects). In the performed univariable regression analyses, except of the aforementioned covariates, the SEF diameter, as well as the SEF–FO and SEF–FS distances were assessed as per their relationship with the respective effect sizes. Moreover, the presence of interrelated moderators was checked to avoid potential multicollinearity issues prior the conduction of the multivariate regression analysis. Due to the limited availability of data about the SEF diameter, and the SEF-FO, and SEF-FS distances in the given dataset, they were not used in this analysis. To detect the presence of publication bias, a Doi plot and a funnel plot were created. The asymmetry of each plot was estimated by calculating the LFK index [9] and Egger’s tests’ p value, respectively. Additionally, to detect the presence of the small-study effect, the phenomenon that smaller studies may show different, often larger effects than large ones [33], a funnel plot of the prevalence against the sample size was constructed and regression-based Egger’s test was estimated. The arithmetic difference between percentages was expressed in percentage point units [39]. Unless otherwise stipulated, the statistical significance was established at p = 0.05 (two-tailed).
Results and discussion
Search results and characteristics of the included studies
A total of 26 studies (n = 6,460 subjects); 23 dried skull (n = 5,590 subjects) and 4 imaging (n = 870 subjects) were included. Thirteen studies (48.2%) were conducted in Asia, nine studies (36.3%) in America, and five (18.5%) in Europe. The majority of articles evaluated the SEF patency (15 studies, 55.6%). Out of them, seven studies (46.6%) reported the use of wires, three (20.0%) the use of bristles, and five (33.4%) the use of other materials, such as metallic probes or endodontic files. The 66.7% of the included studies were estimated as moderate quality and the remaining ones as high quality. The 51.9% of the studies that referred to the SEF had calculated its anteroposterior diameter by utilizing the use of calipers (six studies, 42.9%), DICOM viewers (three studies, 21.4%) or image analysis software (five studies, 35.7%). A list of the included studies is presented in Table 1.
Table 1.
Main characteristics and data outcome of the included studies
| Authors | Year | Continent | Study type | Probing | Total sample | SEF frequency | Dominance | Study size | Study Quality | Conduction of measurements | SEF morphometry (in mm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | B/L | U/L | SEF diameter | SEF–FO | SEF–FS | ||||||||||
| Alves and Deana [1] | 2017 | America | Dried skulls | No | 178 | 57 | 43 | 14 | Bilateral | Small | Moderate | Yes (caliper) | 2.19 | ||
| Bayrak et al. [2] | 2018 | Asia | Imaging | No | 317 | 89 | 22 | 67 | Unilateral | Large | Moderate | Yes (DV) | 2.74 | 2.26 | 11.29 |
| Boyd [3] | 1930 | Europe | Dried skulls | No | 1500 | 548 | 221 | 327 | Unilateral | Small | Moderate | No | |||
| Berlis et al. [4] | 1992 | Europe | Dried skulls | No | 60 | 22 | 9 | 13 | Unilateral | Large | Moderate | Yes (DV) | |||
| Chaisuksunt et al. [5] | 2012 | Asia | Dried skulls | Yes (wire) | 377 | 61 | 16 | 45 | Unilateral | Large | High | Yes (IAS) | 1.59 | 2.42 | |
| Costa do Nascimento et al. [6] | 2018 | America | Dried skulls | No | 194 | 36 | 12 | 24 | Unilateral | Small | Moderate | Yes (caliper) | |||
| Dogan et al. [7] | 2014 | Asia | Dried skulls | No | 31 | 15 | 5 | 10 | Unilateral | Small | Moderate | Yes (caliper) | 2.46 | 3.61 | |
| Ginsberg et al. [9] | 1994 | America | Imaging | No | 123 | 98 | 60 | 38 | Bilateral | Small | Moderate | No | |||
| Görürgöz and Paksoy [10] | 2020 | Asia | Imaging | Yes (wire) | 260 | 190 | 110 | 80 | Bilateral | Large | High | Yes (DV) | 1.75 | 1.39 | 10.32 |
| Gupta et al. [12] | 2005 | Asia | Dried skulls | Yes (bristle) | 35 | 15 | 8 | 7 | Bilateral | Small | Moderate | No | |||
| Gupta et al. [13] | 2014 | Asia | Dried skulls | Yes (bristle) | 200 | 68 | 28 | 40 | Unilateral | Small | Moderate | No | |||
| Jadhav et al. [15] | 2016 | Asia | Dried skulls | Yes (bristle) | 250 | 72 | 44 | 28 | Bilateral | Large | High | No | |||
| Kale et al. [16] | 2009 | Asia | Dried skulls | Yes (other) | 347 | 156 | 87 | 69 | Bilateral | Large | High | No | |||
| Leonel et al. [18] | 2019 | America | Dried skulls | Yes (other) | 1000 | 468 | 254 | 214 | Bilateral | Small | High | No | |||
| Imaging | No | 170 | 77 | 32 | 45 | Unilateral | Large | High | No | ||||||
| Maletin et al. [20] | 2020 | Europe | Dried skulls | No | 26 | 16 | 14 | 2 | Bilateral | Small | Moderate | No | |||
| Martinez et al. [21] | 2014 | America | Dried skulls | No | 53 | 18 | 6 | 12 | Unilateral | Small | Moderate | No | |||
| Murlimanju et al. [23] | 2015 | Asia | Dried skulls | Yes (other) | 78 | 29 | 13 | 16 | Unilateral | Small | High | No | |||
| Natsis et al. [24] | 2018 | Europe | Dried skulls | Yes (wire) | 195 | 78 | 42 | 36 | Bilateral | Large | High | Yes (IAS) | 2.71 | 2.31 | |
| Nayak et al. [25] | 2018 | Asia | Dried skulls | Yes (other) | 30 | 9 | 6 | 3 | Bilateral | Small | High | Caliper (yes) | 1.26 | 1.80 | |
| Ozer and Govsa [26] | 2014 | Asia | Dried skulls | No | 172 | 60 | 16 | 44 | Unilateral | Small | Moderate | Yes (IAS) | 0.97 | 2.38 | 10.59 |
| Raval et al. [28] | 2015 | Asia | Dried skulls | Yes (wire) | 150 | 61 | 29 | 32 | Unilateral | Small | Moderate | Yes (caliper) | 1.05 | ||
| Reymond et al. [29] | 2005 | Europe | Dried skulls | Yes (wire) | 100 | 17 | 5 | 12 | Unilateral | Small | Moderate | Yes (IAS) | |||
| Rossi et al. [30] | 2010 | America | Dried skulls | No | 80 | 32 | 11 | 21 | Unilateral | Small | Moderate | Yes (caliper) | 1.52 | 2.16 | |
| Sharma and Garud [31] | 2011 | Asia | Dried skulls | Yes (wire) | 50 | 31 | 22 | 9 | Bilateral | Small | Moderate | No | |||
| Shinohara et al. [32] | 2010 | America | Dried skulls | Yes (wire) | 400 | 135 | 62 | 73 | Unilateral | Large | Moderate | Yes (IAS) | 0.71 | 2.57 | 11.24 |
| Toledo junior et al. [33] | 2016 | America | Dried skulls | Yes (other) | 84 | 35 | 14 | 21 | Unilateral | Small | Moderate | No | |||
T total, B/L bilateral, U/L unilateral, DV DICOM Viewer, IAS image analysis software
Prevalence of the sphenoidal emissary foramen (SEF)
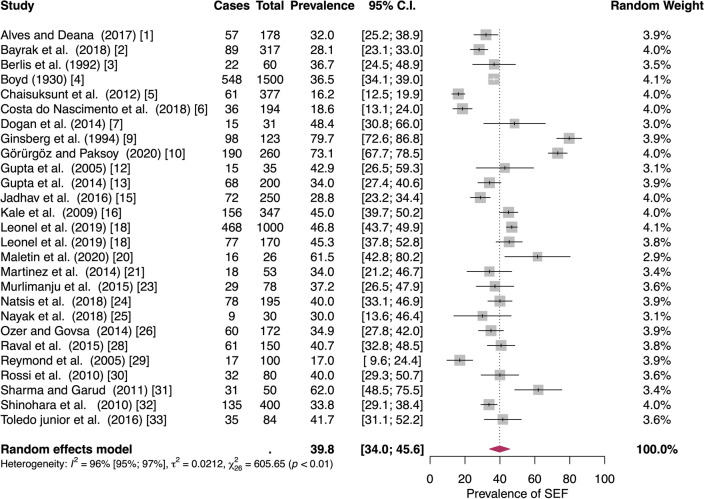
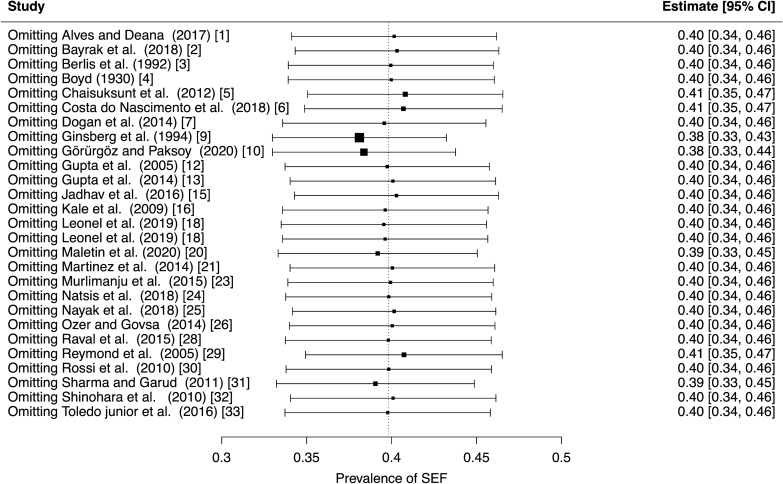
A random-effects model analysis yielded an initial overall SEF prevalence of 39.8% (95% CI 34.0−45.7) (Fig. 2).The estimated heterogeneity was statistically significant (p < 0.001), and of high magnitude (I2 = 95.8%; 95% CI 92.9–97.7). The Baujat plot and the influence diagnostics are presented in Figs. 3 and 4. According to them, even though several studies were initially identified as outliers, the diagnostics indicated that only the Ginbserg et al. [10] study had an influential effect. The forest plot illustrating the results of the leave-one-out analyses is presented in Fig. 5. After the exclusion of the relevant study, the new pooled SEF prevalence was estimated at 38.1% (95% CI 32.8–43.4) with a reduction of 1.2% in the I2 being noticed (I2 = 94.6%; 95% CI 93.2–95.8).
Fig. 2.
Forest plot evaluating the calculated prevalence of the sphenoidal emissary foramina (SEF) using random-effects model
Fig. 3.

Diagnostic plot (Baujat plot) for the detection of heterogeneity sources in meta-analytic data. On the horizontal axis, the contribution of each study to the overall Q-test statistic is displayed
Fig. 4.
Visual representation of the influence diagnostics for each of the included studies. Influential studies are marked as red dots. Abbreviations used—rstudent: studentized deleted residuals; dffits: DFFITS values; cook.d: Cook’s distances; cov.r: covariance ratio; tau2.del: estimated τ2 values; QE.del: estimated Cochran’s Q values
Fig. 5.
Forest plot displaying the re-calculated pooled effects, with one study omitted each time, using the leave-one-out method. The further a box deviates from the reference line, the more pronounced the impact of the corresponding missing study will be on the original summary proportion
Publication bias and small-study effect
Both the produced Doi and funnel plots (Fig. 6) for the assessment of presence of publication bias were assessed as asymmetric implying that bias might be present. However, the estimated LFK index (LFK index = 0.78) and the Egger’s test p value for the quantification of each plots’ asymmetry, respectively, were not deemed consistent with publication bias. As per the small-study effect, according to the data presented in Table 2 as well as on the interpretation of the produced funnel plot (Fig. 6) and the respective Egger’s test p value, the SEF prevalence was not moderated by the sample size. Therefore, no small-study effect was present.
Fig. 6.
Depiction of the produced plots for the detection of publication bias (plots a, b) and small-study effect presence (plot c). The estimation of each plot’s asymmetry was performed by calculating the LFK index for plot (a) and Egger’s tests’ p value for plots (b) and (c)
Table 2.
The results of the subgroup analysis on the differences of the subjects’ continent of origin, type of data, probing, sample size, laterality, and study quality on the estimated prevalence
| Predictor | Moderator (subgroup) | k | Prevalence (95% CI) | QM | QE | |
|---|---|---|---|---|---|---|
| Continent of origin | Europe | 5 | 36.2% (26.7–45.8) | 0.845 | < 0.0001 | |
| America | 8 | 36.4% (28.4–44.4) | ||||
| Asia | 13 | 39.9% (29.7–50.1) | ||||
| Type of data | Imaging | 3 | 48.8% (20.1–77.5) | 0.401 | < 0.0001 | |
| Dried skulls | 23 | 36.4% (31.7–41.0) | ||||
| Probing | No | 11 | 35.8% (30.4–41.1) | 0.508 | < 0.0001 | |
| Yes | 15 | 39.2% (30.7–47.6) | ||||
| Instrument used | Wire | 7 | 40.2% (23.3–57.0) | 0.941 | < 0.0001 | |
| Bristle | 3 | 32.5% (26.7–38.3) | ||||
| Other | 5 | 43.5% (39.3–47.7) | ||||
| Dominance | Unilateral | 16 | 33.1% (28.1–38.1) | 0.016 | < 0.0001 | |
| Bilateral | 10 | 46.0% (36.8–55.3) | ||||
| Sample size | Small | 17 | 37.5% (31.9–43.2) | 0.842 | < 0.0001 | |
| Large | 9 | 38.6% (29.3–48.0) | ||||
| Study quality | Moderate | 17 | 35.7% (31.4–40.1) | 0.473 | < 0.0001 | |
| High | 9 | 40.4% (28.4–52.5) | ||||
| Measurements | No | 12 | 41.5% (36.9–46.2) | 0.198 | < 0.0001 | |
| Yes | 14 | 34.8% (25.6–44.0) | ||||
| Instrument used | Caliper | 6 | 34.0% (24.6—43.5) | 0.084 | < 0.0001 | |
| DICOM Viewer | 3 | 46.0% (13.3—78.8) | ||||
| Image analysis software | 5 | 28.3% (18.2—38.3) | ||||
In bold text, the statistically significant findings are being noted
k number of studies combined, QM p value of the test of moderators, QE p value of the test of residual heterogeneity
Moderator analysis
The results of the subgroup analyses are summarized in Table 2. The SEF prevalence varied significantly only by the type of dominance (p = 0.016). The results of the performed regression analyses display the existence of a statistically significant, and a marginally non-significant association of the reported SEF prevalence with dominance (p = 0.005), and type of data (p = 0.060), respectively (Table 3). Specifically, according to the multivariable regression results, the unilateral dominance was associated with a 13.0 percentage points decrease in the reported SEF prevalence. In other words, when a sample of dried skulls is examined, the reported SEF prevalence will be 13.0% smaller if the frequency of the identified unilateral foramina exceeds the one of the bilateral. This finding highlights the necessity of the simultaneous recording and reporting of the unilateral and bilateral SEF prevalence (laterality-specific prevalence). The performed moderator analyses explained 32.3% of the residual heterogeneity (R2 = 32.3%).
Table 3.
The output of the univariable and multivariable linear meta-regression analyses performed for the association of the sphenoidal emissary foramen (SEF) presence with the studied variables
| Univariable models | Multivariable model | |||||
|---|---|---|---|---|---|---|
| Estimate | p value | 95% CI | Estimate | p value | 95% CI | |
| Continent of origin | ||||||
| America (ref.) | ||||||
| Asia | 0.03 | 0.597 | (− 0.09; 0.15) | |||
| Europe | 0.01 | 0.956 | (− 0.15; 0.16) | |||
| Type of data | ||||||
| Dried skulls (ref.) | ||||||
| Imaging | 0.12 | 0.106 | (− 0.03; 0.27) | 0.13 | 0.060 | (− 0.01; − 0.26) |
| Probing | ||||||
| No (ref.) | ||||||
| Yes | 0.02 | 0.768 | (− 0.09; 0.12) | |||
| Instrument used | ||||||
| Bristle | − 0.02 | 0.778 | (− 0.21; 0.16) | |||
| Wire | 0.03 | 0.705 | (− 0.11; 0.16) | |||
| Other | 0.02 | 0.705 | (− 0.13; 0.18) | |||
| Dominance | ||||||
| Bilateral (ref.) | ||||||
| Unilateral | − 0.13 | 0.010** | (− 0.22; − 0.03) | − 0.13 | 0.005** | (− 0.22; − 0.04) |
| Study sample size | ||||||
| Large (ref.) | ||||||
| Small | − 0.01 | 0.880 | (− 0.12; 0.10) | |||
| Study Quality | ||||||
| High (ref.) | ||||||
| Moderate | − 0.04 | 0.482 | (0.32; 0.49) | |||
| Measurements | ||||||
| No (ref.) | ||||||
| Yes | − 0.08 | 0.140 | (− 0.18; 0.02) | |||
| SEF diameter | 0.02 | 0.787 | (− 0.13; − 0.17) | |||
| SEF–FO distance | − 0.06 | 0.527 | (− 0.27; − 0.15) | |||
| SEF–FS distance | − 0.35 | 0.194 | (− 1.12; − 0.43) | |||
In bold text, the statistically significant findings are being highlighted
Ref reference category, 95% C.I. 95% confidence intervals, SEF diameter sphenoidal emissary foramen’s anteroposterior diameter (measured in mm), SEF–FO distance distance between sphenoidal emissary foramen and foramen ovale (measured in mm), SEF–FS distance distance between sphenoidal emissary foramen and foramen spinosum, ** strong statistical association
Study’s limitations
First, it should be noted that the unidentified heterogeneity remains on moderate levels. This indicates that the reported summary estimates must be interpreted with caution. Moreover, the small number of imaging studies and articles from various geographic locations (e.g., Europe), as well as the lack of a “gold standard” for measuring foramina dimensions and relative distances should be considered. Therefore, more effort should be made toward this direction.
Conclusion
The SEF prevalence is estimated at 38.1%. The unilateral dominance is associated with a decrease in the SEF prevalence. Therefore, laterality-specific estimates should be established and followed for the estimation of the emissary foramina prevalence.
Abbreviations
- FO
Foramen ovale
- FS
Foramen spinosum
- FV
Foramen of Vesalius
- SEF
Sphenoidal emissary foramen
- SEV
Sphenoidal emissary vein
- CI
Confidence intervals
- SEF–FO
Sphenoidal emissary foramen to foramen ovale distance
- SEF–FS
Sphenoidal emissary foramen to foramen spinosum distance
Author contributions
ΜΚ: literature search, manuscript writing, formation of methodology, and statistical meta-analysis; GT: literature search and manuscript editing; MP: formation of methodology, quality assessment, manuscript editing, and supervision; CP: quality assessment, manuscript editing, and supervision; NP: statistical meta-analysis and supervision; PP, FD, KN: manuscript editing and supervision.
Funding
Open access funding provided by HEAL-Link Greece. Open access funding was provided by the Hellenic Academic Libraries Link (HEAL-Link). The authors did not receive support from any organization for the submitted work.
Data availability
Literature and Rstudio data are available if requested.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This declaration is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Maria Piagkou and Michael Kostares equally contributed to the paper.
References
- 1.Alves N, Deana NF. Anatomical study of the Foramen Venosum and its clinical implications. J Anat Soc India. 2017;66:123–126. doi: 10.1016/j.jasi.2017.03.002. [DOI] [Google Scholar]
- 2.Baujat B, Mahé C, Pignon JP, Hill C (2002) A graphical method for exploring heterogeneity in meta‐analyses: application to a meta‐analysis of 65 trials. Stat Med 21 [DOI] [PubMed]
- 3.Bayrak S, Kurşun-Çakmak EŞ, Atakan C, Orhan K. Anatomic study on sphenoidal emissary foramen by using cone-beam computed tomography. J Craniofac Surg. 2018;29:e477–e480. doi: 10.1097/SCS.0000000000004492. [DOI] [PubMed] [Google Scholar]
- 4.Berlis A, Putz R, Schumacher M. Direct and CT measurements of canals and foramina of the skull base. Br J Radiol. 1992;65:653–661. doi: 10.1259/0007-1285-65-776-653. [DOI] [PubMed] [Google Scholar]
- 5.Boyd G (1930) The emissary foramina of the cranium in man and the anthropoids. J Anat 108–121 [PMC free article] [PubMed]
- 6.Chaisuksunt V, Kwathai L, Namonta K, Rungruang T, Apinhasmit W, Chompoopong S. Occurrence of the foramen of vesalius and its morphometry relevant to clinical consideration. Sci World J. 2012;2012:1–5. doi: 10.1100/2012/817454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa do Nascimento J, da Silva Neto E, de Oliveira Ribeiro E, de Almeida Holanda M, Valença M, Oliveira Gomes L (2018) Foramen venosum in macerated skulls from the North-East of Brazil: morphometric study. Eur J Anat 22:17–22
- 8.Dogan N, Fazliogullari Z, Uysal II, Seker M, Karabulut AK. Anatomical examination of the foramens of the middle cranial fossa. Int J Morphol. 2014;32:43–48. doi: 10.4067/S0717-95022014000100008. [DOI] [Google Scholar]
- 9.Furuya-Kanamori L, Barendregt JJ, Doi SAR (2018) A new improved graphical and quantitative method for detecting bias in meta-analysis. JBI Evid Implement 16 [DOI] [PubMed]
- 10.Ginsberg LE, Pruett SW, Chen MYM, Elster AD. Skull-base foramina of the middle cranial fossa: reassessment of normal variation with high-resolution CT. AJNR Am J Neuroradiol. 1994;15(2):283–291. [PMC free article] [PubMed] [Google Scholar]
- 11.Görürgöz C, Paksoy CS. Morphology and morphometry of the foramen venosum: a radiographic study of CBCT images and literature review. Surg Radiol Anat. 2020;42:779–790. doi: 10.1007/s00276-020-02450-6. [DOI] [PubMed] [Google Scholar]
- 12.Gray H. Osteology. 37. New York and London: Churchill Livingstone; 1989. Gray’s Anatomy of Human Body; pp. 267–447. [Google Scholar]
- 13.Gupta N, Ray B, Ghosh S (2005) Anatomic characteristics of foramen vesalius. Kathmandu Univ Med J 2:155–158. doi: 16415612 [PubMed]
- 14.Gupta N, DrA Y, ProfRJ T, Shrivastava A. Incidence of foramen vesalius in adult human North Indian Crania. IOSR J Dent Med Sci. 2014;13:34–38. doi: 10.9790/0853-13553438. [DOI] [Google Scholar]
- 15.Harzing AW (2007) Publish or Perish Publish or Perish, available from https://harzing.com/resources/publish-or-perish
- 16.Henry BM, Tomaszewski KA, Ramakrishnan PK, Roy J, Vikse J, Loukas M, Tubbs RS, Walocha JA. Development of the Anatomical Quality Assessment (AQUA) Tool for the quality assessment of anatomical studies included in meta-analyses and systematic reviews. Clin Anat. 2017;30:6–13. doi: 10.1002/ca.22799. [DOI] [PubMed] [Google Scholar]
- 17.Jadhav S, Ambali M, Zambare B. Sphenoidal emissary foramen and its clinical consideration. Int J Res Med Sci. 2016 doi: 10.18203/2320-6012.ijrms20161978. [DOI] [Google Scholar]
- 18.Kale A, Aksu F, Ozturk A, Gurses IA, Gayretli O, Zeybek FG, Bayraktar B, Zafer A, Onder N (2009) Foramen of Vesalius. 5 [PubMed]
- 19.Khairnar KB, Bhusari P. An anatomical study on the foramen ovale and the foramen spinosum. J Clin Diagn Res. 2013 doi: 10.7860/JCDR/2013/4894.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonel LCPC, Peris-Celda M, Sousa SDG, Haetinger RG, Liberti EA. The sphenoidal emissary foramen and the emissary vein: Anatomy and clinical relevance. Clin Anat. 2020;33:767–781. doi: 10.1002/ca.23504. [DOI] [PubMed] [Google Scholar]
- 21.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, CA, US: Sage Publications Inc; 2001. [Google Scholar]
- 22.Maletin M, Vukovic M, Sekulic M, Drljevic-Todic V. Morphological characteristics of foramen Vesalius in dry adult human skulls. Med Pregl. 2019;72:357–361. doi: 10.2298/MPNS1912357M. [DOI] [Google Scholar]
- 23.Martínez B, Telis O, Laza N, Queirolo M, Rodríguez J, Grillo M. Anatomía topográfica del foramen oval con aplicación al tratamiento percutáneo de la neuralgia trigeminal. Rev Mex Neu- Roci. 2007;8:104–109. [Google Scholar]
- 24.Mortazavi MM, Shane Tubbs R, Riech S, Verma K, Shoja MM, Zurada A, Benninger B, Loukas M, Cohen Gadol AA. Anatomy and pathology of the cranial emissary veins: a review with surgical implications. Neurosurgery. 2012;70:1312–1319. doi: 10.1227/NEU.0b013e31824388f8. [DOI] [PubMed] [Google Scholar]
- 25.Murlimanju B, Reddy G, Latha V, Vasudha V, Rao C, Mangala M (2015) Foramen of Vesalius: Prevalence, Morphology, Embryological Basis and Clinical Implications. J Surg Acad 5
- 26.Natsis K, Piagkou M, Repousi E, Tegos T, Gkioka A, Loukas M. The size of the foramen ovale regarding to the presence and absence of the emissary sphenoidal foramen: is there any relationship between them? Folia Morphol. 2018;77:9. doi: 10.5603/FM.a2017.0068. [DOI] [PubMed] [Google Scholar]
- 27.Nayak G, Pradhan S, Panda SK, Chinara PK. Anatomical study of foramen vesalius. J Evol Med Dent Sci. 2018;7:3847–3850. doi: 10.14260/jemds/2018/862. [DOI] [Google Scholar]
- 28.Ozer MA, Govsa F. Measurement accuracy of foramen of vesalius for safe percutaneous techniques using computer-assisted three-dimensional landmarks. Surg Radiol Anat. 2014;36:147–154. doi: 10.1007/s00276-013-1148-7. [DOI] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raval BB, Singh P, Rajguru J. A morphologic and morphometric study of foramen vesalius in dry adult human skulls of gujarat region. J Clin Diagn Res. 2015 doi: 10.7860/JCDR/2015/11632.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reymond J, Charuta A, Wysocki J (2005) The morphology and morphometry of the foramina of the greater wing of the human sphenoid bone. 7 [PubMed]
- 32.Rossi A, Friere A, Prado F, Caria P, Botacin P. Morphological characteristics of foramen Vesalius and its relationship with clinical implications. J Morphol Sci. 2010;27:26–29. [Google Scholar]
- 33.Schwarzer G, Carpenter JR, Rücker G (2015) Small-Study Effects in Meta-Analysis [DOI] [PubMed]
- 34.Sharma NA, Garud RS (2011) Morphometric evaluation and a report on the aberrations of the foramina in the intermediate region of the human cranial base: A study of an Indian population. 11
- 35.Shinohara AL, de Souza Melo CG, Silveira EMV, Lauris JRP, Andreo JC, de Castro RA. Incidence, morphology and morphometry of the foramen of Vesalius: complementary study for a safer planning and execution of the trigeminal rhizotomy technique. Surg Radiol Anat. 2010;32:159–164. doi: 10.1007/s00276-009-0562-3. [DOI] [PubMed] [Google Scholar]
- 36.Toledo Junior JS, Silva de Lima M, Moreira Correia M, Rodrigues Coutinho R, Magalhães Torres D de F (2016) Foramen Venosum: Prevalence, Patency and Correlation with Cephalic Index. Int J Morphol 34:1328–1332. doi: 10.4067/S0717-95022016000400025
- 37.Viechtbauer W. Conducting meta-analyses in r with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 38.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 39.Wickham K (2003) Math tools for journalists, 2nd ed, professional/professor version. Marion Street Press, Oak Park, IL
- 40.Wood-Jones F (1931) The non-metrical morphological characters of the skull as criteria for racial diagnosis. 17 [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Literature and Rstudio data are available if requested.