Abstract
An international consensus report in 2019 recommended a classification system for limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes (LATE-NC). The suggested neuropathologic staging system and nomenclature have proven useful for autopsy practice and dementia research. However, some issues remain unresolved, such as cases with unusual features that do not fit with current diagnostic categories. The goal of this report is to update the neuropathologic criteria for the diagnosis and staging of LATE-NC, based primarily on published data. We provide practical suggestions about how to integrate available genetic information and comorbid pathologies [e.g., Alzheimer’s disease neuropathologic changes (ADNC) and Lewy body disease]. We also describe recent research findings that have enabled more precise guidance on how to differentiate LATE-NC from other subtypes of TDP-43 pathology [e.g., frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS)], and how to render diagnoses in unusual situations in which TDP-43 pathology does not follow the staging scheme proposed in 2019. Specific recommendations are also made on when not to apply this diagnostic term based on current knowledge. Neuroanatomical regions of interest in LATE-NC are described in detail and the implications for TDP-43 immunohistochemical results are specified more precisely. We also highlight questions that remain unresolved and areas needing additional study. In summary, the current work lays out a number of recommendations to improve the precision of LATE-NC staging based on published reports and diagnostic experience.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00401-022-02524-2.
Keywords: Dementia, Processes, NCI, TDP-43, FTD, Stages, Hippocampal sclerosis, Neuroanatomy, Aging
Introduction
Transactive response DNA-binding protein of 43 kDa (TDP-43) pathology is prevalent in aging brains and is often associated with cognitive impairment or dementia [114]. Age-related TDP-43 pathology and associated clinical features have been described by many investigators over past decades [6, 14, 28, 30, 31, 46, 49, 59, 66, 70, 72, 85, 103, 106, 117, 125, 137, 145, 146, 148, 155, 158, 161], but a consensus nomenclature was lacking until recently. In 2019, a multidisciplinary consensus group suggested terminology for age-related TDP-43 pathologic changes associated with cognitive impairment. The disease was designated “limbic-predominant age-related TDP-43 encephalopathy” (LATE) [114], and guidelines were suggested for post-mortem evaluation and staging of LATE neuropathologic changes (LATE-NC). This terminology has been adopted widely [16, 25, 48, 50–52, 57, 74, 81, 95, 100, 132, 135, 138, 153]. However, diagnostic ambiguities and criticisms of the staging scheme have emerged [22, 61, 115].
At least four shortcomings have been identified in the 2019 LATE-NC guidelines: (1) anatomic regions for sampling were recommended, but the implications of TDP-43 immunopositivity in subregions were not precisely defined; (2) it was not clear how additional information on genetic findings and other pathologies should be incorporated into reports of LATE-NC; (3) there was minimal guidance on how to separate LATE-NC from other TDP-43 proteinopathies; and (4) some cases with TDP-43 pathology in aging could not be readily classified into LATE-NC stages.
The aims of the present paper are to remedy these shortcomings, to provide data to clarify the proper use of the LATE-NC staging system, and to indicate where the diagnosis of LATE-NC may not be appropriate. The overarching goal is to provide additional precision about LATE-NC diagnosis for neuropathologists. These goals are important, since brain autopsy remains the gold-standard for neurodegenerative disease diagnoses. New recommendations and clarifications are proposed for the LATE-NC staging system, guided by published data, findings reported at the LATE 2022 Conference [1] and diagnostic experience.
LATE-NC: recommendations for anatomic sampling and staining
Regarding brain tissue collected at autopsy to assess LATE-NC, this update does not propose additional sampling relative to the 2019 consensus recommendations [114]. However, there is a need to clarify the implications of the immunostaining results within specific regions of interest. Three anatomical regions are recommended for sampling, with the following suggestions (Fig. 1):
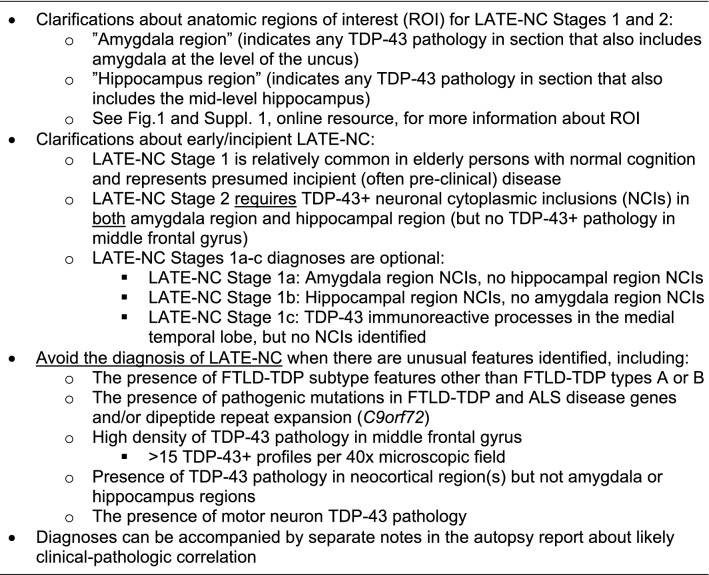
Amygdala region: this refers to the amygdala and surrounding structures at the level of the uncus, including adjacent entorhinal [Brodmann area (BA) 28], transentorhinal (BA35), anterior temporal (BA36) cortices and anterior parts of the hippocampus and the subiculum/presubiculum, sub-pial, subependymal regions, and white matter. The pathology is scored as positive in this region if aberrant TDP-43 immunoreactivity is seen anywhere on the section that contains amygdala and uncus (not just within amygdala proper).
Hippocampus region: this refers to the hippocampus and associated medial temporal cortical structures at the level of the lateral geniculate nucleus. Areas of interest may include fornix, sub-pial region, periventricular region, dentate granule cells, mid-level temporal cortex (BA36), and white matter. This region is considered to be positive if any part of the section has TDP-43 immunoreactive pathology.
Middle frontal gyrus (corresponding roughly to BA46). TDP-43 immunoreactive cytoplasmic inclusions in any part of this section is considered to be positive.
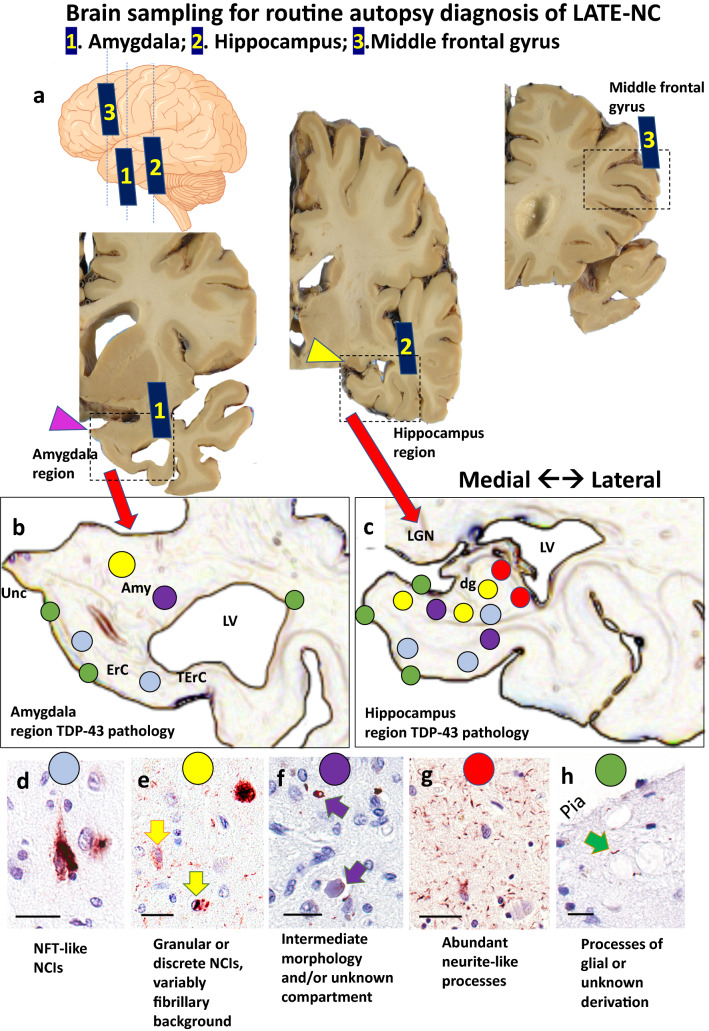
Fig. 1.
Anatomical regions of interest for tissue sampling and typical findings in routine autopsy diagnosis of LATE-NC. At autopsy, tissue portions for sampling include amygdala, mid-level hippocampus, and middle frontal gyrus. The levels of sections are shown in the cartoon form (upper left) with gross photographs of hemi-brains cut in the coronal plane (Panel a). Note that the amygdala is preferably sampled for TDP-43 immunohistochemical staining at the level of the uncus (pink arrowhead, Sect. 1), the hippocampus at the level of the lateral geniculate nucleus (yellow arrowhead, Sect. 2), and the middle frontal gyrus (Sect. 3) is sampled rather than other portions of frontal cortex. Panels b and c are representations of the amygdala region and hippocampal region, showing both the local anatomy and a cursory depiction of the subtypes of TDP-43 pathology that are generally found in those regions with corresponding colored circles in panels d-h. Panel d shows a neuronal TDP-43 + inclusion reminiscent of a neurofibrillary tangle. Panel e depicts a different TDP-43 pathologic appearance with a granular NCI (arrow with red outline), neuronal intranuclear inclusion (arrow with blue outline), and TDP-43 + fibrillary material in the background. This pattern is reminiscent of FTLD-TDP type A. TDP-43 pathology can also be present around vascular components such as capillaries (termed Lin Bodies after Ref. [87]) or in unknown histologic compartments as shown in Panel f. In some regions, the predominant TDP-43 + pathology is fine non-tapering neurite-like processes (Panel g). A different type of TDP-43-immunoreactive cell processes can be seen in the sub-pial region, often near corpora amylacea (arrow in Panel h). Scale bars = 50 microns (d); 30 microns (e); 30 microns (f); 100 microns (g); and, 30 microns (h). Abbreviations: Amyg: amygdala proper; dg: dentate granule layer of hippocampus; ErC: entorhinal cortex; LGN: lateral geniculate nucleus; LV: lateral ventricle; NCI: TDP-43 immunoreactive neuronal cytoplasmic inclusions; NFT: neurofibrillary tangles; TErC: transentorhinal cortex; Unc: uncus
Supplemental File 1 (online resource) provides additional detail on the specific anatomical regions and subregions of interest, as pertains to LATE-NC staging.
The LATE-NC staging guidelines suggested that TDP-43 immunohistochemistry should be performed as part of the neuropathologic evaluation of all older individuals’ brains [114], but there was no specific recommendation on the staining methods used. The antibody used for pathologic diagnosis and associated protocols may affect the ability to detect TDP-43 inclusions [40, 53, 79]. Phosphorylation-specific antibodies, with those against pS409/410 being the most commonly used [44, 120], robustly label pathologic TDP-43 inclusions and do not stain normal (nonphosphorylated) nuclear TDP-43 protein, thereby facilitating identification of aggregates [35] but precluding the visualization of the loss of normal nuclear TDP-43 protein in inclusion bearing cells and nuclear staining as internal positive control. Antibodies that are phosphorylation independent allow for labeling of inclusions (albeit slightly less sensitively), while also enabling assessment of normal nuclear TDP-43 protein [40, 53, 79]. In an informal survey of U.S. Alzheimer’s Disease Research Centers, approximately 2/3rd of the neuropathologists depended on antibodies against the phosphorylated Ser409/Ser410 TDP-43 epitope [71]. More standardization may be achievable in the future, but there is still not a current consensus on a prescribed set of reagents for TDP-43 detection.
LATE-NC: recommendations for pathological staging
LATE-NC staging, like other neurodegenerative disease staging systems [12, 18–20, 140], is based on anatomical regions affected by the neuropathologic changes. Following prior studies which incorporated analyses of TDP-43 immunohistochemical data from more anatomical regions [63, 64, 105, 107, 146, 162], the 2019 consensus guidelines for LATE-NC suggested that TDP-43 pathology progressed in a stereotypical pattern: LATE-NC Stage 1 corresponds to TDP-43 pathology in the amygdala, Stage 2 corresponds to TDP-43 pathology in the amygdala and hippocampus, and Stage 3 corresponds to TDP-43 pathology in the amygdala, hippocampus, and middle frontal gyrus [114]. If other neocortical regions (orbitofrontal cortex, or temporal neocortex) are stained and show neuronal cytoplasmic inclusions (NCIs), but NCIs are not seen in the middle frontal gyrus, this would not represent LATE-NC Stage 3. Some such LATE-NC Stage 2 cases would be expected, because orbitofrontal cortex and temporal neocortex (as well as some other regions) are affected earlier than middle frontal gyrus in the anatomical progression of LATE-NC [64, 105]. While most cases can be readily assigned to a given LATE-NC stage using those criteria, recent reports have described cases that depart from the staging scheme for one or more reasons [21, 29, 41, 62, 143, 147]. Below are described strategies to address various diagnostic challenges in a data-driven and standardized manner.
Brains with TDP-43 + NCI detected in the hippocampal region, but not in the amygdala region
In recently published studies from five separate autopsy series [29, 41, 80, 109, 143], a small subset of cases from each cohort was reported with TDP-43 pathology in the hippocampal formation, but not in the amygdala. This pattern is not accounted for in the original LATE-NC staging guidelines [114]. LATE-NC cases with hippocampal TDP-43 + NCIs, but none in amygdala region, are unusual (< 2% of LATE-NC cases), and the TDP-43 pathology usually involves the anterior hippocampus. These cases lacking detectable amygdala TDP-43 pathology may be due in part to sampling issues, and the clinical implications are unclear. More work is required to confirm that hippocampus-only TDP-43 pathology is appropriately diagnosed as LATE-NC. At present, because such cases have a low burden of TDP-43 pathology overall, and we do not know of an example of an individual with cognitive impairment lacking other pathologies, we recommend that this pattern of TDP-43 pathology be designated LATE-NC Stage 1.
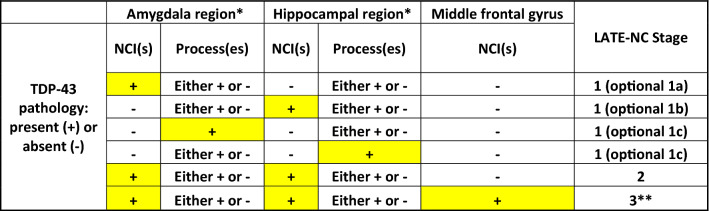
To account for the varied pathologic patterns in LATE-NC Stage 1 cases, a system for differentiating subtypes of LATE-NC Stage 1 cases is now recommended. This classification may be most suitable for academic research centers, and should be considered optional for diagnosticians. In this system, a “conventional” case with TDP-43 immunoreactive NCI(s) in amygdala but not hippocampus would be diagnosed as LATE-NC Stage 1a, whereas an unusual case with NCI(s) in hippocampus but none detected in the amygdala region would be diagnosed as LATE-NC Stage 1b (Table 1).
Table 1.
Specific pathological combinations and corresponding recommendations for LATE-NC staging
*Amygdala region and hippocampal region refer to anatomical areas on the same slide
**See recommendations to distinguish LATE-NC Stage 3 from FTLD-TDP and ALS
Brains with TDP-43 + cell processes, but no TDP-43 + neuronal cytoplasmic inclusions (NCI)
In LATE-NC, TDP-43 proteinopathy is often detected in cellular compartments outside of the neuronal cell body [11, 29, 39, 116]. A recent study focusing on cases with minimal or incipient LATE-NC described cases without NCI, but with short, non-branching TDP-43 immunoreactive structures that frequently localized around corpora amylacea, and/or in aging-related tau astrogliopathy (ARTAG) pathology [29, 37, 110, 128]. Because of the apparent astroglial derivation of some TDP-43 pathology outside of neuronal cell bodies, we use the term “processes”, as opposed to “neurites”, for these common TDP-43-immunoreactive structures. TDP-43-positive processes were detected in the sub-pial zone overlying the corticomedial region of the amygdala and/or in the periventricular white matter subjacent to amygdala. This pattern was common—aberrant TDP-43 was found only in cellular processes in approximately one-third of LATE-NC cases [29]. Another recent study confirmed that there are cases with TDP-43 + processes but lacking TDP-43 + NCIs [80].
We propose that cases with only TDP-43-positive processes, and no NCI in the amgydala and hippocampus regions, should be diagnosed as LATE-NC Stage 1. It is emphasized that LATE-NC Stage 2 requires at least a single NCI to be present in each of both the amygdala and hippocampal regions. In the context of the optional LATE-NC Stage 1 subtyping system as described above, any case with TDP-43 immunoreactive processes only (no NCI) would be LATE-NC Stage 1c, even if the TDP-43 + processes are in the hippocampus region. The various possible neuropathologic changes seen in LATE-NC Stage 1, and their relevant diagnoses, are shown in detail in Table 1. This staging scheme will help clarify diagnostic practice, but we also recognize that more work is required to understand the implications of aberrant TDP-43 protein in processes but not NCIs.
Brains with comorbid Alzheimer’s disease neuropathologic changes (ADNC), Lewy bodies, and/or granulovacuolar degeneration
It has been recognized for over a decade that TDP-43 pathology is often comorbid with ADNC, Lewy body pathology, or both [8, 10, 46, 66, 67]. However, these pathologic features also are commonly seen—even when quite severe—in the absence of each other [51, 52, 88, 113, 147, 153, 154], so they are at least partly independent. Many studies have demonstrated that LATE-NC is associated with additive cognitive impairment for a given amount of other pathologic changes [4, 16, 17, 72, 103, 111, 127]. Therefore, we recommend that descriptions of the individual diseases (and their neuropathologic stages) be rendered as separate line diagnoses on autopsy reports. A potential pitfall for the assessment of LATE-NC is the fact that some phospho-TDP-43 antibodies also detect granulovacuolar degeneration (GVD) [51, 69, 76, 139, 151]—this staining is not considered to represent LATE-NC. For more data and discussion related to immunostaining of GVD, see Refs [51, 69, 76, 77, 141, 151].
Differentiating LATE-NC from other pathologies
Differentiating LATE-NC Stage 3 from FTLD-TDP
TDP-43 was initially identified as a protein that forms abnormal intracellular aggregates in the most common pathology associated with clinical frontotemporal dementia (FTD) syndromes, now termed frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP) and in ALS [122]. The defining feature of FTLD-TDP is the presence of NCIs and dystrophic TDP-43 immunoreactive processes in affected frontotemporal cortices [23, 93, 94, 122]. Differences in the morphologic features, abundance, and laminar distribution of TDP-43 pathology in the cerebral neocortex allow for recognition of at least five subtypes of FTLD-TDP, each with relatively specific clinical and genetic correlations and increasingly recognized molecular differences in the nature of the TDP-43 aggregates [84, 90, 92, 119, 121, 124]. In addition, each FTLD-TDP subtype has a fairly distinct pattern of limbic and subcortical involvement that is helpful in their classification [65, 91]. Nevertheless, potential pitfalls in the FTLD-TDP subtyping have been discussed due to the presence of mixed subtypes (usually FTLD-TDP types A + B), and overall low inter-rater agreement in separating FTLD-TDP types A and B [5, 9, 90].
Cases with TDP-43 pathology mostly restricted to the medial temporal lobes (LATE-NC Stages 1 or 2) do not fulfill criteria for FTLD-TDP and should be classified as LATE-NC. By contrast, boundaries between LATE-NC Stage 3 and FTLD-TDP are more challenging (~ 11% of LATE-NC cases in community-based cohorts were Stage 3 [113]). Ancillary measures, such as neuronal loss and gliosis, as well as superficial laminar spongiosis, involvement of further cortices and subcortical nuclei and white matter in FTLD-TDP, and the morphology of inclusions may help differentiate genuine FTLD-TDP from LATE-NC Stage 3. More work is needed to delineate aspects of biological overlap and differences between LATE-NC and FTLD-TDP, particularly for FTLD-TDP in advanced old age [22].
Based on current knowledge, our recommendations are as follows:
TDP-43 pathology in LATE-NC has histomorphologic features that may be similar to FTLD-TDP type A or less commonly type B [8, 114, 131, 149]. By contrast, all cases with FTLD-TDP types C, D, or E patterns [84] should be classified as FTLD-TDP and not LATE-NC.
The severity of TDP-43 pathology in the middle frontal gyrus has been described as a useful indicator to differentiate LATE-NC Stage 3 from FTLD-TDP [131]. Specifically, more than 15 TDP-43 immunoreactive inclusions (NCIs and/or threads) per high power (40 × objective) microscopic field in the middle frontal gyrus had a high sensitivity and specificity in differentiating FTLD-TDP from LATE-NC [131]. More than 15 TDP-43-immunoreactive pathological structures per 40 × microscopic field in the middle frontal gyrus favor a diagnosis of FTLD-TDP or a descriptive diagnosis of the pathologies observed, rather than LATE-NC.
In cases with substantial TDP-43 pathology in the middle frontal gyrus, it is recommended to expand regions sampled for TDP-43 pathology and to include regions more typically affected in FTLD-TDP, such as subcortical structures, including basal ganglia, medulla (hypoglossal nucleus), and spinal cord. TDP-43 pathology in these regions supports a diagnosis of FTLD-TDP or ALS.
Cases with known pathogenetic mutations in FTLD-TDP-related genes (e.g., GRN, C9orf72, VCP, and others [99, 150]) should not be classified as LATE-NC. Notably, to identify C9orf72 repeat expansion carriers, immunohistochemistry of cerebellum with antibodies against p62 or dipeptide repeat proteins can be used as surrogate markers. This method is recommended in standard histopathologic screening if genetic analyses are not available [89].
Cases with comorbid FTLD-TDP and LATE-NC may exist, but there currently is no consensus about how to definitively diagnose such cases as distinct from FTLD-TDP alone.
Differentiating LATE-NC from amyotrophic lateral sclerosis (ALS)
Another important disease associated with TDP-43 proteinopathy is ALS. A four-tiered staging system of TDP-43 neuropathologic changes in ALS has been described by Brettschneider et al. [20] based on an autopsy cohort of ALS patients with average age at death of 63 years. TDP-43 pathology in the hippocampus region was designated stage 4 and was found in ~ 30% of cases. While, in this study, no significant differences regarding age at disease onset, or age at death, were observed between different stages of TDP-43 pathology, a recent study found medial temporal lobe TDP-43 pathology in 8/8 older ALS cases (> 75 years of age at death) [101], suggesting that in ALS, there may be pathology overlapping with LATE-NC. Yet, the TDP-43 neuropathologic changes in ALS differ from LATE-NC: motor neuron TDP-43 pathology has not been reported in LATE-NC, but is an early site of TDP-43 pathology in ALS [20], and is even seen in some FTLD-TDP cases without clinical ALS [32]. Until methods are available to distinguish TDP-43 pathology associated with LATE-NC from limbic pathology in ALS, we recommend avoiding the term LATE-NC in the context of sporadic and familial ALS.
Brains with unusual TDP-43 pathologies
Research continues to find expanding implications of aberrant TDP-43 protein in human diseases. TDP-43 pathology is now known to occur in more than 20 different conditions spanning neurodegenerative, developmental, trauma-related, myopathic, and even neoplastic disease categories [2, 13, 26, 27, 78, 83, 129, 133, 134, 157]. For example, TDP-43 pathology is often seen in corticobasal degeneration, Perry syndrome, Alexander disease, and the Parkinsonism–dementia complex diseases of Guam and Kii [43, 75, 148, 152, 156]. However, the morphology and anatomical patterns of the TDP-43 pathology in those conditions often differ from that in LATE-NC, so the diagnosis of LATE-NC should be avoided in these conditions. For persons with a clinical history of brain trauma—traumatic brain injury (TBI) and/or chronic traumatic encephalopathy (CTE)–TDP-43 pathologic changes may be seen, but not necessarily related to LATE-NC [3, 60, 96, 97, 126]. More work is required to develop a data-driven consensus of best practices for diagnosing brain trauma-related TDP-43 pathology.
Guidance in autopsy reports
Brain autopsy reports of individuals across the spectrum of dementias convey complex information reflecting the (often multifactorial) nature of the underlying diseases. That complexity, along with technical nomenclatures, can make it difficult, especially for patients’ families and loved ones, to understand the implications of TDP-43 pathology. It may be helpful for practicing neuropathologists to provide interpretive summaries, but we emphasize that this should be a separate comment with a focus on interpretation of findings in nontechnical language [82].
What are the implications of LATE-NC staging results? Clinical–pathological studies demonstrated that LATE-NC is associated with cognitive impairment (often with amnestic features), independent of other disease processes [17, 36, 42, 51, 58, 62, 68, 72, 88, 103, 104, 111, 130, 132, 135]. For example, in an attributable risk analysis based on all observed pathologic changes that may contribute to cognitive impairment in a large community cohort, LATE-NC accounted for more than 15% of identified amnestic dementia risk [16, 114]. Similar to other age-related neurodegenerative disease processes, it is not possible to confidently predict clinical implications of LATE-NC in a given individual, especially if there are multiple concurrent pathologies [24, 98, 108, 109, 112, 143, 146].
The following are possible text templates to complement the diagnostic findings in cases with LATE-NC:
LATE-NC Stage 1 indicates the early/incipient stage of LATE-NC, analogous to early stages of other brain and systemic diseases where pathologies are present before outward clinical signs/symptoms (e.g., Braak NFT Stages I–II in Alzheimer’s disease Neuropathologic Changes). The presence of LATE-NC Stage 1 may be compatible with normal cognition or may have only a relatively small additive impact on cognitive function.
LATE-NC Stage 2 indicates that TDP-43 neuronal cytoplasmic inclusions are detected more broadly in the brain. LATE-NC Stage 2 is usually associated with some impairment of memory or global cognition or both [114], with more cognitive impairment in cases with comorbid hippocampal sclerosis.
LATE-NC Stage 3 indicates the most advanced stage of LATE-NC in terms of neuroanatomic distribution of TDP-43 pathologic burden in the brain. LATE-NC Stage 3 is usually associated with some degree of impairment of memory or global cognitive impairment [114], and with more cognitive impairment in cases with comorbid hippocampal sclerosis.
Future directions and knowledge gaps related to LATE-NC
Considering the major public health impact of LATE-NC, there are many aspects of this disorder that merit further research. Of greatest urgency is the need for a sensitive and specific method to diagnose LATE during life, ideally at early stages of disease, to enable focused recruitment into clinical trials. The involvement of aberrant TDP-43 in a wider range of brain areas, and the role(s) of astrocytes in pathogenesis could also be foci of further scholarship. Systematic neuropathological and clinicopathological studies will inform if the staging system needs modifications to include intermediate stages. Additional pressing research is needed to define and stratify risk factors and interactions with other diseases, including various neurodegenerative and neurovascular disorders (see, for example, Refs [15, 37, 128]). Observations in more diverse communities are critical, since socioeconomic, cultural, and/or ancestral aspects may influence clinical–pathological correlation [55, 109, 123, 146]. (Some recent scholarship has evaluated LATE-NC in cohorts other than Whites [104, 109, 123, 146].)
It may be possible to discover molecular subtypes of LATE-NC using immunophenotyping, transcriptome profiling, and genotyping or other approaches, which has potential implications for prognosis and therapy. Indeed, there has already been significant progress along these lines [119, 143]. In particular, pathologic heterogeneity in LATE-NC has been demonstrated particularly in the early phases of LATE-NC, which occurs in different patterns and appears to originate at one of several different anatomic locations in the amygdala region [29, 62]. Certain parameters and features (e.g., comorbid Lewy body disease) have been associated with differing patterns of LATE-NC [29, 62]. A genetic risk factor for LATE-NC is TMEM106B [33]. Notably, the TMEM106B risk allele frequency was elevated in all identified LATE-NC patterns, and the different TDP-43 pathologic patterns observed in cases with mild pathology tended to converge in more severely affected brains [29]. We conclude that cases meeting criteria for LATE-NC encompass both heterogeneity and meaningful commonalities, but there currently is no agreement on means to differentiate LATE-NC subtypes.
Another area of uncertainty is the relation of LATE-NC to hippocampal sclerosis (HS). HS in the elderly is strongly associated with LATE-NC [7], with 75–90% of HS cases in aging being seen in cases with LATE-NC [34, 38, 47, 103, 106, 118]. The presence or absence of comorbid HS pathology does not affect LATE-NC staging. Further, HS is a diagnostic term that refers to distinctly different disease processes. For example, the diagnosis of HS is commonly applied in the context of persons with epilepsy, where the pathogenesis is very different (and lacks TDP-43 proteinopathy) in comparison to LATE-NC [118, 136, 142]. At autopsy, sensitive detection of HS requires bilateral sampling, because HS in LATE-NC is often unilateral [118, 161]. In hippocampi affected by severe HS, pyramidal cell dropout is extensive, and hippocampal atrophy can be extreme [6]; however, in some individuals with LATE-NC, cell loss is segmental (neuronal loss in some portions of the hippocampus and/or subiculum, but not others) [54]. These factors increase the likelihood of poor inter-rater agreement on the neuropathologic diagnosis of HS, and help explain the differing frequencies of HS reported in various autopsy series [34, 38, 73, 86, 88, 102, 118, 144, 159]. To fully standardize the pathologic diagnosis of HS, especially as it relates to differentiating LATE-NC + HS from other distinct disease processes (e.g., anoxic–ischemic episodes or seizures), will require additional work. Prior studies of HS-related histomorphology [45, 56, 160], and the recent work by Hokkanen et al. [47], provided methodologies to study HS pathologic features systematically.
In conclusion, despite challenges associated with a fast-moving research field, we found opportunities for recommendations to improve the precision of LATE-NC staging based on published reports and diagnostic experience. We emphasize that TDP-43 immunohistochemical assessment should be performed in all brain autopsies of older persons. The diagnosis of LATE-NC Stages requires sampling from three specific brain regions (Fig. 1, and Supplemental File 1, online resource). A specific rubric is presented in Table 1 and overall recommendations in Table 2. Recent literature indicates the scope of the challenge, even for this relatively simple pathologic staging system, as a wide variation of results was reported in the proportion of subjects with LATE-NC Stage 1 in a survey of community-based autopsy cohorts [113]. We hope that the present update will assist in efforts to increase standardization in the diagnosis of LATE-NC. As more observations are made across diverse autopsy cohorts, gathering detailed information on each case, including clinical, radiologic, laboratory, neuropathology, and genetic data, may facilitate future refinements of the classification and staging of LATE-NC.
Table 2.
LATE-NC Stages based on anatomic distribution of TDP-43 pathology: updates and clarifications for routine autopsy diagnoses
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are profoundly grateful to the research participants, caregivers, clinicians, staff, and colleague scientists who contributed to this work.
Abbreviations
- ADNC
Alzheimer’s disease neuropathologic change
- ALS
Amyotrophic lateral sclerosis
- CTE
Chronic traumatic encephalopathy
- FTD
Frontotemporal dementia
- FTLD
Frontotemporal lobar degeneration
- HS
Hippocampal sclerosis
- LATE-NC
Limbic-predominant age-related TDP-43 encephalopathy neuropathologic change
- NCI
Neuronal cytoplasmic inclusions
- TDP-43
Transactive response (TAR) DNA-binding protein of 43 kDa
Funding
We acknowledge National Institutes of Health Grants P30 AG072958 (S.-H. J.W.), P30 AG072977 (M.E.F.), K08 AG065463 (M.E.F.), RF1 AG072080 (M.E.F.), K08 AG 065426 (C.S.L), R01 AG038651 (E.L.A.), UF1 AG057707 (T.J.M and L.W), R01 AG021055 (C.K. & M.C.), P30 AG066519 (UCI ADRC), R01 AG061111 (P.T.N.), R01 AG057187 (P.T.N.), P30 AG072946 (P.T.N.), RF1 NS118584 (M.D.C.), P01 AG066597 (E.B.L.), P30 AG072979 (E.B.L.), U19 AG062418 (E.B.L.), P30 AG072959 (Cleveland ADRC), R01 AG062706 (S.A.S.), R01 AG054449 (M.E.M.), R01 AG075802 (M.E.M.), P30 AG 066507 (J.T.), U19 AG033655 (J.T.), RF1 AG069052 (J.G.R), P30 AG072972 (UC Davis ADRC), U19 AG069701 (M.E.M.), K24 AG053435 (L.T.G.), R01 AG067482 (J.A.S.), R01 AG064233 (J.A.S.), R01 AG022018 (J.A.S.), P30 AG010161/P30 AG072975 (J.A.S.), R01 AG064233 (K.A.), U01 AG061357 (B.N.D.), R01 AG052132 (B.N.D.), R01 AG056519 (B.N.D.), R01 AG062517 (B.N.D.), a research grant from the California Department Of Public Health (19-10611) with partial funding from the 2019 California Budget Act (B.N.D.), K23 AG062750 (H.-S. Y.), U19 AG024904 (K.E.S.), R21 AG078538 (K.E.S.), P30 AG 010133 (B.G. and K.N.), P30 AG062677 (Mayo ADRC), P30 AG066512 (T.W.), UF1 NS125417 (R.C.P.), U01 AG006786 (MCSA), R01 AG034676 (REP), P30 AG066509 (UW ADRC), and U19 AG066567 (ACT Study). Academy of Finland (341007) (L.M.); State funding for university-level health research (TYH2020231, TYH2022316) (L.M.); Liv och Hälsa Foundation (L.M.); Rossy Foundation and the Edmond Safra Philanthropic Foundation (G.G.K.); NOMIS foundation and Alzheimer Forschung Initiative (#21004) (M.N.); and, (#13803) (D.R.T.); UK Medical Research Council (MRC) (MRC/G9901400, U.1052.00.0013, G0900582). Addenbrooke’s Charitable Trust (S.H.), Addenbrooke’s Charitable Trust Grant 900108 (S.H.), Paul G. Allen Foundation (S.H.), ARUK NSG (S.H.), Alzheimer’s Society 554 (AS-PG-2019b-024) (S.H.), and the Nancy and Buster Alvord Endowment (C.D.K.). The Cambridge Brain Bank Laboratory is supported by the National Institute for Health Research, Cambridge Biomedical Research Centre. UK ARUK-PhD2014-19 (S.R.K.H.). Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO: G0F8516N, G065721N) (D.R.T.); Stichting Alzheimer Onderzoek België (SAO-FRA: 2020/017) (D.R.T.); KU-Leuven Internal Funds (Belgium) (C14/17/107; C14/22/132; C3/20/057) (D.R.T.), (PDMT2/21/069) (S.O.T.); BrightFocus Foundation (A2022019F) (S.O.T.). “Miguel Servet” program (CP19/00031) (M.J.G.) and a research grant (PI20/00613) (M.J.G.) of the Instituto de Salud Carlos III-Fondo Europeo de Desarrollo Regional (ISCIII-FEDER). Alzheimer’s Society (AS-JF-18-01; K.M.). Society for the Promotion of Research in Experimental Neurology, Vienna, Austria (K.J.)/Alzheimer’s Research UK (ARUK) doctoral studentship (ARUK-PhD2017-34) (R.M.). AMED under Grant Number JP22wm0425019 (to Y.S.).
Data availability statement
As this is a review/consensus paper, there are no data to be made available.
Declarations
Conflict of interest
E.B.L., D.W.D., M.N., D.R.T., G.H., G.G.K., and P.T.N. are members of the Editorial Board of Acta Neuropathologica and J.A. is the Editor-in-Chief of Acta Neuropathologica, but none of the coauthors were involved in the Editorial handling if this article. D.R.T. received speaker honorary from Novartis Pharma AG (Switzerland) and Biogen (USA), travel reimbursement from GE-Healthcare (UK) and UCB (Belgium), and collaborated with Novartis Pharma AG (Switzerland), Probiodrug (Germany), GE-Healthcare (UK), and Janssen Pharmaceutical Companies (Belgium).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.(2022) LATE 2022 Webinar, 11 February https://www.nia.nih.gov/research/dn/late-2022. Accessed 1 Nov 2022
- 2.Agirre-Beitia G, Moreno-Estebanez A, Gonzalez-Pinto Gonzalez T, Melgar Jimenez B, Campos Rodriguez I, Cabral Martinez L, et al. FOSMN: a possible TDP-43 proteinopathy to consider in a patient with facial sensory symptoms. Neurol Clin Pract. 2020;10:e47–e50. doi: 10.1212/CPJ.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal S, Leurgans SE, James BD, Barnes LL, Mehta RI, Dams-O'Connor K, et al. Association of traumatic brain injury with and without loss of consciousness with neuropathologic outcomes in community-dwelling older persons. JAMA Netw Open. 2022;5:e229311. doi: 10.1001/jamanetworkopen.2022.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal S, Yu L, Nag S, Arfanakis K, Barnes LL, Bennett DA, et al. The association of Lewy bodies with limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes and their role in cognition and Alzheimer’s dementia in older persons. Acta Neuropathol Commun. 2021;9:156. doi: 10.1186/s40478-021-01260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alafuzoff I, Pikkarainen M, Neumann M, Arzberger T, Al-Sarraj S, Bodi I, et al. Neuropathological assessments of the pathology in frontotemporal lobar degeneration with TDP43-positive inclusions: an inter-laboratory study by the BrainNet Europe consortium. J Neural Transm (Vienna) 2015;122:957–972. doi: 10.1007/s00702-014-1304-1. [DOI] [PubMed] [Google Scholar]
- 6.Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol (Berl) 2007;113:245–252. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP type A. Acta Neuropathol. 2015;129:53–64. doi: 10.1007/s00401-014-1358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai T. Significance and limitation of the pathological classification of TDP-43 proteinopathy. Neuropathology. 2014;34:578–588. doi: 10.1111/neup.12138. [DOI] [PubMed] [Google Scholar]
- 10.Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 11.Arnold SJ, Dugger BN, Beach TG. TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol. 2013;126:51–57. doi: 10.1007/s00401-013-1110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attems J, Toledo JB, Walker L, Gelpi E, Gentleman S, Halliday G, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol. 2021;141:159–172. doi: 10.1007/s00401-020-02255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayaki T, Murata K, Kanazawa N, Uruha A, Ohmura K, Sugie K, et al. Myositis with sarcoplasmic inclusions in Nakajo-Nishimura syndrome: a genetic inflammatory myopathy. Neuropathol Appl Neurobiol. 2020;46:579–587. doi: 10.1111/nan.12614. [DOI] [PubMed] [Google Scholar]
- 14.Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Blevins BL, Vinters HV, Love S, Wilcock DM, Grinberg LT, Schneider JA, et al. Brain arteriolosclerosis. Acta Neuropathol. 2020 doi: 10.1007/s00401-020-02235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyle PA, Wang T, Yu L, Wilson RS, Dawe R, Arfanakis K, et al. To what degree is late life cognitive decline driven by age-relatedneuropathologies? Brain. 2021 doi: 10.1093/brain/awab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA, et al. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol. 2019;85:114–124. doi: 10.1002/ana.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 19.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 20.Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buciuc M, Martin PR, Tosakulwong N, Murray ME, Petrucelli L, Senjem ML, et al. TDP-43-associated atrophy in brains with and without frontotemporal lobar degeneration. Neuroimage Clin. 2022;34:102954. doi: 10.1016/j.nicl.2022.102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buciuc M, Whitwell JL, Baker MC, Rademakers R, Dickson DW, Josephs KA. Old age genetically confirmed frontotemporal lobar degeneration with TDP-43 has limbic predominant TDP-43 deposition. Neuropathol Appl Neurobiol: 2021 doi: 10.1111/nan.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlos AF, Tosakulwong N, Weigand SD, Boeve BF, Knopman DS, Petersen RC, et al. Frequency and distribution of TAR DNA-binding protein 43 (TDP-43) pathology increase linearly with age in a large cohort of older adults with and without dementia. Acta Neuropathol. 2022 doi: 10.1007/s00401-022-02434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SH, Choi SM, Kim BC, Song WY, Kim HS, Lee KH. An autopsy-proven case of limbic-predominant age-related TDP-43 encephalopathy. Yonsei Med J. 2020;61:731–735. doi: 10.3349/ymj.2020.61.8.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chompoopong P, Milone M, Niu Z, Cui G, Mer G, Liewluck T. A novel missense HNRNPA1 variant in the PY-NLS domain in a patient with late-onset distal myopathy. Neuromuscul Disord. 2022;32:521–526. doi: 10.1016/j.nmd.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Chornenkyy Y, Fardo DW, Nelson PT. Tau and TDP-43 proteinopathies: kindred pathologic cascades and genetic pleiotropy. Lab Invest. 2019;99:993–1007. doi: 10.1038/s41374-019-0196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corey-Bloom J, Sabbagh MN, Bondi MW, Hansen L, Alford MF, Masliah E, et al. Hippocampal sclerosis contributes to dementia in the elderly. Neurology. 1997;48:154–160. doi: 10.1212/WNL.48.1.154. [DOI] [PubMed] [Google Scholar]
- 29.Cykowski MD, Arumanayagam AS, Powell SZ, Rivera AL, Abner EL, Roman GC, et al. Patterns of amygdala region pathology in LATE-NC: subtypes that differ with regard to TDP-43 histopathology, genetic risk factors, and comorbid pathologies. Acta Neuropathol. 2022 doi: 10.1007/s00401-022-02416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson YS, Raby S, Foulds PG, Robinson A, Thompson JC, Sikkink S, et al. TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol. 2011;122:703–713. doi: 10.1007/s00401-011-0879-y. [DOI] [PubMed] [Google Scholar]
- 31.Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 32.Dickson DW, Josephs KA, Amador-Ortiz C. TDP-43 in differential diagnosis of motor neuron disorders. Acta Neuropathol. 2007;114:71–79. doi: 10.1007/s00401-007-0234-5. [DOI] [PubMed] [Google Scholar]
- 33.Dickson DW, Rademakers R, Nicholson AM, Schneider JA, Yu L, Bennett DA. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology. 2015;85:1354–1355. doi: 10.1212/01.wnl.0000472918.79256.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dugan AJ, Nelson PT, Katsumata Y, Shade LMP, Boehme KL, Teylan MA, et al. Analysis of genes (TMEM106B, GRN, ABCC9, KCNMB2, and APOE) implicated in risk for LATE-NC and hippocampal sclerosis provides pathogenetic insights: a retrospective genetic association study. Acta Neuropathol Commun. 2021;9:152. doi: 10.1186/s40478-021-01250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fearon C, Koch M-L, Hefferman J, Heeney C, Lorigan J, Beausang A, et al. Phosphorylation-dependent TDP-43 antibodies: validation in a neurodegenerative brain bank cohort. J Alzheimer’s Parkinsonism Dement. 2019;3:1–4. [Google Scholar]
- 36.Flanagan ME, Cholerton B, Latimer CS, Hemmy LS, Edland SD, Montine KS, et al. TDP-43 neuropathologic associations in the nun study and the honolulu-asia aging study. J Alzheimers Dis. 2018;66:1549–1558. doi: 10.3233/JAD-180162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forrest SL, Wagner S, Kim A, Kovacs GG. Association of glial tau pathology and LATE-NC in the ageing brain. Neurobiol Aging. 2022;119:77–88. doi: 10.1016/j.neurobiolaging.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Gauthreaux KM, Teylan MA, Katsumata Y, Mock C, Culhane JE, Chen YC, et al. Limbic-predominant age-related TDP-43 encephalopathy: medical and pathologic factors associated with comorbid hippocampal sclerosis. Neurology. 2022;98:e1422–e1433. doi: 10.1212/WNL.0000000000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, et al. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol. 2010;67:1238–1250. doi: 10.1001/archneurol.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goossens J, Vanmechelen E, Trojanowski JQ, Lee VM, Van Broeckhoven C, van der Zee J, et al. TDP-43 as a possible biomarker for frontotemporal lobar degeneration: a systematic review of existing antibodies. Acta Neuropathol Commun. 2015;3:15. doi: 10.1186/s40478-015-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grothe MJ, Moscoso A, Silva-Rodriguez J, Lange C, Nho K, Saykin AJ, et al. Differential diagnosis of amnestic dementia patients based on an FDG-PET signature of autopsy-confirmed LATE-NC. Alzheimers Dement. 2022 doi: 10.1002/alz.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison WT, Lusk JB, Liu B, Ervin JF, Johnson KG, Green CL, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is independently associated with dementia and strongly associated with arteriolosclerosis in the oldest-old. Acta Neuropathol. 2021 doi: 10.1007/s00401-021-02360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, et al. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 44.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatanpaa KJ, Raisanen JM, Herndon E, Burns DK, Foong C, Habib AA, et al. Hippocampal sclerosis in dementia, epilepsy, and ischemic injury: differential vulnerability of hippocampal subfields. J Neuropathol Exp Neurol. 2014;73:136–142. doi: 10.1097/OPX.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 47.Hokkanen SRK, Hunter S, Polvikoski TM, Keage HAD, Minett T, Matthews FE, Group CCS et al. Hippocampal sclerosis, hippocampal neuron loss patterns and TDP-43 in the aged population. Brain Pathol. 2018;28:548–559. doi: 10.1111/bpa.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hokkanen SRK, Kero M, Kaivola K, Hunter S, Keage HAD, Kiviharju A, et al. Putative risk alleles for LATE-NC with hippocampal sclerosis in population-representative autopsy cohorts. Brain Pathol. 2020;30:364–372. doi: 10.1111/bpa.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, et al. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol. 2008;116:215–220. doi: 10.1007/s00401-008-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang W, Zhou Y, Tu L, Ba Z, Huang J, Huang N, et al. TDP-43: from Alzheimer’s disease to limbic-predominant age-related TDP-43 encephalopathy. Front Mol Neurosci. 2020;13:26. doi: 10.3389/fnmol.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunter S, Hokkanen SRK, Keage HAD, Fleming J, Minett T, Polvikoski T, Cambridge City over 75s Cohort c et al. TDP-43 related neuropathologies and phosphorylation state: associations with age and clinical Dementia in the Cambridge city over-75s cohort. J Alzheimers Dis. 2020;75:337–350. doi: 10.3233/JAD-191093. [DOI] [PubMed] [Google Scholar]
- 52.Ichimata S, Yoshida K, Visanji NP, Lang AE, Nishida N, Kovacs GG. Patterns of mixed pathologies in Down syndrome. J Alzheimers Dis. 2022;87:595–607. doi: 10.3233/JAD-215675. [DOI] [PubMed] [Google Scholar]
- 53.Igaz LM, Kwong LK, Xu Y, Truax AC, Uryu K, Neumann M, et al. Enrichment of C-terminal fragments in TAR DNA-binding protein-43 cytoplasmic inclusions in brain but not in spinal cord of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Am J Pathol. 2008;173:182–194. doi: 10.2353/ajpath.2008.080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ighodaro ET, Jicha GA, Schmitt FA, Neltner JH, Abner EL, Kryscio RJ, et al. Hippocampal sclerosis of aging can be segmental: two cases and review of the literature. J Neuropathol Exp Neurol. 2015;74:642–652. doi: 10.1097/NEN.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ighodaro ET, Nelson PT, Kukull WA, Schmitt FA, Abner EL, Caban-Holt A, et al. Challenges and considerations related to studying dementia in blacks/African Americans. J Alzheimers Dis. 2017;60:1–10. doi: 10.3233/JAD-170242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ihara R, Vincent BD, Baxter MR, Franklin EE, Hassenstab JJ, Xiong C, et al. Relative neuron loss in hippocampal sclerosis of aging and Alzheimer’s disease. Ann Neurol. 2018;84:741–753. doi: 10.1002/ana.25344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jamerlan A, An SSA. The influence of Abeta-dependent and independent pathways on TDP-43 proteinopathy in Alzheimer’s disease: a possible connection to LATE-NC. Neurobiol Aging. 2020;95:161–167. doi: 10.1016/j.neurobiolaging.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 58.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. 2016;139:2983–2993. doi: 10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jellinger KA. Hippocampal sclerosis: a common pathological feature of dementia in very old humans. Acta Neuropathol. 1994;88:599. doi: 10.1007/BF00296500. [DOI] [PubMed] [Google Scholar]
- 60.Johnson VE, Stewart W, Trojanowski JQ, Smith DH. Acute and chronically increased immunoreactivity to phosphorylation-independent but not pathological TDP-43 after a single traumatic brain injury in humans. Acta Neuropathol. 2011;122:715–726. doi: 10.1007/s00401-011-0909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Josephs KA, Mackenzie I, Frosch MP, Bigio EH, Neumann M, Arai T, et al. LATE to the PART-y. Brain. 2019;142:e47. doi: 10.1093/brain/awz224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Josephs KA, Murray ME, Tosakulwong N, Weigand SD, Serie AM, Perkerson RB, et al. Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol. 2019;137:227–238. doi: 10.1007/s00401-018-1951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, et al. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 2014;127:441–450. doi: 10.1007/s00401-013-1211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol. 2016;131:571–585. doi: 10.1007/s00401-016-1537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, et al. TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol. 2015;78:697–709. doi: 10.1002/ana.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, et al. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014;127:811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kadokura A, Yamazaki T, Kakuda S, Makioka K, Lemere CA, Fujita Y, et al. Phosphorylation-dependent TDP-43 antibody detects intraneuronal dot-like structures showing morphological characters of granulovacuolar degeneration. Neurosci Lett. 2009;463:87–92. doi: 10.1016/j.neulet.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 70.Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology. 2009;29:566–573. doi: 10.1111/j.1440-1789.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 71.Katsumata Y, Fardo DW, Kukull WA, Nelson PT. Dichotomous scoring of TDP-43 proteinopathy from specific brain regions in 27 academic research centers: associations with Alzheimer’s disease and cerebrovascular disease pathologies. Acta Neuropathol Commun. 2018;6:142. doi: 10.1186/s40478-018-0641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keage HA, Hunter S, Matthews FE, Ince PG, Hodges J, Hokkanen SR, et al. TDP-43 pathology in the population: prevalence and associations with dementia and age. J Alzheimers Dis. 2014;42:641–650. doi: 10.3233/JAD-132351. [DOI] [PubMed] [Google Scholar]
- 73.Kero M, Raunio A, Polvikoski T, Tienari PJ, Paetau A, Myllykangas L. Hippocampal sclerosis in the oldest old: a finnish population-based study. J Alzheimers Dis. 2018;63:263–272. doi: 10.3233/JAD-171068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein HU, Trumpff C, Yang HS, Lee AJ, Picard M, Bennett DA, et al. Characterization of mitochondrial DNA quantity and quality in the human aged and Alzheimer’s disease brain. Mol Neurodegener. 2021;16:75. doi: 10.1186/s13024-021-00495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kokubo Y, Morimoto S, Sasaki R, Hasegawa M, Ishiura H, Tsuji S, et al. An immigrant family with Kii amyotrophic lateral sclerosis/parkinsonism-dementia complex. Neurol Sci. 2022;43:1423–1425. doi: 10.1007/s10072-021-05737-7. [DOI] [PubMed] [Google Scholar]
- 76.Koper MJ, Tome SO, Gawor K, Belet A, Van Schoor E, Schaeverbeke J, et al. LATE-NC aggravates GVD-mediated necroptosis in Alzheimer’s disease. Acta Neuropathol Commun. 2022;10:128. doi: 10.1186/s40478-022-01432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koper MJ, Van Schoor E, Ospitalieri S, Vandenberghe R, Vandenbulcke M, von Arnim CAF, et al. Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol. 2020;139:463–484. doi: 10.1007/s00401-019-02103-y. [DOI] [PubMed] [Google Scholar]
- 78.Koyano S, Yagishita S, Tada M, Doi H, Uchihara T, Tanaka F. Parallel appearance of polyglutamine and transactivation-responsive DNA-binding protein 43 and their complementary subcellular localization in brains of patients with spinocerebellar ataxia type 2. J Neuropathol Exp Neurol. 2022;81:535–544. doi: 10.1093/jnen/nlac032. [DOI] [PubMed] [Google Scholar]
- 79.Kwong LK, Irwin DJ, Walker AK, Xu Y, Riddle DM, Trojanowski JQ, et al. Novel monoclonal antibodies to normal and pathologically altered human TDP-43 proteins. Acta Neuropathol Commun. 2014;2:33. doi: 10.1186/2051-5960-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lachner C, Day GS, Camsari GB, Kouri N, Ertekin-Taner N, Boeve BF et al (2022) Cancer and Vascular Comorbidity Effects on Dementia Risk and Neuropathology in the Oldest- Old. J Alzheimers Dis. 90(1):405-417. 10.3233/JAD-220440 [DOI] [PMC free article] [PubMed]
- 81.Latimer CS, Burke BT, Liachko NF, Currey HN, Kilgore MD, Gibbons LE, et al. Resistance and resilience to Alzheimer's disease pathology are associated with reduced cortical pTau and absence of limbic-predominant age-related TDP-43 encephalopathy in a community-based cohort. Acta Neuropathol Commun. 2019;7:91. doi: 10.1186/s40478-019-0743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee EB. Integrated neurodegenerative disease autopsy diagnosis. Acta Neuropathol. 2018;135:643–646. doi: 10.1007/s00401-018-1827-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee EB, Lee VM, Trojanowski JQ, Neumann M. TDP-43 immunoreactivity in anoxic, ischemic and neoplastic lesions of the central nervous system. Acta Neuropathol. 2008;115:305–311. doi: 10.1007/s00401-007-0331-5. [DOI] [PubMed] [Google Scholar]
- 84.Lee EB, Porta S, Michael Baer G, Xu Y, Suh E, Kwong LK, et al. Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotemporal degeneration. Acta Neuropathol. 2017;134:65–78. doi: 10.1007/s00401-017-1679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leverenz JB, Agustin CM, Tsuang D, Peskind ER, Edland SD, Nochlin D, et al. Clinical and neuropathological characteristics of hippocampal sclerosis: a community-based study. Arch Neurol. 2002;59:1099–1106. doi: 10.1001/archneur.59.7.1099. [DOI] [PubMed] [Google Scholar]
- 86.Leverenz JB, Lipton AM. Clinical aspects of hippocampal sclerosis. Handb Clin Neurol. 2008;89:565–567. doi: 10.1016/S0072-9752(07)01252-3. [DOI] [PubMed] [Google Scholar]
- 87.Lin WL, Castanedes-Casey M, Dickson DW. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol. 2009;68:1167–1176. doi: 10.1097/NEN.0b013e3181baacec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lopez OL, Kofler J, Chang Y, Berman SB, Becker JT, Sweet RA, et al. Hippocampal sclerosis, TDP-43, and the duration of the symptoms of dementia of AD patients. Ann Clin Transl Neurol. 2020;7:1546–1556. doi: 10.1002/acn3.51135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mackenzie IR, Frick P, Neumann M. The neuropathology associated with repeat expansions in the C9ORF72 gene. Acta Neuropathol. 2014;127:347–357. doi: 10.1007/s00401-013-1232-4. [DOI] [PubMed] [Google Scholar]
- 90.Mackenzie IR, Neumann M. Reappraisal of TDP-43 pathology in FTLD-U subtypes. Acta Neuropathol. 2017;134:79–96. doi: 10.1007/s00401-017-1716-8. [DOI] [PubMed] [Google Scholar]
- 91.Mackenzie IR, Neumann M. Subcortical TDP-43 pathology patterns validate cortical FTLD-TDP subtypes and demonstrate unique aspects of C9orf72 mutation cases. Acta Neuropathol. 2020;139:83–98. doi: 10.1007/s00401-019-02070-4. [DOI] [PubMed] [Google Scholar]
- 92.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McAleese KE, Walker L, Erskine D, Johnson M, Koss D, Thomas AJ, et al. Concomitant LATE-NC in Alzheimer’s disease is not associated with increased tau or amyloid-beta pathological burden. Neuropathol Appl Neurobiol. 2020;46:722–734. doi: 10.1111/nan.12664. [DOI] [PubMed] [Google Scholar]
- 96.McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melikyan ZA, Corrada MM, Leiby AM, Sajjadi SA, Bukhari S, Montine TJ, et al. Cognitive resilience to three dementia-related neuropathologies in an oldest-old man: a case report from the 90+ study. Neurobiol Aging. 2022;116:12–15. doi: 10.1016/j.neurobiolaging.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mol MO, van Rooij JGJ, Wong TH, Melhem S, Verkerk A, Kievit AJA, et al. Underlying genetic variation in familial frontotemporal dementia: sequencing of 198 patients. Neurobiol Aging. 2021;97:148e149–148e116. doi: 10.1016/j.neurobiolaging.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 100.Montine TJ, Corrada MM, Kawas C, Bukhari S, White L, Tian L, et al. Association of cognition and dementia with neuropathologic changes of Alzheimer disease and other conditions in the oldest-old. Neurology. 2022 doi: 10.1212/WNL.0000000000200832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murakami A, Koga S, Sekiya H, Oskarsson B, Boylan K, Petrucelli L, et al. Old age amyotrophic lateral sclerosis and limbic TDP-43 pathology. Brain Pathol. 2022 doi: 10.1111/bpa.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murray ME, Bieniek KF, Banks Greenberg M, DeJesus-Hernandez M, Rutherford NJ, van Blitterswijk M, et al. Progressive amnestic dementia, hippocampal sclerosis, and mutation in C9ORF72. Acta Neuropathol. 2013;126:545–554. doi: 10.1007/s00401-013-1161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128:411–421. doi: 10.1007/s00401-014-1302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nag S, Barnes LL, Yu L, Wilson RS, Bennett DA, Schneider JA. Limbic-predominant age-related TDP-43 encephalopathy in Black and White decedents. Neurology. 2020;95:e2056–e2064. doi: 10.1212/WNL.0000000000010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nag S, Yu L, Boyle PA, Leurgans SE, Bennett DA, Schneider JA. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol Commun. 2018;6:33. doi: 10.1186/s40478-018-0531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, et al. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol. 2015;77:942–952. doi: 10.1002/ana.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nag S, Yu L, Wilson RS, Chen EY, Bennett DA, Schneider JA. TDP-43 pathology and memory impairment in elders without pathologic diagnoses of AD or FTLD. Neurology. 2017;88:653–660. doi: 10.1212/WNL.0000000000003610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nascimento C, Di Lorenzo Alho AT, Bazan Conceicao Amaral C, Leite REP, Nitrini R, Jacob-Filho W, et al. Prevalence of transactive response DNA-binding protein 43 (TDP-43) proteinopathy in cognitively normal older adults: systematic review and meta-analysis. Neuropathol Appl Neurobiol. 2018;44:286–297. doi: 10.1111/nan.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM, et al. Higher prevalence of TDP-43 proteinopathy in cognitively normal asians: a clinicopathological study on a multiethnic sample. Brain Pathol. 2016;26:177–185. doi: 10.1111/bpa.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nelson PT, Abner EL, Patel E, Anderson S, Wilcock DM, Kryscio RJ, et al. The amygdala as a locus of pathologic misfolding in neurodegenerative diseases. J Neuropathol Exp Neurol. 2018;77:2–20. doi: 10.1093/jnen/nlx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nelson PT, Brayne C, Flanagan ME, Abner EL, Agrawal S, Attems J, et al. Frequency of LATE neuropathologic change across the spectrum of Alzheimer’s disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts. Acta Neuropathol. 2022 doi: 10.1007/s00401-022-02444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019 doi: 10.1093/brain/awz099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, et al. Reply: LATE to the PART-y. Brain. 2019;142:e48. doi: 10.1093/brain/awz226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nelson PT, Gal Z, Wang WX, Niedowicz DM, Artiushin SC, Wycoff S, et al. TDP-43 proteinopathy in aging: Associations with risk-associated gene variants and with brain parenchymal thyroid hormone levels. Neurobiol Dis. 2019;125:67–76. doi: 10.1016/j.nbd.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang WX, Neltner JH, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 2013;126:161–177. doi: 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Neumann M, Frick P, Paron F, Kosten J, Buratti E, Mackenzie IR. Antibody against TDP-43 phosphorylated at serine 375 suggests conformational differences of TDP-43 aggregates among FTLD-TDP subtypes. Acta Neuropathol. 2020;140:645–658. doi: 10.1007/s00401-020-02207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neumann M, Lee EB, Mackenzie IR. Frontotemporal lobar degeneration TDP-43-immunoreactive pathological subtypes: clinical and mechanistic significance. Adv Exp Med Biol. 2021;1281:201–217. doi: 10.1007/978-3-030-51140-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 123.Nguyen ML, Huie EZ, Whitmer RA, George KM, Dugger BN. Neuropathology studies of dementia in US persons other than non-hispanic whites. Free Neuropathol. 2022 doi: 10.17879/freeneuropathology-2022-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nishihira Y, Gefen T, Mao Q, Appin C, Kohler M, Walker J, et al. Revisiting the utility of TDP-43 immunoreactive (TDP-43-ir) pathology to classify FTLD-TDP subtypes. Acta Neuropathol. 2019;138:167–169. doi: 10.1007/s00401-019-02024-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pao WC, Dickson DW, Crook JE, Finch NA, Rademakers R, Graff-Radford NR. Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord. 2011;25:364–368. doi: 10.1097/WAD.0b013e31820f8f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Postupna N, Rose SE, Gibbons LE, Coleman NM, Hellstern LL, Ritchie K, et al. The delayed neuropathological consequences of traumatic brain injury in a community-based sample. Front Neurol. 2021;12:624696. doi: 10.3389/fneur.2021.624696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Power MC, Mormino E, Soldan A, James BD, Yu L, Armstrong NM, et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol. 2018;84:10–22. doi: 10.1002/ana.25246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prater KE, Latimer CS, Jayadev S. Glial TDP-43 and TDP-43 induced glial pathology, focus on neurodegenerative proteinopathy syndromes. Glia. 2022;70:239–255. doi: 10.1002/glia.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Riku Y, Iwasaki Y, Ishigaki S, Akagi A, Hasegawa M, Nishioka K, et al. Motor neuron TDP-43 proteinopathy in progressive supranuclear palsy and corticobasal degeneration. Brain. 2022 doi: 10.1093/brain/awac091. [DOI] [PubMed] [Google Scholar]
- 130.Robinson JL, Corrada MM, Kovacs GG, Dominique M, Caswell C, Xie SX, et al. Non-Alzheimer’s contributions to dementia and cognitive resilience in the 90+ study. Acta Neuropathol. 2018 doi: 10.1007/s00401-018-1872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robinson JL, Porta S, Garrett FG, Zhang P, Xie SX, Suh E, et al. Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration. Brain. 2020;143:2844–2857. doi: 10.1093/brain/awaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Robinson JL, Richardson H, Xie SX, Suh E, Van Deerlin VM, Alfaro B, et al. The development and convergence of co-pathologies in Alzheimer’s disease. Brain. 2021;144:953–962. doi: 10.1093/brain/awaa438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sakuwa M, Adachi T, Suzuki Y, Yoshida K, Fukuda H, Miura H, et al. First Japanese autopsy case showing LRRK2 mutation G2019S and TDP-43 proteinopathy. Parkinsonism Relat Disord. 2021;91:85–87. doi: 10.1016/j.parkreldis.2021.09.009. [DOI] [PubMed] [Google Scholar]
- 134.Saveri P, Magri S, Maderna E, Balistreri F, Lombardi R, Ciano C, et al. DNAJB2-related Charcot-Marie-Tooth disease type 2: pathomechanism insights and phenotypic spectrum widening. Eur J Neurol. 2022;29:2056–2065. doi: 10.1111/ene.15326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smirnov DS, Salmon DP, Galasko D, Edland SD, Pizzo DP, Goodwill V, et al. TDP-43 pathology exacerbates cognitive decline in primary age-related tauopathy. Ann neurol. 2022 doi: 10.1002/ana.26438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sommer W. Erkrankung des Ammon’s horn als aetiologis ches moment der epilepsien. Arch Psychiatr Nurs. 1880;10:631–675. doi: 10.1007/BF02224538. [DOI] [Google Scholar]
- 137.Tan RH, Kril JJ, Fatima M, McGeachie A, McCann H, Shepherd C, et al. TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain. 2015;138:3110–3122. doi: 10.1093/brain/awv220. [DOI] [PubMed] [Google Scholar]
- 138.Tazwar M, Evia AM, Tamhane AA, Ridwan AR, Leurgans SE, Bennett DA, et al. Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is associated with lower R2 relaxation rate: an ex-vivo MRI and pathology investigation. Neurobiol Aging. 2022;117:128–138. doi: 10.1016/j.neurobiolaging.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thal DR, Del Tredici K, Ludolph AC, Hoozemans JJ, Rozemuller AJ, Braak H, et al. Stages of granulovacuolar degeneration: their relation to Alzheimer's disease and chronic stress response. Acta Neuropathol. 2011;122:577–589. doi: 10.1007/s00401-011-0871-6. [DOI] [PubMed] [Google Scholar]
- 140.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/WNL.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 141.Thal DR, von Arnim C, Griffin WS, Yamaguchi H, Mrak RE, Attems J, et al. Pathology of clinical and preclinical Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 2013;263(Suppl 2):S137–145. doi: 10.1007/s00406-013-0449-5. [DOI] [PubMed] [Google Scholar]
- 142.Thom M. Hippocampal sclerosis: progress since Sommer. Brain Pathol. 2009;19:565–572. doi: 10.1111/j.1750-3639.2008.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tome SO, Vandenberghe R, Ospitalieri S, Van Schoor E, Tousseyn T, Otto M, et al. Distinct molecular patterns of TDP-43 pathology in Alzheimer’s disease: relationship with clinical phenotypes. Acta Neuropathol Commun. 2020;8:61. doi: 10.1186/s40478-020-00934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Trieu T, Sajjadi SA, Kawas CH, Nelson PT, Corrada MM. Risk factors of hippocampal sclerosis in the oldest old: the 90+ study. Neurology. 2018;91:e1788–e1798. doi: 10.1212/WNL.0000000000006455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Troncoso JC, Kawas CH, Chang CK, Folstein MF, Hedreen JC. Lack of association of the apoE4 allele with hippocampal sclerosis dementia. Neurosci Lett. 1996;204:138–140. doi: 10.1016/0304-3940(96)12331-4. [DOI] [PubMed] [Google Scholar]
- 146.Uchino A, Takao M, Hatsuta H, Sumikura H, Nakano Y, Nogami A, et al. Incidence and extent of TDP-43 accumulation in aging human brain. Acta Neuropathol Commun. 2015;3:35. doi: 10.1186/s40478-015-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Uemura MT, Robinson JL, Cousins KAQ, Tropea TF, Kargilis DC, McBride JD, et al. Distinct characteristics of limbic-predominant age-related TDP-43 encephalopathy in Lewy body disease. Acta Neuropathol. 2021 doi: 10.1007/s00401-021-02383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, et al. Concomitant TAR-DNA-binding in Alzheimer disease and protein 43 pathology is present corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Van Mossevelde S, Engelborghs S, van der Zee J, Van Broeckhoven C. Genotype-phenotype links in frontotemporal lobar degeneration. Nat Rev Neurol. 2018;14:363–378. doi: 10.1038/s41582-018-0009-8. [DOI] [PubMed] [Google Scholar]
- 151.Van Schoor E, Koper MJ, Ospitalieri S, Dedeene L, Tome SO, Vandenberghe R, et al. Necrosome-positive granulovacuolar degeneration is associated with TDP-43 pathological lesions in the hippocampus of ALS/FTLD cases. Neuropathol Appl Neurobiol. 2021;47:328–345. doi: 10.1111/nan.12668. [DOI] [PubMed] [Google Scholar]
- 152.Walker AK, Daniels CM, Goldman JE, Trojanowski JQ, Lee VM, Messing A. Astrocytic TDP-43 pathology in Alexander disease. J Neurosci. 2014;34:6448–6458. doi: 10.1523/JNEUROSCI.0248-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang SJ, Guo Y, Ervin JF, Lusk JB, Luo S. Neuropathological associations of limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) differ between the oldest-old and younger-old. Acta Neuropathol. 2022;144:45–57. doi: 10.1007/s00401-022-02432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wegiel J, Flory M, Kuchna I, Nowicki K, Wegiel J, Ma SY, et al. Developmental deficits and staging of dynamics of age associated Alzheimer’s disease neurodegeneration and neuronal loss in subjects with Down syndrome. Acta Neuropathol Commun. 2022;10:2. doi: 10.1186/s40478-021-01300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia aging study participants. Ann NY Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 156.Wider C, Dickson DW, Stoessl AJ, Tsuboi Y, Chapon F, Gutmann L, et al. Pallidonigral TDP-43 pathology in perry syndrome. Parkinsonism Relat Disord. 2009;15:281–286. doi: 10.1016/j.parkreldis.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yamashita R, Beck G, Yonenobu Y, Inoue K, Mitsutake A, Ishiura H, et al. TDP-43 proteinopathy presenting with typical symptoms of Parkinson’s disease. Mov Disord. 2022 doi: 10.1002/mds.29048. [DOI] [PubMed] [Google Scholar]
- 158.Zabar Y, Carson KA, Troncoso JC, Kawas CH. Dementia due to hippocampal sclerosis: clinical features and comparison to Alzheimer’s disease. Neurology. 1998;50(4):A59–A60. [Google Scholar]
- 159.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–370. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]
- 160.Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L, et al. Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol. 2005;57:896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zarow C, Weiner MW, Ellis WG, Chui HC. Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample. Brain Behav. 2012;2:435–442. doi: 10.1002/brb3.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang X, Sun B, Wang X, Lu H, Shao F, Rozemuller AJM, et al. Phosphorylated TDP-43 staging of primary age-related tauopathy. Neurosci Bull. 2018 doi: 10.1007/s12264-018-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As this is a review/consensus paper, there are no data to be made available.