Abstract
Currently, the term “heart failure with preserved left ventricular ejection fraction (HFpEF)” is based on echocardiographic parameters and clinical symptoms combined with elevated or normal levels of natriuretic peptides. Thus, “HFpEF” as a diagnosis subsumes multiple pathophysiological entities making a uniform management plan for “HFpEF” impossible. Therefore, a more specific characterization of the underlying cardiac pathologies in patients with preserved ejection fraction and symptoms of heart failure is mandatory. The present proposal seeks to offer practical support by a standardized echocardiographic workflow to characterize specific diagnostic entities associated with “HFpEF”. It focuses on morphological and functional cardiac phenotypes characterized by echocardiography in patients with normal or preserved left ventricular ejection fraction (LVEF). The proposal discusses methodological issues to clarify why and when echocardiography is helpful to improve the diagnosis. Thus, the proposal addresses a systematic echocardiographic approach using a feasible algorithm with weighting criteria for interpretation of echocardiographic parameters related to patients with preserved ejection fraction and symptoms of heart failure. The authors consciously do not use the diagnosis “HFpEF” to avoid misunderstandings.
Graphical abstract
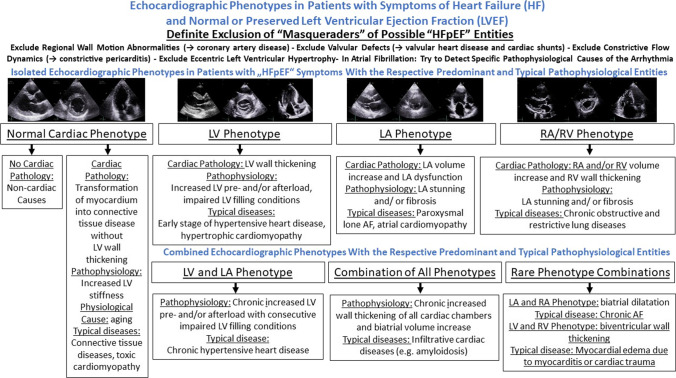
Central illustration: Scheme illustrating the characteristic echocardiographic phenotypes and their combinations in patients with “HFpEF” symptoms with respect to the respective cardiac pathology and pathophysiology as well as the underlying typical disease
Keywords: Echocardiography, HFpEF, Ventricular elasticity, Left ventricular hypertrophy, Speckle tracking, Diagnostic workflow
Introduction
The analysis of left ventricular (LV) function in patients with symptoms of heart failure (HF) is usually performed by transthoracic echocardiography (TTE) as the primary imaging technique in clinical cardiology [1, 2]. Many HF patients with high normal or elevated natriuretic peptides present with a preserved or normal LV ejection fraction (LVEF) above 50% and are currently classified as “heart failure with preserved LVEF” (HFpEF) [3–7]. However, HFpEF should not be considered as a diagnosis, but rather as a syndrome [8]. Multiple pathologic entities may present with the same phenotype “HFpEF”, but with different comorbidities and varying outcomes [9]. Thus, the real diagnostic challenge of TTE in these patients is not to merely measure LVEF, but to characterize the underlying cardiac pathologies and to precisely obtain a specific diagnosis.
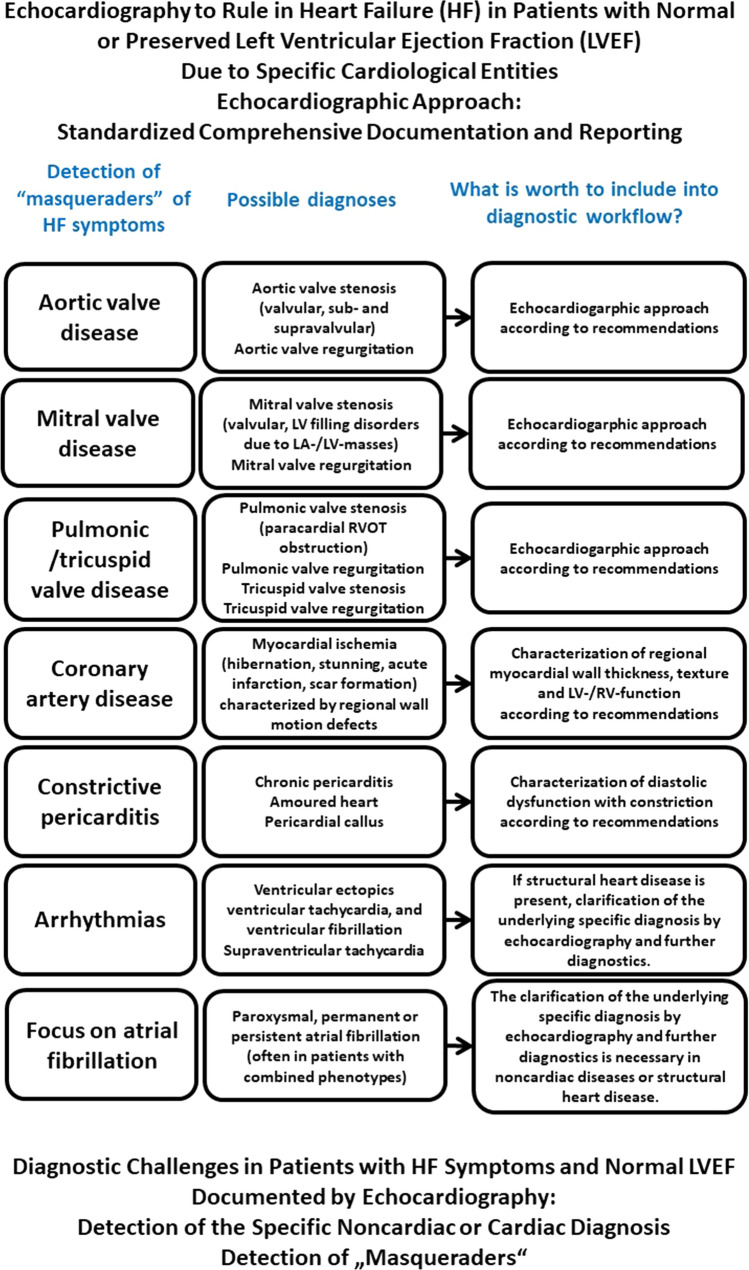
“Diastolic HF” was initially defined as “an increased resistance to filling in one or both ventricles, leading to symptoms of pulmonary congestion due to an inappropriate upward shift of the diastolic pressure–volume relation” [3]. Later it was replaced by the term “HFpEF” [5, 6]. The incidence and prevalence of “HFpEF” symptoms approximately constitute half of all HF patients [2, 7]. Recently, diagnostic algorithms have been introduced to characterize patients with preserved LVEF and HF symptoms by the hypernym “HFpEF” as a common diagnosis despite different underlying pathologies and treatment options, as well as varied prognosis [7, 9, 10]. The “HFpEF” algorithms are based on clinical complaints, laboratory findings, echocardiographic data and/or invasive hemodynamic measurements [4, 7], which often do not lead to the specific underlying diagnosis [11–18]. If cardiac diagnoses like heart valve disease, significant coronary artery disease, pericardial constriction, et cetera are detected, these specific diagnoses are labeled as “HFpEF masqueraders” and must be excluded [7]. Arrhythmias—most often symptoms due to specific cardiac diagnoses—are also described as “HFpEF” masqueraders, except for atrial fibrillation (AF) which is very frequently observed in patients with “HFpEF” symptoms [19, 20]. To be fair, the exclusion of “HFpEF masqueraders” at best results in the diagnosis “HFpEF” with unknown origin. However, there are several other cardiologic diagnoses like myocarditis, myocardial infarction with non-obstructive coronary artery disease, restrictive and infiltrative cardiomyopathies contributing to the dilemma of multiple components of etiological variety in patients with “HFpEF” symptoms [21–26]. Furthermore, non-cardiac diseases—especially pulmonary diseases, anemia, diabetes, systemic infections, consuming neoplasms, obesity, and frailty—complicate the multifactorial hodgepodge of “HFpEF” as a diagnosis [27–32].
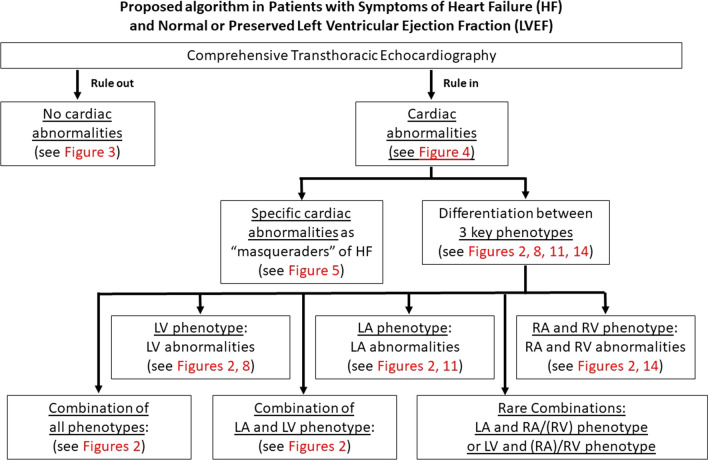
Thus, the present expert proposal focuses on a diagnostic TTE workflow to overcome diagnostic challenges and to increase options for the detection of specific cardiac entities in patients with HF symptoms and preserved LVEF (Fig. 1). With respect to conventional and modern echocardiographic tools the authors try to highlight, why, when, and how to implement special echocardiographic features into clinical practice. In addition, the proposal underlines new aspects, which are presumably worth to be included into the workflow.
Fig. 1.
Scheme illustrating the echocardiographic workflow to characterize patients with “HFpEF” symptoms due to cardiac phenotypes
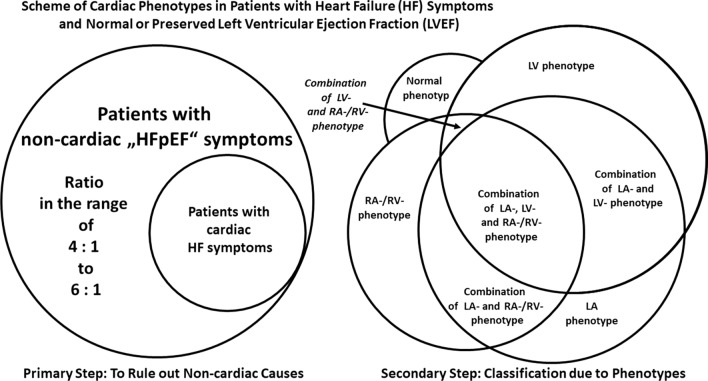
TTE guiding to diagnose specific cardiac pathologies commonly reflects the initial contact of patients undergoing diagnostic cardiological procedures prior to laboratory findings, prior to invasive measurements, and sometimes even prior to interpretation of the electrocardiogram. Thus, the focus of a detailed morphological and functional characterization of cardiac entities by TTE plays a key role of cardiac diagnostics. With respect to morphological and functional alterations of cardiac chambers, the following cardiac phenotypes are outlined (Fig. 2):
The normal cardiac phenotype which can be associated with increased LV stiffness in patients with HFpEF symptoms,
The LV (= left ventricular) phenotype which is associated with morphological or functional abnormalities of the LV wall,
The LA (left atrial) phenotype which is solely associated with an increase of LA size and consecutive impairment of LA function,
The RA (= right atrial) and RV (= right ventricular) phenotype which is solely associated with morphological and functional abnormalities of right cardiac chambers,
The combination of LA and LV phenotype,
The combination of all three phenotypes and
Rare combinations of LA and RA/(RV) phenotype or LV and (RA)/RV phenotype.
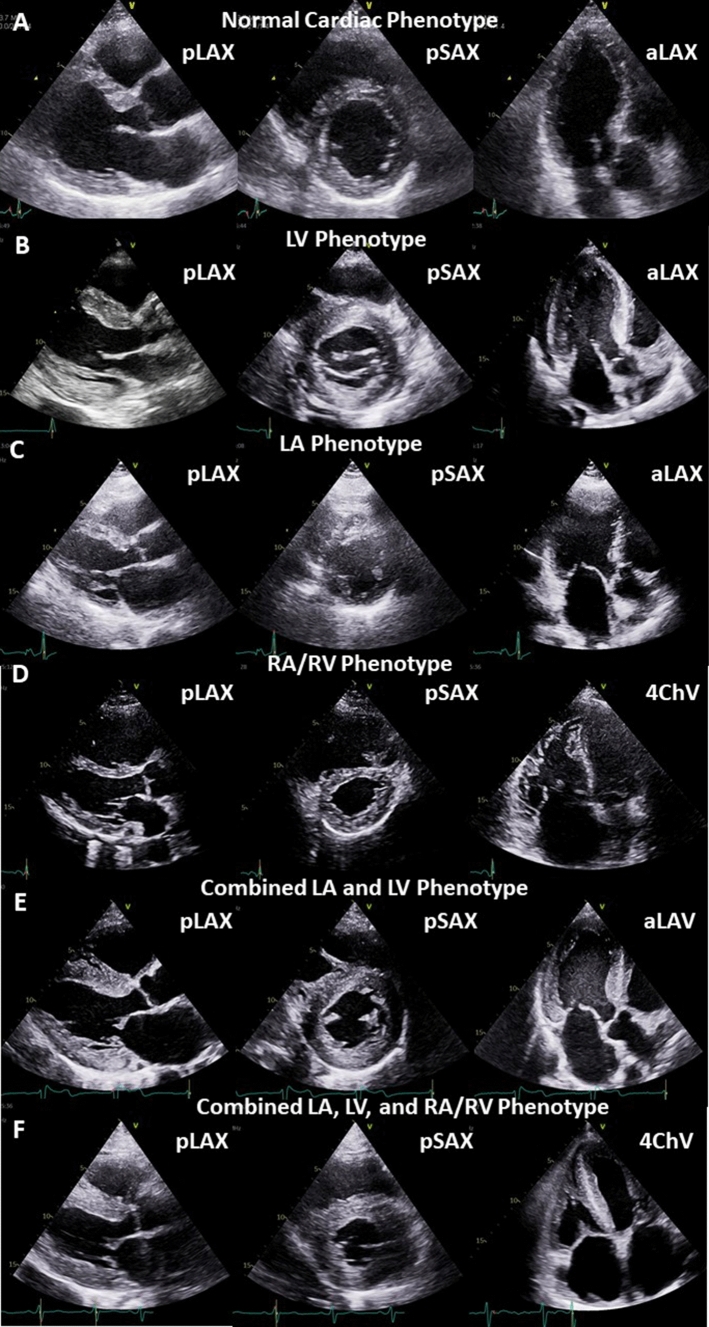
Fig. 2.
Illustration of cardiac phenotypes in patients with “HFpEF” symptoms by echocardiography. The rows illustrate representative parasternal long and short axis views (pLAX, pSAX) and apical long axis views (aLAX) or apical 4-chamber views (4ChV) of the respective phenotypes. In A the normal cardiac phenotype is shown, in B the isolated LV phenotype (in compensated hypertensive heart disease—short history), in C the isolated LA phenotype (in paroxysmal lone atrial fibrillation), in D the isolated RA/RV phenotype (in chronic pulmonary hypertension due to repetitive pulmonary thromboembolism), in E the combined LA and LV phenotype (in compensated hypertensive heart disease—long history), and in F the combined LA, LV, and LA/RV phenotype (in amyloidosis)
The introduction of isolated echocardiographic phenotypes is proposed with respect to diagnostic systematics to focus on the predominant morphological or functional cardiac abnormality. The classification of patients with “HFpEF” symptoms according to these morphological and functional cardiac entities is still challenging due to the complex interactions of pathophysiological factors on the heart. Hence, majority of patients with “HFpEF” symptoms have combinations of these echocardiographic phenotypes.
The characteristic echocardiographic phenotypes and their respective combinations are depicted with respect to the representative diseases in the central illustration (central illustration). In addition, the cardiac pathology and pathophysiology as well as typical representative diseases are highlighted to demonstrate the diagnostic pathway depending on echocardiographic morphological classification.
Targets of echocardiography in patients with heart failure and preserved ejection fraction
A proper LVEF assessment is the first diagnostic step to identify HF patients with preserved LVEF. The recommended quantitative LVEF assessment is mostly performed by biplane planimetry using the modified Simpson method [33]. The two-dimensional approach from the apical four- and two-chamber view often results in underestimation of LV cavity size by apical foreshortening. Nowadays, standardization of apical LV views can be ensured using triplane or multidimensional TTE [33–35]. Thus, these modern modalities should be generally preferred, especially in the presence of regional wall motion abnormalities and pathological LV geometry. According to recent recommendations the parameter LVEF is still predominantly used to differentiate between normal, preserved, mildly, moderately, or severely reduced LV function [2]. Total LV stroke volume (LVSVtot) measured by 2D planimetry or 3D volumetry reflects effective (systemic) LV stroke volume (LVSVeff) if patients with significant aortic and/or mitral regurgitation are excluded. Stroke volume can also be estimated using Doppler echocardiography in the LV and right ventricular (RV) outflow tract (LVOT, RVOT). LVSVtot and LVSVeff should be in the same range if no relevant valvular heart disease is present and can be counterchecked to verify hemodynamic plausibility of the measurements [33–35]. In case of limited acoustic windows, optimization of LV endocardial border detection by contrast echocardiography should be considered for LV volume assessment [33, 35, 36].
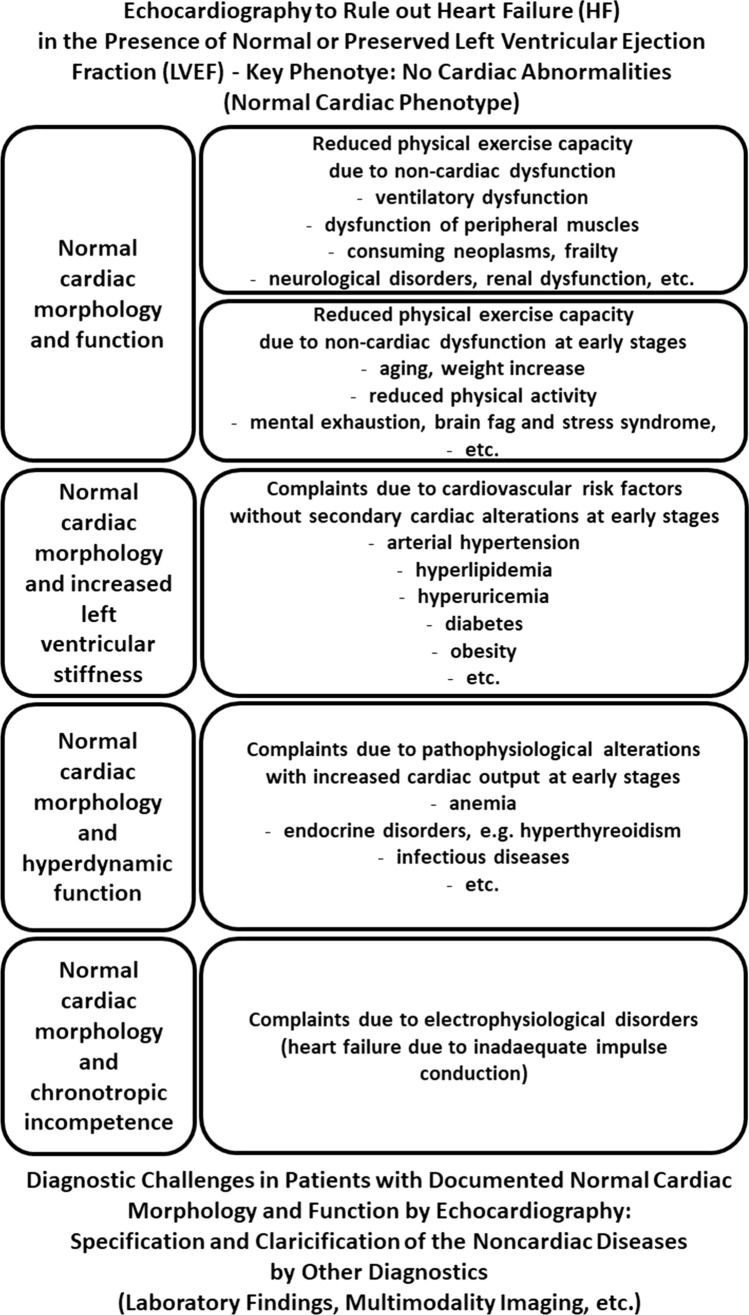
The first integral component of HF diagnostics by TTE in patients with “HFpEF” symptoms (predominantly dyspnea or disability) is to rule-out or to rule-in HF as the reason for clinical symptoms [2]. Cardiac morphology and function should be classified in relation to the cardiac phenotypes and their combinations to structure the echocardiographic workflow cardiac in patients with “HFpEF” symptoms (Fig. 2). If no abnormalities are detected by conventional echocardiography, a “normal cardiac phenotype” refers to a non-cardiac entity of the “HFpEF” symptoms (Figs. 3, 4). The pathophysiological reason for “HFpEF” symptoms in “normal cardiac phenotype” patients can be explained by the mismatch between oxygen supply and oxygen demand due to high output stages, chronotropic incompetence, and/or subclinical stages of unknown cardiac diseases. Non-cardiac disease-related “HFpEF” symptoms might be caused by a high cardiac output (CO) due anemia, hyperthyroidism, liver cirrhosis, infectious diseases, and/or obesity due to different pathophysiologies. Notably, the measurement of CO is mandatorily in patients with dyspnea and normal cardiac phenotype if LV hypercontractility and brady- or tachycardia is observed. The problem of the echocardiographic “normal cardiac phenotype” is to miss early echocardiographic signs related to LV and left atrial (LA) wall thickness, stiffness, and function. Thus, this scenario describes the diagnostic challenge to detect early or preclinical disease stages with subtle echocardiographic signs of pathological cardiac phenotypes.
Fig. 3.
Scheme of echocardiographic workflow describing the tool to rule out cardiac manifestations as the primary cause of “HFpEF” symptoms
Fig. 4.
Scheme of the ratio between patients with noncardiac and cardiac HF symptoms (left)—scheme of the interactions/combinations between cardiac phenotypes in patients with “HFpEF” symptoms
The proposed pathological echocardiographic phenotypes can be subdivided into a LV phenotype, a LA phenotype, and a combined right atrial (RA) and RV phenotype (Figs. 1, 2). The diagnostic aspects of these pathological phenotypes in patients with “HFpEF” symptoms are described in detail in the following paragraphs. They address the second step of TTE after the rule-in of HF as the cause of the symptoms. We believe that this proposed classification of echocardiographic phenotypes will help to improve the identification of a specific underlying diagnosis for HF. The third task of echocardiography in HF patients is to monitor specific treatment effects during follow up and to estimate individual patient’s prognosis.
The echocardiographic characterization of patient with “HFpEF” symptoms using recent “HFpEF” algorithms: what is useful?
“New” paradigms—e. g. the identification of a systemic proinflammatory state induced by comorbidities as the cause of myocardial structural and functional alterations—have been proposed to explain the genesis of “HFpEF” symptoms [10, 11] and new scores have been introduced to overcome the challenges of diagnostics in patients with “HFpEF” symptoms [13–18].
After LVEF determination the second pivotal finding by TTE in patients with “HFpEF” symptoms is the increased ratio of E/E´ (E = early mitral flow velocity, E´ = early tissue Doppler lengthening velocity of the myocardium near to the mitral annulus) [4, 7]. Interestingly, increased NT-proBNP- and BNP levels (NT-proBNP = N-terminal-pro brain natriuretic peptide, BNP = brain natriuretic peptide) are not necessarily measured in patients characterized by these echocardiographic criteria. Invasive hemodynamic measurements of PCWP, LVEDP (PCWP = pulmonary capillary wedge pressure, LVEDP = left ventricular end diastolic pressure), the time constant of ventricular relaxation τ and LV chamber stiffness are proposed as an alternative to prove cardiac cause of “HFpEF” symptoms, but often not performed [2, 4, 7]. Thus, echocardiography should implement additional parameters beside the documentation of E´, E/E´, and systolic pulmonary artery pressure (sPAP) to characterize diastolic dysfunction (DD) in patients with “HFpEF” symptoms [37–42].
According to recent recommendations, the second target in patients with “HFpEF” symptoms is the detection of the “masqueraders” (Fig. 5), as described above [4, 7]. The list of “masqueraders” might be complemented by all specific diagnoses which can be detected by a comprehensive echocardiography to minimize the usage of “HFpEF” with unknown origin as a final diagnosis. In addition, the underlying diagnoses in the presence of arrhythmias—including AF—should also be clarified in patients with HF symptoms and preserved LVEF.
Fig. 5.
Scheme of the echocardiographic workflow in the presence of “HFpEF masqueraders”. “HFpEF masqueraders” are specific detected diagnoses in patients with “HFpEF” symptoms
After ruling out patients with non-cardiac causes and excluding those with a known specific cardiac diagnosis the remaining cohort of patients is composed of patients with hypertensive heart diseases, infiltrative/storage diseases, and unknown specific diagnoses. Because clinical and echocardiographic abnormalities can be observed in subgroups of this patient cohort several phenotypes have also been introduced in the literature, i. e. phenotypes associated with LA abnormalities, with pulmonary hypertension and pulmonary disease in the presence of RV dysfunction, with obesity combined with cardiac abnormalities, and with the ischemia/microvascular dysfunction [39, 43–46]. However, several further entities like aging, myocardial LA and LV stiffness, systemic hypertension, high output stages, and lone AF can be added illustrating the diversity within this remaining cohort of patients with HF symptoms and preserved LVEF.
Thus, we like to propose the following phenotypes, solely described by echocardiographic findings: the LV phenotype, the LA phenotype, and the combined RA and RV phenotype (Fig. 2).
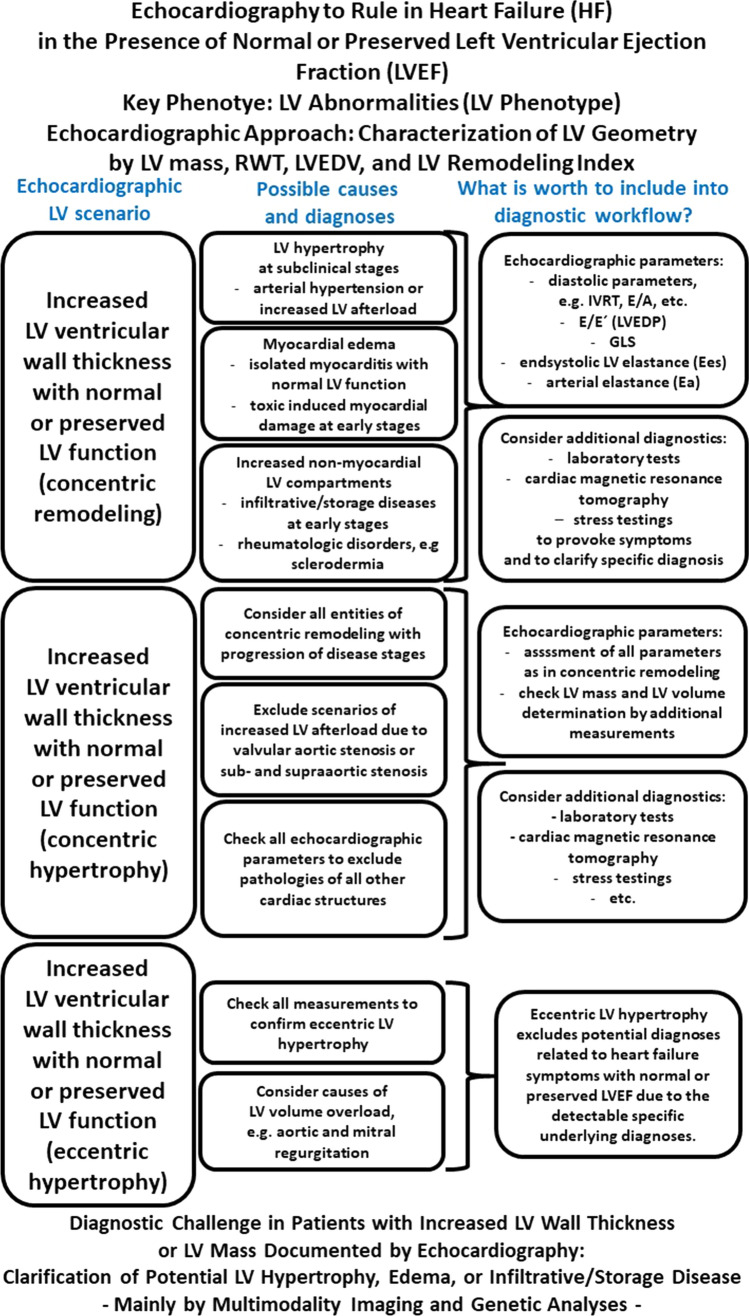
The LV phenotype: abnormalities of LV volumes, LV function, LV geometry, and LV mass
Conventional echocardiographic analyses of LV wall thickness, LV volumes and LVEF
This phenotype addresses LV abnormalities characterized by TTE. Despite LV planimetry or LV volumetry is possible in native 2D- and 3D echocardiography with adequate image quality endocardial contour delineation can often be improved by LV opacification using contrast to avoid underestimation of LV volumes [33–36, 47]. Modern modalities of triplane and 3D echocardiography are strongly recommended to avoid foreshortening which leads to smaller LV volumes and underestimation of LV function. Triplane and 3D echocardiography modalities are particularly useful in patients with regional wall motion abnormalities and pathological LV geometry.
The conventional echocardiographic M-Mode and 2D parameters characterizing LV dimensions, LV wall thickness, relative wall thickness (RWT), LV mass are most useful and reproducible in normal LV geometry and during normal cardiac conditions [33, 48]. If LV abnormalities are detectable and if the acoustic window is appropriate, LV wall thickness, LV volumes, LV wall stress and LV remodeling index can also be assessed by 3D echocardiography to distinguish between normal conditions, LV remodeling, concentric and eccentric LV hypertrophy [33–36, 47]. Standardization of M-Mode measurements can be achieved by anatomical M-Mode in biplane image acquisition to provide appropriate sectional planes through the LV center rather than LV secants, and LV measurements perpendicular to the LV long axis at the level of the mitral valve (MV) leaflet tips. A high spatial resolution using the best acoustic window usually enables the exclusion of right and left ventricular trabecula for appropriate delineation of LV wall diameters.
Strain analyses of GLS and myocardial work
Specific echocardiographic modalities should be used to demarcate subclinical and/or subtle findings as a screening tool in this predefined patient cohort. Global longitudinal strain (GLS) is nowadays an established parameter to characterize LV function as well as prognosis [49, 50]. Global circumferential and global radial strain (GCS, GRS) are also detectable by speckle tracking echocardiography, but are still not generally used. Regional myocardial dysfunction due to local ischemia, scar, hypocontractility or dyssynchrony can be visualized and quantified by the segmental 2D strain curves, which are often showing disease specific deformation patterns. When compared to the conventional LVEF assessment, the quantification of GLS is much more sensitive for the detection of subtle impairment of LV function like in early stages of infiltrative/storage diseases—especially in patients with amyloidosis—as many pathologies tend to primarily affect the longitudinal LV deformation [51, 52]. The conventional evaluation of LV longitudinal deformation by M-mode measurement of mitral annular plane systolic excursion (MAPSE) is a valid surrogate marker for GLS, but without regional/segmental information, thus GLS by 2DS should be preferred [53]. The different strain distribution patterns are associated with different underlying pathologies. The presence of subclinical fibrosis, e.g. in hypertensive heart disease, toxic, immune, inflammatory, infiltrative, metabolic, genetic and endomyocardial myocardial pathologies, typically affects more the basal and mid LV segments, [7, 49, 54], while the “apical sparing” pattern with a preserved regional strain only in the apical segments is typically seen in patients with cardiac amyloidosis. Predominant alterations of regional circumferential and radial strain are observed in patients with myocardial edema due to myocarditis or cardiotoxic agents [55–57]. Thus, the analysis of the systolic LV contraction patterns should include the analysis of all interacting compounds of LV deformation.
LV function must be considered in relation to LV contraction, which is strongly dependent on pre- and afterload. Myocardial work can be non-invasively analyzed by pressure–strain loops to (partially) overcome the load-dependent limitations of LVEF and GLS. The parameter global work index (GWI), global constructive work (GCW), global wasted work (GWW), and global work efficiency (GWE) can be determined, if blood pressure is added to the strain analysis measured simultaneous to the TTE [58, 59]. The pressure–strain loop area reflects myocardial metabolic demand and oxygen consumption providing information about myocardial energetics [58, 59]. In patients with “HFpEF” symptoms and normal cardiac phenotype or LV phenotype differentiation between hypertensive and coronary heart disease is facilitated because myocardial work incorporates deformation and loading conditions into the analysis [58–61]. Thus, strain patterns with regional decreased longitudinal strain correspond to myocardial work patterns, whereas normalization of myocardial work patterns can be observed in hypertensive heart disease.
Analysis of LV stiffness, LV elastance, and arterial elastance
Doppler and tissue Doppler echocardiographic parameters have been evaluated in comparison to invasive measurements to estimate diastolic function and LV filling pressures favoring the echocardiographic parameter of the lateral E´ [62]. However, the estimation of LV filing pressures solely by echocardiographic parameters has several limitations, especially in patients with regional LV wall motion abnormalities [63, 64]. In patients with “HFpEF” symptoms, a further approach to detect subtle alterations of the LV myocardium might be the assessment of diastolic stiffness, myocardial relaxation, and resulting LV contractility by the analysis of LV end-diastolic volume (LVEDV) in relation to LVEDP [65–67]. The invasive LV pressure–volume relationship is recognized as the gold standard to analyze these parameters. It requires the generation of several pressure–volume loops in different preload states. The approximately linear relationship of their end-systolic pressure–volume points represents the end-systolic pressure volume relationship (ESPVR). The slope of the ESPVR defines the end-systolic elastance (Ees), which serves as a measure of myocardial contractility (Fig. 6). Compared to LVEF or GLS, Ees exhibits a significant correlation to increased global myocardial afterload characterized by arterial elastance (Ea). The increased steepness and left-shift of ESPVR with increasing inotropy represents the myocardial homeometric autoregulation known as Anrep effect [68–70]. Graphically, Ea is reflected by the negative slope between the end-systolic pressure–volume point and the end-diastolic volume at a LV pressure of 0 mmHg (Fig. 6). Ea incorporates arterial compliance and vascular resistance but is also affected by heart rate. In healthy individuals, Ea and Ees are well connected to provide optimal mechanical ventricular-arterial coupling with an Ea/Ees ratio between 0.5 and 1.0 [71]. The indirect measurement of Ees has been developed using a single-beat non-invasive approach to calculate Ees and Ea from pressure and volume measurements during one cardiac cycle [72, 73]. Specifically, the pressure–volume loop is computed from LVEDV and LV end-systolic volume (LVESV), measured by TTE, and blood pressure, measured non-invasively. For Ees calculation, systolic time intervals, defined by pre-ejection period and systolic ejection time, are determined from pulsed-waved Doppler in the LVOT with simultaneous ECG recording. Non-invasive single beat pressure–volume analysis has been applied to study the pathophysiology, prognosis [65–67] as well as the effect of therapeutic HF interventions [74–76]. However, due to the complexity of non-invasive Ea and Ees determination this method is not commonly used and needs further evaluation.
Fig. 6.
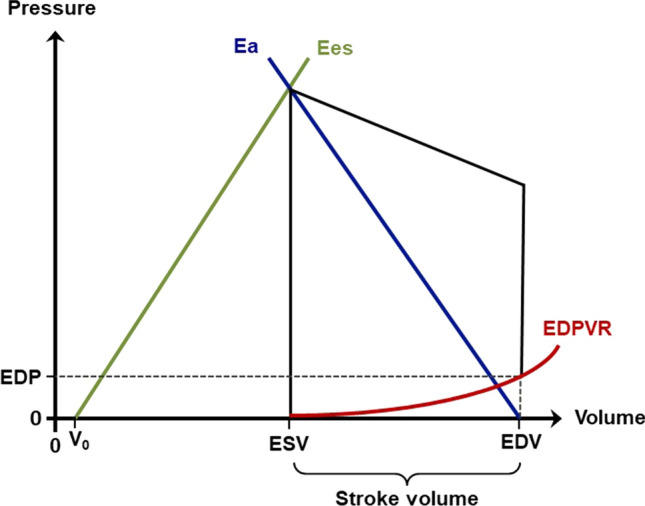
Scheme of a non-invasive LV pressure volume loop to explain the calculations of the end-systolic pressure volume relationship (ESPVR) by its slope which defines the end-systolic elastance (Ees), and by the slope between the end-systolic pressure–volume point and the end-diastolic volume at a LV pressure of 0 mmHg, which defines arterial elastance (Ea) representing LV afterload. EDP, end-diastolic pressure; EDPVR, end-diastolic pressure volume relationship; EDV, end-diastolic volume; ESV, end-systolic volume; V0, x-axis intercept of Ees
In HF patients with reduced ejection fraction (HFrEF), Ees is reduced due to impaired myocardial contraction, while Ea might be elevated despite normal blood pressure. Hence, Ea/Ees increases in HFrEF patients, indicating ineffective arterial-ventricular coupling (Fig. 7) [66, 71]. An important hemodynamic characteristic in patients with normal LV size and normal or increased LV wall thickness and “HFpEF” symptoms (normal cardiac phenotype and LV phenotype) and in HFrEF patients is the response in LV ejection to changes in afterload. In HFrEF patients, LV stroke volume is more sensitive for afterload changes than in the non-failing heart or in this defined phenotype of patients with “HFpEF” symptoms—which can be labelled as an increased LV stiffness phenotype—because the slope of ESPVR (Ees) is flat (Fig. 7). Thus, afterload reduction, for instance using vasodilators, reduces Ea, which is frequently elevated in HFrEF patients, but lowers blood pressure only slightly. Thus, LVSVtot increases. In increased LV stiffness phenotype Ees is high or normal because LV contractility is preserved (Fig. 7). Vasodilators reduce Ea and blood pressure significantly but LVSVtot would remain relatively constant [77]. Both patients with hypertensive heart disease and with “HFpEF” symptoms show increased Ea values, and Ees increases to compensate for elevated Ea. Thus, ventricular-arterial coupling remains in the normal range. When HF symptoms occur, patients with “HFpEF” symptoms are characterized by increased myocardial stiffness and reduced myocardial contractility—both impact Ees—keeping Ees at a high level. Hence, Ees does not specifically characterize patients with “HFpEF” symptoms nor does it predict outcome [65].
Fig. 7.
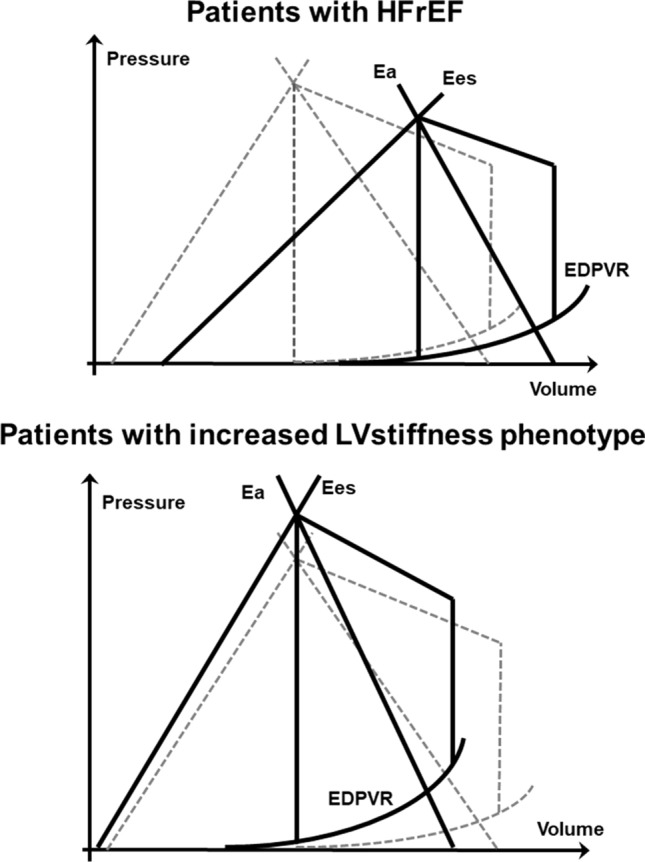
Characteristic pressure–volume loops in patients with heart failure with reduced (HFpEF; top)and preserved (HFpEF; bottom) ejection fraction. In HFrEF patients, end-systolic elastance (Ees) is reduced and LV volumes are increased, thus LV pressure–volume loop is right-ward shifted. In patients with “HFpEF” symptoms and increased LV stiffness, LV end-diastolic volume is reduced due to concentric remodeling, which leads to a leftward shifted end-diastolic pressure volume relationship (EDPVR), i. e. increased LV filling. Ea is frequently elevated, Ees normal or increased. The pressure–volume curves of a non-failing heart with its EDPVR, Ees and Ea is drawn with dotted lines. The schemes explain the increase of LV stroke volume (LVSVtot) in HFrEF patients and the minor changes of LVSVtot in patients with “HFpEF” symptoms with afterload reduction
The single-beat method to assess Ees and Ea allows also to calculate the end-diastolic pressure–volume relationship (EDPVR) (Fig. 6) [78]. EDPVR is built from echocardiographic measurement of LVEDVs and the calculation of LVEDP, derived from Doppler and tissue Doppler (E/E’) parameters [78]. Thus, the increased LV stiffness phenotype is also characterized by increased diastolic stiffness and impaired myocardial relaxation, leading to reduced LVEDVs at similar LVEDPs compared to patients with hypertension without HF symptoms and healthy controls [79]. In consequence, the EDPVR curve is leftward-shifted in patients with “HFpEF” symptoms; in contrast, EDPVR is rightward-shifted in HFrEF patients compared to non-heart failure patients (Fig. 7). Both alterations of EDPVR have prognostic implications [67, 80].
In summary, non-invasive single-beat analysis of the LV pressure–volume relationship is based on proper measurements by TTE in combination with simultaneous non-invasive blood pressure measurements [72, 73, 78]. It allows to evaluate LV systolic and diastolic function, LV afterload, and ventricular–arterial coupling. The pressure–volume analysis of the LV provides important insights into the specific pathophysiology and hemodynamics in patients with “HFpEF” symptoms defining a LV stiffness sub-phenotype. The analysis of LV stiffness might be helpful to detect subclinical stages of cardiac diseases, but still needs validation in further clinical studies.
Table 1 summarizes the echocardiographic parameters, which are important to differentiate between the normal cardiac phenotype and the LV phenotype. The parameters are listed including normal ranges and cut offs [33, 72, 81–88], methodological aspects of TTE determination, their importance to be determined, and their reasons, why it is worth to determine the respective parameter in clinical routine. The intention of the tables is to list these important echocardiographic parameters for the sake of completeness as an easy reference for the user. Thus, the authors do not mandatory propose to measure of all these parameters.
Table 1.
Echocardiographic parameters characterizing patients with heart failure (HF) symptoms and normal or preserved left ventricular ejection fraction (LVEF) in normal cardiac phenotype and the left ventricular (LV) phenotype
| Echocardiographic parameter | normal ranges—cut offs | Methodological aspects | Mandatory to determine (methods) | Why worth to do in routine |
|---|---|---|---|---|
| LV parameters | ||||
| LVEDD—left ventricular end diastolic diameter (mm) |
♂ 36–56 cut off ≤ 58 ♀ 35–51 cut off ≤ 52 |
2D or anatomical M-Mode: avoid oblique measurements using conventional M-Mode, define the LV level at MV leaflet tips | Yes | To estimate LV size |
| LVESD—left ventricular end sytolic diameter (mm) |
♂ 21–41 cut off ≤ 40 ♀ 21–37 cut off ≤ 35 |
2D or anatomical M-Mode: see above | Yes | To estimate LV size |
| LVEDV—left ventricular end diastolic volume (ml) |
♂ 72–204 cut off ≤ 204 ♀ 61–144 cut off ≤ 144 [81] |
2D planimetry (biplane or triplane) or 3D volumetry: if regional wall motion defects are present use triplane or 3D volumetry, avoid foreshortening | Yes | To estimate LVEDV—especially in LV remodeling |
| LVESV—left ventricular end systolic volume (ml) |
♂ 28–83 cut off ≤ 83 ♀ 21–61 cut off ≤ 61 [81] |
2D planimetry (biplane or triplane) or 3D volumetry: see above | Yes | To estimate LVEF and total LVSV |
| LVSVtot (total left ventricular stroke volume) = LVSVeff (effective left ventricular stroke volume) |
♂ 41–115 cut off > 41 ♀ 36–88 cut off > 36 [81] |
2D planimetry (biplane or triplane) or 3D volumetry: see above | Yes | To estimate LVEF and total LVSV, which corresponds to effective LVSV, if AV and MV are normal |
| DLVOT (mm)—diameter of the left ventricular outflow tract (LVOT) |
♂ 18–26 ♀ 17–23 [85] |
2D imaging—biplane imaging preferred with respect to verifiable standardization | Yes—under certain conditions | To estimate LVSVeff and cardiac output (CO) |
| Cardiac output (CO) determined by LVSVeff using pulsed waved (pw) Doppler x heart rate (ml/l)—LVSVeff = 0.785 × DLVOT2 × VTILVOT (velocity time integral determined in the LVOT) |
♂ 3.5–8.2 ♀ 3.3–7.3 [88] |
Proper positioning of the pw sample volume in relation to the DLVOT is mandatory—angulation of the cursor parallel to the blood stream is mandatory | Yes—under certain conditions |
To estimate LVSVeff and CO To compare LVSVtot and LVSVeff |
| LVEF—left ventricular ejection fraction (%) |
♂ 49–67 cut off ≥ 55 ♀ 51–79 cut off ≥ 52 |
2D planimetry (biplane or triplane) or 3D volumetry: see above | Yes | To estimate normal or preserved LVEF |
| LVEDV/BSA (body surface area) (ml/m2) |
♂ 41–97 cut off < 75 ♀ 39–82 cut off < 62 |
Determination of BSA by body height and body weight, 2D planimetry (biplane or triplane) or 3D volumetry: see above | Yes—under certain conditions | To adjust LV volumes in extreme conditions |
| LVESV/BSA (ml/m2) |
♂ 16–42 cut off < 32 ♀ 14–35 cut off < 25 |
Determination of BSA by body height and body weight, 2D planimetry (biplane or triplane) or 3D volumetry: see above | Yes—under certain conditions | To adjust LV volumes in extreme conditions |
| EDLVs—enddiastolic septal LV wall thickness (mm) |
♂6–12 cut off < 12 ♀ 5–11 cut off < 11 [85] |
2D or anatomical M-Mode: avoid oblique measurements, avoid inclusion of RV and LV trabecula, define the LV level at MV leaflet tips | Yes | To detect or exclude LV hypertrophy |
| EDLVp—enddiastolic posterior LV wall thickness (mm) |
♂ 6–13 cut off < 13 ♀ 6–12 cut off < 12 [85] |
2D or anatomical M-Mode: see above | Yes | To detect or exclude LV hypertrophy |
| RWT (relative wall thickness) = 2 EDLVp/LVEDD - |
♂ 0.24 – 0.42 cut off < 0.42 ♀ 0.22 – 0.42 cut off < 0.42 [33] |
2D or anatomical M-Mode: see above | Yes | To detect remodeling and to distinguish between concentric and eccentric LVH |
| LV mass (g)—linear method (Cube formula)—3D analysis (3D based formula) (formula see [8]) |
♂ 88–224 cut off ≤ 224 ♀ 67–162 cut off ≤ 162 |
2D or anatomical M-Mode or 3D volumetry: if image quality is good, 3D assessment is preferred | Yes—under certain conditions | To determine severity of LVH |
| LV mass/BSA (g/m2)—linear method (Cube formula)—3D analysis (3D based formula) (formula see [8]) |
♂ 49–115 cut off ≤ 102 ♀ 43–95 cut off ≤ 88 |
Determination of BSA by body height and body weight, 3D volumetry: if image quality is good, 3D assessment is preferred | Yes | To determine severity of LVH |
|
LVRI—left ventricular remodeling index (g/ml) = LV mass/LVEDV (formula see [4]) |
0.79–1.27 cut off < 0.79 (dcm) cut off > 1.27 (lvh) [83] |
2D or anatomical M-Mode or by 3D volumetry | Yes—under certain conditions | To detect dilative or hypertrophic cardiomyopathy |
| Ees—LV elastance (mmHg/ml) (formula see [17]) |
7–20 cut off < 20 [72] |
Blood pressure measurement and verifiable LV volume measurements are necessary | Optional—but helpful to detect subclinical states of cardiac diseases | To detect LV stiffness—especially in symptomatic patients with normal LV geometry or increased LV wall thickness |
| Ea—arterial elastance (mmHg/ml) (formula see [17]) |
− 4 to − 14 cut off > − 14 [72] |
Blood pressure measurement and verifiable LV volume measurements are necessary | Optional—but helpful to detect subclinical states of cardiac diseases | To detect arterial stiffness (and see above) |
| MAPSE (mm) |
♂ 10–18 cut off > 8 ♀ 8–20 cut off > 8 [82] |
Assessment is possible by postprocessing of 2D cineloops of the 4ChV, not useful in MR and septal or lateral myocardial infarction | Optional—but helpful to detect subclinical states of cardiac diseases | To detect reduced longitudinal LV function, increased LV stiffness, and early stages of systolic dysfunction |
| MAPSE/TAPSE |
♂ 0.45–0.81 cut off > 0.45 ♀ 0.36–1.00 cut off > 0.36 [82] |
Assessment is possible by postprocessing of 2D cineloops of the 4ChV, not useful in MR, TR and septal or lateral myocardial or right ventricular infarction | Optional—but helpful to detect subclinical states of cardiac diseases | To detect LV fibrosis and interventricular abnormal mitral and tricuspid annular motion |
| GLS (%)—global longitudinal strain |
♂ − 17–27 cut off > − 17 ♀–18–28 cut off > -18 [87] |
LV strain analysis is only reliable in patients with adequate image quality. Avoid artefact tracking due to minor image quality | Yes | To detect reduced longitudinal LV function and pathological LV strain patterns at early stages |
| GWI (mmHg%)—global work index |
♂ 1270–2428 cut off > 1270 ♀ 1310–2538 cut off > 1310 [86] |
Blood pressure measurement and respective image quality for LV strain analysis are prerequisites | Optional—but helpful to detect subclinical states of cardiac diseases | To distinguish between CAD and HHD |
| GCW (mmHg%)—global constructive work |
♂1650–2807 cut off > 1650 ♀ 1543–2924 cut off > 1543 [86] |
Blood pressure measurement and respective image quality for LV strain analysis are prerequisites | Optional—but helpful to detect subclinical states of cardiac diseases | To distinguish between CAD and HHD. To detect asynchrony |
| GWW (mmHg%)—global wasted work |
♂ 94–271 cut off < 238 ♀ 74–278 cut off < 239 [86] |
Blood pressure measurement and respective image quality for LV strain analysis are prerequisites | Optional—but helpful to detect subclinical states of cardiac diseases | To distinguish between CAD and HHD. To detect asynchrony |
| GWE (mmHg%)—global work efficiency |
♂ 88–97 cut off > 88 ♀ 90–97 cut off > 90 [86] |
Blood pressure measurement and respective image quality for LV strain analysis are prerequisites | Optional—but helpful to detect subclinical states of cardiac diseases | To distinguish between CAD and HHD. To detect asynchrony |
For each echocardiographic parameter the normal ranges (if age dependent for elderlies more than 60 years) (and cut offs), methodological aspects, the importance of its determination, and the value to determine it in routine are listed. Mandatory parameters to be determined in clinical practice are labeled in bold print
LVEDD left ventricular end diastolic diameter, LVESD left ventricular end systolic diameter, LVEDV left ventricular end diastolic volume, LVESV left ventricular end systolic volume, LVSVtot total left ventricular stroke volume, LVSVeff effective left ventricular stroke volume, LVOT left ventricular outflow tract, DLVOT diameter of the LVOT, CO cardiac output, VTILVOT velocity time integral determined in the LVOT, LVEF left ventricular ejection fraction, BSA body surface area, EDLVs enddiastolic septal LV wall thickness, EDLVp enddiastolic posterior LV wall thickness, RWT relative wall thickness, LVRI left ventricular remodeling index, Ees LV elastance, Ea arterial elastance, MAPSE mitral annular plane systolic excursion, TAPSE tricuspid annular plane systolic excursion, GLS global longitudinal strain, GWI global work index, GCW global constructive work, GWW global wasted work, GWE global work efficiency
Figure 8 summarizes the echocardiographic workflow in patients with “HFpEF” symptoms presenting normal cardiac phenotype or LV phenotype.
Fig. 8.
Scheme of the echocardiographic workflow in patients with “HFpEF” symptoms to characterize the echocardiographic LV phenotype and to exclude normal cardiac phenotype
The LA phenotype: abnormalities of left atrial (LA) volumes and LA function (diastolic dysfunction and increased LV filling pressures)
Increased stiffness of the LV wall impedes LV filling and increases LA pressure. This leads to the distinct morphological and functional LA alterations: the “LA phenotype” (Fig. 2).
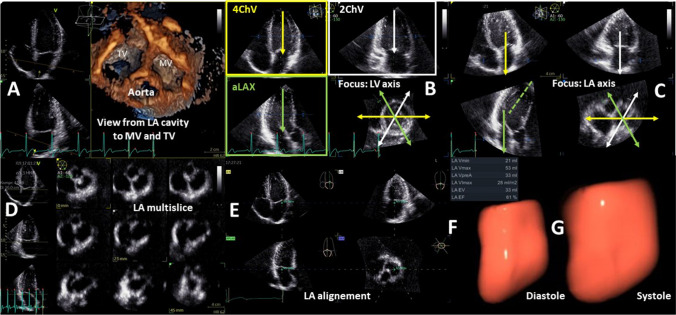
The chronic increase in LA pressure results in progressive LA dilatation with increased volumes. Traditionally, LA size is measured in the anterior–posterior dimension from parasternal views. However, in pathologic states the LA frequently enlarges predominantly in the long-axis direction (base-apex). Therefore, the assessment of LA volume during pathological conditions should at least be performed by biplane planimetry from apical views. Volumetry of the complete LA by 3D TTE is preferred because centering of the longitudinal LA dimensions in standardized apical LV views may lead to foreshortening and systematic over- or underestimation of LA volumes when compared to 3D data sets (Fig. 9).
Fig. 9.
Illustration about the necessity for 3D-LA assessment. A presents the enface view from the LA cavity to the MV and TV. In B the apical 3D views (4ChV, 2ChV—apical 4- and 2-chamber view; aLAX—apical long axis view) within the 3D data set are centered to the LV illustrating the axis deviation of the LA mainly in the aLAX. In C the LA long axis is centered to document the deviation of LV an LA center line. In D a multisclice presentation of the 3D data set illustrate the complete acquisition of the LA volume. In E the LA alignment for LA volume determination is shown. In F the diastolic LA volume, in G the systolic LA volume is presented by a 3D volume calculation
Biplane LA volume measurement indexed to body surface area (BSA) is the currently recommended standard. The obtained LA volume index (LAVI) has been associated with cardiovascular outcomes [33]. A maximum LAVI > 34 ml/m2 documents an increased LA volume [2, 4, 7, 33, 35, 48]. Different echocardiographic parameters of LA function can be described by conventional LA volume measurements: the maximum LA volume (LAVmax) measured at LV end systole (onset of MV opening), the minimum LA volume (LAVmin) at LV end diastole (prior to MV closure), and the LA volume prior to LA contraction at the onset of P wave (LAVpreA). The echocardiographic parameters characterizing LA function and the respective calculations are listed in Table 2 [53, 89].
Table 2.
Echocardiographic parameters characterizing LA function and their calculations by conventional LA volume and LA strain determination
| Echocardiographic parameter | LA function (LA strain) | Calculation (by volumes) |
|---|---|---|
|
Total LA emptying fraction (%) Normal range: 63–71 [53] |
Global LA reservoir function |
(LAVmax − LAVmin)/LAVmax |
|
Expansion index Normal range: 171–250 [53] |
Reservoir Index (no strain data available) [91] |
(LAVmax − LAVmin)/LAVmin |
|
Passive LA emptying fraction (%) Normal range: 38–49 [53] |
LA conduit function |
(LAVmax − LAVpreA)/LAVmax |
|
Active LA emptying fraction (%) Normal range: 35–48 [53] |
LA booster pump function |
(LAVpreA—LAVmin) / LAVpreA |
LA—left atrial, LAVmax maximum LA volume, LAVmin minimum LA volume
Of all LA volume measurements, maximum LAVI (LAVImax) has the most important prognostic value [90–92]. However, minimum LAVI (LAVImin) might be better to characterize LA function during sinus rhythm because it may better reflect elevated LVEDP and PCWP than LAVImax [93, 94]. The main practical challenge to assess LAVImin is the higher error-proneness in comparison to LAVImax due to inappropriate LA centering in the respective sectional 2D planes [91, 95–97]. The geometric assumptions of the LA size by 2D echocardiography usually cause an underestimation of LA volumes in comparison to LA volume measurements by 3D echocardiography [53, 91, 97, 98] underlining the necessity of 3D echocardiography for a better LA volume assessment. With respect to 3D echocardiography a higher threshold for the maximum LAVI above 40 ml/m2 has been proposed (instead of 34 ml/m2 by 2D) [53, 91, 97, 98]. In chronic AF threshold for maximum LAVI are higher than a LAVImax of 40 ml/m2 in the literature [2, 91].
LA function can presumably be more objectively assessed by analysis of longitudinal LA strain. However, atrial strain is to a large extent determined by ventricular strains and modulated by the atrial and ventricular volumes. LA strain (LAS) is analyzed using apical 2D four-chamber views by triggering the beginning of the QRS complex [53]. Global average LA reservoir (LASr), LA conduit (LASr minus average LAS value at the onset of the p-wave), and LA contraction strain (LASct = LASr − LA conduit strain) are extracted from the respective strain curves. In patients with atrial fibrillation, LAS analysis was limited to the investigation of LASr and LAScd, as proposed by the recent EACVI recommendations [53, 85, 89, 90].
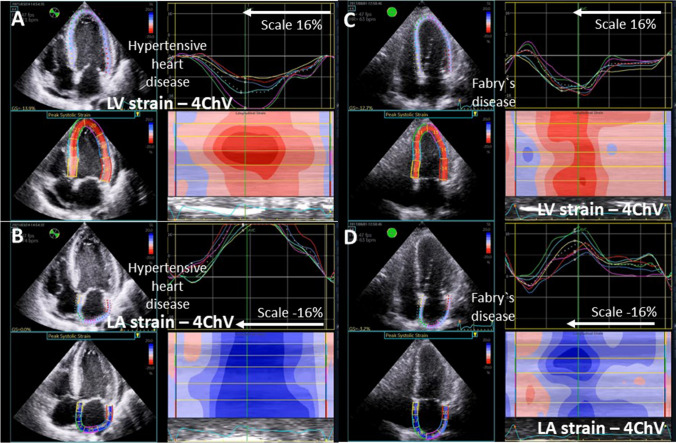
LAS provides valuable additive information for the differential diagnosis between infiltrative/storage diseases like amyloidosis, hypertrophic cardiomyopathy and other types of LA and LV wall thickening including edema [99–101] (Fig. 10). Two distinct mechanisms can theoretically lead to LA dysfunction in infiltrative/storage diseases: firstly, the increased LA pressure induced by LV dysfunction and secondly, the direct accumulation of pathological deposits in the LA wall. Thus, hypertrophic cardiomyopathy as a primary LV disease affects LA dysfunction to a lesser degree than e.g. infiltrative/storage diseases. The diagnostic strength of LAS has been shown to be better than classical LV strain features like “apical sparing” [99–101]. Recent data suggests that LA dysfunction plays an essential role in the clinical course of patients with “HFpEF” symptoms due to early stages of infiltrative/storage diseases followed by DD and AF [99–101].
Fig. 10.
Illustration about advanced strain assessment in patients with “HFpEF” symptoms. In A, a quad screen of 4ChV (4-chamber view) documents LV strain analysis by presenting the tracking LV area, the respective strain graphs of the regional LV strain, the corresponding maximum strain values, and the color-coded curved strain M-Mode in a patient with hypertensive heart disease. Reduced mid-basal strain and normal mid-apical strain values are documented. In B, a comparable quad screen of the 4ChV for LA strain analysis is shown documenting LA strain values higher than 16%. In C, a comparable quad screen of 4ChV for LV strain analysis in a patient with Fabry’s disease illustrates reduced reginal LV strain values in all LV segments. In D, a comparable quad screen of 4ChV for LA strain analysis in the Fabry patient disease illustrates the possibility of early detection of LA dysfunction by the reduced LA strain values
The clinical symptoms and the impaired exercise capacity in patients with “HFpEF” symptoms can mainly be explained by DD due to increased LVEDP at rest and/or during exercise. Historically, DD has initially been graded mainly according to the pw transmitral inflow E/A ratio. A high E/A ratio ≥ 2 with a short E-wave deceleration time (Edt) < 140 ms indicates severely impaired diastolic function due to increased LVEDP, however rarely documented at compensated stages. The frequently observed E/A ratio < 0.8–1 with a normal or prolonged Edt can be classified as abnormal relaxation and may indicate a slightly elevated LVEDP with normal LA pressure and no evidence for pulmonary congestion at rest [102–104]. However, the diagnostic value of the E/A ratio is severely limited by its bimodal behavior with the inability to distinguish between a normal and elevated filling pressure if the E/A is between 0.8 and 2. This limitation may be overcome by integrating pw Doppler information from pulmonary venous inflow. The duration of atrial reversal flow in the pulmonary vein is longer than the duration of forward transmitral atrial flow (A-wave) in the presence of elevated LVEDP [102–104]. Elevated LV filling pressures will also progressively shorten isovolumic relaxation time (IVRT) < 60–80 ms, which is typically obtained by a cw Doppler placed in-between the transmitral inflow and LV outflow [105]. The recommended and more practical alternative to estimate LVEDP can be performed by tissue Doppler imaging of the E´ wave in the basal septal and lateral LV segments. An average E/E’ ratio > 15 calculated with the mean E´ velocity of the basal septal and lateral LV segments indicates a significantly elevated LVEDP and is a major criterion in the HFA-PEFF score. However, several coexisting conditions such as the presence of left bundle branch block, valvular regurgitation, regional wall motion abnormalities affect the E/E’ ratio. If E/E´ratio is measured within the grey zone between 9 to 14, further echocardiographic parameters can be determined to estimate the degree of DD [4, 34, 41, 55, 84, 102–107]. The elevated LVEDP is transmitted through the pulmonary vasculature and will chronically lead to an increase in pulmonary pressure with mild to moderate postcapillary pulmonary hypertension. This can be identified by an increase in the tricuspid peak regurgitant (TR) velocity > 2.8 m/s reflecting an estimated systolic pulmonary artery pressure (sPAP) > 35 mmHg. These hemodynamic results may importantly vary with changing volume conditions.
All echocardiographic parameters defining DD are changing with age. An age-related decrease in the E/A ratio, mitral annular velocities, septal and lateral E’ has been observed in many population-based studies [108–110]. Also, mitral annular velocities decrease significantly during a lifetime. Elevated LV filling pressures and DD are not synonymous but refer to abnormal mechanical LV properties. Thus, patients with “HFpEF” symptoms usually have a higher sPAP, E/E’ ratio, and LAVI than patients with LV wall thickening presumably due to arterial hypertension without HF signs [111].
Patients with “HFpEF” symptoms and sole LA abnormalities are rare.
Table 3 summarizes the echocardiographic parameters, which are important to characterize the LA phenotype. The parameters are listed including normal ranges and cut offs [84, 85, 89, 102, 103, 110–113], methodological aspects of TTE determination, their importance to be determined, and their reasons, why it is worth to determine the respective parameter in clinical routine. Specific parameters can be helpful to detect functional LA abnormalities—especially in subclinical stages of possible cardiac diseases.
Table 3.
Echocardiographic parameters characterizing patients with heart failure (HF) symptoms and normal or preserved left ventricular ejection fraction (LVEF) in left atrial (LA) phenotype
| Echocardiographic parameter | Normal ranges—cut offs | Methodological aspects | Mandatory to determine (methods) | Why worth to do in routine |
|---|---|---|---|---|
| LA parameters and parameters of diastolic function | ||||
| LAD—left atruial diameter (mm) |
♂ 31–39 Cut off < 39 ♀ 28–37 Cut off < 37 [85] |
“Old” parameter, which can only be used in normal LA geometry | No—only if LA dimensions are documented as normal | To document LA dimension—if LA geometry is normal |
| LAD/BSA (mm/m2) |
♂ 13–23 Cut off < 23 ♀ 14–24 Cut off < 24 [85] |
“Old” parameter, which can only be used in normal LA geometry | No—only if LA dimensions are documented as normal | To document LA dimension—if LA geometry is normal |
| LAVImax—maximum LA volume indexed to BSA (ml/m2) |
♂ 18–35 Cut off < 39 ♀ 18–36 Cut off < 38 (Cut off < 34) |
Avoid foreshortening, prefer triplane analysis or 3D volumetry. Increased LA volume predicts increased LV filling pressure | Yes | To document chronic diseases due to impaired LV filling |
| LAVImin—minimum LA volume indexed to BSA (ml/m2) |
♂ 8–18 Cut off < 18 ♀ 18–18 Cut off < 18 [6] |
Avoid foreshortening, prefer triplane analysis or 3D volumetry. Increased LAVImin predicts impaired active LA contractility | Yes—under certain conditions | To document impact on active LA contraction on global LA function |
| Total LA emptying fraction (LAEF) (%) |
51–61 Cut off > 38 [112] |
Avoid foreshortening, prefer triplane analysis or 3D volumetry. Reduced LA emptying fraction indicates LA dysfunction | Yes-—under certain conditions | To characterize LA function |
|
Average LA reservoir strain—reservoir LAS (%): LA reservoir function |
31–42 Cut off > 23 [89] |
LA strain analysis is only possible if image quality is adequate. 4ChV is usually used for LA strain analysis | Yes—especially in normal or LV phenotype—helpful to detect subclinical states of cardiac diseases | To characterize global LA function |
|
Passive LA conduit strain—passive LAS (%): LA conduit function |
15–23 Cut off > 11 [89] |
LA strain analysis is only possible if image quality is adequate. 4ChV is usually used for LA strain analysis | Yes—especially in normal or LV phenotype—helpful to detect subclinical states of cardiac diseases | To characterize passive LA filling properties |
|
Active LAS contraction strain—active LAS (%): LA contraction function |
14–21 Cut off > 8 [89] |
LA strain analysis is only possible if image quality is adequate. 4ChV is usually used for LA strain analysis | Yes—especially in normal or LV phenotype—helpful to detect subclinical states of cardiac diseases | To characterize active LA contractility |
| LA stiffness—E/E´ devided by LAEF (%−1) |
0.13–0.17 Cut off < 0.27 [89] |
Standardize the Doppler assessment with respect to breathing to ensure comparability in follow-ups | No—especially in normal or LV phenotype—helpful in suspected infiltrative/storage diseases | To detect causes of LA stiffness by conventional parameters |
| LA stiffness—E/E´ devided by LAS (%−1) |
0.18–0.29 Cut off < 0.55 [89] |
Standardize the Doppler assessment with respect to breathing to ensure comparability in follow-ups | No—especially in normal or LV phenotype—helpful in suspected infiltrative/storage diseases | To detect causes of LA stiffness using speckle tracking |
| E-peak E-wave velocity (cm/sec) |
♂ 42–116 Cut off > 42 ♀ 43–115 Cut off > 43 [110] |
Acquire the pw Doppler spectra using Duplex mode to control correct positioning of the sample volume at the level of mitral valve (MV) coaptation | Yes—to differentiate between normal, abnormal relaxation, pseudo-normal, and restrictive | E reflects LA-LV gradient during early diastole |
| Peak A-wave velocity (cm/sec) |
♂ 25–93 Cut off > 25 ♀ 29–93 Cut off > 29 [110] |
Acquire the pw Doppler spectra using Duplex mode to control correct positioning of the sample volume | Yes—to differentiate between normal, abnormal relaxation, pseudo-normal, and restrictive | A reflects LA-LV gradient during late diastole |
| Transmitral A-duration (msec) |
100–176 Cut off > 100 |
Acquire an additional pw Doppler spectrum of blood LV inflow at the level of mitral anulus (MA). Time speed must be100mm/sec to ensure sufficient temporal resolution | Yes—especially in normal or LV phenotype | To be able to compare forward and retrograde LA blood flow during LA contraction |
| Transmitral E/A ratio |
♂ 0.62–2.34 cut off > 0.62 ♀ 0.32–2.44 cut off > 0.32 [110] |
Acquire the pw Doppler spectrum with sharp contours (possibly highest Doppler frequencies) with a sample volume in the central blood stream of LV inflow | Yes—- to differentiate between normal, abnormal relaxation, pseudo-normal, and restrictive | To distinguish between impaired LV relaxation, pseudonormal conditions, and LV restriction |
| Edt—E-wave deceleration time (msec) |
♂ 78–302 cut off > 78 ♀ 99–275 cut off > 99 [110] |
Acquire the pw Doppler spectrum with sharp contours (possibly highest Doppler frequencies) with a sample volume in the central blood stream of LV inflow | Yes- especially in normal or LV phenotype | To detect impaired LV relaxation and LV stiffness |
| IVRT—isovolumetric relaxation time (msec) |
73–101 cut off ≤ 70 |
Acquire an additional pw Doppler spectrum with the sample volume positioned at the anterior mitral leaflet. IVRT estimates relaxation (τ) | Yes—especially in normal or LV phenotype | Prolonged in impaires relaxation; shortened if LAP increases |
| L-wave (transmitral velocity spectrum and tissue doppler spectra) |
Qualitative sign of diastolic dysfunction |
The L-wave is documented in the transmitral pw Doppler spectrum and in the LV tissue Doppler spectra | Yes | If present, indicates increased LVEDP |
| Peak E´-velocity basal septal (cm(sec) |
♂ 6–11 Cut off > 6 ♀ 5–10 Cut off > 5 [110] |
Acquire the pw tissue Doppler spectra using Duplex mode to control proper sample volume positioning of. Try to center the LV septum for optimal image quality | Yes | E´ includes LV relaxation, restoring forces and LV filling pressure |
| Peak E´-velocity basal lateral [cm (sec)] |
♂ 5–16 Cut off > 5 ♀ 5–14 Cut off > 5 [110] |
Acquire the pw tissue Doppler spectra using Duplex mode to control proper sample volume positioning. Try to center the later LV segment for optimal image quality | Yes | E´ includes LV relaxation, restoring forces and LV filling pressure |
| Average E/E´ratio |
< 8 normal 8–14 borderline > 14 pathological |
Check the respective positions of the sample volumes and standardize both documentation to comparable breathing periods | Yes | To estimate LVEDP. E/E’reflects normal or pathological relaxation |
| TE´-E—time interval between E´- and E-onset (msec) |
0–8 Cut off > 8 [113] |
The estimation is within the limit of detection. Significant differences of time intervals in Doppler spectra can be detected by intervals > 20 ms | Optional—it can be used as a qualitative sign of diastolic dysfunction | TE´-E can distinguish between restriction (prolonged) and constriction (normal) |
| IVRT/TE´-E ratio |
> 2 [113] |
The estimation should only be performed if TE´-E is > 20 | Optional—but helpful to detect increased LVEDP | If ratio is < 2, PCWP and LAP are increased |
| ArD—retrograde pulmonary vein Ar-duration (msec) |
53–173 Cut off Ar < A |
Acquire pw Doppler spectrum using low pulse repetition frequency (LPRF). Prefer time speed of the spectrum at 100 mm/sec or more to ensure sufficient temporal resolution | Yes—especially in normal or LV phenotype | Prolonged ArD indicates diastolic dysfunction an increased VEDP |
| Peak Ar velocity (cm/s) |
− 11to − 39 Cut off < 35 |
Acquire pw Doppler spectrum using LPRF. Try to increase contour sharpness by increasing Doppler frequencies in the range of LPRF | Yes—especially in normal or LV phenotype | Increased Ar velocity indicates increased VEDP |
| Transmitral A duration–Ar duration |
0–20 cut Off < 30 |
For optimal documentation of sample volume position at the levels of MV coaptation and MA acquire both spectra using Duplex mode | Yes—especially in normal or LV phenotype |
Prolonged A—Ar indicates increased LVEDP |
| Vp—left ventricular diastolic flow propagation (cm/sec) |
Cut off ≥ 50 |
Vp correlates with LV relaxation and the invasive parameter (τ. Adjust color Doppler setting and prefer time speed of the spectrum at 100 mm/sec or more | Yes—especially in normal or LV phenotype | Decreased Vp indicates LVEDP increase |
| E/Vp ratio (sec) |
Cut off ≤ 2.5 |
Perform Measure E and Vp in transmitral pw spectra and mitral flow color M-modes at standardized comparable breathing intervals | Yes—especially in normal or LV phenotype | E/Vp correlates with LAP and PCWP. Decreased E/Vp indicates increases of LAP and PCWP |
For each echocardiographic parameter the normal ranges (and cut offs), methodological aspects, the importance of its determination, and the value to determine it in routine are listed. Mandatory parameters to be determined in clinical practice are labeled in bold print
LAD left atrial diameter, BSA body surface area, LAVImax maximum LA volume indexed to BSA, LA left atrial, LAVImin minimum LA volume indexed to BSA, LAEF total LA emptying fraction, LAS left atrial strain, E maximum early mitral flow velocity, E´ maximum early tissue Doppler lengthening velocity of the myocardium near to the mitral anulus, A maximum forward transmitral atrial flow velocity, Edt E-wave deceleration time, IVRT isovolumetric relaxation time, L flow velocity peak of transmitral flow during diastasis, TE´-E Time interval between E´ and E-onset, ArD retrograde pulmonary vein flow duration, Vp left ventricular diastolic flow propagation
Figure 11 summarizes the echocardiographic workflow in patients with “HFpEF” symptoms presenting the LA phenotype.
Fig. 11.
Scheme of the echocardiographic workflow in patients with “HFpEF” symptoms to characterize the echocardiographic LA phenotype
The RA/RV phenotype: abnormalities of right atrial (RA) volumes and RA function as well as of right ventricular (RV) volumes, RV function, and RV geometry
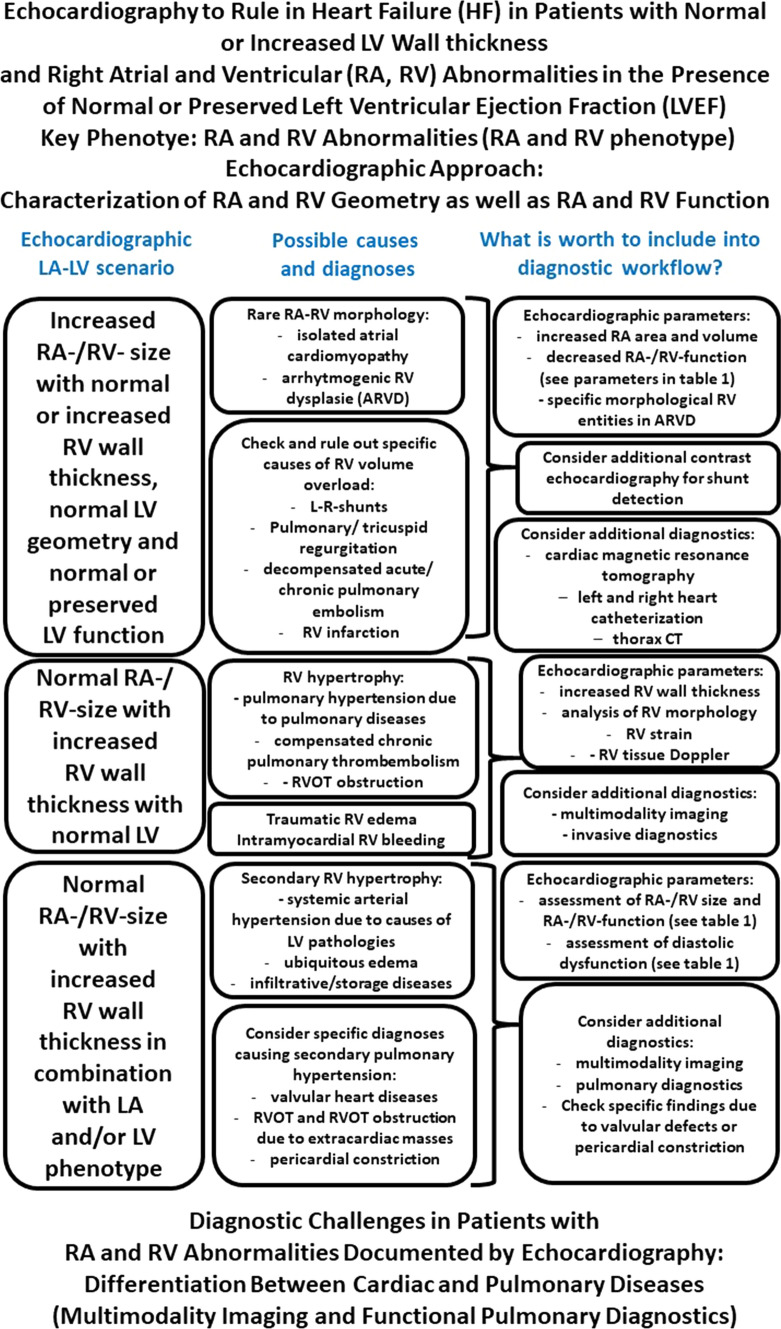
In patients with “HFpEF” symptoms abnormalities of RA and RV morphology and function can be induced by several factors. The constellation of pathological echocardiographic findings and its interpretation encompasses RV wall thickening, RA and RV volume, RV contractility and deformation as well as tricuspid and pulmonary valvular function to differentiate between RV volume and RV pressure overload [114, 115]. Increased RV contractility combined with increased RV volume primarily refers to compensated staged with increased RV volume load, increased RV contractility combined with RV wall thickening and normal RV volumes primarily refers to compensated stages with RV pressure load. Thus, these echocardiographic features can lead to diagnoses like tricuspid regurgitation or left–right shunts due to increased RV volume load or to diagnoses like primary or secondary pulmonary hypertension due to increased RV pressure load [114, 115]. The interpretation of the echocardiographic findings is more challenging and difficult at decompensated stages.
Due to secondary pulmonary hypertension or infiltrative/storage diseases the RA/RV phenotype is often linked to the LA phenotype and the LV phenotype. The isolated RA/RV phenotype in patients with “HFpEF” symptoms normally characterizes primary pulmonary hypertension due to specific lung diseases or rare diseases like arrhythmogenic RV dysplasia. Especially in this scenario, the non-invasive determination of pulmonary vascular resistance by echocardiography is recommended (Table 4). The combination of the RA/RV phenotype with the other echocardiographic phenotypes is observed in almost all other cardiac diseases.
Table 4.
Echocardiographic parameters characterizing patients with heart failure (HF) symptoms and normal or preserved left ventricular ejection fraction (LVEF) in the combined right atrial (RA) and right ventricular (RV) phenotype
| Echocardiographic parameter | Normal ranges—cut offs | Methodological aspects | Mandatory to determine (methods) | Why worth to do in routine |
|---|---|---|---|---|
| RV parameters | ||||
| Enddiastolic RV free wall thickness (mm) |
3–5 Cutoff < 5 [124] |
Parasternal or subcostal short axis views of the RVOT can be used. Parasternal assessment is influenced by near field, subcostal assessment by far field | Yes |
To detect or exclude RV hypertrophy Increased RV wall thickness indicates RV pressure overload |
| FAC (%)—fractional area change of the RV (assessed by RV focused 4ChV) |
Cut off < 35 [115] |
Estimation is error prone to individual RV topography in relation to LV in the 4ChV. FAC can only be interpreted if no or only mild TR is present | No—usually too error-prone, only if 3D acquisition is not possible | To estimate RV size and RV function |
| 3D RVEDV—RV enddiastolic volume (ml) |
♂ 98–141 Cut off > 98 ♀ 75–110 Cut off > 75 [125] |
RV volumes should be measured by 3D echocardiography 2D parameters (areas, diameter) are too error prone due to improper standardization of the RV in respective sectional planes |
Yes—if image quality is adequate | To estimate RVEDV—especially in abnormal RV geometry |
| 3D RVESV—RV endsystolic volume (ml) |
♂ 37–67 Cut off > 37 ♀ 27–49 Cut off > 27 [125] |
RV volumes should be measured by 3D echocardiography 2D parameters (areas, diameter) are too error prone due to improper standardization of the RV in respective sectional planes |
Yes—if image quality is adequate | To estimate RVEF and total RVSV |
| 3D RVSVtot—total RV stroke volume (ml) |
♂ 53–83 Cut off > 53 ♀ 42–67 cut off > 42 [125] |
RV volumes should be measured by 3D echocardiography 2D parameters (areas, diameter) are too error prone due to improper standardization of the RV in respective sectional planes |
Yes—if image quality is adequate | To estimate RVEF and total RVSV, which corresponds to effective RVSV, if PV and TV are normal |
| DRVOT (mm)—diameter of the right ventricular outflow tract (RVOT) |
♂ 17—27 ♀ 17—27 [33] |
3D imaging is preferred with respect to verifiable standardization. Diameter of the pulmonary trunk can be used as an alternative | Yes—under certain coditions | To estimate RVSVeff and cardiac output (CO) |
|
Cardiac output (CO) determined by RVSVeff (effective RV stroke volume) using pulsed waved (pw) Doppler x heart rate (ml/l) - LVSVeff = 2 × DRVOT x VTIRVOT where VTIRVOT is velocity time integral of flow velocities determined in the right ventricular outflow tract |
♂ 3.5–8.2 ♀ 3.3–7.3 [88] |
Proper positioning of the pw sample volume in relation to the DRVOT is mandatory (if pulmonary trunc as an alternative is used, position of the sample volume must be adjusted—angulation of the cursor parallel to the blood stream is mandatory (subcostal imaging should be considered | Yes- under certain coditions |
To estimate RVSVeff and CO To compare LVSVeff and RVSVeff |
| 3D RVEF—RV ejection fraction (%) |
♂ 54–59 Cut off > 54 ♀ 56–62 cut off > 56 [125] |
RV volumes should be measured by 3D echocardiography 2D parameters (areas, diameter) are too error prone due to improper standardization of the RV in respective sectional planes |
Yes—if image quality is adequate | To estimate normal or preserved LVEF |
| RVEDV/BSA (ml/m2) |
♂ 55 – 68 Cut off > 55 ♀ 48—60 Cut off > 48 [125] |
RV volumes should be measured by 3D echocardiography 2D parameters (areas, diameter) are too error prone due to improper standardization of the RV in respective sectional planes |
Optional—by 3D volumetry | To adjust LV volumes in extreme conditions |
| RVESV/BSA (ml/m2) |
♂ 22–32 Cut off > 22 ♀ 19–15 Cut off > 19 [125] |
RV volumes should be measured by 3D echocardiography 2D parameters (areas, diameter) are too error prone due to improper standardization of the RV in respective sectional planes |
Optional—by 3D volumetry | To adjust LV volumes in extreme conditions |
| TAPSE (mm) |
♂ 17–29 cut off > 17 ♀ 17–27 Cut off > 17 |
Assessment is possible by postprocessing of 2D cineloops of the 4ChV, not useful in TR and shunts | Optional—but helpful to detect subclinical states of cardiac diseases | To detect RV dysfunction in the presence of normal TV or only mild TR |
| RV free wall peak pw S ‘ velocity (cm/sec) |
♂ 8—19 cut off > 8 ♀ 9—17 Cut off > 9 [110] |
Acquire the pw tissue Doppler spectra using Duplex mode to control proper sample volume positioning of. Try to center the RV free wall for optimal image quality | Optional—but helpful in the normal and atrial phenotype | To estimate RV contractility |
| RV-IVRT = Free wall RV total isovolumetric relaxation time by pw tissue Doppler |
0–36 Cut off ≤ 36 [123] |
Acquire the pw tissue Doppler spectra with time speed of 100 mm/sec | Optional—but helpful in the normal and atrial phenotype | To estimate RV relaxation |
| TVI RV free wall strain (%) |
27–31 Cut off > 17 [115] |
Acquire three consecutive RR-intervals to detect artefacts and drifting | Optional—but helpful in the normal and atrial phenotype | To characterize longitudinal RV deformation |
| RV free wall GLS (%) |
25–32 Cut off > 18 [16] |
Ensure the full myocardial tracking of all RV myocardial layers | Optional—but helpful in the normal and atrial phenotype | To characterize longitudinal RV deformation |
|
RIMP by pw RIMP = (TCO–ET)/ET -right index of myocardial performance |
0.21–0.43 cut off < 0.43 |
Acquire transpulmonic and transtricuspid pw Doppler spectra at the same heart rate to be comparable | Yes—especially in the RV phenotype | Increased RIMP indicates RV dysfunction and indicates reduced filling and ejection intervals |
|
RIMP by TVI RIMP = (IVRT + IVCT)/ET |
0.22–0.54 cut off > 0.54 |
Acquire the pw tissue Doppler spectra with time speed of 100 mm/sec. The isovolumetric time intervals can only be determined with high temporal resolution | Yes—especially in the RV phenotype | Increased RIMP indicates RV dysfunction and indicates reduced filling and ejection intervals |
| VmaxTR–TR systolic peak velocity (m/sec) |
Cut off ≤ 2.8 |
VmaxTR only reflects RV pressure if pulmonary stenosis is excluded and if RV is not decompensated | Yes | To estimate RVESP |
| sPAP = 4 × VmaxTR2 plus estimated RAP (right atrial pressure)- estimated systolic pulmonary artery pressure (mmHg) - |
Cut off ≤ 30 |
RV pressures can only be estimated if pulmonary stenosis is excluded and if RV is not decompensated | Yes | To estimate RVESP |
| edVmaxPR—PR end-diastolic peak velocity (m/sec) |
No normal ranges in the literature—if normal RVEDP-values of 10–15 mmHg are assumed, cut off < 1.2 |
Acquire a transpulmonic cw Doppler spectrum for assessment | Yes—especially in the RV phenotype | To estimate RVEDP (= dPAP) |
| dPAP = 4 × edVmaxPR2 plus estimated RAP—estimated enddiastolic pulmonary artery pressure (mmHg) |
No normal ranges in the literature, according to invasive assessment, normal dPAP < 10–15 mmHg |
Acquire a transpulmonic cw Doppler spectrum for assessment | Yes—especially in the RV phenotype | To estimate RVEDP (= dPAP) |
| RAVImax (ml/m2) |
♂: Cut off < 30 ♀: cut off > 28 [7] |
Estimation is error prone to individual RA topography in the 4ChV | No—usually too error-prone, only if 3D acquisition is not possible | To estimate RA dysfunction and chronic RV diseases due to increased RV filling pressures |
| Collapse index inferior caval vein (%) |
Cut off > 50 [115] |
Ensure centering of the inferior caval vein (VCI) during longitudinal scanning. Biplane scanning should be preferred to document the VCI simultaneously in a short axis view | Yes | To estimate right atrial pressure (RAP) and systemic congestion. Decreased values indicate RAP increase |
|
Pulmonary vascular resistance = PVR (msec−1) = (PEP/AcT)/(PEP + RVET), where PEP is preejection period (= time interval between TR onset and onset of systolic pulmonary flow), AcT is time interval between onset and peak pulmonary flow, and RVET is right ventricular ejection time |
Cut off < 2.5 [114] |
This index estimates PVR and correlates with wood units (WU). The methodological advantage is the robustness of Doppler time intervals | Optional—especially in RV phenotype | To estimate PVR |
| PVR (cm−1) = (10 × VmaxTR)/VTIRVOT, where VTIRVOT is velocity time integral of flow velocities determined in the right ventricular outflow tract |
Cut off < 2 [114] |
This index estimate PVR and correlates with wood units (WU). Avoid methodological errors by acquiring the pw Doppler spectra. (adjust cursor and sample volume position) | Optional—especially in RV phenotype | To estimate PVR |
| PVR—(mmHg min cm−1) = sPAP/(heart rate x VTIRVOT) |
Cut off > 0.076 [114] |
This index estimates PVR. Increased index predicts severely increased PVR | Optional—especially in RV phenotype | To estimate PVR |
For each echocardiographic parameter the normal ranges (and cut offs), methodological aspects, the importance of its determination, and the value to determine it in routine are listed. Mandatory parameters to be determined in clinical practice are labeled in bold print
RV right ventricular, FAC fractional area change of the RV, 4ChV 4-chamber view, RVEDV RV enddiastolic volume, RVESV RV endsystolic volume, RVSVtot total RV stroke volume, RVOT right ventricular outflow tract, DRVOT diameter of the RVOT, CO cardiac output, RVSVeff effective RV stroke volume, VTIRVOT velocity time integral of flow velocities determined in the RVOT, RVEF RV ejection fraction, BSA body surface area, TAPSE tricuspid annular plane systolic excursion, S´- maximum systolic tissue Doppler velocity of the myocardium near to the tricuspid anulus at the RV free wall, RV-IVRT free wall RV total isovolumetric relaxation, TVI tissue velocity imaging, RIMP right index of myocardial performance, TCO time interval between tricuspid valve closure and opening, ET ejection time, IVRT isovolumetric relaxation time, IVCT isovolumetric contraction time, TR tricuspid regurgitation, VmaxTR TR systolic peak velocity, sPAP systolic pulmonary artery pressure, RAP right atrial pressure, PR pulmonary regurgitation, edVmaxPR PR end-diastolic peak velocity, dPAP enddiastolic pulmonary artery pressure, RAVImax maximum RA volume indexed to BSA, VCI inferior caval vein, PVR pulmonary vascular resistance, PEP pre-ejection period, AcT time interval between onset and peak pulmonary flow, RVET RV ejection time, VTIRVOT velocity time integral of flow velocities determined in the RVOT
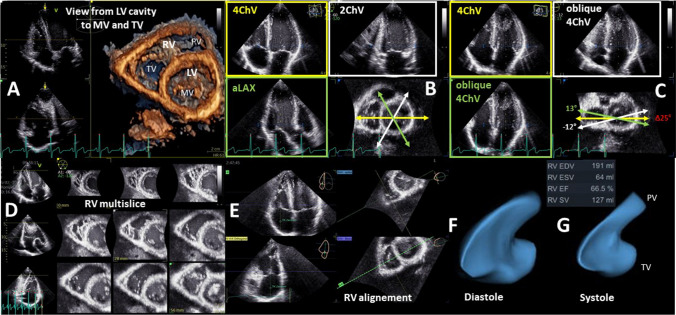
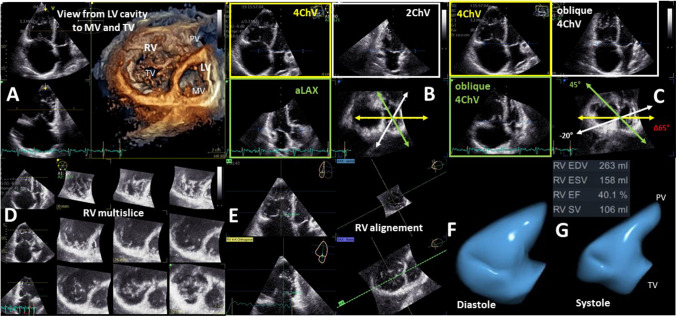
The determination of the echocardiographic RV parameters presented in Table 4 should be considered if RV abnormalities are observed in patients with “HFpEF” symptoms [114, 115]. RV wall thickness, the tricuspid annular plane systolic excursion (TAPSE), sPAP, and RV strain are relatively robust parameters [33, 102, 103, 114, 115] whereas estimations of RV volumes resulting on 2D measurements are often error prone due to problems to standardize the sectional planes through the right ventricle [116–118]. Thus, 3D RV volume assessment is always recommended if possible—and 2D distance measurements to characterize RV volume should be avoided [33, 118]. Measurements of the RV fractional area change (FAC) determined by 2D planimetry of the end diastolic and end systolic RV area in an apical four chamber view focused to the right ventricle are often misleading due to methodology of standardization [116–118]. Thus, estimation of RV function should be performed by the assessment of RV ejection fraction (RVEF) by 3D echocardiography or by RV strain analysis (Figs. 12, 13).
Fig. 12.
Illustration about the necessity for 3D-RV assessment—normal conditions. A presents the enface view from the LV cavity to the MV and TV. In B, the apical 3D views (4ChV, 2ChV—apical 4- and 2-chamber view; aLAX—apical long axis view) within the 3D data set are centered to the LV illustrating the standardized 4ChV by representative angular distance of 60° between aLAX, 2ChV, and 4ChV. In C, oblique 4ChV are shown to illustrate that even during normal conditions the deviation of non-standardized 4ChVs for 2D measurements is within a Δ of 25°. In D, a multisclice presentation of the 3D data set illustrate the complete acquisition of the RV volume. In E, the RV alignment for RV volume determination is shown. In F the diastolic RV volume, in G the systolic RV volume is presented by a 3D volume calculation
Fig. 13.
Illustration about the necessity for 3D-RV assessment -pathological conditions. A presents the enface view from the LV cavity to the MV and TV. In B the apical 3D views (4ChV, 2ChV—apical 4- and 2-chamber view; aLAX—apical long axis view) within the 3D data set are centered to the LV illustrating the standardized 4ChV by representative angular distance of 60° between aLAX, 2ChV, and 4ChV. In C oblique 4ChV are shown to illustrate that during pathological RV conditions the deviation of non-standardized 4ChVs for 2D measurements is within a Δ of 65°. In D a multisclice presentation of the 3D data set illustrate the complete acquisition of the RV volume. In E the RV alignment for RV volume determination is shown. In F the diastolic RV volume, in G the systolic RV volume is presented by a 3D volume calculation
RV strain should be measured in a RV-focused apical four chamber visualizing the RV free wall in the center of the scanning sector. Sector size should be minimized to increase frame rate for tissue Doppler measurements of the free RV wall [49, 50, 119]. Speckle tracking of the RV wall also like of the LV wall requires frame rates between 40 and 80/min. Then, RV strain can be analyzed in the basal, medial, and apical RV segments. In addition to RV strain, RA strain can be analyzed semi-automatically after manual determination of the endocardial borders. Global RA reservoir strain was assessed by analyzing the maximum excursion of the respective strain curve representing average peak strain values of all RA segments [50, 119, 120]. In addition, RA strain during passive RV filling (RA conduit strain) and during peak atrial contraction (RA active strain) can be determined. Comparable to LA strain RA conduit strain can be calculated by the difference of RA global strain and average RA strain value at the onset of the p-wave, whereas RA active strain can be calculated by the difference of RA global strain and RA reservoir strain. Reference values of mean RA reservoir strain, RA conduit and contraction strain are 44.9 ± 11.6%, 27.1 ± 9.5%, and 17.0 ± 5.9%, respectively [121, 122].
Table 4 summarizes the echocardiographic parameters, which are important to characterize the RA/RV phenotype. The parameters are listed including normal ranges and cut offs [33, 85, 87, 91, 113, 117, 118, 126–128], methodological aspects of TTE determination, their importance to be determined, and their reasons, why it is worth to determine the respective parameter in clinical routine. Specific parameters can be helpful to detect functional RA and RV abnormalities—especially in combination with pathological LA and LV findings.
Figure 14 summarizes the echocardiographic workflow in patients with “HFpEF” symptoms presenting the RA/RV phenotype.
Fig. 14.
Scheme of the echocardiographic workflow in patients with “HFpEF” symptoms to characterize the echocardiographic RA/RV phenotype
The role of diastolic stress echocardiography in patients with “HFpEF” symptoms
Sensitivity of resting echocardiographic parameters is limited to detect DD as the cause of “HFpEF” symptoms. Only 34%–60% of DD patients characterized by TTE have been confirmed with invasively proven DD in patients with preserved LVEF [62, 126]. Thus, artificial intelligence is proposed to predict HF in asymptomatic patients with normal cardiac phenotype, changes of diastolic filling properties and diastolic abnormalities with structural cardiac findings [127]. Thus, asymptomatic patients at rest might provide “HFpEF” symptoms due to DD during stress. The detection of DD development during physical diastolic stress echocardiography [2, 7] requires a proper standardization of the acquisition of Doppler spectra with respect to comparable breathing state and comparable sample volume positions at the respective stress levels. Besides these methodological challenges, increasing stress levels are normally limited due to the fusion of E- and A-wave with increasing heart rate. In consequence, DD detection by diastolic stress echocardiography should be confirmation by invasive hemodynamic exercise testing [7, 128, 129]. However, invasive hemodynamic studies are rarely performed to confirm an elevated PCWP in patients with “HFpEF” symptoms in clinical practice. Adding the criterion “exercise E/E’ > 14” to detect DD by diastolic stress echocardiography improved the sensitivity to 90%, but decreased specificity from 92 to 71% [126]. The hemodynamic response to exercise in patients with “HFpEF” symptoms was analyzed documenting that E/E’ at rest was higher in HF patients with small LV size and normal LA size group than in healthy controls, but that this difference did not reflect a difference in PCWP [128]. Furthermore, E/E’ did not significantly increase during stress in both groups and did not correlate with invasively measured PCWP at peak stress. It was concluded that increased arterial stiffness is presumably one of the key mechanisms of “HFpEF” symptoms [128, 129]. Stress testing in cardiology, however, is mainly used to detect coronary artery disease by stress-induced LV wall motion abnormalities, but it might obviously contribute to distinguish between cardiac and non-cardiac causes of “HFpEF” symptoms [2, 7].
Summary
The present proposal provides an echocardiographic focused algorithm for patients with HF symptoms and preserved LVEF. In line with recent recommendations [2, 7], distinct cardiac phenotypes characterized by TTE are introduced as predominant gatekeepers for further diagnostic steps to clarify the specific underlying diagnoses. The algorithm starts with ruling out patients with non-cardiac causes for HF symptoms because edema has a low sensitivity of 20% and dyspnea on exertion a low specificity of 17% to be caused by cardiac failure [1]. In addition, a comparison of invasively measured PCWP with clinical HF classification according to the New York Heart Association (NYHA) showed in 61% (NYHA II) and in 14% (NYHA III) a normal PCWP < 16 mmHg, and biomarkers like NT-pro BNP showed no correlation to PCWP at rest [130]. Thus, symptoms suggestive of “HFpEF” may in some patients represent non-HF comorbidities” [131, 132]. The morphological classification of patients with “HFpEF” symptoms into the normal cardiac phenotype, the LV phenotype, the LA phenotype, and the combined RA/RV phenotype is the main challenge of the algorithm to detect the underlying pathologies responsible for “HFpEF” symptoms. The systematic echocardiographic approach emphasizes conventional, but uncommon parameters like Ees and Ea, but also modern parameters like RV volume, LA strain and myocardial work—determined by 3D echocardiography and deformation imaging—to improve echocardiographic detection of specific cardiac diagnosis in patients with “HFpEF” symptoms. The assessment of LA and LV stiffness might be particularly useful to detect subclinical pathologic entities in an apparently normal cardiac phenotype with borderline morphological abnormalities. For example, LA dysfunction might precede LA remodeling which is better predicted by LASr enables to distinguish asymptomatic patients with DD from those with HF symptoms [130]. Presumably, additional diastolic stress testing by dynamic stress echocardiography can further improve the diagnostic accuracy. In summary, the proposed diagnostic TTE workflow highlights the necessity to detect a specific diagnosis of non-cardiac and cardiac diseases. The common use of “HFpEF” as a diagnosis should be avoided.
Appendix
Conference discussion
Prof. Dr. Andreas Hagendorff (Leipzig): The “3. Mitteldeutscher Echokardiographie Kongress” in Leipzig had focused the topic “HFpEF” at the debate “focus on echocardiographic clinical trials: can we improve the HFA-PEFF algorithm by echocardiography? “and at the scientific session “heart failure with preserved or normal ejection fraction: a special echocardiographic challenge”. As in 2020 the congress in 2021 and the discussions about this expert proposal were again web based. To introduce into the following virtual conference discussion the president of our working group will start:
Please, tell us about the main diagnostic problem in patients with “HFpEF” symptoms in echocardiography.
Dr. Andreas Helfen (Lünen): According to recent recommendations the echocardiographic characterization of patients with “HFpEF” symptoms is mainly based on clinical symptoms, normal LV size and increased E/E´ as a surrogate parameter of increased LVEDP. However, it is well known that non-cardiac as well as cardiac diseases can be the underlying causes of the described symptoms. Thus, the multiple non-cardiac diseases with symptoms of reduced exercise capacity, edema and dyspnea on exertion should not be integrated into the definition of the “HFpEF” symptoms because cardiac morphology and function as well as PCWP can be within normal ranges in these patients. Thus, heart failure does not exist as the basic problem in this cohort. The uncertainty to clearly define patients with “HFpEF” symptoms according to the described criteria is potentiated by the increased incidence of the clinical symptoms with aging and obesity.
Prof. Dr. Andreas Hagendorff (Leipzig): How can we untangle the Gordian knot of “HFpEF” symptoms?
Dr. Stephan Stöbe (Leipzig): The answer runs like a common thread through the expert proposal. It has consequently been avoided to use “HFpEF” as a diagnosis. “HFpEF” is definitively not a diagnosis. It can only describe a syndrome or a symptom complex, which can be caused by several different pathologies. We need to focus on the specific underlying diagnoses in patients with “HFpEF” symptoms which is the central take home message of the expert proposal.
Prof. Dr. Andreas Hagendorff (Leipzig): Please explain the rationale to avoid the diagnosis “HFpEF” using an example.
Prof. Dr. Dariusch Haghi (Ludwigshafen): The increase of LV wall thickening is an excellent example to explain the importance of a specific diagnosis. LV wall thickening can be induced by hypertrophy of the myocardial fibers i. e. in hypertensive heart disease, aortic valvular stenosis, or hypertrophic cardiomyopathy, by edema i. e. in myocarditis and thoracic trauma, or by accumulation of pathological deposits i. e. in amyloidosis, Gaucher’s or Fabry’s disease. All these specific diagnoses require specific treatment and have different prognoses. Thus, we cannot put all these patients in one cohort to analyze therapeutic effects. It is like using cough as a diagnosis instead of a symptom. Cough due to lung cancer obviously needs another treatment than cough due to neuroses.
Prof. Dr. Andreas Hagendorff (Leipzig): How to improve diagnostic accuracy by echocardiography in patients with “HFpEF” symptoms? Which echocardiographic parameters are helpful?
Priv.-Doz. Dr. Ole Breithardt (Kassel): Echocardiography—especially TTE—offers a plenty of parameters which can be measured. However, a camel is a horse designed by committee. Thus, we must focus on conventional and presumably on a few modern parameters to characterize patients with “HFpEF” symptoms. In the tables we listed the echocardiographic parameters concerning the “HFpEF” scenario almost completely. However, we strongly recommend—of course—not all of them in clinical practice. But how to use uncommon parameters—when applicable under special conditions—if they are not described. Thus, the tables of the expert proposal are informative, helpful and provide orientation.
Prof. Dr. Andreas Hagendorff (Leipzig): Do you think the classification into cardiac phenotypes is useful?
Dr. Jan Knierim (Berlin): The characterization of cardiac morphology and function during real life conditions is the strength of echocardiography. Thus, it is reasonable to start with a descriptive approach of cardiac morphology and function when performing echocardiography. A lot of parameters are age- and gender-dependent. These and further factors must be integrated in a clinical and pathophysiological interpretation of the findings determined by a comprehensive echocardiography. Based on a structured echocardiographic investigation it might be possible to be more successful to detect specific diagnoses.
Prof. Dr. Andreas Hagendorff (Leipzig): This might be especially the problem in patients with “HFpEF” symptoms with the normal cardiac phenotype. How to handle this problem?
Dr. Roland Brandt (Bad Nauheim): This is definitively the most difficult issue in these patients, because either we have overlooked marginal pathological cardiac findings, or the patient has non-cardiac reasons for the symptoms. This scenario reflects the border between general screening and analyzing specific cohorts. General screening is only successful, if the screening tool is frequent and the screening test is highly sensitive and specific. On the other side we want to detect several cardiac diseases at early stages, because we often have good treatment options, i. e. in amyloidosis and Fabry’s diseases.
Prof. Dr. Andreas Hagendorff (Leipzig): Considering this problem, which new or conventional echocardiographic parameters should be determined?
Priv.-Doz. Dr. Daniel Lavall (Leipzig): End systolic LV elastance and arterial elastance are important parameters to understand myocardial function in different HF types. They allow comprehensive evaluation of LV performance for scientific purposes. Due to the complex calculation of these parameters, strain-based measurements such as strain-work analysis, LV and LA strain may be more helpful for rapid, easy, and reproducible assessment of myocardial function in patients with sign and/or symptoms of HF.
Prof. Dr. Andreas Hagendorff (Leipzig): With respect to DD how to analyze LA morphology nowadays?
Priv.-Doz. Dr. Christoph Sinning (Hamburg): The conventional approach analyzing LA volumes and the parameter LAVI is the biplane planimetry using 2D echocardiography. Due to the deviation of the longitudinal LA and LV axis the respective atrial volumes are often underestimated by 2D echocardiography. Thus, if possible, we should use 3D volumetry of the cardiac cavities by TTE in our echo labs.
Prof. Dr. Andreas Hagendorff (Leipzig): What do you think about the coincidence of AF in patients with “HFpEF” symptoms? Do we need different interpretations of the echocardiographic parameters of LA volumes and DD?
Priv.-Doz. Dr. Ertunc Altiok (Aachen): AF is often observed in patients with “HFpEF” symptoms, because increasing LA pressure, impairing LV filling, and stretching LA wall will trigger AF development. However, like the term “HFpEF” AF is mostly a symptom of an underlying disease, which must be characterized by a specific diagnosis. The only exception is lone AF. AF—especially chronic AF—of course, influences LA morphology and function. Thus, LA volume cut offs are different in chronic AF patients in comparison to patients with sinus rhythm.
Prof. Dr. Andreas Hagendorff (Leipzig): If we focus on RA and RV analysis, how to improve echocardiographic workflow?
Dr. Nicolas Merke (Berlin): There are at least to main important aspects to focus. Firstly, due to the bipyramidal anatomy of the right ventricle we are unable to standardize 2D assessments for RV volume analysis. Thus, we must implement 3D echocardiography for verifiable assessment of RV volumes, Secondly, cardiologists should assess the non-invasive estimation of pulmonary vascular resistance (PVR) in clinical routine to differentiate the causes of pulmonary hypertension. The easiest approach is the formula PVR (cm−1) = (10 × VmaxTR)/VTIRVOT, where VTIRVOT is velocity time integral of flow velocities determined in the right ventricular outflow tract.
Prof. Dr. Andreas Hagendorff (Leipzig): Do you think that the structural echocardiographic approach in patients with “HFpEF” symptoms described in the present proposal might substantially improve workflow in clinical routine?
Prof. Dr. Fabian Knebel (Berlin): Systematic and structured procedures always have the chance for improvement. The important message of this expert proposal is not to be content to diagnose “HFpEF”. We must focus on the underlying diseases of the “HFpEF” syndrome using all echocardiographic tools. To solve this challenge echocardiography provides more than E/E’. Especially modern tools like deformation imaging and 3D TEE, but also “old” conventional parameters like LV elastance and PVR as well as diastolic stress tests should be integrated into a comprehensive echocardiography.
Prof. Dr. Andreas Hagendorff (Leipzig): Carsten, as the coauthor of the HFA-PEFF diagnostic algorithm published 2019 in the European Heart Journal, do you agree with the proposed echocardiographic implementations to analyze patients with “HFpEF” symptoms by TEE?
Prof. Dr. Carsten Tschöpe (Berlin): This expert proposal includes a lot of information—perhaps too much for physicians who are not experts in echocardiography. However, the variability of diseases in patients with “HFpEF” favours a more detailed echocardiographic analysis than estimating LVEDP by E/E´ and parameters of DD. As I said in the paper of 2019 “HFpEF” is a syndrome and needs final clarification by a specific diagnosis. Thus, I can agree to prepone the step 4 in our HFA-PEFF algorithm if we start with TTE. The echocardiographic workflow can be more successful in detecting specific diagnoses in patients with “HFpEF” symptoms by a structured approach as introduced in this expert proposal.
Prof. Dr. Andreas Hagendorff (Leipzig): To come to an end in our discussion, just a few words to therapeutic options in patients with “HFpEF” symptoms.
Priv.-Doz. Dr. Sebastian Ewen (Homburg/Saar): The answer is easy. Specific findings and diagnoses require specific treatment. Thus, prior to treatment the diagnosis is needed. We often treat symptoms, but it is always better to treat the underlying disease than only the symptoms. Thus, if we can address specific cohorts of patients with “HFpEF” symptoms to specific diseases i. e. due to hypertensive heart disease we can analyze therapeutical effects in these patient cohorts better than analyzing a mixture of patients with a variety of underlying diseases with varying prognoses. However, to finalize our discussion, Andreas, what is your final summary to the “HFpEF” topic.
Prof. Dr. Andreas Hagendorff (Leipzig): I think the most important message of the expert proposal for the old and young generation of physicians is not to use the term “HFpEF” as a diagnosis anymore. The detection of a specific diagnosis is not only the main target in diagnostics. It also reflects the physician’s competence.
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare that they have no competing interest.
References
- 1.Davie AP, Francis CM, Caruana L, Sutherland GR, McMurray JJ. Assessing diagnosis in heart failure: which features are any use? Q J Med. 1997;90(5):335–339. doi: 10.1093/qjmed/90.5.335. [DOI] [PubMed] [Google Scholar]
- 2.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 3.Brutsaert DL, Sys SU, Gillebert TC. Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol. 1993;22(1):318–325. doi: 10.1016/0735-1097(93)90850-z. [DOI] [PubMed] [Google Scholar]
- 4.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging. 2020;13(1 Pt 2):245–257. doi: 10.1016/j.jcmg.2018.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Seferovic P, Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40(40):3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 8.Braunwald E. Heart failure with preserved ejection fraction: a stepchild no more! Eur Heart J. 2021;42(38):3900–3901. doi: 10.1093/eurheartj/ehab601. [DOI] [PubMed] [Google Scholar]
- 9.Segar MW, Patel KV, Ayers C, Basit M, Tang WHW, Willett D, Berry J, Grodin JL, Pandey A. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur J Heart Fail. 2020;22(1):148–158. doi: 10.1002/ejhf.1621. [DOI] [PubMed] [Google Scholar]
- 10.Paulus WJ, Zile MR. From systemic inflammation to myocardial fibrosis: the heart failure with preserved ejection fraction paradigm revisited. Circ Res. 2021;128(10):1451–1467. doi: 10.1161/CIRCRESAHA.121.318159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ. H2FPEF score: at last, a properly validated diagnostic algorithm for heart failure with preserved ejection fraction. Circulation. 2018;138(9):871–873. doi: 10.1161/CIRCULATIONAHA.118.035711. [DOI] [PubMed] [Google Scholar]
- 13.Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manolis AS, Manolis AA, Manolis TA, Melita H. Sudden death in heart failure with preserved ejection fraction and beyond: an elusive target. Heart Fail Rev. 2019;24(6):847–866. doi: 10.1007/s10741-019-09804-2. [DOI] [PubMed] [Google Scholar]
- 15.Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O'Meara E, Ryan JJ, Shah SJ, Mullens W, Zile MR, Lam CSP, McMurray JJV, Solomon SD; PARAGON-HF Investigators Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2019;74(23):2858–2873. doi: 10.1016/j.jacc.2019.09.063. [DOI] [PubMed] [Google Scholar]
- 16.Barandiarán Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca HP, Henkens M, Heymans S, Beussink-Nelson L, Shah SJ, van Empel VPM. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(3):413–421. doi: 10.1002/ejhf.1614. [DOI] [PubMed] [Google Scholar]
- 17.Fayol A, Wack M, Livrozet M, Carves JB, Domengé O, Vermersch E, Mirabel M, Karras A, Le Guen J, Blanchard A, Azizi M, Amar L, Bories MC, Mousseaux E, Carette C, Puymirat E, Hagège A, Jannot AS, Hulot JS. Aetiological classification and prognosis in patients with heart failure with preserved ejection fraction. ESC Heart Fail. 2021 doi: 10.1002/ehf2.13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders-van Wijk S, Barandiarán Aizpurua A, Brunner-La Rocca HP, Henkens MTHM, Weerts J, Knackstedt C, Uszko-Lencer N, Heymans S, van Empel V. The HFA-PEFF and H2 FPEF scores largely disagree in classifying patients with suspected heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23(5):838–840. doi: 10.1002/ejhf.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasic-Szpak E, Marwick TH, Donal E, Przewlocka-Kosmala M, Huynh Q, Gozdzik A, Woznicka AK, Jankowska EA, Ponikowski P, Kosmala W. Prediction of AF in heart failure with preserved ejection fraction: incremental value of left atrial strain. JACC Cardiovasc Imaging. 2021;14(1):131–144. doi: 10.1016/j.jcmg.2020.07.040. [DOI] [PubMed] [Google Scholar]
- 20.Nicoli CD, O’Neal WT, Levitan EB, Singleton MJ, Judd SE, Howard G, Safford MM, Soliman EZ. Atrial fibrillation and risk of incident heart failure with reduced versus preserved ejection fraction. Heart. 2021 doi: 10.1136/heartjnl-2021-319122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson MD, Wei J, Bairey Merz CN. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur Heart J. 2018;39(10):850–852. doi: 10.1093/eurheartj/ehx818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira NL, Grogan M, Dec GW. Spectrum of restrictive and infiltrative cardiomyopathies: part 1 of a 2-part series. J Am Coll Cardiol. 2018;71(10):1130–1148. doi: 10.1016/j.jacc.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Pereira NL, Grogan M, Dec GW. Spectrum of restrictive and infiltrative cardiomyopathies: part 2 of a 2-part series. J Am Coll Cardiol. 2018;71(10):1149–1166. doi: 10.1016/j.jacc.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Del Buono MG, Montone RA, Camilli M, Carbone S, Narula J, Lavie CJ, Niccoli G, Crea F. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(13):1352–1371. doi: 10.1016/j.jacc.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rush CJ, Berry C, Oldroyd KG, Rocchiccioli JP, Lindsay MM, Touyz RM, Murphy CL, Ford TJ, Sidik N, McEntegart MB, Lang NN, Jhund PS, Campbell RT, McMurray JJV, Petrie MC. Prevalence of coronary artery disease and coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6(10):1130–1143. doi: 10.1001/jamacardio.2021.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha A, Rahman H, Webb A, Shah AM, Perera D. Untangling the pathophysiologic link between coronary microvascular dysfunction and heart failure with preserved ejection fraction. Eur Heart J. 2021;42(43):4431–4441. doi: 10.1093/eurheartj/ehab653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristensen SL, Jhund PS, Lee MMY, Køber L, Solomon SD, Granger CB, Yusuf S, Pfeffer MA, Swedberg K, McMurray JJV; CHARM Investigators and Committees Prevalence of prediabetes and undiagnosed diabetes in patients with HFpEF and HFrEF and associated clinical outcomes. Cardiovasc Drugs Ther. 2017;31(5–6):545–549. doi: 10.1007/s10557-017-6754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, Di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, Di Lenarda A. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community-based study. Eur J Heart Fail. 2018;20(9):1257–1266. doi: 10.1002/ejhf.1202. [DOI] [PubMed] [Google Scholar]
- 29.Streng KW, Nauta JF, Hillege HL, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Zannad F, Damman K, van der Meer P, Voors AA. Non-cardiac comorbidities in heart failure with reduced, mid-range and preserved ejection fraction. Int J Cardiol. 2018;271:132–139. doi: 10.1016/j.ijcard.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Bell DSH, Goncalves E. Heart failure in the patient with diabetes: Epidemiology, aetiology, prognosis, therapy and the effect of glucose-lowering medications. Diabetes Obes Metab. 2019;21(6):1277–1290. doi: 10.1111/dom.13652. [DOI] [PubMed] [Google Scholar]
- 31.Gevaert AB, Boen JRA, Segers VF. Van Craenenbroeck EM (2019) Heart failure with preserved ejection fraction: a review of cardiac and noncardiac pathophysiology. Front Physiol. 2019;10:638. doi: 10.3389/fphys.2019.00638.eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hulot JS, Livrozet M. HFpEF: should we consider diabetic patients separately?: the cardiomyocytes say yes. J Am Coll Cardiol. 2021;77(4):420–422. doi: 10.1016/j.jacc.2020.11.051. [DOI] [PubMed] [Google Scholar]
- 33.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 34.Hagendorff A, Fehske W, Flachskampf FA, Helfen A, Kreidel F, Kruck S, La Rosée K, Tiemann K, Voigt JU, von Bardeleben RS, Zahn R, Knebel F. Manual on indications and performance of echocardiography—update 2020 of the German Cardiac Society. Kardiologe. 2020;14:396–431. doi: 10.1007/s12181-020-00402-3. [DOI] [Google Scholar]
- 35.Hagendorff A, Helfen A, Flachskampf FA, Ewen S, Sebastian Kruck S, La Rosee K, Knierim J, Voigt JU, Kreidel F, Fehske W, Brandt R, Zahn R, Knebel F. Manual on indications and performance of specific echocardiographic applications. Kardiologe. 2021;15:595–641. doi: 10.1007/s12181-021-00509-1. [DOI] [Google Scholar]
- 36.Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P, Edvardsen T, Lancellotti P, EACVI Scientific DocumentsCommitteefor2014–16 and 2016–18 Clinical practice of contrast echocardiography: recommendation by the European Association of cardiovascular imaging (EACVI) 2017. Eur Heart J Cardiovasc Imaging. 2017;18:1205–1205a. doi: 10.1093/ehjci/jex182. [DOI] [PubMed] [Google Scholar]
- 37.Omar AM, Bansal M, Sengupta PP. Advances in echocardiographic imaging in heart failure with reduced and preserved ejection fraction. Circ Res. 2016;119(2):357–374. doi: 10.1161/CIRCRESAHA.116.309128. [DOI] [PubMed] [Google Scholar]
- 38.Čelutkienė J, Plymen CM, Flachskampf FA, de Boer RA, Grapsa J, Manka R, Anderson L, Garbi M, Barberis V, Filardi PP, Gargiulo P, Zamorano JL, Lainscak M, Seferovic P, Ruschitzka F, Rosano GMC, Nihoyannopoulos P. Innovative imaging methods in heart failure: a shifting paradigm in cardiac assessment. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(12):1615–1633. doi: 10.1002/ejhf.1330. [DOI] [PubMed] [Google Scholar]
- 39.Obokata M, Reddy YNV, Borlaug BA. The role of echocardiography in heart failure with preserved ejection fraction: what do we want from imaging? Heart Fail Clin. 2019;15(2):241–256. doi: 10.1016/j.hfc.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis GA, Pearce K, Williams SG, Schelbert EB, Macnab A, Miller CA. The utility of cardiovascular imaging in heart failure with preserved ejection fraction: diagnosis, biological classification and risk stratification. Heart Fail Rev. 2021;26(3):661–678. doi: 10.1007/s10741-020-10047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, Flachskampf FA, Galderisi M, Gerber BL, Gimelli A, Klein AL, Knuuti J, Lancellotti P, Mascherbauer J, Milicic D, Seferovic P, Solomon S, Edvardsen T, Popescu BA. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2021 doi: 10.1093/ehjci/jeab154. [DOI] [PubMed] [Google Scholar]
- 42.Støylen A, Daae AS. Physiological significance of pre- and post-ejection left ventricular tissue velocities and relations to mitral and aortic valve closures. Clin Physiol Funct Imaging. 2021;41(5):443–451. doi: 10.1111/cpf.12721. [DOI] [PubMed] [Google Scholar]
- 43.Kasner M, Sinning D, Lober J, Post H, Fraser AG, Pieske B, Burkhoff D, Tschöpe C. Heterogeneous responses of systolic and diastolic left ventricular function to exercise in patients with heart failure and preserved ejection fraction. ESC Heart Failure. 2015;2(3):121–132. doi: 10.1002/ehf2.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, Pitt B, Pfeffer MA, Solomon SD. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132(5):402–414. doi: 10.1161/CIRCULATIONAHA.115.015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tschöpe C, Birner C, Böhm M, Bruder O, Frantz S, Luchner A, Maier L, Störk S, Kherad B, Laufs U. Heart failure with preserved ejection fraction: current management and future strategies: expert opinion on the behalf of the Nucleus of the “Heart Failure Working Group” of the German Society of Cardiology (DKG) Clin Res Cardiol. 2018;107(1):1–19. doi: 10.1007/s00392-017-1170-6. [DOI] [PubMed] [Google Scholar]
- 46.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten CFJ, Vannan MA, Zamorano JL, Zoghbi WA, American Society of Echocardiography, European Association of Echocardiography EAE/ASE recommendations for image acquisition and display using three dimensional echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13:1–46. doi: 10.1093/ehjci/jer316. [DOI] [PubMed] [Google Scholar]
- 47.Rigolli M, Anandabaskaran S, Christiansen JP, Whalley GA. Bias associated with left ventricular quantification by multimodality imaging: a systematic review and meta-analysis. Open Heart. 2016;3(1):388. doi: 10.1136/openheart-2015-000388(eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson S, Rana B, Oxborough D, Steeds R, Monaghan M, Stout M, Pearce K, Harkness A, Ring L, Paton M, Akhtar W, Bedair R, Bhattacharyya S, Collins K, Oxley C, Sandoval J, Schofield MBChBR, Siva A, Parker K, Willis J, Augustine DX. A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: the British Society of Echocardiography minimum dataset. Echo Res Pract. 2020;7(4):G59–G93. doi: 10.1530/ERP-20-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amzulescu MS, De Craene M, Langet H, Pasquet A, Vancraeynest D, Pouleur AC, Vanoverschelde JL, Gerber BL. Myocardial strain imaging: review of general principles, validation, and sources of discrepancies. Eur Heart J Cardiovasc Imaging. 2019;20(6):605–619. doi: 10.1093/ehjci/jez041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voigt JU, Cvijic M. 2- and 3-dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. 2019;12(9):1849–1863. doi: 10.1016/j.jcmg.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 51.Karlsen S, Dahlslett T, Grenne B, Sjøli B, Smiseth O, Edvardsen T, Brunvand H. Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc Ultrasound. 2019;17(1):18. doi: 10.1186/s12947-019-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Čelutkienė J, Pudil R, López-Fernández T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J, Cohen-Solal A, Farmakis D, Tocchetti CG, von Haehling S, Barberis V, Flachskampf FA, Čeponienė I, Haegler-Laube E, Suter T, Lapinskas T, Prasad S, de Boer RA, Wechalekar K, Anker MS, Iakobishvili Z, Bucciarelli-Ducci C, Schulz-Menger J, Cosyns B, Gaemperli O, Belenkov Y, Hulot JS, Galderisi M, Lancellotti P, Bax J, Marwick TH, Chioncel O, Jaarsma T, Mullens W, Piepoli M, Thum T, Heymans S, Mueller C, Moura B, Ruschitzka F, Zamorano JL, Rosano G, Coats AJS, Asteggiano R, Seferovic P, Edvardsen T, Lyon AR. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC) Eur J Heart Fail. 2020;22(9):1504–1524. doi: 10.1002/ejhf.1957. [DOI] [PubMed] [Google Scholar]
- 53.Badano LP, Miglioranza MH, Mihaila S, Peluso D, Xhaxho J, Marra MP, Cucchini U, Soriani N, Iliceto S, Muraru D. Left atrial volumes and function by three-dimensional echocardiography: reference values, accuracy, reproducibility, and comparison with two-dimensional echocardiographic measurements. Circ Cardiovasc Imaging. 2016 doi: 10.1161/CIRCIMAGING.115.004229. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka H. Efficacy of echocardiography for differential diagnosis of left ventricular hypertrophy: special focus on speckle-tracking longitudinal strain. J Echocardiogr. 2021;19(2):71–79. doi: 10.1007/s12574-020-00508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stöbe S, Hagendorff A, Gutberlet M, Tayal B. Myocardial work: a modern tool to detect possible compensation mechanism of deformation in acute myocarditis with preserved left ventricular function. J Cardiovasc Echogr. 2020;30(4):206–210. doi: 10.4103/jcecho.jcecho_48_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stöbe S, Richter S, Seige M, Stehr S, Laufs U, Hagendorff A. Echocardiographic characteristics of patients with SARS-CoV-2 infection. Clin Res Cardiol. 2020;109(12):1549–1566. doi: 10.1007/s00392-020-01727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofrichter P, Hagendorff A, Laufs U, Fikenzer S, Hepp P, Marshall RP, Tayal B, Stöbe S. Analysis of left ventricular rotational deformation by 2D speckle tracking echocardiography: a feasibility study in athletes. Int J Cardiovasc Imaging. 2021;37(8):2369–2386. doi: 10.1007/s10554-021-02213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, Haugaa KH, Opdahl A, Fjeld JG, Gjesdal O, Edvardsen T, Smiseth OA. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: a non-invasive index of myocardial work. Eur Heart J. 2012;33(6):724–733. doi: 10.1093/eurheartj/ehs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Gjesdal O, Edvardsen T, Smiseth OA. Assessment of wasted myocardial work: a novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am J Physiol Heart Circ Physiol. 2013;305(7):H996–1003. doi: 10.1152/ajpheart.00191.2013. [DOI] [PubMed] [Google Scholar]
- 60.Galli E, John-Matthwes B, Rousseau C, Schnell F, Leclercq C, Donal E. Echocardiographic reference ranges for myocardial work in healthy subjects: a preliminary study. Echocardiography. 2019;36(10):1814–1824. doi: 10.1111/echo.14494. [DOI] [PubMed] [Google Scholar]
- 61.Ilardi F, D'Andrea A, D'Ascenzi F, Bandera F, Benfari G, Esposito R, Malagoli A, Mandoli GE, Santoro C, Russo V, Crisci M, Esposito G, Cameli M, On Behalf of The Working Group of Echocardiography of The Italian Society of Cardiology Sic Myocardial work by echocardiography: principles and applications in clinical practice. J Clin Med. 2021;10(19):4521. doi: 10.3390/jcm10194521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschöpe C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116(6):637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 63.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 64.Jones R, Varian F, Alabed S, Morris P, Rothman A, Swift AJ, Lewis N, Kyriacou A, Wild JM, Al-Mohammad A, Zhong L, Dastidar A, Storey RF, Swoboda PP, Bax JJ, Garg P. Meta-analysis of echocardiographic quantification of left ventricular filling pressure. ESC Heart Fail. 2021;8(1):566–576. doi: 10.1002/ehf2.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and Ventricular Systolic Stiffening in Hypertensive Heart Disease. Insights Into the Pathogenesis of Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2009;54(5):410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, Fang JC, Sweitzer NK, Borlaug BA, Kass DA, St. John Sutton M, Cappola TP. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62(13):1165–1172. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwarzl M, Ojeda F, Zeller T, Seiffert M, Becher PM, Munzel T, Wild PS, Blettner M, Lackner KJ, Pfeiffer N, Beutel ME, Blankenberg S, Westermann D. Risk factors for heart failure are associated with alterations of the LV end-diastolic pressure–volume relationship in non-heart failure individuals: data from a large-scale, population-based cohort. Eur Heart J. 2016;37(23):1807–1814. doi: 10.1093/eurheartj/ehw120. [DOI] [PubMed] [Google Scholar]
- 68.von Anrep G. On local vascular reactions and their interpretation. J Physiol. 1912;45(5):318–327. doi: 10.1113/jphysiol.1912.sp001554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarnoff SJ, Mitchell JH, Gilmore JP, Remensnyder JP. Homeometric autoregulation in the heart. Circ Res. 1960;8:1077–1091. doi: 10.1161/01.res.8.5.1077. [DOI] [PubMed] [Google Scholar]
- 70.Reil JC, Reil GH, Kovács Á, Sequeira V, Waddingham MT, Lodi M, Herwig M, Ghaderi S, Kreusser MM, Papp Z, Voigt N, Dobrev D, Meyhöfer S, Langer HF, Maier LS, Linz D, Mügge A, Hohl M, Steendijk P, Hamdani N. CaMKII activity contributes to homeometric autoregulation of the heart: A novel mechanism for the Anrep effect. J Physiol. 2020;598(15):3129–3153. doi: 10.1113/JP279607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borlaug BA. Heart rate reduction: It is not just for ventricles anymore. J Am Coll Cardiol. 2013;62(21):1986–1989. doi: 10.1016/j.jacc.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 72.Shishido T, Hayashi K, Shigemi K, Sato T, Sugimachi M, Sunagawa K. Single-beat estimation of end-systolic elastance using bilinearly approximated time-varying elastance curve. Circulation. 2000;102(16):1983–1989. doi: 10.1161/01.cir.102.16.1983. [DOI] [PubMed] [Google Scholar]
- 73.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PYA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38(7):2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 74.Reil JC, Tardif JC, Ford I, Lloyd SM, O’Meara E, Komajda M, Borer JS, Tavazzi L, Swedberg K, Böhm M. Selective heart rate reduction with ivabradine unloads the left ventricle in heart failure patients. J Am Coll Cardiol. 2013;62(21):1977–1985. doi: 10.1016/j.jacc.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 75.Lavall D, Reil JC, Segura Schmitz L, Mehrer M, Schirmer SH, Böhm M, Laufs U. Early hemodynamic improvement after percutaneous mitral valve repair evaluated by noninvasive pressure–volume analysis. J Am Soc Echocardiogr. 2016;29(9):888–898. doi: 10.1016/j.echo.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 76.Lavall D, Mehrer M, Schirmer SH, Reil JC, Wagenpfeil S, Böhm M, Laufs U. Long-term hemodynamic improvement after transcatheter mitral valve repair. J Am Soc Echocardiogr. 2018;31(9):1013–1020. doi: 10.1016/j.echo.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 77.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction: Implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59(5):442–451. doi: 10.1016/j.jacc.2011.09.062. [DOI] [PubMed] [Google Scholar]
- 78.Klotz S, Hay I, Dickstein ML, Yi GH, Wang J, Maurer MS, Kass DA, Burkhoff D. Single-beat estimation of end-diastolic pressure–volume relationship: a novel method with potential for noninvasive application. Am J Physiol - Hear Circ Physiol. 2006;291(1):H403–412. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- 79.Lam CSP, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County. Minnesota Circulation. 2007;115(15):1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hieda M, Sarma S, Hearon CM, MacNamara JP, Dias KA, Samels M, Palmer D, Livingston S, Morris M, Levine BD. One-year committed exercise training reverses abnormal left ventricular myocardial stiffness in patients with stage B heart failure with preserved ejection fraction. Circulation. 2021;144(12):934–946. doi: 10.1161/CIRCULATIONAHA.121.054117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernard A, Addetia K, Dulgheru R, Caballero L, Sugimoto T, Akhaladze N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, Ilardi F, Lopez T, de la Morena G, Popescu BA, Penicka M, Ozyigit T, David Rodrigo Carbonero J, van de Veire N, Stephan Von Bardeleben R, Vinereanu D, Luis Zamorano J, Martinez C, Magne J, Cosyns B, Donal E, Habib G, Badano LP, Lang RM, Lancellotti P. 3D echocardiographic reference ranges for normal left ventricular volumes and strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18(4):475–483. doi: 10.1093/ehjci/jew284. [DOI] [PubMed] [Google Scholar]
- 82.Bruhl SR, Chahal M, Khouri SJ. A novel approach to standard techniques in the assessment and quantification of the interventricular systolic relationship. Cardiovasc Ultrasound. 2011;9(42):54. doi: 10.1186/1476-7120-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Castro S, Caselli S, Maron M, Pelliccia A, Cavarretta E, Maddukuri P, Cartoni D, Di Angelantonio E, Kuvin JT, Patel AR, Pandian NG. Left ventricular remodelling index (LVRI) in various pathophysiological conditions: a real-time three-dimensional echocardiographic study. Heart. 2007;93(2):205–209. doi: 10.1136/hrt.2006.093997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, Donal E, Sade LE, Ernande L, Garbi M, Grapsa J, Hagendorff A, Kamp O, Magne J, Santoro C, Stefanidis A, Lancellotti P, Popescu B, Habib G; 2016–2018 EACVI Scientific Documents Committee; 2016–2018 EACVI Scientific Documents Committee Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2017;18(12):1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 85.Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez De Diego JJ, Hagendorff A, Henri C, Hristova K, Lopez T, Magne J, De La Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging. 2014;15(6):680–690. doi: 10.1093/ehjci/jet284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manganaro R, Marchetta S, Dulgheru R, Ilardi F, Sugimoto T, Robinet S, Cimino S, Go YY, Bernard A, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, López-Fernández T, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, van de Veire N, Von Bardeleben RS, Vinereanu D, Zamorano JL, Rosca M, Calin A, Moonen M, Magne J, Cosyns B, Galli E, Donal E, Carerj S, Zito C, Santoro C, Galderisi M, Badano LP, Lang RM, Oury C, Lancellotti P. Echocardiographic reference ranges for normal non-invasive myocardial work indices: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2019;20(5):582–590. doi: 10.1093/ehjci/jey188. [DOI] [PubMed] [Google Scholar]
- 87.Sugimoto T, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, Caballero L, Akhaladze N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, Lopez T, de la Morena G, Popescu BA, Moonen M, Penicka M, Ozyigit T, Rodrigo Carbonero JD, van de Veire N, von Bardeleben RS, Vinereanu D, Zamorano JL, Go YY, Rosca M, Calin A, Magne J, Cosyns B, Marchetta S, Donal E, Habib G, Galderisi M, Badano LP, Lang RM, Lancellotti P. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18(8):833–840. doi: 10.1093/ehjci/jex140. [DOI] [PubMed] [Google Scholar]
- 88.Rusinaru D, Bohbot Y, Djelaili F, Delpierre Q, Altes A, Serbout S, Kubala M, Maréchaux S, Tribouilloy C. Normative reference values of cardiac output by pulsed-wave doppler echocardiography in adults. Am J Cardiol. 2021;140:128–133. doi: 10.1016/j.amjcard.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 89.Sugimoto T, Robinet S, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, Caballero L, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, Lopez T, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, van de Veire N, Von Bardeleben RS, Vinereanu D, Zamorano JL, Go YY, Marchetta S, Nchimi A, Rosca M, Calin A, Moonen M, Cimino S, Magne J, Cosyns B, Galli E, Donal E, Habib G, Esposito R, Galderisi M, Badano LP, Lang RM, Lancellotti P; NORRE Study Echocardiographic reference ranges for normal left atrial function parameters: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2018;19(6):630–638. doi: 10.1093/ehjci/jey018. [DOI] [PubMed] [Google Scholar]
- 90.Badano LP, Nagueh SF, Muraru D. Left atrial function: an overlooked metrics in clinical routine echocardiography. Eur J Heart Fail. 2019;21(7):901–903. doi: 10.1002/ejhf.1475. [DOI] [PubMed] [Google Scholar]
- 91.Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(15):1961–1977. doi: 10.1016/j.jacc.2019.01.059. [DOI] [PubMed] [Google Scholar]
- 92.Thomas L, Muraru D, Popescu BA, Sitges M, Rosca M, Pedrizzetti G, Henein MY, Donal E, Badano LP. Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr. 2020;33(8):934–952. doi: 10.1016/j.echo.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 93.Prasad SB, Guppy-Coles K, Stanton T, Armstrong J, Krishnaswamy R, Whalley G, Atherton JJ, Thomas L. Relation of left atrial volumes in patients with myocardial infarction to left ventricular filling pressures and outcomes. Am J Cardiol. 2019;124(3):325–333. doi: 10.1016/j.amjcard.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 94.Shin SH, Claggett B, Inciardi RM, Santos ABS, Shah SJ, Zile MR, Pfeffer MA, Shah AM, Solomon SD. Prognostic value of minimal left atrial volume in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10(15):e019545. doi: 10.1161/JAHA.120.019545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, Tsang TS. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10(2):282–286. doi: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 96.Wu VC, Takeuchi M, Kuwaki H, Iwataki M, Nagata Y, Otani K, Haruki N, Yoshitani H, Tamura M, Abe H, Negishi K, Lin FC, Otsuji Y. Prognostic value of LA volumes assessed by transthoracic 3D echocardiography: comparison with 2D echocardiography. JACC Cardiovasc Imaging. 2013;6(10):1025–1035. doi: 10.1016/j.jcmg.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Russo C, Jin Z, Homma S, Rundek T, Elkind MSV, Sacco RL, Di Tullio MR. LA phasic volumes and reservoir function in the elderly by real-time 3D echocardiography: normal values, prognostic significance, and clinical correlates. JACC Cardiovasc Imaging. 2017;10(9):976–985. doi: 10.1016/j.jcmg.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bajraktari G, Bytyci I, Henein MY. Left atrial structure and function predictors of recurrent fibrillation after catheter ablation: a systematic review and meta-analysis. Clin Physiol Funct Imaging. 2020;40(1):1–13. doi: 10.1111/cpf.12595. [DOI] [PubMed] [Google Scholar]
- 99.Brand A, Frumkin D, Hübscher A, Dreger H, Stangl K, Baldenhofer G, Knebel F. Phasic left atrial strain analysis to discriminate cardiac amyloidosis in patients with unclear thick heart pathology. Eur Heart J Cardiovasc Imaging. 2021;22(6):680–687. doi: 10.1093/ehjci/jeaa043. [DOI] [PubMed] [Google Scholar]
- 100.Frumkin D, Mattig I, Laule N, Al Daas M, Canaan-Kühl S, Knebel F, Stangl K, Brand A. Comparative analysis of phasic left atrial strain and left ventricular posterolateral strain pattern to discriminate Fabry cardiomyopathy from other forms of left ventricular hypertrophy. Echocardiography. 2021;38(11):1870–1878. doi: 10.1111/echo.15224. [DOI] [PubMed] [Google Scholar]
- 101.Huntjens PR, Zhang KW, Soyama Y, Karmpalioti M, Lenihan DJ, Gorcsan J., 3rd Prognostic utility of echocardiographic atrial and ventricular strain imaging in patients with cardiac amyloidosis. JACC Cardiovasc Imaging. 2021;14(8):1508–1519. doi: 10.1016/j.jcmg.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 102.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 103.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 104.Nagueh SF. Diastology: 2020—a practical guide. Echocardiography. 2020;37(11):1919–1925. doi: 10.1111/echo.14742. [DOI] [PubMed] [Google Scholar]
- 105.Abudiab MM, Chebrolu LH, Schutt RC, Nagueh SF, Zoghbi WA. Doppler Echocardiography for the estimation of LV filling pressure in patients with mitral annular calcification. JACC Cardiovasc Imaging. 2017;10(12):1411–1420. doi: 10.1016/j.jcmg.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 106.Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL, Nagueh SF. Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol. 2017;69(15):1937–1948. doi: 10.1016/j.jacc.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 107.Lancellotti P, Galderisi M, Edvardsen T, Donal E, Goliasch G, Cardim N, Magne J, Laginha S, Hagendorff A, Haland TF, Aaberge L, Martinez C, Rapacciuolo A, Santoro C, Ilardi F, Postolache A, Dulgheru R, Mateescu AD, Beladan CC, Deleanu D, Marchetta S, Auffret V, Schwammenthal E, Habib G, Popescu BA. Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-filling study. Eur Heart J Cardiovasc Imaging. 2017;18(9):961–968. doi: 10.1093/ehjci/jex067. [DOI] [PubMed] [Google Scholar]
- 108.Munagala VK, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Association of newer diastolic function parameters with age in healthy subjects: a population-based study. J Am Soc Echocardiogr. 2003;16(10):1049–1056. doi: 10.1016/S0894-7317(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 109.Dalen H, Thorstensen A, Vatten LJ, Aase SA, Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ Cardiovasc Imaging. 2010;3(5):614–622. doi: 10.1161/CIRCIMAGING.109.926022. [DOI] [PubMed] [Google Scholar]
- 110.Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez de Diego JJ, Oliva MJ, Hagendorff A, Hristova K, Lopez T, Magne J, Martinez C, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Bernard A, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging. 2015;16(9):1031–1041. doi: 10.1093/ehjci/jev083. [DOI] [PubMed] [Google Scholar]
- 111.Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Figliozzi S, Georgiopoulos G, Pateras K, Sianis A, Previtero M, Tondi L, Petropoulos Ι, Bragato RM, Papachristidis A, Condorelli G, Takeuchi M. Normal ranges of left atrial volumes and ejection fraction by 3D echocardiography in adults: a systematic review and meta-analysis. Int J Cardiovasc Imaging. 2022 doi: 10.1007/s10554-021-02520-9. [DOI] [PubMed] [Google Scholar]
- 113.Rivas-Gotz C, Khoury DS, Manolios M, Rao L, Kopelen HA, Nagueh SF. Time interval between onset of mitral inflow and onset of early diastolic velocity by tissue Doppler: a novel index of left ventricular relaxation: experimental studies and clinical application. J Am Coll Cardiol. 2003;42(8):1463–1470. doi: 10.1016/s0735-1097(03)01034-9. [DOI] [PubMed] [Google Scholar]
- 114.Milan A, Magnino C, Veglio F. Echocardiographic indexes for the non-invasive evaluation of pulmonary hemodynamics. J Am Soc Echocardiogr. 2010;23(3):225–239. doi: 10.1016/j.echo.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 115.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 116.Maffessanti F, Muraru D, Esposito R, Gripari P, Ermacora D, Santoro C, Tamborini G, Galderisi M, Pepi M, Badano LP. Age-, body size-, and sex-specific reference values for right ventricular volumes and ejection fraction by three-dimensional echocardiography: a multicenter echocardiographic study in 507 healthy volunteers. Circ Cardiovasc Imaging. 2013;6(5):700–710. doi: 10.1161/CIRCIMAGING.113.000706. [DOI] [PubMed] [Google Scholar]
- 117.Muraru D, Spadotto V, Cecchetto A, Romeo G, Aruta P, Ermacora D, Jenei C, Cucchini U, Iliceto S, Badano LP. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur Heart J Cardiovasc Imaging. 2016;17(11):1279–1289. doi: 10.1093/ehjci/jev309. [DOI] [PubMed] [Google Scholar]
- 118.Muraru D, Badano LP, Nagata Y, Surkova E, Nabeshima Y, Genovese D, Otsuji Y, Guida V, Azzolina D, Palermo C, Takeuchi M. Development and prognostic validation of partition values to grade right ventricular dysfunction severity using 3D echocardiography. Eur Heart J Cardiovasc Imaging. 2020;21(1):10–21. doi: 10.1093/ehjci/jez233. [DOI] [PubMed] [Google Scholar]
- 119.Muraru D, Haugaa K, Donal E, Stankovic I, Voigt JU, Petersen SE, Popescu BA, Marwick T. Right ventricular longitudinal strain in the clinical routine: a state-of-the-art review. Eur Heart J Cardiovasc Imaging. 2022 doi: 10.1093/ehjci/jeac022. [DOI] [PubMed] [Google Scholar]
- 120.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, D'Hooge J, Donal E, Fraser AG, Marwick T, Mertens L, Popescu BA, Sengupta PP, Lancellotti P, Thomas JD, Voigt JU; Industry representative Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 121.Brand A, Bathe M, Oertelt-Prigione S, Seeland U, Rücke M, Regitz-Zagrosek V, Stangl K, Knebel F, Stangl V, Dreger H. Right heart function in impaired left ventricular diastolic function: 2D speckle tracking echocardiography-based and Doppler tissue imaging-based analysis of right atrial and ventricular function. Echocardiography. 2018;35(1):47–55. doi: 10.1111/echo.13745. [DOI] [PubMed] [Google Scholar]
- 122.Brand A, Bathe M, Hübscher A, Baldenhofer G, Hättasch R, Seeland U, Oertelt-Prigione S, Rücke M, Regitz-Zagrosek V, Stangl K, Dreger H, Stangl V, Knebel F. Normative reference data, determinants, and clinical implications of right atrial reservoir function in women assessed by 2D speckle-tracking echocardiography. Echocardiography. 2018;35(10):1542–1549. doi: 10.1111/echo.14092. [DOI] [PubMed] [Google Scholar]
- 123.Bréchot N, Gambotti L, Lafitte S, Roudaut R. Usefulness of right ventricular isovolumic relaxation time in predicting systolic pulmonary artery pressure. Eur J Echocardiogr. 2008;9(4):547–554. doi: 10.1093/ejechocard/jen121. [DOI] [PubMed] [Google Scholar]
- 124.Matsukubo H, Matsuura T, Endo N, Asayama J, Watanabe T, Furukawa K, Kunishjge H, Katsume H, Ijichi H. Echocardiographic measurement of right ventricular wall thickness. A new application of subxiphoid echocardiography. Circulation. 1977;56(2):278–284. doi: 10.1161/01.cir.56.2.278. [DOI] [PubMed] [Google Scholar]
- 125.Wang S, Wang S, Zhu Q, Wang Y, Li G, Kong F, Yang J, Ma C. Reference values of right ventricular volumes and ejection fraction by three-dimensional echocardiography in adults: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:709863. doi: 10.3389/fcvm.2021.709863.eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017;135(9):825–838. doi: 10.1161/CIRCULATIONAHA.116.024822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kobayashi M, Huttin O, Magnusson M, Ferreira JP, Bozec E, Huby AC, Preud'homme G, Duarte K, Lamiral Z, Dalleau K, Bresso E, Smaïl-Tabbone M, Devignes MD, Nilsson PM, Leosdottir M, Boivin JM, Zannad F, Rossignol P, Girerd N; STANISLAS Study Investigators Machine learning-derived echocardiographic phenotypes predict heart failure incidence in asymptomatic individuals. JACC Cardiovasc Imaging. 2022;15(2):193–208. doi: 10.1016/j.jcmg.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 128.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56(11):855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 129.Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20(9):1303–1311. doi: 10.1002/ejhf.1220. [DOI] [PubMed] [Google Scholar]
- 130.Hummel YM, Liu LCY, Lam CSP, Fonseca-Munoz DF, Damman K, Rienstra M, van der Meer P, Rosenkranz S, van Veldhuisen DJ, Voors AA, Hoendermis ES. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail. 2017;19(12):1651–1660. doi: 10.1002/ejhf.957. [DOI] [PubMed] [Google Scholar]
- 131.Kapłon-Cieślicka A, Laroche C, Crespo-Leiro MG, Coats AJS, Anker SD, Filippatos G, Maggioni AP, Hage C, Lara-Padrón A, Fucili A, Drożdż J, Seferovic P, Rosano GMC, Mebazaa A, McDonagh T, Lainscak M, Ruschitzka F, Lund LH; Heart Failure Association (HFA) of the European Society of Cardiology (ESC) and the ESC Heart Failure Long-Term Registry Investigators Is heart failure misdiagnosed in hospitalized patients with preserved ejection fraction? From the European society of cardiology—heart failure association Eurobservational research programme heart failure long-term registry. ESC Heart Fail. 2020;7:2098–2112. doi: 10.1002/ehf2.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kapłon-Cieślicka A, Kupczyńska K, Dobrowolski P, Michalski B, Jaguszewski MJ, Banasiak W, Burchardt P, Chrzanowski Ł, Darocha S, Domienik-Karłowicz J, Drożdż J, Fijałkowski M, Filipiak KJ, Gruchała M, Jankowska EA, Jankowski P, Kasprzak JD, Kosmala W, Lipiec P, Mitkowski P, Mizia-Stec K, Szymański P, Tycińska A, Wańha W, Wybraniec M, Witkowski A, Ponikowski P, "Club 30" Of The Polish Cardiac Society OBO On the search for the right definition of heart failure with preserved ejection fraction. Cardiol J. 2020;27:449–468. doi: 10.5603/CJ.a2020.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]