Abstract
Data of the diabetes mellitus patients is essential in the study of diabetes management, especially when employing the data-driven machine learning methods into the management. To promote and facilitate the research in diabetes management, we have developed the ShanghaiT1DM and ShanghaiT2DM Datasets and made them publicly available for research purposes. This paper describes the datasets, which was acquired on Type 1 (n = 12) and Type 2 (n = 100) diabetic patients in Shanghai, China. The acquisition has been made in real-life conditions. The datasets contain the clinical characteristics, laboratory measurements and medications of the patients. Moreover, the continuous glucose monitoring readings with 3 to 14 days as a period together with the daily dietary information are also provided. The datasets can contribute to the development of data-driven algorithms/models and diabetes monitoring/managing technologies.
Subject terms: Public health, Machine learning, Metabolomics, Data mining
| Measurement(s) | blood glucose |
| Technology Type(s) | Continuous Glucose Monitoring System |
| Sample Characteristic - Organism | Homo sapiens |
Background & Summary
Diabetes is a chronic disease that could lead to cardiovascular disease, neuropathy, retinopathy, kidney failure and even mortality. Rapid socioeconomic changes and unhealthy lifestyle habits have led to the increasing prevalence of diabetes worldwide. Type 1 diabetes mellitus (T1DM) and Type 2 diabetes mellitus (T2DM) are the two main types of diabetes. T1DM is a chronic autoimmune disease resulting from destruction or damaging of the pancreatic beta cells1. T2DM is caused by insulin resistance and relative insulin deficiency2. T1DM accounts for only 5–10% of all diabetes worldwide, but varies geographically with the annual incidence of adult-onset T1DM about 1 per 100,000 in China3, while T2DM is the most common subtype of diabetes, accounting for over 90% of all the diabetes worldwide and in China3,4. It is shown that good blood glucose (BG) control significantly reduces the development or progression of chronic complications in T1DM and T2DM5–7. Thus, BG measurement plays a key part in diabetes care, which allows patients to adjust their food intake, physical activity and medications with the help of physicians (clinicians)8. Self-monitoring of blood glucose (SMBG) is a measurement that uses blood to collect blood glucose information at many time points9. Recently, a continuous glucose monitoring (CGM) technology is used to continuously monitor the BG levels in more or less real time10,11.
The use of CGM technology makes it possible to obtain a large amount of continuous BG data. However, there were relatively few publicly available BG datasets, as the data may have ethical restrictions and privacy concerns. There have been many studies12,13 on the BG prediction using different datasets. A rigorous literature review12 was conducted to develop a compact guide regarding machine learning methods on BG prediction in T1DM. The review included 55 papers from 2000 to 2018 and showed their subject, type of input, data source, input pre-processing methods, machine learning algorithms, prediction horizon and performance metrics. A systematical review13 on the literature from 2014 to 2020 was performed to study the data-based algorithms and models using real data for BG and hypoglycaemia prediction in T1/T2DM. The existing datasets in T1/T2DM for the BG prediction have been listed in the review. However, the T2DM datasets are much less studied than the T1DM datasets, e.g., 6 of 63 publications included T2DM in the review13. For real data, the data size was relatively small. In the review13, 27 papers (42.9%) present small samples (n < 10), 19 papers (30.2%) with small-medium samples (n = 11–50) and 17 papers (27%) with relatively large samples (n > 50). In another review for T1DM12, 51.7% were with small samples, 29.3% with small-medium samples, 17.2% with simulated data and 1.7% with samples over 50 patients. Another limitation pointed out by the reviews was the low free access data availability. Most data are credentialed or not accessible due to ethical restrictions and data privacy. We summarized recently studied and popular T1DM and T2DM datasets in Table 1.
Table 1.
A summary on existing diabetes data in the literature.
| Datasets | Type | Study period (days) | No.of patients | Data Availability | CGM /CBG | Food | Exercise | Insulin Use | Published Year |
|---|---|---|---|---|---|---|---|---|---|
| UVA/Padova14,16–18,41,42 | T1DM | customized | 30 | Open43 | ✓/✓ | ✓ | × | ✓ | 2018 |
| OhioT1DM18,19,21,22,44 | T1DM | 56 | 12 | Credentialed45 | ✓/✓ | ✓ | ✓ | ✓ | 2020 |
| D1NAMO23 | T1DM | 4 | 9 | Credentialed46 | ✓/× | ✓ | ✓ | ✓ | 2018 |
| ABC4D18,24 | T1DM | 180 | 10 | not accessible | ✓/× | ✓ | × | ✓ | 2020 |
| Weinstock25 | T1DM | 12 | 201 | not accessible | ✓/× | × | × | ✓ | 2016 |
| KDD1826 | T1DM | 1095 | 40 | Open47 | ✓/× | × | × | × | 2018 |
| Yang29 | T1/T2DM | 1–7 | 49/51 | not accessible | ✓/× | N/A | N/A | N/A | 2018 |
| Maryland27 | T2DM | 365 | N/A | not accessible | ×/✓ | × | × | ✓ | 2015 |
| Maastricht study28,30 | T2DM | 2 | 851 | Credentialed48 | ✓/✓ | × | ✓ | × | 2021 |
CBG, capillary blood glucose; CGM, continuous glucose monitoring; N/A, not available; T1DM, Type 1 diabetes mellitus; T2DM, Type 2 diabetes mellitus.
In T1DM, both real and simulated patient data in silico were well studied. Simulators can conveniently provide and customize detailed data of virtual diabetic patients from their dietary and treatment strategies. UVA/Padova T1DM simulator14 was widely employed, which was approved by Food and Drug Administration (FDA) and provided 30 different virtual patients freely. Virtual diabetes simulators were studied in tasks such as glycemic events identification, BG control15 and predictions14,16–18. The simulators were able to generate as many BG instances as possible for each patient14.
As a public dataset, OhioT1DM18–22 was a comprehensive dataset of real T1DM patients in the United States, which was publicly released by Ohio University and contained data of 12 real patients. Compared to the OhioT1DM, D1NAMO23 dataset focused on diabetes management. This dataset was composed of 20 real healthy people and nine real T1DM patients with additional patient information such as BG measurements, food pictures, breathing signals and accelerometer outputs. A clinical data18,24 including 10 T1DM adults from the ABC4D project using CGM sensors was used in a deep learning framework for accurate glucose forecasting. Weinstock25 collected diabetes-related data from adult type 1 diabetes (> = 60 years of age, diabetes duration > = 20 years). This dataset consisted of 14 days’ CGM data, information of insulin, other medications and patient demographics from 201 patients. This dataset was proposed to analyze the risk factors that can cause severe hypoglycemia in old patients. Fox et al.26 collected CGM records from 40 T1DM patients over three years (data size > = 1900 days of BG measurements, > = 550k distinct glucose measurements) and developed a deep multi-output forecasting algorithm.
T2DM datasets were less common than T1DM datasets27,28. A CGM data from both the T1DM and T2DM patients were employed to predict future BG levels for preventing hyperglycemia or hypoglycemia29, which was collected over a period ranging from 1.3 to 7 days. The Maryland data27 contained 56,000 SMBG data points collected in a 1-year prospective study. In this study, patients were treated with a variety of medications, including oral antihyperglycemic agents and insulin. The Maastricht Study28,30, an observational, prospective, population-based cohort study, focused on the aetiology, pathophysiology, complications and comorbidities of T2DM, and was characterized by an extensive phenotyping approach.
The existing diabetes data are used not only in BG prediction31, but also in other diabetes-related fields, such as the generation of BG control strategies15 and the study of the influence of external factors on blood glucose level. However, the limitations of many diabetes datasets in terms of the number of patients, the racial regions where they are collected, and the types of diabetes mellitus have led to the restrictions in diabetes-related research.
It is known that dietary intake, exercise and medication are the main factors affecting the BG level32,33. The collection on these external information is therefore essential in the datasets, which is a tedious task. More specifically, eating habits are quite influenced by ethnic groups and regions, e.g., the Chinese dietary habits are very complicated34. Therefore, two datasets from T1DM and T2DM patients in Shanghai, China with dietary information, clinical characteristics, laboratory measurements and medications of the patients were constructed. To the best of our knowledge, these are the first publicly available datasets to include rich information for people with T1DM and T2DM in China. The datasets could contribute to the research in data-driven machine learning.
Methods
Study population
A registry study on Diabetes Data Registry and Individualized Lifestyle Intervention (DiaDRIL) was initiated in Shanghai East Hospital and Shanghai Fourth People’s Hospital affiliated to Tongji University since 2019. The aims of this project were to provide evidence for personalized lifestyle recommendations and optimize glycemic control.
In this study, the patients were recruited from DiaDRIL in Shanghai East Hospital (September 2019 to March 2021) and Shanghai Fourth Peopleś Hospital (June 2021 to November 2021), respectively. The inclusion criteria were as follows: patients with diagnosed diabetes according to the 1999 World Health Organization (WHO) criteria; more than 18 years of age, willing to sign the informed consent form and with CGM recording for at least 3 days. Patients were excluded if they reported alcohol or drug abuse, were unable to comply with the study, or were not suitable to attend this study judged by the investigators. Data was anonymous to protect the sensitive information of the patients.
Clinical and laboratory measurements
A standard questionnaire was conducted by trained research staff to obtain demographic information. Information on diagnosis and treatment of diabetes, duration of diabetes, laboratory measurements, comorbidities and pharmacologic treatments were collected from medical records. Each patient underwent a physical examination including measurement of height and weight. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Each patient wore a flash glucose monitoring device (FreeStyle Libre H, Abbott Diabetes Care, Witney, UK) to measure interstitial glucose levels continuously for up to 14 days. CGM glucose data were automatically stored on the sensor every 15 minutes. The data can be obtained by scanning the glucose sensor with the reader and uploaded using the device software. Available laboratory measurements (≤6 months before or after CGM) including glucose metabolism, lipid profile and renal function were obtained from medical records. Any dietary intake including the exact time at consumption and weighed food record was reported by the patients. Hypoglycemic medications during CGM were also recorded.
This study was approved by the Ethics Committee of Shanghai Fourth People’s Hospital and Shanghai East Hospital affiliated to Tongji University in accordance with the Declaration of Helsinki. The informed consent was obtained from all the patients.
CGM parameters
Time in range (TIR), one of the critical CGM-derived metrics, reflects the glucose variability and evaluates the quality of glycemic control35. It is associated with microvascular complications and macrovascular outcomes of diabetes. TIR is defined as the percentage of time spent in the target glucose range of 70–180 mg/dL. Time below range (TBR) and time above range (TAR) are the percentage of time when blood glucose is below 70 mg/dL and above 180 mg/dL, respectively. For most patients with T1DM or T2DM, the recommended CGM targets by the Advanced Technologies & Treatments for Diabetes (ATTD) consensus were ≥70% for TIR, ≤25% for TAR and ≤4% for TBR36.
Analysis for CGM data
A clinical important task in diabetes management is the prevention of hypo/hyperglycemic events37. The algorithms to prevent the hpyo/hyperglycemic events can be obtained by generating hpyo/hyperalerts on the basis of ahead-of-time prediction of glucose concentration by using past CGM data and suitable time-series models.
Auto-correlation38 represents the degree of similarity between a given time series and a lagged version of itself over successive time intervals. It can help to uncover hidden patterns in data. Additionally, analyzing the autocorrelation function (ACF) and partial autocorrelation function (PACF) in conjunction is necessary for selecting the appropriate time-series models, e.g., ARIMA39.
where xt is the observation at time t, k is lag, E is the expected value operator, μ is the mean and σ2 is the variance of the time series. ρk can show the correlation between two observations with a lag k in the time series.
Data Records
The datasets ShanghaiT1DM and ShanghaiT2DM comprise two folders named “Shanghai_T1DM” and “Shanghai_T2DM” and two summary sheets named “Shanghai_T1DM_Summary” and “Shanghai_T2DM_Summary”. The datasets can be downloaded through Figshare repository40.
The “Shanghai_T1DM” folder and “Shanghai_T2DM” folder contain 3 to 14 days of CGM data corresponding to 12 patients with T1DM and 100 patients with T2DM, respectively. Of note, for one patient, there might be multiple periods of CGM recordings due to different visits to the hospital, which were stored in different excel tables. In fact, collecting data from different periods in one patient can reflect the changes of diabetes status during the follow-up. The excel table is named by the patient ID, period number and the start date of the CGM recording. Thus, for 12 patients with T1DM, there are 8 patients with 1 period of the CGM recording and 2 patients with 3 periods, totally equal to 16 excel tables in the “Shanghai_T1DM” folder. As for 100 patients with T2DM, there are 94 patients with 1 period of CGM recording, 6 patients with 2 periods, and 1 patient with 3 periods, amounting to 109 excel tables in the “Shanghai_T2DM” folder. Overall, the excel tables include CGM BG values every 15 minutes, capillary blood glucose (CBG) values, blood ketone, self-reported dietary intake, insulin doses and non-insulin hypoglycemic agents. The blood ketone was measured when diabetic ketoacidosis was suspected with a considerably high glucose level. Insulin administration includes continuous subcutaneous insulin infusion using insulin pump, multiple daily injections with insulin pen, and insulin that were given intravenously in case of an extremely high BG level.
Each excel table in the “Shanghai_T1DM” folder and “Shanghai_T2DM” folder contains the following data fields: <Date> Recording time of the CGM data. <CGM> CGM data recorded every 15 minutes. <CBG> CBG level measured by the glucose meter. <Blood ketone> Plasma-hydroxybutyrate measured with ketone test strips (Abbott Laboratories, Abbott Park, Illinois, USA). <Dietary intake> Self-reported time and weighed food intake <Insulin dose-s.c.> Subcutaneous insulin injection with insulin pen. <Insulin dose-i.v.> Dose of intravenous insulin infusion. <Non-insulin hypoglycemic agents> Hypoglycemic agents other than insulin. <CSII-bolus insulin> Dose of insulin delivered before a meal through insulin pump. <CSII-basal insulin> The rate (iu/per hour) at which basal insulin was continuously infused through insulin pump.
The summary sheets summarize the clinical characteristics, laboratory measurements and medications of the patients included in this study, with each row corresponding to one excel table in “Shanghai_T1DM” and “Shanghai_T2DM” folders. Clinical characteristics include patient ID, gender, age, height, weight, BMI, smoking and drinking history, type of diabetes, duration of diabetes, diabetic complications, comorbidities as well as occurrence of hypoglycemia. Laboratory measurements contain fasting and 2-hour postprandial plasma glucose/C-peptide/insulin, hemoglobin A1c (HbA1c), glycated albumin, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, creatinine, estimated glomerular filtration rate, uric acid and blood urea nitrogen. Both hypoglycemic agents and medications given for other diseases before the CGM reading were also recorded.
Technical Validation
The characteristics of the Chinese diabetes datasets
The detailed characteristics of the patients in the ShanghaiT1DM and ShanghaiT2DM datasets were summarized in Table 2. The age of the ShanghaiT1DM group and the ShanghaiT2DM group was 57.8 ± 11.1 and 60.2 ± 13.7 years, respectively. There was no statistically significant difference in age between the ShanghaiT1DM group and ShanghaiT2DM group. This is because most of the patients (10/12) in the ShanghaiT1DM group belonged to a subtype of T1DM called “latent autoimmune diabetes in adults”, which is characterized by slow autoimmune β-cell destruction and an older mean age at onset of diabetes1. Women accounted for 58.3% of the ShanghaiT1DM group and 44% of the ShanghaiT2DM group, respectively. Besides, data concerning fasting plasma glucose, 2-hour postprandial plasma glucose and HbA1c were comparable between the two groups. However, the ShanghaiT2DM group had higher BMI values than the ShanghaiT1DM group (p < 0.05).
Table 2.
The characteristics of the T1DM and T2DM patients in the ShanghaiT1DM and ShanghaiT2DM.
| Characteristics | ShanghaiT1DM (n = 12) | ShanghaiT2DM (n = 100) | p value |
|---|---|---|---|
| Age, years | 57.83 ± 11.12 | 60.17 ± 13.71 | 0.571 |
| Women, n (%) | 7 (58.3%) | 44 (44.0%) | 0.346 |
| BMI, kg/m2 | 20.95 [17.87–24.21] | 23.69 [22.12–25.54] | 0.017 |
| Duration of diabetes, years | 8.50 [2.25–16.75] | 7.00 [1.00–14.75] | 0.614 |
| Fasting plasma glucose, mg/dL | 184.08 [117.00–262.35] | 158.40 [126.00–194.40] | 0.410 |
| 2-hour postprandial plasma glucose, mg/dL | 297.00 [248.76–348.84] | 250.65 [196.16–317.88] | 0.218 |
| HbA1c, mmol/mol | 71 [63–122] | 69 [54–97] | 0.223 |
Data are presented as mean ± SD, median [interquartile range], or number(percentage%). BMI, body mass index; HbA1c, hemoglobin A1c; T1DM, Type 1 diabetes mellitus; T2DM, Type 2 diabetes mellitus.
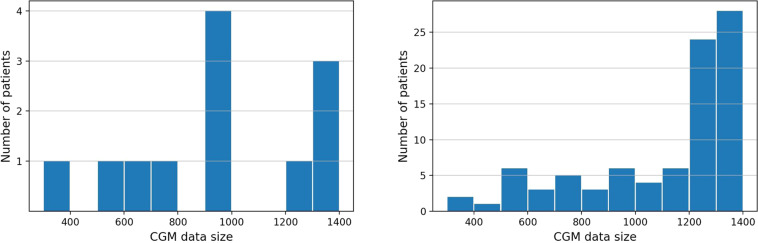
To show the size of these two datasets more intuitively, we listed the patient’s type, the study period, sampling interval of CGM devices, number of patients, total number of recording files and total CGM measurements of the ShanghaiT1DM and ShanghaiT2DM in Table 3. For a given patient, he or she may have more than one recording period. In Fig. 1, we showed the number of recording files with different CGM data size in days in the ShanghaiT1DM and ShanghaiT2DM. The collected CGM data size varied from 3 days to 14 days.
Table 3.
General characteristics of the datasets.
| Datasets | Type of diabetes | Study period (days) | Monitoring interval (minutes) | No. of patients | No. of recording file | Total CGM measurements |
|---|---|---|---|---|---|---|
| ShanghaiT1DM | T1DM | 4–14 | 15 | 12 | 16 | 15,695 |
| ShanghaiT2DM | T2DM | 3–14 | 15 | 100 | 109 | 112,475 |
| SimulatorT1DM | T1DM | 56 | 5 | 30 | unlimited | 482,610 |
| OhioT1DM | T1DM | 56 | 5 | 12 | 12 | 191,605 |
CGM, continuous glucose monitoring; T1DM, Type 1 diabetes mellitus; T2DM, Type 2 diabetes mellitus; No., number.
Fig. 1.
The number of recording files with different CGM data size in days (a) ShanghaiT1DM dataset (b) ShanghaiT2DM dataset.
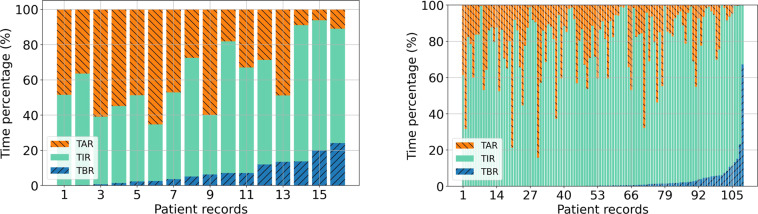
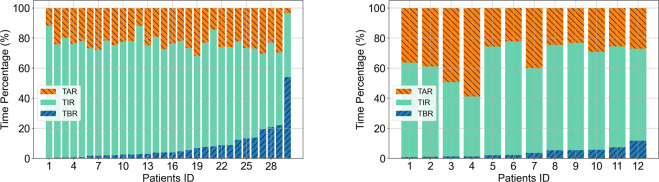
We summarized the hypo/hyperglycemia events and calculated the auto-correlation coefficient on the BG values of the two datasets in time series. Hypoglycemia and hyperglycemia events are two potential risk factors for complications in diabetes. Hence, the time percentages of hypoglycemia (TBR) and hyperglycemia (TAR) events for each patient were calculated in Fig. 2. The horizontal axis represented each recording file of the patients with an order of TBR increasing, while the vertical axis represented the percentage of time (TAR, TIR and TBR) during the data collection period. The higher values of the TAR and TBR indicated that the patient’s condition was more serious. To give a clearer view of the TBR, TIR and TAR in the two datasets, we calculated the mean ± standard deviation of these values for the two datasets. For the ShanghaiT1DM, the mean ± standard deviation of the TIR were 54.7 ± 14.5% and 77.7 ± 18.1% for the ShanghaiT2DM. We noted that the average TIR was higher in T2DM patients than in T1DM patients (Fig. 2).
Fig. 2.
The average percentage of TBR (time below range), TIR (time in range) and TAR (time above range) for CGM in two datasets. (a) ShanghaiT1DM: TAR (37.8 ± 18.8%), TIR (54.7 ± 14.5%), TBR (7.5 ± 7.0%). (b) ShanghaiT2DM: TAR (20.0 ± 18.4%), TIR (77.7 ± 18.1%), TBR (2.4 ± 7.2%). Data are presented as mean ± SD.
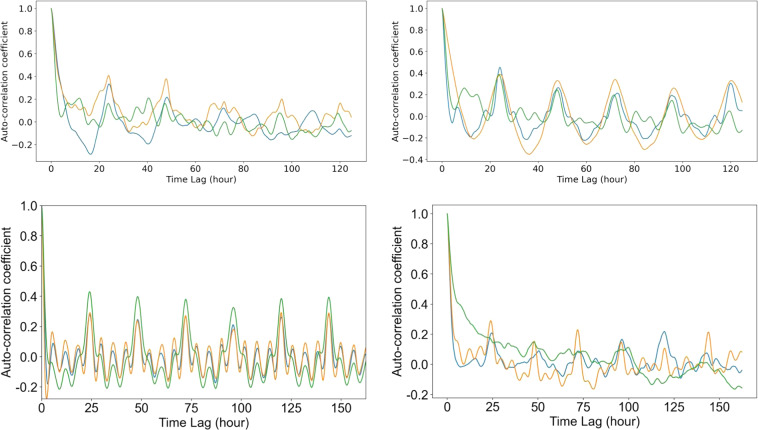
Besides, as the collection on individual patient’s behavior information in each dataset was different, we randomly chose three patients from each dataset for the auto-correlation graph of the BG time series in Fig. 3. The auto-correlation coefficients identify seasonality and trend in time series data. It can be found that patients in ShanghaiT2DM (Fig. 3b) showed a more noticeable 24-hour periodic pattern than those in ShanghaiT1DM (Fig. 3a).
Fig. 3.
Auto-correlation coefficient of randomly picked three patients from the (a) ShanghaiT1DM, (b) ShanghaiT2DM, (c) SimulatorT1DM and (d) OhioT1DM.
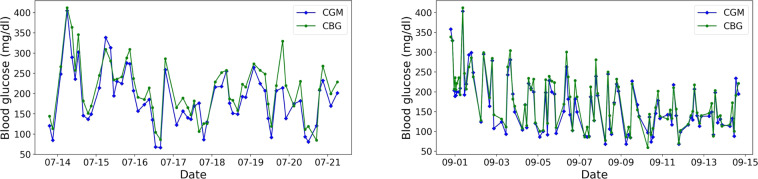
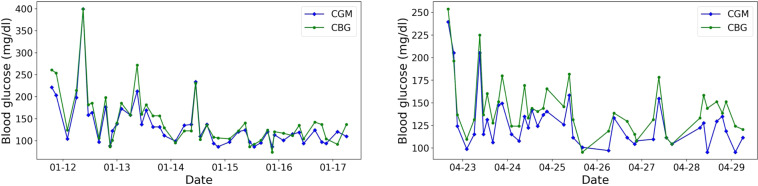
Since there might be discrepancy in BG levels by different blood glucose monitoring methods, we conducted a comparative analysis of the blood glucose measured by the CGM and CBG in Fig. 4, 5. The collection of the CBG was more sparse than that of the CGM, we only plotted the time stamps with both of the measurements. Two patients were randomly selected from each dataset. The results showed that the CBG values were usually greater than those of CGM readings.
Fig. 4.
Randomly selected patients (a) 1008_0_20210713 and (b) 1003_0_20210831 in the ShanghaiT1DM for the distributions of glucose values of CGM readings and CBG. (CGM, continuous glucose monitoring; CBG, capillary blood glucose).
Fig. 5.
Randomly selected patients (a) 2010_0_20220111 and (b) 2022_0_20210419 in the ShanghaiT2DM for the distributions of glucose values of CGM readings and CBG. (CGM, continuous glucose monitoring; CBG, capillary blood glucose).
Comparison to other datasets
There have been widely used datasets such as the SimulatorT1DM and the OhioT1DM (see Table 3). In order to show more specifically the difference between the newly constructed datasets and other existing data, the comparisons were performed in Table 3, figs. 3c,d & 6.
Fig. 6.
The average percentage of TBR (time below range), TIR (time in range) and TAR (time above range) for CGM (continuous glucose monitoring) in two datasets. (a) SimulatorT1DM: TAR (22.9 ± 5.9%), TIR (69.1 ± 10.2%),TBR (8.0 ± 10.5%). (b) OhioT1DM: TAR (33.4 ± 11.1%),TIR (62.6 ± 9.9%),TBR (4.0 ± 3.1%). Data are presented as mean ± SD.
The auto-correlation coefficients of the ShanghaiT1DM (Fig. 3a) and OhioT1DM (Fig. 3d) indicated that the two real T1DM datasets shared similar trend and periodic pattern, which made it possible to combine the two datasets together in certain research. The SimulatorT1DM (Fig. 3c) had strong regularity as it was simulated.
Achieving higher TIR has been shown to reduce the percentages of time in the hypoglycemic and hyperglycemic range and complications of diabetes. In Fig. 6, we found that the patients in the OhioT1DM had lower mean TBR values compared to those in the ShanghaiT1DM (Fig. 2), which means that they have better control of hypoglycemia. In addition, patients in the ShanghaiT2DM (Fig. 2) had the highest mean TIR values, which suggests that people with T2D have better glycemic control overall than people with T1D. The virtual patients from the UVA/Padova (Fig. 6) had worse control of hypoglycemia, which may be due to the fact that the glycemic control strategy of the virtual patients was based on a fixed formula and therefore could not produce a timely response to the hypoglycemia. By comparing the ShanghaiT1DM and OhioT1DM (Fig. 6), we found that the standard deviations of TBR, TIR and TAR in the ShanghaiT1DM were higher than those in the OhioT1DM.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 61972286, 82070913), the Shanghai Municipal Science and Technology Major Project (2021SHZDZX0100), the Natural Science Foundation of Shanghai, China (Grant No. 20ZR1460500, 22511104300), the Shanghai Science and Technology Development Funds (Grant No. 20ZR1446000, 22410713200), the Fundamental Research Funds for the Central Universities and the Research fund from Shanghai Fourth People’s Hospital (sykyqd01801, SY-XKZT-2021-1001). Finally, thanks Ms. Xiongbaixue Yan for her previous efforts on the management of the project.
Author contributions
Q.Z., J.Z. and C.W. had the initial idea for this study. C.L., X.S., B.C. and C.W. established the datasets, i.e., ShanghaiT1DM and ShanghaiT2DM. Y. Liang verified the food data. Q.P. and J.Z. designed and performed the technical validation. J.Z., X.S. and Q.Z. drafted the paper. J.L., C.W. and W.Rao jointly supervised the work. All authors participated in verifying the data and revising the manuscript.
Code availability
The code for the analysis of the datasets and the generation of the figures and tables can be accessed in the Figshare repository40, which is a JUPYTER notebook named “data_analysis.ipynb”. The script can be executed with Python 3.6 and allows for reproducibility and code reuse.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qinpei Zhao, Jinhao Zhu, Xuan Shen, Chuwen Lin.
These authors jointly supervised this work: Jiangfeng Li, Weixiong Rao, Congrong Wang.
Contributor Information
Jiangfeng Li, Email: lijf@tongji.edu.cn.
Weixiong Rao, Email: wxrao@tongji.edu.cn.
Congrong Wang, Email: crwang@tongji.edu.cn.
References
- 1.American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45:S17–S38. doi: 10.2337/dc22-S002. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 3.IDF DIABETES ATLAS, 10th edn. (Brussels: International Diabetes Federation, 2021).
- 4.Chinese Diabetes Society Guideline for the prevention and treatment of type 2 diabetes mellitus in china (2020 edition) Chin J Diabetes Mellitus. 2021;13:315–409. doi: 10.3760/cma.j.cn115791-20210221-00095. [DOI] [Google Scholar]
- 5.Diabetes Control and Complications Trial Research Group et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 7.Holman R, Paul S, Bethel M, Matthews D, Neil H. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Introduction: Standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S1–S2. doi: 10.2337/dc22-Sint. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EM. Self-monitoring of blood glucose: The basics. Clinical Diabetes. 2002;20:45–47. doi: 10.2337/diaclin.20.1.45. [DOI] [Google Scholar]
- 10.Bao Y, et al. Chinese clinical guidelines for continuous glucose monitoring (2018 edition) Diabetes/metabolism research and reviews. 2019;35:e3152. doi: 10.1002/dmrr.3152. [DOI] [PubMed] [Google Scholar]
- 11.Galindo RJ, Aleppo G. Continuous glucose monitoring: the achievement of 100 years of innovation in diabetes technology. Diabetes Research and Clinical Practice. 2020;170:108502. doi: 10.1016/j.diabres.2020.108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woldaregay AZ, et al. Data-driven modeling and prediction of blood glucose dynamics: Machine learning applications in type 1 diabetes. Artificial Intelligence in Medicine. 2019;98:109–134. doi: 10.1016/j.artmed.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Felizardo V, Garcia NM, Pombo N, Megdiche I. Data-based algorithms and models using diabetics real data for blood glucose and hypoglycaemia prediction-a systematic literature review. Artificial Intelligence in Medicine. 2021;118:102120. doi: 10.1016/j.artmed.2021.102120. [DOI] [PubMed] [Google Scholar]
- 14.Visentin R, et al. The UVA/Padova type 1 diabetes simulator goes from single meal to single day. J Diabetes Sci Technol. 2018;12:273–281. doi: 10.1177/1932296818757747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu, J. et al. Reinforcement learning for diabetes blood glucose control with meal information. In Wei, Y., Li, M., Skums, P. & Cai, Z. (eds.) Bioinformatics Research and Applications, 80–91 (Springer International Publishing, Cham, 2021).
- 16.Pompa M, Panunzi S, Borri A, De Gaetano A. A comparison among three maximal mathematical models of the glucose-insulin system. PloS one. 2021;16:e0257789. doi: 10.1371/journal.pone.0257789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contreras I, Oviedo S, Vettoretti M, Visentin R, Veh J. Personalized blood glucose prediction: A hybrid approach using grammatical evolution and physiological models. PloS one. 2017;12:e0187754. doi: 10.1371/journal.pone.0187754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K, Liu C, Zhu T, Herrero P, Georgiou P. GluNet: A deep learning framework for accurate glucose forecasting. IEEE Journal of Biomedical and Health Informatics. 2020;24:414–423. doi: 10.1109/JBHI.2019.2931842. [DOI] [PubMed] [Google Scholar]
- 19.Marling, C. & Bunescu, R. The OhioT1DM dataset for blood glucose level prediction: Update 2020. In CEUR workshop proceedings, vol. 2675, 71 (NIH Public Access, 2020). [PMC free article] [PubMed]
- 20.Marling, C. & Bunescu, R. C. The OhioT1DM dataset for blood glucose level prediction. In KHD@ IJCAI (2018). [PMC free article] [PubMed]
- 21.Xie J, Wang Q. Benchmarking machine learning algorithms on blood glucose prediction for type I diabetes in comparison with classical time-series models. IEEE Transactions on Biomedical Engineering. 2020;67:3101–3124. doi: 10.1109/TBME.2020.2975959. [DOI] [PubMed] [Google Scholar]
- 22.Martinsson J, Schliep A, Eliasson B, Mogren O. Blood glucose prediction with variance estimation using recurrent neural networks. Journal of Healthcare Informatics Research. 2020;4:1–18. doi: 10.1007/s41666-019-00059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubosson F, et al. The open D1NAMO dataset: A multi-modal dataset for research on non-invasive type 1 diabetes management. Informatics in Medicine Unlocked. 2018;13:92–100. doi: 10.1016/j.imu.2018.09.003. [DOI] [Google Scholar]
- 24.Reddy, M. et al. Clinical safety and feasibility of the advanced bolus calculator for type 1 diabetes based on case-based reasoning: A 6-week nonrandomized single-arm pilot study. Diabetes Technol Ther487 (2016). [DOI] [PubMed]
- 25.Weinstock RS, et al. Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care. 2016;39:603–610. doi: 10.2337/dc15-1426. [DOI] [PubMed] [Google Scholar]
- 26.Fox, I., Ang, L., Jaiswal, M., Pop-Busui, R. & Wiens, J. Deep multi-output forecasting: Learning to accurately predict blood glucose trajectories. In Proceedings of the 24th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, 1387–1395 (2018).
- 27.Sudharsan B, Peeples M, Shomali M. Hypoglycemia prediction using machine learning models for patients with type 2 diabetes. Journal of Diabetes Science & Technology. 2015;9:86. doi: 10.1177/1932296814554260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Doorn WP, et al. Machine learning-based glucose prediction with use of continuous glucose and physical activity monitoring data: The maastricht study. PloS one. 2021;16:e0253125. doi: 10.1371/journal.pone.0253125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Li L, Shi Y, Xie X. An ARIMA model with adaptive orders for predicting blood glucose concentrations and hypoglycemia. IEEE Journal of Biomedical and Health Informatics. 2018;23:1251–1260. doi: 10.1109/JBHI.2018.2840690. [DOI] [PubMed] [Google Scholar]
- 30.Schram MT, et al. The maastricht study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. European Journal of Epidemiology. 2014;29:439–451. doi: 10.1007/s10654-014-9889-0. [DOI] [PubMed] [Google Scholar]
- 31.Zhu T, Yao X, Li K, Herrero P, Georgiou P. Blood glucose prediction for type 1 diabetes using generative adversarial networks. CEUR Workshop Proceedings. 2020;2675:90–94. [Google Scholar]
- 32.Pan X, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 33.Tuomilehto J, et al. Finnish diabetes prevention study group. prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 34.Mora N, Golden SH. Understanding cultural influences on dietary habits in asian, middle eastern, and latino patients with type 2 diabetes: A review of current literature and future directions. Curr Diab Rep. 2017;17:126. doi: 10.1007/s11892-017-0952-6. [DOI] [PubMed] [Google Scholar]
- 35.Danne T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battelino T, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparacino G, et al. Glucose concentration can be predicted ahead in time from continuous glucose monitoring sensor time-series. IEEE Transactions on Biomedical Engineering. 2007;54:931–937. doi: 10.1109/TBME.2006.889774. [DOI] [PubMed] [Google Scholar]
- 38.Yin, J. et al. Experimental study of multivariate time series forecasting models. In Proceedings of the 28th ACM International Conference on Information and Knowledge Management, 2833–2839 (2019).
- 39.Zhang GP. Time series forecasting using a hybrid arima and neural network model. Neurocomputing. 2003;50:159–175. doi: 10.1016/S0925-2312(01)00702-0. [DOI] [Google Scholar]
- 40.Zhao Q, 2022. Diabetes Datasets, ShanghaiT1DM and ShanghaiT2DM. figshare. [DOI]
- 41.Turksoy K, et al. Meal detection in patients with type 1 diabetes: a new module for the multivariable adaptive artificial pancreas control system. IEEE Journal of Biomedical and Health Informatics. 2015;20:47–54. doi: 10.1109/JBHI.2015.2446413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haidar A. The artificial pancreas: How closed-loop control is revolutionizing diabetes. IEEE Control Systems Magazine. 2016;36:28–47. doi: 10.1109/MCS.2016.2584318. [DOI] [Google Scholar]
- 43.Xie, J. Simglucose v0.2.1.https://github.com/jxx123/simglucose (2018).
- 44.Veh J, Contreras I, Oviedo S, Biagi L, Bertachi A. Prediction and prevention of hypoglycaemic events in type-1 diabetic patients using machine learning. Health Informatics Journal. 2020;26:703–718. doi: 10.1177/1460458219850682. [DOI] [PubMed] [Google Scholar]
- 45.Marling, C. & Bunescu, R. OhioT1DM, http://smarthealth.cs.ohio.edu/OhioT1DM-dataset.html (2020). [PMC free article] [PubMed]
- 46.Dubosson F, 2018. The open D1NAMO dataset: A multi-modal dataset for research on non-invasive type 1 diabetes management. Zenodo. [DOI]
- 47.Fox, I., Ang, L., Jaiswal, M., Pop-Busui, R. & Wiens, J. Learning to accurately predict blood glucose trajectories, https://github.com/igfox/multi-output-glucose-forecasting (2018).
- 48.Stehouwer, C. et al. Maastricht study, https://www.demaastrichtstudie.nl/research (2014).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
Data Availability Statement
The code for the analysis of the datasets and the generation of the figures and tables can be accessed in the Figshare repository40, which is a JUPYTER notebook named “data_analysis.ipynb”. The script can be executed with Python 3.6 and allows for reproducibility and code reuse.