Abstract
Dietary restriction (DR) delays aging and the onset of age-associated diseases. However, it is yet to be determined whether and how restriction of specific nutrients promote longevity. Previous genome-wide screens isolated several Escherichia coli mutants that extended lifespan of Caenorhabditis elegans. Here, using 1H-NMR metabolite analyses and inter-species genetics, we demonstrate that E. coli mutants depleted of intracellular glucose extend C. elegans lifespans, serving as bona fide glucose-restricted (GR) diets. Unlike general DR, GR diets don’t reduce the fecundity of animals, while still improving stress resistance and ameliorating neuro-degenerative pathologies of Aβ42. Interestingly, AAK-2a, a new AMPK isoform, is necessary and sufficient for GR-induced longevity. AAK-2a functions exclusively in neurons to modulate GR-mediated longevity via neuropeptide signaling. Last, we find that GR/AAK-2a prolongs longevity through PAQR-2/NHR-49/Δ9 desaturases by promoting membrane fluidity in peripheral tissues. Together, our studies identify the molecular mechanisms underlying prolonged longevity by glucose specific restriction in the context of whole animals.
Subject terms: Molecular biology, Ageing, Feeding behaviour
Although diet modulates aging, little is known about whether and how nutrient regulates longevity. Here, the authors show that glucose-restricted diets prolong longevity through series of conserved factors, such as neuronal AMPK, neuropeptide, AdipoR, PPARα, and Δ9 desaturases by promoting membrane fluidity.
Introduction
Reduced nutrient intake, known as dietary restriction (DR) delays aging and the onsets of age-associated diseases in multiple species1–6. A lower intake of particular nutrients rather than reduced calorie intake seems critical for its health benefits, with the restriction of specific amino acids playing a significant role7. However, aging studies using diets with defined nutrient composition are limited, because even simple animal models, such as worms and flies feed on complex nutrient source, Escherichia coli, and yeast, respectively. In addition, the specific and(or) common players are implicated in promoting longevity by different nutrient restrictions such as carbohydrates, amino acids, and fat. Thus they function in highly complicated manners when whole diets are restricted. Therefore, the contribution of a specific nutrient to longevity and the molecular mechanisms involved are poorly understood. To dissect the role of specific nutrients in modulating longevity at the organismal level, developing a DR regimen for particular nutrient-restricted diets is essential.
Glucose, the primary energy source of most living organisms, is one of the best-studied carbohydrates that affect aging. Increased glucose intake accelerates aging in various organisms, including yeasts, worms, flies, and mammals. Glucose-rich diets shorten the lifespan of Caenorhabditis elegans by affecting the activity of pro-longevity proteins, including AMP-activated protein kinase (AMPK)8, and the FOXO transcription factor9 and through toxic metabolite generation10. By contrast, 2-deoxy-glucose, a non-metabolizable glucose analog, extends the lifespan by hermetic generation of reactive oxygen species (ROS)8. However, to our knowledge, a bona fide glucose-restricted dietary regimen for animal models does not exist at present, that enable the studies on lifespan regulation by glucose-specific restriction.
C. elegans is a widely used model organism for aging research11, because many genes and signaling pathways that regulate longevity are conserved. In C. elegans, DR-mediated longevity requires multiple factors, including sirtuins12, SKN-113, PHA-414, AMPK15, DAF-1615, SEK-116, and HSF-117. Interestingly, the extent of their requirement for DR-induced longevity varies, depending on each regimen18. Thus, the mechanisms through which they extend lifespan can differ.
Among DR regulators, AMPK is a highly conserved protein kinase, which regulates energy homeostasis by increasing energy production, and reducing energy wastage under low energy conditions. In unicellular eukaryotes, such as yeasts, AMPK directly senses the intracellular energy status and modulates fitness in response to nutritional conditions19. However, in multicellular eukaryotes, AMPK is ubiquitously expressed and has evolved to perform specific and overlapping functions depending on the tissues, such as neuronal and peripheral tissues. In mice, hypothalamic AMPK integrates signals from the peripheral tissues through hormones, including leptin and ghrelin, which in turn modulates feeding as well as metabolism20. In peripheral tissues, such as the liver and muscles, AMPK autonomously regulates fatty acid oxidation, lipogenesis, glucose uptake, gluconeogenesis, and protein synthesis through the phosphorylation of various targets, including ACC, HMGR, GS, and mTOR19. These indicate the divergent evolution of AMPK into sensor and effector to control whole-body energy homeostasis. However, whether AMPK promotes the organismal longevity mainly in peripheral tissues or through endocrine signaling initiated in neurons remains poorly understood. C. elegans provides a genetically tractable system for studying the physiological roles of AMPK in the context of the whole animal. Similar to mammals, in C. elegans, the two genes aak-1 and aak-2 separately encode the catalytic α subunit of AMPK. Thus far, aak-2 was shown to rescue most AMPK-deficient phenotypes, such as regulation of feeding, fat metabolism, dauer maintenance, and longevity21. Of note, aak-2 is required for only a subset of the DR regimen, in which a diluted bacteria was provided on agar plates in C. elegans (sDR)18. Previously, neuronal AMPK was suggested to modulate longevity non-cell autonomously by inhibiting neuronal CRTC-122. However, direct evidence is lacking, as neuronal rescue of constitutively active AMPK failed to extend the lifespan22.
The nervous system is the central integrator of information obtained both internally and externally, and communicates with peripheral tissues through secreted signaling molecules. Neuropeptides are small peptides produced and released by the neurons. They play a variety of roles in regulation of food intake, metabolism, reproduction, social behavior, aging, learning and memory23–27.
Mounting evidence suggests that lipid metabolism has an important role in aging. Direct supplementation of polyunsaturated fatty acids (PUFAs), such as arachidonic acid, di‐homo‐γ‐linoleic acid, and α‐linolenic acid can extend lifespan28,29 and improves resistance to starvation, oxidative stress, and heat stress30. Dietary supplementation with monounsaturated fatty acids (MUFAs), such as oleic, palmitoleic, or cis‐vaccenic acids, is sufficient to increase lifespan31,32. Further, MUFAs are abundant in long-lived C. elegans mutants and the plasma of long-lived humans33,34, suggesting that unsaturated fatty acids (UFA) may be beneficial for longevity across species. However, the mechanism involved remains unknown.
The composition of fatty acids in the cell membrane greatly influences membrane properties, such as fluidity35. Saturated fatty acids make the membranes rigid, whereas unsaturated fatty acids promote fluidity, which consequently affects cellular physiology impinging upon cell-to-cell communication, intracellular signaling, organellar homeostasis, and membrane dynamics36. Interestingly glucose-rich diets promote the saturation of fatty acids37, hence reducing membrane fluidity, which may contribute to glucose toxicity38. Of note, membrane fluidity has been reported to decrease with age in several tissues, including intestinal cells, macrophages, lymphocytes, neurons, the heart, skeletal muscles and blood vessels in mammals39–41, suggesting that the maintenance of membrane fluidity may promote longevity.
Recently, C. elegans and its bacterial diet have become a powerful model system to study the effects of diets on animal life history traits42,43. In this study, using 1H-NMR metabolite analyses coupled with inter-species genetic analyses, we have demonstrated that glucose-depleted E. coli mutants extend the lifespan and prolong the healthspan of C. elegans. Interestingly, neuronal AAK-2a, a new isoform of AMPK is necessary and sufficient for this GR-mediated longevity. Mechanistically, GR/neuronal AAK-2a prolongs longevity through a series of actions including neuropeptide, PAQR-2/AdipoR, NHR-49/PPARα, and Δ9 desaturases. Further neuronal AAK-2a regulates fat metabolism and promotes membrane fluidity non-cell autonomously. In summary, we provide the molecular mechanisms by which organisms respond to GR condition to promote longevity using conserved factors across species.
Results
Glucose-depleted bacteria prolong longevity in C. elegans
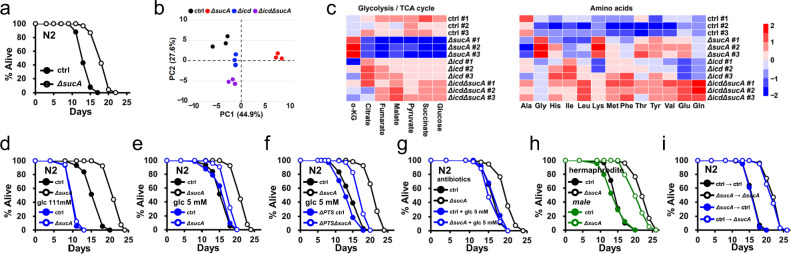
Previously, we isolated several E. coli mutants that prolonged C. elegans longevity44, including ΔsucA E. coli. The sucA gene encodes α-ketoglutarate (αKG) decarboxylase, the E1 subunit of the αKG dehydrogenase complex that catalyzes the conversion of αKG to succinyl-CoA in the tricarboxylic acid (TCA) cycle. Consistent with previous screen data, the lifespan of N2 control animals was significantly extended by feeding ΔsucA E. coli mutants compared with control E. coli K12 parental strains (Fig. 1a; Supplementary Table 1a). This lifespan extension was completely suppressed by wild-type sucA+ complementation (Supplementary Fig. 1a; Supplementary Table 1b), demonstrating that E. coli ΔsucA mutation causally extended the lifespan of C. elegans. Because the TCA cycle functions as a metabolic hub for energy production and a source of biosynthetic precursors, we asked whether the impaired TCA cycle flux in E. coli could extend the lifespan of C. elegans. We evaluated the lifespans of C. elegans that fed six additional mutants of E. coli TCA cycle (Fig. 2a), including ΔacnA, ΔacnB, Δicd, ΔsucC, ΔsdhC, and Δmdh. Interestingly, none of these mutants affected the C. elegans lifespan (Supplementary Table 1c), suggesting that the simple impairment of TCA cycle in E. coli did not causally extend the lifespan of C. elegans. To systemically study molecular cues that promoted C. elegans longevity, we compared the intracellular metabolites extracted from ΔsucA, Δicd and control E. coli using proton nuclear magnetic resonance (1H-NMR) (Supplementary Table 2). A Δicd E. coli was included as an additional negative control because TCA cycle impairment likely changed the levels of various metabolites which were not associated with C. elegans longevity. Principal component analysis (PCA) revealed that the metabolite profile of ΔsucA E. coli was quite distinct from that of wild-type E. coli (Fig. 1b). By contrast, the metabolome of Δicd E. coli resembled that of wild-type E. coli with minor differences (Fig. 1b), suggesting that metabolite changes by ΔsucA mutation might causally extend the lifespan of the animals. Among the glycolysis-TCA cycle intermediates, ΔsucA E. coli accumulated 7-fold more αKG and contained less glucose and TCA cycle intermediates, compared to control E. coli (Fig. 1c; Supplementary Table 2). Regarding amino acids, glycine and lysine accumulation increased by 6.67 and 2.93-folds respectively in ΔsucA E. coli (Fig. 1c; Supplementary Table 2). As reported45, glutamate was the most abundant amino acid in wild-type E. coli (Supplementary Table 2), supporting the validity of our metabolite analysis.
Fig. 1. Glucose-depleted E. coli mutants promote longevity of C. elegans.
a Lifespan showing that ΔsucA Escherichia coli mutants extend lifespan of Caenorhabditis elegans (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). E. coli K12 parental strain (BW25113) is indicated as ctrl. Results from one of three independent experiments are shown. b PCA analysis of metabolites from control, Δicd, ΔicdΔsucA and ΔsucA E. coli. Metabolites from three biological repeats are analyzed using 1H-NMR. PC1 and PC2 indicate principal component 1 and principal component 2. Each point represents an independent biological sample. c Heat map analysis of metabolite quantification data. The row represents the sample and the column represents metabolite. Metabolites significantly decreased are displayed in blue, whereas metabolites significantly increased are displayed in red. The brightness of each color corresponds to the magnitude of the difference when compared with average values. The glycolysis-TCA metabolites and amino acids are presented. For total metabolites data, see also Supplementary Table 2. d Lifespan showing that 2% glucose (111 mM) treatment completely suppress the lifespan extension by GR diets (P = 0.3318, P value determined by two-tailed Student’s t test). Results from one of three independent experiments are shown. e, f Lifespan showing that glucose supplementation (5 mM) shortens lifespan extension by ΔsucA E. coli mutants (e) and by ΔPTSGlcΔsucA E. coli mutants (f) (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from representative experiments are shown with additional repeats. g Lifespan showing that 5 mM glucose treatment significantly reduce the lifespan extension by ΔsucA E. coli (P = 0.0197, P value determined by two-tailed Student’s t test). All E. coli strains are metabolically inactivated by antibiotics, ampicillin and kanamycin. Results from representative experiments are shown with additional repeats. h Lifespan showing that ΔsucA E. coli extend lifespan in both hermaphrodites and male C. elegans (P < 1.0 × 10−10, P < 1.0 × 10−10, respectively, P value determined by two-tailed Student’s t test). Results from representative experiments are shown with additional repeats. i Lifespan showing that adult stage diets determine the lifespan of C. elegans. Adult-only GR diets sufficiently extend lifespan (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from one of three independent experiments are shown. Source data are provided as a Source Data file.
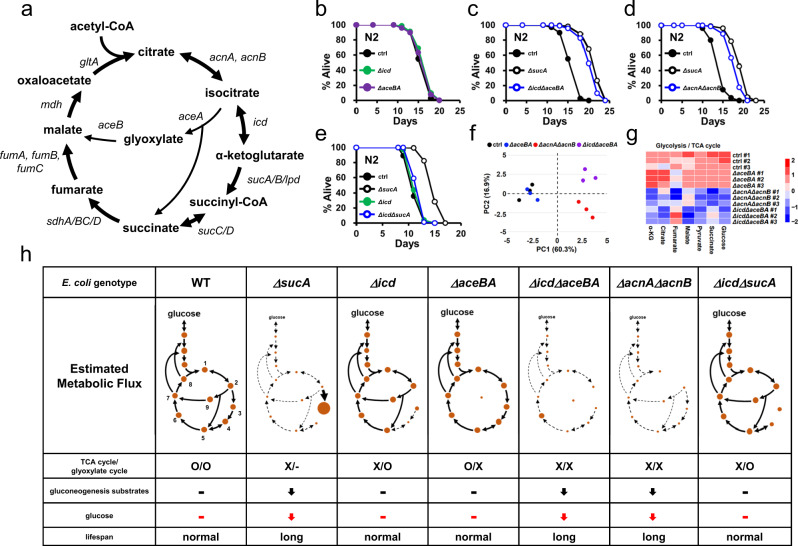
Fig. 2. E. coli mutants simultaneously impaired in TCA and glyoxylate cycle prolong the longevity of C. elegans.
a The TCA and glyoxylate cycles in E. coli. Metabolites are indicated in bold and genes encoding metabolic enzymes are indicated in italic. b–e Lifespan showing the effects of E. coli TCA and (or) glyoxylate cycle mutations on C. elegans longevity. Neither Δicd nor ΔaceBA E. coli mutants affect lifespan (b) while ΔicdΔaceBA E. coli triple mutants extend lifespan (c). ΔacnAΔacnB E. coli double mutants extended lifespan (d). Δicd mutation abolished lifespan extension by ΔsucA E. coli mutants (e). Results from representative experiments are shown with additional repeats. f PCA analysis of metabolites from control, ΔaceBA, ΔicdΔaceBA, and ΔacnAΔacnB E. coli. Metabolites from three biological repeats are analyzed using 1H-NMR. PC1 and PC2 indicate principal component 1 and principal component 2. Each point represents an independent biological sample. g Heat map analysis of metabolite quantification data. The row represents the sample and the column represents metabolite. Metabolites significantly decreased are displayed in blue, whereas metabolites significantly increased are displayed in red. The brightness of each color corresponds to the magnitude of the difference when compared with average values. For total metabolites data, see also Supplementary Table 3. h The estimated metabolic fluxes of gluconeogenesis, TCA, and glyoxylate cycles in E. coli mutants. The thickness of lines corresponds to the magnitude of metabolic fluxes. The dotted line represents weak metabolic flux. Circle size represents the level of metabolic intermediates. Numbers indicate citrate (1), isocitrate (2), αKG (3), succinyl-CoA (4), succinate (5), fumarate (6), malate (7), oxaloacetate (8), and glyoxylate (9) in WT panel. The effects on C. elegans lifespan are also indicated. Source data are provided as a Source Data file.
Previously, αKG was reported to extend the lifespan of C. elegans by inhibiting ATPase and TOR46, suggesting that the accumulated αKG in ΔsucA E. coli might cause the lifespan extension of C. elegans. First, we performed a series of lifespan assays with αKG supplementation as follows; 2, 4, 6, 8, or 12 mM treatment from L1 larvae or adults; treatment every other day; and treatment on standard E. coli B strain OP50 or K12 strains. However, αKG did not extend the C. elegans lifespan under the assay conditions we tested (Supplementary Fig. 1b; Supplementary Table 1d). Because bacterial growth was greatly enhanced and the bacteria made a thick lawn by αKG treatment, E. coli might have consumed αKG and abolished the beneficial effects of αKG. To exclude this possibility, we used ΔkgtP E. coli mutants, which were unable to uptake extracellular αKG and grew similarly to control E. coli upon αKG treatment. However, αKG failed to extend the lifespan of worms fed ΔkgtP E. coli (Supplementary Fig. 1c; Supplementary Table 1e). Lastly, we asked whether C. elegans could uptake αKG from media. When supplemented in various concentrations (0, 2, 4, 10 mM), αKG levels in C. elegans increased in a dose-dependent manner (Supplementary Fig. 1d). Together, these data strongly suggested that the accumulated αKG in ΔsucA E. coli did not causally promote longevity in C. elegans. Another enriched metabolite, glycine was reported to promote longevity in C. elegans in a methionine metabolism-dependent manner, as glycine failed to promote longevity in metr-1(ok521) and sams-1(ok3033) mutants47. Notably, ΔsucA E. coli extended the lifespan of both metr-1(ok521) and sams-1(ok3033) mutants (Supplementary Fig. 1e, and 1f; Supplementary Table 1f). Lysine was shown to extend the lifespan in a particular regimen such as liquid medium48. To validate the effect of lysine on longevity, we tested whether supplemented lysine could extend the lifespan on a standard solid agar medium in wild-type C. elegans. Lysine did not extend lifespan on agar medium at the concentrations previously known to increase lifespan (Supplementary Fig. 1g, h; Supplementary Table 1g), indicating that ΔsucA E. coli promotes longevity through a distinct mechanism.
Glucose is the primary energy source for most organisms. Increased glucose intake accelerates aging and the onset of age-associated diseases, such as diabetes and hypertension49. However, the effect on aging by glucose restriction is relatively unknown due to the lack of relevant models. To evaluate whether low glucose levels in ΔsucA E. coli causally extended the lifespan of C. elegans, we first asked whether glucose supplementation could suppress lifespan extension by ΔsucA E. coli. As reported9, 2% glucose (111 mM) significantly shortened the lifespan of control N2 animals (Fig. 1d; Supplementary Table 1h). Further, 2% glucose completely suppressed the lifespan extension by ΔsucA E. coli (Fig. 1d). Notably, glucose robustly enhanced the growth of control and ΔsucA E. coli, which could indirectly affect C. elegans survival50. To exclude this possibility, we tested whether a low concentration of glucose could specifically reduce lifespan extension mediated by ΔsucA E. coli. Notably, 5 mM glucose suppressed lifespan extension by ΔsucA E. coli with a minor effect on C. elegans lifespan when treated on control E. coli (Fig. 1e; Supplementary Table 1i). Once transported into E. coli, glucose is converted into various metabolites, which may affect the lifespan of C. elegans. To exclude this possibility, we generated ΔPTSGlc (ΔptsH-ΔptsI-Δcrr) mutants in a wild-type or ΔsucA background, which were deficient in glucose uptake9,51. Importantly, 5 mM glucose reduced lifespan extension mediated by ΔPTSGlcΔsucA E. coli to a similar extent as ΔsucA E. coli (Fig. 1f; Supplementary Table 1j). Further, we found that glucose supplementation suppressed lifespan extension by ΔsucA E. coli that was metabolically inactivated by antibiotics (Fig. 1g; Supplementary Table 1k).
Previously glucose-induced toxicity in paqr-2 C. elegans that is compromised in lipid metabolism38. Interestingly this glucose toxicity requires the conversion of glucose into saturated fatty acids (SFA) by E. coli, as ΔPTSGlc E. coli mutants prevent the toxic glucose effects on paqr-2 C. elegans. Although we show that glucose still reduce the lifespan of N2 animals on ΔPTSGlcΔsucA E. coli as well as metabolically inactivated ΔsucA E. coli, glucose depleted ΔsucA E. coli may contain less SFA and more unsaturated FA (UFA). This change of FA composition in E. coli could extend lifespan of C. elegans. To test this idea, we measured the FA composition of ΔsucA E. coli using UPLC/QTOF mass spectrometry. Of note, the SFA/UFA ratio in phosphatidylethanolamine is not lower in ΔsucA E. coli. It is slightly higher, compared to the control (Supplementary Fig. 1i; Supplementary Table 1l), suggesting that the FA composition in ΔsucA E. coli did not causally promote longevity of C. elegans. Taken altogether, glucose abrogated lifespan extension by ΔsucA E. coli by directly acting on C. elegans.
Sex-differences in aging manipulations are recognized across species, including worms52, flies53, and mouse54,55. Interestingly, C. elegans males are almost unresponsive to several DR regimens56. To investigate whether our glucose restricted (GR) regimens could be applicable to two sexes, we performed lifespan assays using male C. elegans on GR diets vs. AL diets. Similar to C. elegans hermaphrodites, ΔsucA E. coli extended the lifespan of male C. elegans (Fig. 1h; Supplementary Table 1m). Finally, to investigate when ΔsucA E. coli act to prolong longevity, we conducted diet-switch experiments. After larval development, the worms were transferred from wild-type control E. coli to ΔsucA E. coli or vice versa. Interestingly, adult-only ΔsucA E. coli diets sufficiently extended the lifespan (Fig. 1i; Supplementary Table 1n). By contrast, developmental stage-only ΔsucA E. coli diets exhibited no lifespan extension (Fig. 1i; Supplementary Table 1n).
Overall, these data suggest that the glucose-depleted ΔsucA E. coli mutants prolong the longevity of C. elegans, serving as GR diets and adult-only GR diets sufficiently prolong the lifespan of C. elegans.
E. coli mutants simultaneously impaired in TCA and glyoxylate cycle prolong the longevity of C. elegans
In the laboratory, C. elegans feed E. coli that grows on peptone-based nematode growth media plates, which lacks carbohydrates, such as glucose. Therefore, E. coli must synthesize the six-carbon sugar to make various cellular macromolecules from non-sugar compounds, such as amino acids. Amino acids are converted to glycolysis/TCA cycle intermediates, which could serve as substrates for gluconeogenesis. Notably, metabolites from central carbon metabolism, including glucose, pyruvate, succinate, fumarate, and malate were significantly less in ΔsucA E. coli, compared to the control (Fig. 1c; Supplementary Table 2), thereby implying lowered gluconeogenesis. By contrast, in TCA cycle-deficient Δicd E. coli, the levels of these metabolites were comparable to those in control (Fig. 1c; Supplementary Table 2). These levels of metabolites could be due to glyoxylate bypass, which enables alternative metabolic cyclic flux by converting isocitrate to succinate and malate (Fig. 2a). Therefore, we hypothesized that impairment of the glyoxylate cycle in Δicd E. coli should extend the lifespan of C. elegans, as it mimicked a reduced gluconeogenesis in ΔsucA E. coli. The E. coli genome contains the aceB-aceA operon, which encodes the glyoxylate cycle enzymes, malate synthase and isocitrate lyase, respectively (Fig. 2a). We generated ΔaceBA mutants in either wild-type or Δicd mutant backgrounds. While the lifespans of C. elegans fed on TCA cycle-deficient Δicd or glyoxylate cycle-deficient ΔaceBA E. coli were comparable to that on control E. coli (Fig. 2b; Supplementary Table 1o), ΔicdΔaceBA triple mutants remarkably extended the lifespan of C. elegans, similarly to ΔsucA E. coli (Fig. 2c; Supplementary Table 1o). This suggests that E. coli mutants, which are deficient in both TCA and glyoxylate cycle, prolong C. elegans lifespan by serving as low-glucose diets.
Aconitase converts citrate to isocitrate, being a shared enzyme in the TCA and glyoxylate cycle. Therefore, we reasoned that aconitase-deficient E. coli should extend C. elegans lifespan. E. coli contains two aconitase encoding genes, acnA and acnB (Fig. 2a). Previously, we showed that single mutants of either gene did not affect the lifespan of the animals (Supplementary Table 1c). We generated a ΔacnAΔacnB double mutant and assessed its effects on C. elegans lifespan. Surprisingly, it significantly extended C. elegans lifespan, similarly to ΔsucA E. coli (Fig. 2d; Supplementary Table 1p). To validate the metabolic features of newly generated E. coli mutants, we analyzed the intracellular metabolites of ΔacnAΔacnB, ΔicdΔaceBA, ΔaceBA E. coli using 1H-NMR. Consistent with our idea, two E. coli mutants that extend the lifespan of C. elegans exhibit similar metabolite profiles to ΔsucA E. coli, with low level of glucose and TCA intermediates (Fig. 2f; Supplementary Table 3). In contrast, the metabolite profile of ΔaceBA mutants only deficient in the glyoxylate cycle resembles that of control. Thus, C. elegans lifespan is extended by E. coli mutants with a simultaneous impairment of TCA and glyoxylate cycle.
Pyruvate, a central metabolite in glycolysis and TCA cycle, is reduced in all E. coli mutants that extend the lifespan of C. elegans (Fig. 1c; Fig. 2g; Supplementary Table 2). Next we asked if pyruvate could suppress the lifespan extension by ΔsucA E. coli similarly with glucose. To our surprise, unlike glucose, pyruvate did not restore the growth retardation of ΔsucA E. coli to normal (Supplementary Fig. 1j). This implies that supplemented pyruvate might not be actively converted to glucose in ΔsucA E. coli, potentially due to active consumption by glycolysis and TCA cycle. Consequently, pyruvate did not abolish the lifespan extension by ΔsucA E. coli (Supplementary Fig. 1k; Supplementary Table 1q). Glycerol enters into gluconeogenesis via dihydroxyacetone phosphate and the upper part of glycolysis57. Recently it has been reported that glycerol is a better substrate for gluconeogenesis than pyruvate in fasting mice58. Therefore, we asked whether glycerol could suppress lifespan extension by ΔsucA E. coli. Unlike pyruvate, glycerol enhanced the growth of ΔsucA E. coli (Supplementary Fig. 1j) and the lifespan extension by ΔsucA was significantly reduced by glycerol (Supplementary Fig. 1l; Supplementary Table 1r). These data demonstrate that ΔsucA-induced longevity can be suppressed by metabolite that is easily converted to glucose.
Isocitrate is a branch point metabolite, which is converted to αKG through the TCA cycle or to succinate and malate through the glyoxylate cycle (Fig. 2a). Given the low level of succinate and malate and the high level of αKG in ΔsucA mutants (Fig. 1c; Supplementary Table 2), TCA cycle flux must override that of the glyoxylate cycle in ΔsucA E. coli. If that is the case, the inhibition of TCA cycle flux in ΔsucA E. coli by introducing an Δicd mutation can facilitate the glyoxylate cycle (Fig. 2h) and, in turn, abolish the lifespan extension by ΔsucA mutation. As hypothesized, the metabolite profile of the ΔicdΔsucA double mutants became similar to those of the control and Δicd mutants (Fig. 1b; Supplementary Table 2), recovering the levels of TCA intermediates and glucose (Fig. 1c). Of note, Δicd mutation completely abolished the lifespan extension by ΔsucA E. coli (Fig. 2e; Supplementary Table 1s). The levels of metabolites and metabolic fluxes in six respective E. coli mutants are presented in Fig. 2h. Hereafter, ΔsucA E. coli and wild-type E. coli will be referred to as GR diets and ad libitum (AL) diets, respectively, in this study. Taken together, these results demonstrate that E. coli mutants impaired in both TCA and glyoxylate cycle serve as low glucose diets and promote longevity in C. elegans.
GR ameliorates age-associated pathologies with no trade-off of fecundity
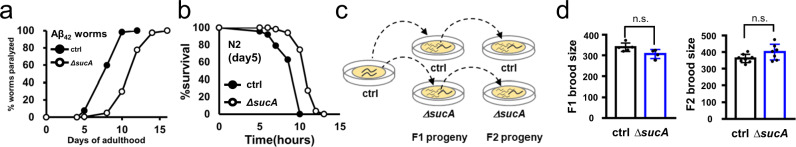
DR not only extends the lifespan but also delays various age-associated diseases, including neurodegenerative diseases59. We tested whether GR diets could ameliorate age-associated pathologies using Alzheimer’s disease (AD) model animals. The 42 amino acids of beta amyloid (Aβ42) play a pivotal role in the pathogenesis of AD60. C. elegans expressing human Aβ42 exhibited an early decline in physical activity61. As expected, we found that GR diets substantially delayed age-associated paralysis (Fig. 3a; Supplementary Table 1t). Further, C. elegans grown on GR diets maintained health with aging, as determined by enhanced stress resistance (Fig. 3b; Supplementary Table 1u). By contrast to its beneficial effects, DR often reduced fecundity likely due to a soma/germline trade-off7. However, interestingly GR diets were not generally dietary restricted, as brood size of C. elegans feeding GR diets was comparable with that on AL diets during two consecutive generations (Fig. 3c, d; Supplementary Table 1v). Taken together, our findings reveal that GR diets improve many indices of healthspan without loss of fecundity.
Fig. 3. GR delays Age-associated pathologies with no germline trade-off.
a Paralysis assay showing that GR diets delayed age-associated paralysis in Aβ42 animals (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from one of three independent experiments are shown. b Survival assay showing that GR diets enhance heat stress resistance of worms at day 5 (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from representative experiments are shown with additional repeats. c, d Brood size analysis during consecutive generations on the indicated diets (c). GR diets do not reduce F1 brood size (AL diets: n = 5, GR diets: n = 4, P = 0.0532, P value determined by two-tailed Student’s t test) and F2 brood size (AL diets: n = 8, GR diets: n = 7, P = 0.0653, P value determined by two-tailed Student’s t test) (d). Results from representative experiment are shown one of two independent repeats. Error bars indicate mean ± s.d. Source data are provided as a Source Data file.
New AMPK isoform, AAK-2a mediates GR lifespan extension
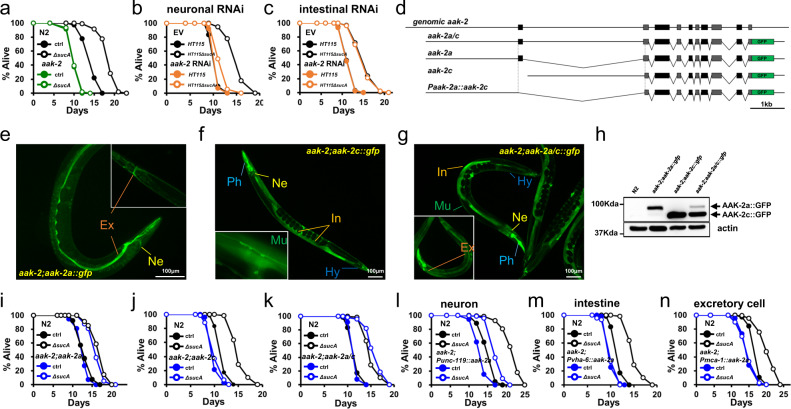
The non-metabolizable glucose analog, 2-deoxy glucose (2-DG) inhibits glucose metabolism, indicating its potential as a DR mimetic. Given that antioxidant, N-acetyl cysteine (NAC), suppressed the lifespan extension by 2-DG, hormesis, a beneficial effect of mild stress, is critical for 2-DG-induced longevity in C. elegans8. However, we found that NAC did not suppress lifespan extension by GR diets (Supplementary Fig. 2a; Supplementary Table 4a), suggesting that a distinct mechanism was involved. Therefore, to gain insight into the pro-longevity mechanism of GR diets, we asked if GR diets extended lifespan through known DR effectors, including FOXA, FOXO, TOR, HIF, AMPK, Sirtuin, HSF, SEK-1, and NRF2 as well as other longevity pathway components. C. elegans mutants tested were as follows, pha-4(RNAi), daf-16(mgDf50), raga-1(ok386), rict-1(ft7), hif-1(ia4), aak-2(ok524), sir-2.1(ok434), hsf-1(sy441), skn-1(zu135), sek-1(km4), pdr-1(gk448), pink-1(ok3538), and isp-1(qm150). We found that most DR effectors were dispensable for GR-induced longevity (Supplementary Fig. 2b–i; Supplementary Table 4b). In addition, stress response and mitochondrial longevity pathway were not implicated (Supplementary Fig. 2j–m; Supplementary Table 4b). Interestingly, GR-mediated longevity was completely abolished only in aak-2(ok524) mutants (Fig. 4a; Supplementary Table 4c). An aak-2 encodes the catalytic α-subunit of AMPK, which rescues most AMPK-deficient phenotypes in C. elegans62, indicating that AMPK is specifically required for GR-induced lifespan extension. AAK-2 is expressed in various tissues including neurons, muscles, excretory cells, and intestinal cells62. Although AMPK performs specific and overlapping functions in organismal energy homeostasis depending on the expressed tissues63, it is relatively unknown whether AMPK activity, in the neurons or peripheral tissues (or both), plays a critical role in longevity in the context of whole animals. Interestingly, although GR-mediated longevity was completely suppressed in aak-2(ok524) mutants, it was not affected by aak-2 RNAi (Supplementary Fig. 2n; Supplementary Table 4d). The efficiencies of aak-2 RNAi were confirmed by the reduced transcript of aak-2 (Supplementary Fig. 2p) and the GFP intensity in AAK-2::GFP animals (Supplementary Fig. 2q, r). Because C. elegans neurons are refractory to RNAi64,65, this data suggests that neuronal AMPK activity is crucial for GR-mediated longevity. Consistently, aak-2 RNAi impaired lifespan extension by GR diets in rrf-3(pk1426) mutants that were hyper-sensitive to RNAi66 (Supplementary Fig. 2o; Supplementary Table 4e). To further validate this data, we utilized neuron-specific RNAi transgenic strain, TU3401 which express the dsRNA transporter in neurons67 and intestine-specific RNAi strain, VP303 which enable RNAi only in the intestine68, for aak-2 knockdown. As shown in Fig. 4b, the knockdown of aak-2 in neuronal cells significantly reduced GR-mediated lifespan extension (Supplementary Table 4f). By contrast, aak-2 RNAi in intestinal cells did not affect GR-mediated lifespan extension (Fig. 4c; Supplementary Table 4f), similarly to that in N2. These data support that AMPK play an essential role in GR-induced longevity exclusively in neurons. According to expressed sequence tag database, C. elegans expresses at least two isoforms of aak-2 using distinct upstream promoters and start sites, generating AAK-2a and AAK-2c isoforms (Fig. 4d). Thus far, an AAK-2 isoform-specific study has not been conducted, such as the tissue-specific expressions and potential distinct functions of isoforms. To evaluate the role of AMPK isoforms in GR-mediated longevity, next we generated transgenic animals specifically expressing AAK-2a, AAK-2c, or AAK-2a/c tagged with GFP using each endogenous promoter in an aak-2(ok524) background (Fig. 4d). AAK-2c was expressed in various tissues, including the pharynx, intestine, muscles, hypodermis, and neurons (Fig. 4f), consistent with previous AAK-2 expression62. This implies that previous AMPK studies utilized the AAK-2c isoform. Interestingly AAK-2a was expressed in only a few head neurons and excretory cells (Fig. 4e), with the combined expression patterns being observed in aak-2(ok524);aak-2a/c::gfp animals (Fig. 4g). To verify the existence and the levels of each isoform, we detected AAK-2 protein in transgenic animals expressing either of or both isoforms, using western blotting. Consistent with GFP intensities, the protein level of AAK-2c is much higher than that of AAK-2a (Fig. 4h). As AAK-2a is larger with additional 62 amino acids at its N-terminus, two discrete bands of AAK-2a and AAK-2c were detected in AAK-2a/c transgenic animals (Fig. 4h), demonstrating that two different AAK-2 isoforms are endogenously expressed in C. elegans.
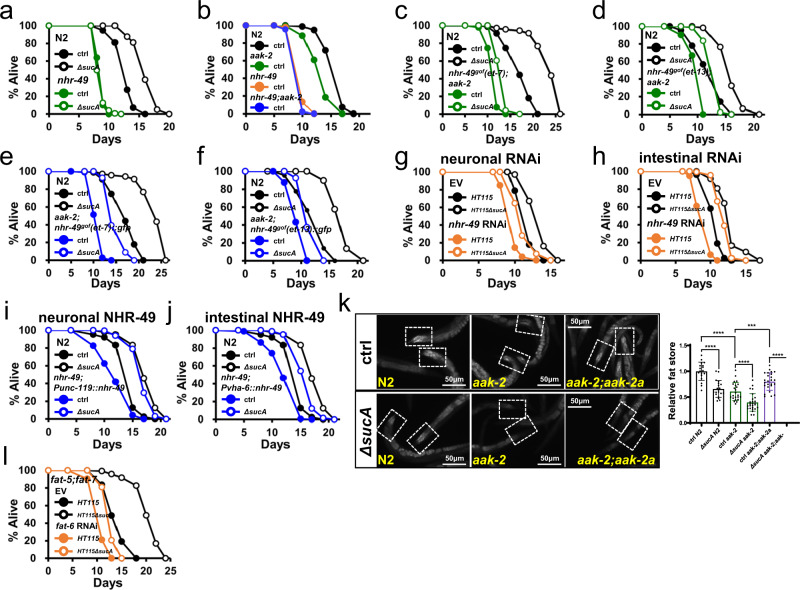
Fig. 4. New AMPK isoform, AAK-2a modulates GR-mediated longevity in neurons.
a Lifespan showing that aak-2(ok524) abolishes GR-induced longevity (P = 1, P value determined by two-tailed Student’s t test). Results from one of three biological independent experiments are shown. b, c Lifespan showing that neuronal AAK-2 is required for GR-induced longevity. Tissue-specific RNAi animals are used for neuronal aak-2 RNAi knockdown (b) and intestinal aak-2 RNAi knockdown (c). Results from one of three biological independent experiments are shown. d Structure of aak-2+ gene and aak-2 isoform::gfp constructs. Coding regions are indicated as closed boxes; noncoding regions as line; introns spliced-out in DNA constructs as ∨. Locus of start codon for AAK-2 isoforms are indicated as dotted lines. e–g GFP image showing the expression of AAK-2 isoforms. GFP images are taken using aak-2(ok524);aak-2 isoform::gfp animals. AAK-2a is expressed in the head neurons (Ne) and excretory cells (Ex) (e). AAK-2c is expressed in multiple tissues, including the intestine (In), pharynx (Ph), muscles (Mu), hypodermis (Hy), and neuron (Ne) (f). AAK-2a/c transgenic animals show the combined expression patterns (g). Representative images obtained from at least four biological independent repeats. h Immunoblotting analysis showing the existence and relative levels of AAK-2 isoforms in the indicated animals. Worm lysates were detected with anti-GFP (top panel), and anti-α-tubulin (bottom panel). Representative images obtained from four biological independent repeats. i–k Lifespans showing contribution to GR-induced longevity by AAK-2 isoform(s). The lifespans of aak-2(ok524) expressing AAK-2 isoform(s) under endogenous promoter are shown; AAK-2a (i), AAK-2c (j) and AAK-2a/c (k). Results from one of three biological independent experiments are shown. l–n The lifespans of aak-2(ok524) transgenic animals expressing AAK-2a isoform in indicated tissues; AAK-2a in the neurons (l), the intestine (m) and the excretory cells (n). Results from one of three biological independent experiments are shown. Source data are provided as a Source Data file.
To determine which AMPK isoform(s) was required for GR-induced longevity, we explored the lifespan of wild-type N2, aak-2(ok524), and aak-2(ok524) mutants expressing either isoform or both, which were fed either AL or GR diets. Surprisingly, AAK-2a fully rescued GR-induced longevity (Fig. 4i; Supplementary Table 4g), whereas the widely expressed AAK-2c was dispensable for GR-induced longevity (Fig. 4j; Supplementary Table 4h). The lifespans of aak-2(ok524) transgenic animals expressing both AAK-2a/c isoforms were comparable to that of aak-2(ok524);aak-2a transgenic animals (Figs. 4i, and 4k; Supplementary Table 4i). Importantly aak-2(ok524);aak-2a transgenic animals lived longer compared to aak-2(ok524); aak-2c transgenic animals on control diets (Supplementary Fig. 3a; Supplementary Table 4j), demonstrating that the AAK-2a isoform plays a critical role not only in GR induced longevity but also in the normal lifespan. To further determine whether aak-2a is sufficient for wild-type aak-2+ for lifespan regulation, we generated aak-2+;aak-2a::gfp animals and compared their lifespans with aak-2(ok524);aak-2a::gfp animals on AL as well as GR diets. We found that their lifespans were comparable on either AL or GR diets (Supplementary Fig. 3b; Supplementary Table 4k), indicating that the AAK-2a isoform was sufficient for endogenous AMPK-mediated lifespan regulation. Notably, aak-2(ok524); aak-2c transgenic animals lived longer than aak-2 mutant animals (Supplementary Fig. 3a; Supplementary Table 4j). This indicates that the AAK-2c isoform also has a role in longevity on AL diets, although its role is minor. Together, our data reveal that the new AMPK isoform AAK-2a plays the major role in longevity. Since AAK-2a exhibited a distinct tissue expression pattern and contained an additional amino acids at N-terminus, compared with AAK-2c (Fig. 4d), next we asked what determined GR-mediated lifespan extension by AMPK. We generated transgenic animals expressing AAK-2c::GFP using aak-2a promoter in aak-2(ok524) background (Supplementary Fig. 3c). Notably, GR diets extended the lifespan of these animals (Supplementary Fig. 3d; Supplementary Table 4l), demonstrating that the expression pattern of AAK-2 determined the GR-mediated lifespan extension. AAK-2a is expressed in the neurons and excretory cells (Fig. 4e). Although our data strongly support the neuronal function of AAK-2a in GR-induced longevity, it was shown that AAK-2 in excretory cells plays roles in longevity69 and germ cell proliferation70. In their studies, the sulp-5 promoter was used to drive aak-2 expression in the excretory cells. Therefore, to further explore in which cells AMPK functioned for GR-mediated lifespan extension, we generated a series of aak-2(ok524); tissue-specific aak-2a::gfp animals using the pan-neuronal Punc-119, Prab-3; excretory Pmca-1, Psulp-5; and intestinal Pvha-6 promoters (Supplementary Fig. 3e–i) and measured their lifespans on AL vs. GR diets. Consistent with our tissue-specific RNAi lifespan data, neuronal AAK-2a restored lifespan extension by GR diets, whereas AAK-2a in intestine did not (Fig. 4l, m; Supplementary Fig. 3j; Supplementary Table 4m).
The role of excretory cells was more complex, as Psulp-5::aak-2a transgene rescued GR-induced longevity while Pmca-1::aak-2a did not (Fig. 4n; Supplementary Fig. 3k; Supplementary Table 4m, n). Of note, in contrast to the exclusive expression in excretory cells by Pmca-1 (Supplementary Fig. 3g), Psulp-5 promoter driven AAK-2 is not only in excretory cells but also in neurons (Supplementary Fig. 3h)71. To knock down AAK-2a specifically in the excretory cells, we took advantage of RNAi feeding, which is refractory in neurons. As expected, gfp RNAi abolished the expression of AAK-2 only in excretory cells in aak-2;Psulp-5::aak-2a animals, while having no effect on neuronal aak-2a expression (Supplementary Fig. 3l). Importantly, this RNAi did not suppress GR mediated longevity (Supplementary Fig. 3m, n; Supplementary Table 4o), demonstrating that AAK-2 in neurons is sufficient to extend lifespan by GR diets.
Taken together, we have demonstrated that a new AMPK isoform, AAK-2a modulates GR-mediated longevity in a neuron-dependent manner.
AAK-2a modulate longevity non-cell autonomously via neuropeptide signaling
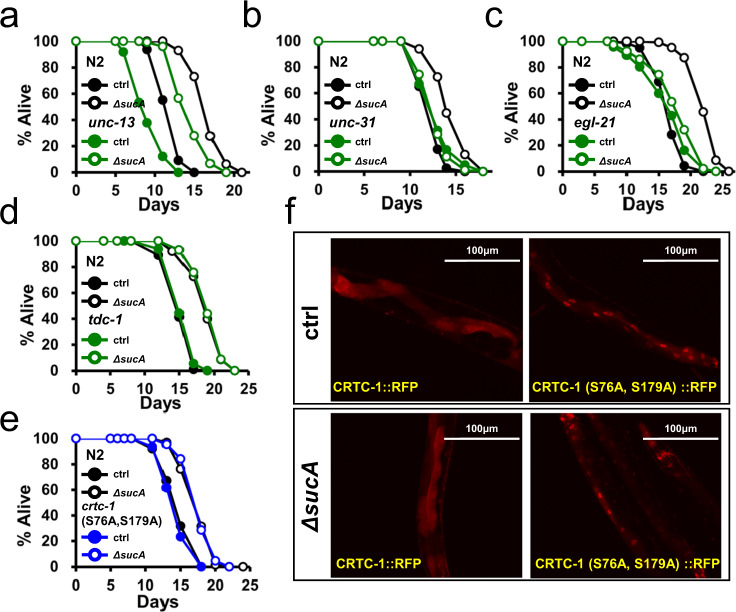
In mammals, hypothalamic AMPK modulates feeding, thermogenesis as well as fat metabolism in peripheral tissues in an endocrine manner72. In C. elegans, constitutively active AMPK (CA-AMPK) was reported to extend the lifespan22. CA-AMPK inhibited CRTC-1 in the neurons, thereby inhibiting pro-aging catecholamine signaling to prolong longevity. However, the neuronal function of AMPK in longevity remains elusive, as neuron-specific expression of CA-AMPK was unable to extend lifespan22. Since AAK-2a is only required in the neurons to mediate GR-induced longevity, we first asked which endocrine activity was required for GR-mediated lifespan extension. We utilized the unc-13 mutants, which were defective in neurotransmitter release through small clear vesicles73, and unc-31 mutants, defective in release of dense core vesicles that mainly contained neuropeptides73. Interestingly, GR diets extended the lifespans of multiple unc-13 mutant alleles, including e51, s69, e450, and e1091 (Fig. 5a, and Supplementary Fig. 4a–c; Supplementary Table 5a). By contrast, unc-31 (e928) completely abolished the GR-mediated lifespan extension (Fig. 5b; Supplementary Table 5b). Next, the lifespan extension by GR diets was significantly impaired in egl-21 (n476), carboxypeptidase E mutants (Fig. 5c; Supplementary Table 5c), which was deficient in neuropeptide processing74. Thus, GR/neuronal AAK-2a modulates longevity non-cell autonomously through neuropeptides. To further exclude the possibility that neurotransmitters are involved in GR-mediated longevity, we measured the lifespan of various neurotransmitter signaling mutants, including mutants for octopamine; tdc-1(ok914), serotonin; tph-1(n4622), and dopamine; cat-2(e1112). Notably, in contrast to the dependence on TDC-1 in CA-AMPK/CRTC-1-mediated longevity22, GR diets extended the lifespan of all tested mutants, including tdc-1(ok914) mutants (Fig. 5d, Supplementary Fig. 4d, and 4e; Supplementary Table 5d). Lastly, we asked whether GR diets extended the lifespan of CRTC-1 (S76A, S179A) mutants, in which CRTC-1 is constitutively nuclear and refractory to the lifespan extension by CA-AAK-273. Notably, GR diets extended the lifespan of CRTC-1(S76A, S179A) animals (Fig. 5e; Supplementary Table 5e), demonstrating that GR-mediated pro-longevity signaling is dominant over the CRTC-1-mediated pro-aging signaling. GR diets did not affect the nuclear/cytoplasmic localization of CRTC-1, further supporting the independence of CRTC-1 in GR-mediated longevity (Fig. 5f). Together, our data demonstrate that GR/neuronal AMPK prolongs longevity non-cell autonomously through neuropeptide signaling.
Fig. 5. GR promotes longevity non-cell autonomously via pro-longevity neuropeptide.
a Lifespan showing that GR diets extend lifespan of unc-13 (e51) (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from one of three biological independent experiments are shown. b Lifespan showing that GR diets fail to extend lifespan of unc-31(e928) (P = 0.3647, P value determined by two-tailed Student’s t test). Results from one of three biological independent experiments are shown. c Lifespan showing that GR-induced longevity is significantly shortened in egl-21(n476) animals (P = 0.006, P value determined by two-tailed Student’s t test). Results from one of three biological independent experiments are shown. d Lifespan showing that GR diets extend the lifespan of tdc-1(ok914) mutant animals (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from one of three biological independent experiments are shown. e Lifespan showing that GR diets extend the lifespan of CRTC-1(S76A, S179A) animals (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from one of three biological independent experiments are shown. f GR diets do not affect the nuclear/cytoplasmic localization of CRTC-1::RFP and CRTC-1(S76A, S179A)::RFP. Representative images obtained from three biological independent repeats. Source data are provided as a Source Data file.
GR-mediated lifespan extension requires NHR-49
Organelle homeostasis was often associated with AMPK-mediated longevity, as CA-AMPK did not extend the lifespan of mitochondrial dynamics mutants and peroxisome-depleted animal, such as fzo-1 (tm1133), drp-1 (tm1108), and prx-5 RNAi animals respectively75. We asked whether regulators of organelle dynamics were required for GR-mediated longevity. Surprisingly, GR diets extended the lifespans of drp-1(tm1108), fzo-1(tm1133), and prx-5 RNAi animals (Supplementary Fig. 5a–c; Supplementary Table 6a), demonstrating that GR prolongs longevity through different mechanisms. In response to low nutrient intake, AMPK remodels fat metabolism through peroxisome proliferator-activated receptor alpha (PPARα) across species76,77. In C. elegans, NHR-49, a functional PPARα orthologue, was shown to be required for CA-AMPK-mediated longevity22. We, therefore, asked whether NHR-49 was required for GR-mediated longevity. As shown in Fig. 6a, nhr-49(nr2041) completely abolished GR-mediated longevity (Supplementary Table 6b).
Fig. 6. NHR-49 is required for GR-induced longevity.
a Lifespan showing that NHR-49 is required for GR-induced longevity. nhr-49 (nr2041) completely abolished GR-induced longevity (P = 1, P value determined by two-tailed Student’s t test). Results from one of three independent experiments are shown. b Lifespans showing that the effects of aak-2 and nhr-49 mutation on lifespan are not additive. The mean lifespan of nhr-49;aak-2 is comparable to that of nhr-49 animals (P = 1, P value determined by two-tailed Student’s t test). Results from one of three biological independent experiments are shown. c, d Lifespans showing that nhr-49 gain-of-function (gof) mutations, et7 (c) and et13 (d), in aak-2(ok524) animals gain the GR-mediated longevity (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from one of three biological independent experiments are shown. e, f Lifespans showing that nhr-49gof transgenes, et7 (e) and et13 (f), rescued GR induced longevity in aak-2 mutants (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from representative experiments are shown with additional repeats. g, h Lifespans showing that neither neuronal nor intestinal nhr-49 RNAi reduced the lifespan extension by GR diets (P < 1.0 × 10−10, Student’s t test). Tissue-specific RNAi animals are used for neuronal nhr-49 RNAi knockdown (g) and intestinal nhr-49 RNAi knockdown (h). Results from representative experiments are shown with additional repeats. i, j Lifespans showing that restoring NHR-49 to either neuron or intestine fully restored the lifespan extension by GR in nhr-49 mutants (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). The lifespans of nhr-49(nr2041) expressing NHR-49 in the indicated tissue are shown; pan-neuronal nhr-49 (i) and intestinal nhr-49 (j). Results from one of three independent experiments are shown. k Fat store of animals feeding AL vs. GR diets. Fixed worms are stained with Nile red dye. Intensity of posterior parts of intestine are quantified as fat contents (dotted boxes) using ImageJ software (***P < 0.001, ****P < 0.0001, P value determined by two-tailed Student’s t test). Error bars indicate standard error of mean (SEM). Results from representative experiments are shown with additional repeats. (n ≥ 18 for each condition). l Lifespan showing that Δ9 desaturases are implicated in GR-induced longevity. Lifespan extension by GR diets are significantly reduced in fat-5(tm420); fat-7(wa36); fat-6(RNAi) animals. Results from one of three independent experiments are shown. Source data are provided as a Source Data file.
Both AMPK and NHR-49 control energy homeostasis, lipid metabolism and longevity22,78. However, their relative interactions remain elusive. Since either aak-2 or nhr-49 mutation shortened normal lifespans on control diets (Figs. 4a and 6a), we asked if the effects on lifespan by either mutation are additive or not. An aak-2 mutation in the nhr-49(nr2041) mutants did not further reduce the lifespan of nhr-49(nr2041) animals (Fig. 6b; Supplementary Table 6d), suggesting that AAK-2 and NHR-49 might function in the same pathway. For a genetic epistasis analysis, we introduced the nhr-49 gain-of-function (gof) mutations, et7 and et13, in aak-2(ok524) animals. Despite the unresponsiveness of aak-2 mutants to GR diets, nhr-49gof;aak-2 animals lived longer on GR diets, compared to AL diets (Fig. 6c, d; Supplementary Table 6e). Furthermore, the nhr-49 gof(et7 and et13) transgenes conferred the GR-induced longevity to aak-2 mutant animals (Fig. 6e, f; Supplementary Table 6f). This data suggests that nhr-49 functions at least genetically downstream of aak-2 for GR-induced longevity. Due to lack of direct evidence, our genetic data do not exclude the possibility that AAK-2a and NHR-49 function to promote metabolic remodeling for GR-induced longevity in parallel.
Given that neuronal AMPK activity was sufficient for GR-mediated longevity, we next asked in which tissues NHR-49 functioned for GR-mediated longevity. To this end, we used tissue-specific RNAi sensitive strains for nhr-49 knockdown in neurons and intestinal cells. Surprisingly, none of the tissue-specific knockdowns attenuated GR-induced longevity (Fig. 6g, h; Supplementary Table 6g), suggesting that residual NHR-49 activity in tissues unaffected by RNAi was sufficient to extend the lifespan by GR. To test this, we selectively restored NHR-49 activity to neuronal or intestinal cells of nhr-49(nr2041) mutants using Punc-119/Prab-3 and Pvha-6, respectively. Unlike neuron-specific AMPK activity for GR-induced longevity, either neuronal or intestinal rescue of NHR-49 restored GR-induced longevity in nhr-49(nr2041) mutants (Fig. 6i, j; Supplementary Fig. 5h; Supplementary Table 6h). Interestingly, there are multiple publications that NHR-49 in a single cell type is sufficient for a non-cell autonomous effect on lifespan22,79,80. These imply that some of downstream effects of NHR-49 are non-cell autonomous. As previously suggested81, it is possible that NHR-49 in one tissue functions non-cell autonomously via lipid exchange between distal cells. Taken altogether, we demonstrate that NHR-49 modulates GR-induced longevity, communicating between the tissue of expression and the rest of body in the organismal context.
Δ9 desaturases are required for GR-induced longevity
Under low energy status, AMPK maintains energy homeostasis through remodeling fat metabolisms across species82. NHR-49 functions as a key modulator of these processes by regulating genes involved in fat oxidation and fatty acid desaturation77,79. First, we measured the fat content of animals on AL vs. GR diets using Nile red and Oil Red O staining. Wild-type control N2 animals stored less fat on GR diets (Fig. 6k; Supplementary Fig. 5f), likely due to enhanced fat usage. As reported83, aak-2 mutants stored significantly less fat on AL diets, compared to N2 control (Fig. 6k; Supplementary Fig. 5f). However, fat content in aak-2(ok524) mutants was further reduced by GR diets compared to AL diets (Fig. 6k; Supplementary Fig. 5f), suggesting that enhanced fat usage under GR conditions was independent of AAK-2 and might not directly cause lifespan extension. Notably, AAK-2a restored fat storage in the intestine (Fig. 6k; Supplementary Fig. 5f), demonstrating that neuronal AMPK enhanced fat stores in peripheral tissues non-cell autonomously. To validate fat content analyzed by staining methods, we conducted biochemical assays for triacylglycerol (TAG) levels. Consistent with dye staining data, GR diets reduced TAG levels in all tested animals, including wild-type control, aak-2(ok524) mutants, and aak-2(ok524);aak-2a transgenic animals (Supplementary Fig. 5g). To examine whether GR-enhanced fat oxidation might not causally extend lifespan, GR-induced longevity was assessed when fat oxidation was inhibited. For stored fat to be mobilized, TAG is hydrolyzed by adipose triglyceride lipase 1 (ATGL-1) and hormone sensitive lipase homolog (HOSL-1) in C. elegans84. We found that GR diets similarly extended C. elegans lifespan whether atgl-1 or hosl-1 was knocked down or not (Supplementary Fig. 5i, j; Supplementary Table 6i). The mitochondrial carnitine palmitoyltransferase-1 (CPT-1) transports long chain fatty acids into the mitochondria for fatty acid oxidation85. The cpt-1 RNAi did not affect GR-induced longevity (Supplementary Fig. 5k; Supplementary Table 6j). Together, these data demonstrate that enhanced fat oxidation accompanied by GR diets does not causally mediate lifespan extension by GR.
In addition to β-oxidation, NHR-49 promotes fatty acid desaturation via Δ9 desaturases77. To investigate whether fatty acid desaturation might be required for GR-mediated longevity, we assessed their involvement in GR-induced longevity using Δ9 desaturase mutants. C. elegans has one C16 Δ9 desaturase, FAT-5, and two C18 Δ9 desaturases, FAT-6 and FAT-786. Due to their potential redundancy, we measured the lifespans on AL vs. GR diets of all possible combinations using mutants or RNAi as follows; fat-5(tm420), fat-6(tm331), fat-7(wa36), fat-5(tm420);fat-6(tm331), fat-5(tm420);fat-7(wa36), fat-7(wa36);fat-6(RNAi), and fat-5(tm420);fat-7(wa36);fat-6(RNAi). Interestingly the slow growth phenotype of fat-6(tm331);fat-7(wa36) double mutants87 was significantly exacerbated by GR diets, implying that fat remodeling was essential under GR conditions. As fat-5;fat-6;fat-7 triple mutant is lethal88, fat-6 was post-developmentally knocked down by RNAi in fat-5(tm420);fat-7(wa36) animals. GR diets extended the lifespan of single fat mutants and all three double mutant (or RNAi) combinations, similar to control animals (Supplementary Fig. 5l–q; Supplementary Table 6k). However, we found that fat-6 RNAi reduced the GR-mediated longevity in fat-5(tm420);fat-7(wa36) animals by 61.03 % (Fig. 6l; Supplementary Table 6l), demonstrating that fatty acid desaturation was implicated in GR-mediated longevity. Notably, oleic acid, a monounsaturated fatty acid (MUFA), is associated with lifespan extension in C. elegans under various conditions, such as ER stress, germ cell loss, altered chromatin, and low temperature31,32,89,90. Given these pro-longevity effects of MUFA, our data demonstrate that GR prolongs longevity by remodeling the fat composition through Δ9 desaturases.
GR enhances membrane fluidity to prolong longevity
UFAs are abundant fatty acids in cell membranes, critical for the membrane fluidity with their physical properties, double bonds, interfering with the tight stacking of membrane lipids35. Any one of C16 or C18 Δ9 desaturases functions sufficiently for GR-mediated longevity (Supplementary Fig. 5l–q; Supplementary Table 6h), implying that a specific lipid species might not be necessary for GR-mediated longevity. Instead, any type of UFA could mediate the beneficial effects by GR. In C. elegans glucose supplementation promotes the saturation of membrane lipids, hence membrane rigidity37, which might contribute to high glucose-induced short lifespan. In addition, membrane fluidity decreases with age in mammals40,41. Therefore, we hypothesized that GR diets might enhance membrane fluidity to promote longevity. To address this, we measured in vivo membrane fluidity in animals fed AL vs. GR diets using fluorescence recovery after photobleaching (FRAP). Using FRAP on transgenic worms expressing GFP incorporated in the intestinal membrane37, membrane fluidity is directly determined as the rate and capacity of fluorescence recovery in an area bleached by a laser. Remarkably, GR diets promoted membrane fluidity in wild-type background as shown by shorter Thalf, compared to AL diets (Fig. 7a; supplementary videos 1, 2). Moreover, the maximum recovered fluorescence was greater on GR diets. This indicates that not only fluidity is improved but also mobile membrane fraction increases by GR diets. C-Laurdan dye is commonly used to measure membrane fluidity91–93. It exhibits the spectral shift in emission spectrum according to the levels of membrane order, enabling a straightforward method to monitor the membrane fluidity. We found that the spectrum of C-Laurdan was shifted from solid ordered phases to liquid-disordered phases by GR diets, as shown by generalized polarization (GP) index94 (Fig. 7b, c). Taken all, FRAP and C-Laurdan analyses reveal that GR diets promote membrane fluidity in C. elegans. Given that fatty acid desaturases were implicated in GR-induced longevity, we asked whether GR diets increased levels of unsaturated fatty acids in C. elegans. Using UPLC/QTOF mass spectrometry, we observed a slight increase in PUFA in free fatty acids by GR diets, although it was not significant (Supplementary Fig. 6a; Supplementary Table 7). Although GR diets caused no statistically significant change in the level of individual fatty acids in phosphatidylethanolamine (PE) and phosphatidylglycerol (PC), some PCs in which the acyl groups contained multiple double bonds were increased by GR diets, such as PC (34:2), (36:5), (38:3), (38:5) and (40:5) (Supplementary Fig. 6d; Supplementary Table 7). Given that the SFA content of cellular membranes is resistant to dietary challenges95, these small changes might collectively contribute to GR-induced membrane fluidity.
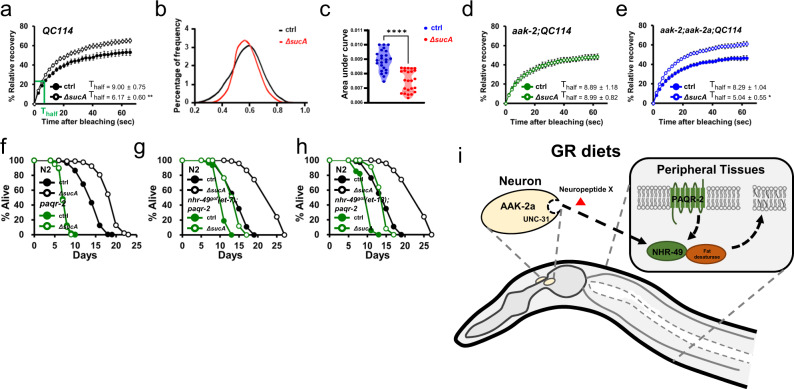
Fig. 7. GR diets promote membrane fluidity.
a FRAP analysis showing that GR diets enhance the membrane fluidity. Intestinal membrane fluidity is measured using control animals expressing membrane incorporated GFP (QC114). Average Thalf values (the time when half of maximum recovery is reached) are presented. Error bars indicate SEM. Results from representative experiments are shown with additional repeats (n ≥ 9 for each condition). See also Supplementary Movie 1-2. b, c C-Laurdan staining showing that GR diets promote membrane fluidity in C. elegans. Distribution of the average GP index values are shown from multiple images for AL vs. GR diets (n = 23, 24 respectively) (b). Reduced area under the curve (AUC) values demonstrate that C. elegans on GR diets has more fluidic membrane (P < 0.0001, P value determined by two-tailed Student’s t test). d, e FRAP analysis showing that GR diets enhance the membrane fluidity in an AAK-2a dependent manner. Intestinal membrane fluidity is measured using animals expressing membrane incorporated GFP (QC114) in aak-2(ok524) (d) and aak-2(ok524);aak-2a::gfp animals (e). Average Thalf values (the time when half of maximum recovery is reached) are presented. Error bars indicate SEM. Results from representative experiments are shown with additional repeats (n ≥ 8 for each condition). See also Supplementary Movie 3–6. f Lifespan showing that paqr-2 is required for GR-induced longevity. paqr-2(tm3410) completely abolished GR-induced longevity (P = 0.653, P value determined by two-tailed Student’s t test). Results from one of three independent experiments are shown. g, h Lifespans showing that nhr-49 (gof) mutations, et7 (g) and et13 (h), in paqr-2(tm3410) animals gain the GR-mediated longevity (P < 1.0 × 10−10, P value determined by two-tailed Student’s t test). Results from representative experiments are shown with additional repeats. i Model showing that GR prolongs longevity non-cell autonomously by promoting membrane fluidity through neuronal AMPK/neuropeptide/PAQR-2/NHR-49/Δ9 desaturase. Source data are provided as a Source Data file.
Importantly, GR-enhanced membrane fluidity was completely abrogated in aak-2(ok524) mutants, with no effect on the rate and capacity of fluorescence recovery by GR diets (Fig. 7d; supplementary videos 3, 4). Thus, AMPK is a crucial modulator of membrane fluidity in response to glucose restriction. This is in contrast to the fact that GR further enhanced fat oxidation even in the aak-2(ok524) background (Fig. 6k). Next, we asked whether the AAK-2a isoform could rescue the GR-induced membrane fluidity. Remarkably, aak-2(ok524);aak-2a animals completely restored both the recovery rate and maximum capacity of the membrane fluidity in intestinal cells (Fig. 7e; supplementary videos 5, 6). These data suggest that GR/neuronal AMPK prolongs longevity by promoting the membrane fluidity in peripheral tissues. PAQR-2 is an orthologue of mammalian adiponectin receptor AdipoR2 and is crucial to control membrane fluidity37,96. Therefore, we asked whether PAQR-2 was required for GR-mediated longevity. Remarkably, introducing the paqr-2(tm3410) mutation completely abolished GR-mediated longevity (Fig. 7f; Supplementary Table 6m), indicating that adjusting membrane fluidity is critical for GR-mediated longevity.
Of note, adipoR2 is shown to regulate lipid metabolism via PPARα signaling pathways in mice95,97. To investigate whether NHR-49gof could bypass the requirement of PAQR-2 in GR induced longevity, we introduced the nhr-49 gain-of-function (gof) mutations, et7 and et13, in paqr-2(tm3410) animals. Similar to nhr-49gof;aak-2 animals, nhr-49gof;paqr-2 animals exhibited lifespan extension by GR diets (Fig. 7g, h; Supplementary Table 6n). This data suggests that NHR-49 may function at least genetically downstream of PAQR-2 for GR-induced longevity. Of note, the nhr-49 mutations in paqr-2 mutants severely impaired the general fitness of C. elegans, resulting in the inability to conduct lifespan assays. These data imply that nhr-49 and paqr-2 may play non-overlapping roles in general worm health. Taken altogether, we demonstrate that GR prolongs longevity by promoting membrane fluidity via signaling including neuronal AMPK, neuropeptide, PAQR-2/AdipoR, NHR-49/PPARα, and Δ9 desaturases (Fig. 7i).
Discussion
Dietary restriction delays aging and aging-associated diseases in multiple organisms. Nonetheless, its unsustainability and possible side-effects limit DR application for humans. Therefore, lowered intake of particular nutrients could be a realistic alternative for DR-mediated health benefits. However, studies of the effects on longevity by defined nutrients have been limited, due to the lack of a relevant model.
Glucose is the best known nutrient to affect aging and aging-associated diseases. However, the molecular mechanisms underlying its effects on aging are poorly understood. It has been proposed that a glucose-rich diet activates the IIS pathway9, generates toxic advanced glycation end products10, and promotes the saturation of membrane lipids37, which may collectively mediate glucose toxicity. Glucose is known to reduce the lifespan in C. elegans9. Glucose likely functions directly on worms, as most of its effects still remain when supplied on ΔPTSGlc E. coli deficient in glucose uptake. However, glucose toxicity is abrogated in paqr-2 mutant C. elegans when the dietary E. coli is unable to convert the glucose into SFAs38. Therefore, at least in certain circumstances, glucose toxicity can be caused by gut microbes. In this study, glucose supplementation significantly increased the saturation of free fatty acids in C. elegans when fed ΔPTSGlc E. coli (Supplementary Fig. 6e), demonstrating that glucose could directly affect the lipid composition of C. elegans.
Studies on the longevity effects of GR diets are even less common. To mimic GR diets, the inhibitor of glycolysis 2-DG, or knocking down/blocking glycolytic enzyme has been utilized8. However, 2-DG represents only a subset of GR-induced phenotypes and exhibits glycolysis-independent effects, such as cell cycle arrest, apoptosis, ER stress, and AKT phosphorylation98–101. Therefore, a bona fide GR regimen will be valuable as it enables studies on the exact molecular mechanisms by which glucose modulates longevity and can provide a novel target for developing a GR mimetic.
Our previous genome-wide lifespan screens identified several E. coli mutants that extend the lifespan of C. elegans44. Among them, we demonstrate that ΔsucA E. coli mutants extend C. elegans longevity by serving as GR diets. Further, GR diets recapitulate DR benefits, such as delayed onset of Aβ42-induced pathologies and enhanced stress resistance. In contrast to DR, GR diets did not exhibit any loss of fitness such as the slow growth and lowered fecundity.
In mammals, the brain utilizes ~20% of the entire glucose supply102, suggesting that neurons are sensitive to glucose availability and should convey the signal to peripheral tissues to maintain organismal energy homeostasis. In this study, we demonstrate that a new AMPK isoform, AAK-2a, plays a pivotal role in the neurons, orchestrating GR-induced organismal responses in C. elegans. GR/neuronal AAK-2a prolongs longevity non-cell autonomously via pro-longevity neuropeptide signaling. Moreover, the GR-induced pro-longevity signal is dominant over the CA-AMPK/CRTC-1-mediated pro-aging signal22, as the GR diets extend the lifespan of constitutively active CRTC-1 (S76A, S179A) animals.
GR/AAK-2a mediated longevity requires NHR-49. NHR-49/PPARα orthologue regulates fat metabolism with MDT-15, C. elegans orthologue of mammalian mediator subunit MED1578,103 and SREBP ortholog SBP-1104. MDT-15 was also required for GR-mediated longevity (Supplementary Fig. 5d; Supplementary Table 6c). However, when sbp-1 knocked down post-developmentally, ΔsucA E. coli reproducibly extend the lifespan (Supplementary Fig. 5e; Supplementary Table 6o). These data imply that sbp-1 might not be involved in GR-induced longevity. Due to insufficient RNAi knock-down issues, however, we cannot rule out the possibility that SBP-1 is still involved in GR longevity.
While AAK-2 functions exclusively in neurons for GR-induced longevity, either neuronal or intestinal NHR-49 is sufficient for GR-induced longevity. Interestingly, similar to NHR-49, the expression of paqr-2 in a single tissue or alternatively in any one tissue is sufficient to suppress systemic paqr-2 mutant phenotypes81. The authors show that cell membrane homeostasis is maintained non-cell autonomously via lipid exchange between distant cells. It is possible that NHR-49 in one tissue modulates the systemic effect of GR-induced longevity in a similar manner. This could provide a plausible explanation that rescue of NHR-49 in either neurons or intestinal cells is sufficient for GR-mediated longevity.
In peripheral tissues, GR diets promote the fluidity of cellular membranes, which also requires neuronal AAK-2a. Since lipid regulators such as PAQR-2/AdipoR2, NHR-49/PPARα, and Δ9 desaturases are required for GR-induced longevity, GR diets promote whole-body lipid homeostasis including membrane fluidity.
Of interest, the membrane fluidity decreases in various cells from aged mice39,40 and human diabetes patients105, suggesting that the maintenance of membrane fluidity may promote longevity across species. Further, the PAQR-2 pathway functions not only for SFA tolerance106 but also for GR-induced longevity. Taken together, our data provide a regulatory network showing how organisms activate an intrinsic pro-longevity program in response to environmental factors, such as GR (Fig. 7i). Given the conservation of factors involved in GR-mediated longevity, our studies deepen understanding on aging and age-associated diseases and identify potential therapeutic targets to improve the healthspan in humans.
Methods
C. elegans
C. elegans strains were maintained at 20 °C on E. coli using standard cultivation techniques107, unless otherwise described. C. elegans strains were purchased from the Genetic Genome Center or were gifts from other laboratories. The transgenic animals were generated using standard microinjection methods108. Briefly, to generate aak-2 transgenic animals, aak-2 DNA construct was injected into aak-2;unc-119 double mutants along with co-injection marker, unc-119+ (200 ng/μl). Double mutants were generated using standard genetic methods as described below. In brief, to generate nhr-49; aak-2 double mutants, aak-2 males were mated to nhr-49 hermaphrodites. Several F1 progenies were singled on individual plates. F2 progenies were selected and tested for homozygous aak-2 mutation by PCR. From multiple F3 progenies, homozygous nhr-49 mutants were selected by PCR. The primers used for genotyping are listed in Supplementary Table 8. All strains were backcrossed at least four times to control strains. The strains are listed in Supplementary Table 9.s
E. coli strains
E. coli mutants were sourced from an E. coli single-gene deletion mutant library (Keio collection). Double or quadruple E. coli mutants were generated as previously described109.
DNA construction
To generate the aak-2a promoter construct, a SphI/XbaI fragment containing the 2.4 kb upstream region of aak-2a was cloned into the pPD95.75 vector. To generate the aak-2a cDNA constructs, aak-2a cDNA was cloned into the pUC18 vector using mutagenic primers with XbaI/KpnI restriction sites. The aak-2a cDNA was subcloned into pPD95.75-Paak-2a using XbaI/KpnI. To generate the aak-2c promoter construct, a PstI/SalI fragment containing the 3.6 kb upstream region of aak-2c was cloned into the pUC18-aak-2a vector. The Paak-2c::aak-2c ORF was subcloned into pPD95.75 using PstI/KpnI. The aak-2c promoter was cloned into pPD95.75 aak-2a to make aak-2a/c using NheI/XbaI. To generate tissue specific expression vectors, 1.3 kb unc-119 promoter was amplified using mutagenic oligos containing HindIII/SphI and cloned into pPD95.75 vector. The 2.4 kb mca-1 promoter containing SphI/PstI and 1.2 kb vha-6 promoter containing HindIII/SalI were cloned into pPD95.75. An aak-2a cDNA was subcloned into each tissue specific expression vector. To generate tissue specific NHR-49 expression vectors, a nhr-49 cDNA was subcloned into pPD95.75Punc-119, pPD95.75Prab-3 and pPD95.75Pvha-6 vectors using SalI/SmaI. The primers used for the DNA constructs are listed in Supplementary Table 8.
Lifespan assay
Lifespan assays were performed at 25 °C, unless mentioned otherwise. Synchronized worms were prepared by 3 hour-egg laying or egg preparation followed by L1 arrest. The worms were allowed to grow for several days until they reached the young adult stage. Synchronized young adult stage worms were treated with 0.1 mg/mL FUDR to prevent the proliferation of the progeny. The worms were scored as dead or alive by tapping them with a platinum wire every 2–3 day. The worms that died of vulval rupture were excluded from the analysis. Lifespan plates were freshly prepared with overnight culture of E. coli in LB media. For glucose-depleted E. coli such as ΔsucA, ΔacnAΔacnB, ΔicdΔaceBA, overnight culture was concentrated 15 times before seeding on NGM plates, to prevent worms from starving during lifespan assays. All lifespan assays were repeated at least three times, unless mentioned otherwise. Statistical analyses and p-values were calculated using the log-rank (Mantel–Cox) method, through the OASIS application (http://sbi.postech.ac.kr/oasis2/). Glucose, αKG, lysine, pyruvate, or glycerol was freshly dissolved in distilled water, followed by filter sterilization. All supplements were added on plates one day before use.
Paralysis assay
For the paralysis assay, CL2006 animals expressing the human Aβ42 were utilized as previously described61,110. In brief, synchronized L1 worms were grown on the BW25113 control E. coli (AL diets) or ΔsucA E. coli mutants (GR diets) until they reached the young adults at 20 °C. To paralyze worms, plates were moved to 25 °C and monitored until all worms were paralyzed. The worms were scored every other day as paralyzed when they did not respond upon tapping. One of representative data was shown from 3 biological replicates.
Brood size assay
Brood size assays were conducted as previously reported44. In brief, total 12 L4 stage N2 worms were moved to individual plates seeded with AL or GR diets and allowed to lay eggs for 24 h at 20 °C. Each worm was transferred to a new plate every 24 h for 5 d. The number of progeny was counted when the worms reached the L4 stage. For consecutive brood size analysis, the same procedures were repeated using worms previously grown on AL diets vs. GR diets. One of representative data was shown from three biological replicates.
Thermotolerance assay
Thermotolerance aassay was conducted as previously reported111. In brief, synchronized wild-type N2 worms were grown at 20 °C, until they reached the L4 stage, followed by an upshift to 37 °C. The plates were removed at the indicated times and the worms were allowed to recover for ~12 h. The plates were then scored and discarded. Heat stress-induced mortality was determined by tapping the worms with a platinum wire to check motility. Statistical analyses was calculated through the OASIS application (http://sbi.postech.ac.kr/oasis2/). One of representative data was shown from three biological replicates.
RNAi knockdown
Ahringer RNAi collection was used for RNAi knockdown of specific genes. If necessary, RNAi vectors were constructed using classical cloning techniques. RNAi knockdown conducted as previously reported44. In brief, HT115 bacteria with L4440 vector encoded target-gene sequence were grown at 37 °C overnight in LB media, with 50 μg/mL ampicillin and 10 μg/mL tetracycline. 1% overnight culture was inoculated in LB media containing 50 μg/mL ampicillin, and incubated for 3 hours. The culture was concentrated 10-fold and used to seed NGM plates containing 50 μg/mL ampicillin and 1 mM IPTG. For tissue-specific RNAi experiments, TU3410 and VP303 were used for neuron- and intestine-specific RNAi, respectively.
Metabolite analysis
Bacteria were cultured overnight in LB media, harvested, and washed with M9 solution two times. Thereafter, the bacteria were diluted with M9 solution and seeded on 90 mm NGM plates. After 3–4 day of growth, the bacteria were harvested using a scraper, suspended in pre-chilled 2X quenching solution (−20 °C, 40% ethanol, 0.8% NaCl), and centrifuged at −20 °C and 3,000 g for 10 min. After discarding the supernatant, the bacterial pellet was frozen using liquid nitrogen and stored at −80 °C until further analysis. Polar metabolites were extracted from cells using three freeze-thaw cycles in the presence of 2 mL of cold 80% aqueous methanol. After centrifugation, the supernatant was transferred to a new EP tube and 500 μL of chloroform and 1 mL of distilled water were added to the remaining pellet. Next, the solution was vortexed and centrifuged, and then, the supernatant was combined with the supernatant obtained from the previous step. The supernatant containing polar metabolites was vacuum dried and resuspended in 600 μL of 0.1 M sodium phosphate-buffered deuterium oxide (pH 7.0) containing 0.1 mM 3-(trimethylsilyl) propionic-2,2,3,3-d4 acid (TSP-d4) (Sigma Aldrich). 1H-NMR spectra were measured using an 800-MHz NMR instrument. Briefly, One-dimensional (1D) nuclear Overhauser enhancement spectroscopy (NOESY)-PRESAT pulse sequence was applied to suppress the residual signal in water. Total 256 transients were collected into 64,000 data points using a spectral width of 16393.4 Hz with a relaxation delay of 4.0 s and an acquisition time of 2.00 s. NMR spectra were phased and baseline corrected using Chenomx NMR suite version 7.1 (Chenomx Inc., Edmonton, Alberta, Canada). Identification of the metabolites was performed using 2D NMR TOCSY and HSQC and spiking experiments. Quantification of metabolite was accomplished using an 800MNz library to determine the concentration of individual compounds using sodium 3- (trimethylsilyl) propionate- 2,2,3,3- d4 (TSP) as an internal standard. Data file was transferred to MATLAB (R2006a; Mathworks, Inc., Natick, MA, USA) and all spectra were aligned by correlation optimized warping (COW) method112.
Lipidomic analysis
Lipids were extracted from samples using three freeze-thaw cycles in the presence of 1.6 mL of cold methanol/chloroform mixture (1/1, v/v). After centrifugation, the supernatant was transferred to a new EP tube. Next, the supernatant containing the lipids was nitrogen dried and resuspended in 400 μL (for C. elegans) and 4 mL (for E. coli) of 80% aqueous isopropanol. Lipids were measured using a Xevo G2-XS Q/TOF mass spectrometry (MS) system coupled with ultra-performance liquid chromatography (UPLC) system (Waters, Milford, MA, USA). An acquity UPLC CSH C18 column (1.7 μm × 2.1 × 100 mm) was used for lipid separation. The mobile phase in positive mode was consisted of 10 mM ammonium formate and 0.1% formic acid in water:acetonitrile (4:6 v/v, solvent A) and 0.1% formic acid in isopropyl alcohol:acetonitrile (9:1 v/v, solvent B). The mobile phase in negative mode was consisted of 10 mM ammonium formate in water:acetonitrile (6:4 v/v, solvent A) and isopropyl alcohol:acetonitrile (9:1 v/v, solvent B). Samples were eluted at 0.40 ml/min for 20 min. The eluate was analyzed with electrospray ionization (ESI) in positive and negative modes. Mass range was 50–1200 m/z. Leucine-enkephalin ([M + H] + : m/z 556.2771 and [M-H] − : m/z 554.2615) was used as a reference in the lock-spray and introduced by a lock-spray at 5 μL/min for accurate mass acquisition. Raw UPLC/QTOF MS spectral data were preprocessed using Progenesis QI software (Waters, Milford, MA, USA) and normalized to the total ion sum. Lipids were identified using Lipid Maps (www.lipidmaps.org), Human Metabolome (www.hmdb.ca), and Metlin (metlin.scripps.edu) databases. Identification was confirmed using MS/MS pattern and retention time of lipid standards (Avanti Polar Lipids, Alabaster, USA and Sigma-Aldrich, St Louis, USA).
Fat staining
For Nile red staining, the worms were synchronized by seeding the eggs on to fresh plates and grown until the L4 stage. The worms were then harvested using the M9 buffer, washed three times with the M9 buffer, and fixed in 1% paraformaldehyde for 10 min. The worms were then freeze–thawed three times using cold ethanol on dry ice. After three washes with the M9 buffer, the worms were dehydrated using 60% isopropanol for 2 min. The fixed worms were incubated in Nile red solution (3 μg/mL Nile red in 60% isopropanol) for 30 min and rehydrated using three washes of the M9 buffer. For Oil Red O staining, the worms were synchronized by seeding the eggs onto fresh plates and grown until the day 1 adult stage. The worms were then harvested using the M9 buffer, washed three times with the M9 buffer, and fixed in 1% paraformaldehyde for 10 min. After three washes with the M9 buffer, the worms were dehydrated using 60% isopropanol for 2 min. The worms were stained in 400μl of saturated Oil Red O solution overnight on a shaker at RT. A minimum of 20 animals was quantified for each conditions. Fat was visualized using the Nikon ECLIPSE Ni-U microscope. Fat stores were quantified using the ImageJ software.
Triglycerides assay
Day 1 synchronized adult worms were harvested using the M9 buffer, washed three times with the M9 buffer and suspended in NP40/ddH2O (5% NP40). Samples were sonicated at 20% power, for 30 s. To solubilize triglyceride, lysates were incubated at 90 °C for 5 min, then cooled down at room temperature for 5 min, followed by centrifugation at 24,000 × g for 10 min. Aqueous phases of the samples were collected. Triglycerides were measured using the Triglyceride assay Kit (ab65336, Abcam), according to manufacturer’s instructions. The amount of triglyceride was normalized with protein concentration (BCA protein kit, 23228, Pierce). One of representative data was shown from two biological replicates.
αKG assay
Worms were grown on NGM plates supplemented with α-KG. Day 3 synchronized adult worms were harvested, followed by washing three times with M9 buffer and sonication (30 s, 20% power). After sonication, lysed worms were centrifuged at 24,000 × g for 10 min at 4 °C.
Aqueous phases of the samples were collected. αKG concentration was measured using the αKG Assay Kit (ab83431, Abcam), according to manufacturer’s instructions. The colorimetric signal was measured using the Synergy HT Multi-detection Microplate Reader. One of representative data were shown from two biological replicates.
Western blot analysis
The mixed stage worms were collected and washed three times with M9 buffer. Worms were resuspended in RIFA buffer containing protease inhibitor (1 mM PMSF, 1 μg/mL leupeptin and aprotinin) and phosphatase inhibitors cocktail (4906837001, Roche), followed by sonication. After sonication, lysed worms were centrifuged at 24,000 × g for 10 min at 4 °C. Aqueous phases of the samples were collected. The lysates were boiled in SDS sample buffer. Worm lysates were then resolved on 8% SDS-PAGE and western-blotted using antibody against to GFP (1:1000 diluted, ab290, Abcam), Goat anti-rabbit IgG HRP conjugated (1:5000, #31460, ThermoFisher) and β-actin (1:5000, ab133626, Abcam). β-actin served as the endogenous controls for normalization.
Quantitative RT-PCR
RNA preparation and cDNA synthesis were performed according to standard protocols. Total RNA was isolated using an RNeasy Plus Mini Kit (Qiagen). Total RNA (2 μo) was used for cDNA synthesis with a SuperScriptII cDNA synthesis kit (Thermo Fisher Scientific). Quantitative RT-PCR was performed using StepOnePlusTM (Applied Biosystems). in a total reaction volume of 25 μL containing cDNA, primers, and SYBR Master Mix (AppliedBiosystems). Data were normalized to actin mRNA levels in each reaction. The sequences of the PCR primers are listed in Supplementary Table 8
Fluorescence recovery after photobleaching (FRAP) analysis
FRAP assays were performed using animals expressing the membrane-associated GFP (Pglo-1::GFP-CAAX) in intestinal cells with a Zeiss LSM800 confocal microscope and Zen software (Zeiss). The intestinal membranes of L2-3 stage worms were photobleached over circular region (0.75 μm radius) using a 488 nm power laser with 70% laser power transmission for 5 s. The recovered fluorescence of bleached region was recorded every 2.0 s for 90 s. The Thalf value was defined as the time the fluorescence intensity reached to 50% of maximum fluorescence recovery. Images were acquired with 16 bits image depth and 1024 × 1024 resolution using ~0.76 μs pixel dwell settings.
C-Laurdan staining
Live C. elegans was stained in M9 buffer containing 10mM C-Laurdan dye (N-Methyl-N-[6-(1-oxododecyl)-2-naphthalenyl]glycine) (TOCRIS) for 2 h. Images were acquired Zeiss LSM800 confocal microscope and Zen software (Zeiss). Samples were excited with a 405 nm laser and the emission recorded between 400 and 460 nm (ordered phase) and between 470 and 530 nm (disordered phase). Pictures were acquired with 16 bits image depth and 512 × 512 resolution, using a pixel dwell of ~1.52 μs. generalized polarization(GP) values were analyzed using automated ImageJ macro, according to published guidelines91.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We are grateful to Kyuhyung Kim for advice and critical comments on the manuscript. Some of the strains were kindly provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources. This research was supported by the National Research Foundation of Korea (NRF) funded by the Korean Government (Ministry of Science and ICT) (2021R1A2C100891912), the National Research Council of Science & Technology (NST) grant by the Korea government (MSIT) (CRC22011-200), Korea Basic Science Institute (C270200) and the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program (1711134076).
Source data
Author contributions
E.-S.K. designed and supervised the project. J.-H.J., J.-S.H., S.-H.P., M.P., M.-G.S., and E.-S.K. performed lifespan assays. J.-H.J., J.-S.H., M.-G.S., and E.-S.K. generated E. coli mutants and transgenic animals. J.-H.J. performed fat analysis. J.-H.J. and S.M.L. performed FRAP experiments. Y.J., N.K., M.S.K., C.-A.K., and G.-S.H. performed metabolite analysis. E.-S.K., S.K., K.-P. L., K.-S.K., and Y.R.Y. analyzed data. E.-S.K. wrote the paper. All authors read and edited the paper.
Peer review
Peer review information
Nature Communications thanks Bénédicte Elena-Herrmann and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The main data supporting the results are available within this article as well as its Supplementary information. Source data including the individual P values, whole western blot image and lifespan raw data are provided with this paper. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jin-Hyuck Jeong, Jun-Seok Han, Youngae Jung.
Contributor Information
Geum-Sook Hwang, Email: gshwang@kbsi.re.kr.
Eun-Soo Kwon, Email: eunsoo.kwon@kribb.re.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-35952-z.
References
- 1.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5:155–171. [PubMed] [Google Scholar]
- 2.Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14:2135–2137. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- 3.Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech. Ageing Dev. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 5.Kealy RD, et al. Effects of diet restriction on life span and age-related changes in dogs. J. Am. Vet. Med. Assoc. 2002;220:1315–1320. doi: 10.2460/javma.2002.220.1315. [DOI] [PubMed] [Google Scholar]
- 6.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz TJ, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Murphy CT, Kenyon C. Glucose shortens the life span of C. elegans by downregulating DAF-16/FOXO activity and aquaporin gene expression. Cell Metab. 2009;10:379–391. doi: 10.1016/j.cmet.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlotterer A, et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes. 2009;58:2450–2456. doi: 10.2337/db09-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 12.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 13.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 14.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 15.Greer EL, et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamoli M, et al. Polyunsaturated fatty acids and p38-MAPK link metabolic reprogramming to cytoprotective gene expression during dietary restriction. Nat. Commun. 2020;11:4865. doi: 10.1038/s41467-020-18690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinkraus KA, et al. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkewitz K, et al. Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell. 2015;160:842–855. doi: 10.1016/j.cell.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riera CE, et al. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell. 2014;157:1023–1036. doi: 10.1016/j.cell.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, et al. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/S0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 25.Tachibana T, Tsutsui K. Neuropeptide control of feeding behavior in birds and its difference with mammals. Front Neurosci. 2016;10:485. doi: 10.3389/fnins.2016.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libert S, et al. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315:1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- 27.Hussey R, et al. Oxygen-sensing neurons reciprocally regulate peripheral lipid metabolism via neuropeptide signaling in Caenorhabditis elegans. PLoS Genet. 2018;14:e1007305. doi: 10.1371/journal.pgen.1007305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Rourke EJ, Kuballa P, Xavier R, Ruvkun G. omega-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev. 2013;27:429–440. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi W, et al. The omega-3 fatty acid alpha-linolenic acid extends Caenorhabditis elegans lifespan via NHR-49/PPARalpha and oxidation to oxylipins. Aging Cell. 2017;16:1125–1135. doi: 10.1111/acel.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda Y, et al. 10-Hydroxy-2-decenoic acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015;2015:425261. doi: 10.1155/2015/425261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han S, et al. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature. 2017;544:185–190. doi: 10.1038/nature21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee D, et al. MDT-15/MED15 permits longevity at low temperature via enhancing lipidostasis and proteostasis. PLoS Biol. 2019;17:e3000415. doi: 10.1371/journal.pbio.3000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shmookler Reis RJ, et al. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging (Albany NY) 2011;3:125–147. doi: 10.18632/aging.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Covarrubias V. Lipidomics in longevity and healthy aging. Biogerontology. 2013;14:663–672. doi: 10.1007/s10522-013-9450-7. [DOI] [PubMed] [Google Scholar]
- 35.Maulucci G, et al. Fatty acid-related modulations of membrane fluidity in cells: detection and implications. Free Radic. Res. 2016;50:S40–S50. doi: 10.1080/10715762.2016.1231403. [DOI] [PubMed] [Google Scholar]
- 36.Sunshine H, Iruela-Arispe ML. Membrane lipids and cell signaling. Curr. Opin. Lipido. 2017;28:408–413. doi: 10.1097/MOL.0000000000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svensk E, et al. Caenorhabditis elegans PAQR-2 and IGLR-2 protect against glucose toxicity by modulating membrane lipid composition. PLoS Genet. 2016;12:e1005982. doi: 10.1371/journal.pgen.1005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devkota R, et al. The adiponectin receptor AdipoR2 and its Caenorhabditis elegans homolog PAQR-2 prevent membrane rigidification by exogenous saturated fatty acids. PLoS Genet. 2017;13:e1007004. doi: 10.1371/journal.pgen.1007004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egawa J, Pearn ML, Lemkuil BP, Patel PM, Head BP. Membrane lipid rafts and neurobiology: age-related changes in membrane lipids and loss of neuronal function. J. Physiol. 2016;594:4565–4579. doi: 10.1113/JP270590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wahnon R, Mokady S, Cogan U. Age and membrane fluidity. Mech. Ageing Dev. 1989;50:249–255. doi: 10.1016/0047-6374(89)90103-6. [DOI] [PubMed] [Google Scholar]
- 41.Lee SM, et al. FABP3-mediated membrane lipid saturation alters fluidity and induces ER stress in skeletal muscle with aging. Nat. Commun. 2020;11:5661. doi: 10.1038/s41467-020-19501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson E, et al. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 2014;156:1336–1337. doi: 10.1016/j.cell.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 43.Yilmaz LS, Walhout AJ. Worms, bacteria, and micronutrients: an elegant model of our diet. Trends Genet. 2014;30:496–503. doi: 10.1016/j.tig.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin MG, et al. Bacteria-derived metabolite, methylglyoxal, modulates the longevity of C. elegans through TORC2/SGK-1/DAF-16 signaling. Proc. Natl Acad. Sci. USA. 2020;117:17142–17150. doi: 10.1073/pnas.1915719117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett BD, et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 2009;5:593–599. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chin RM, et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature. 2014;510:397–401. doi: 10.1038/nature13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu YJ, et al. Glycine promotes longevity in Caenorhabditis elegans in a methionine cycle-dependent fashion. PLoS Genet. 2019;15:e1007633. doi: 10.1371/journal.pgen.1007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards C, et al. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015;16:8. doi: 10.1186/s12863-015-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chia CW, Egan JM, Ferrucci L. Age-related changes in glucose metabolism, hyperglycemia, and cardiovascular risk. Circ. Res. 2018;123:886–904. doi: 10.1161/CIRCRESAHA.118.312806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Portal-Celhay C, Bradley ER, Blaser MJ. Control of intestinal bacterial proliferation in regulation of lifespan in Caenorhabditis elegans. BMC Microbiol. 2012;12:49. doi: 10.1186/1471-2180-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Q, et al. Comparison of individual component deletions in a glucose-specific phosphotransferase system revealed their different applications. Sci. Rep. 2015;5:13200. doi: 10.1038/srep13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCulloch D, Gems D. Sex-specific effects of the DAF-12 steroid receptor on aging in Caenorhabditis elegans. Ann. N. Y Acad. Sci. 2007;1119:253–259. doi: 10.1196/annals.1404.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Q, et al. Sexual dimorphism in the nutritional requirement for adult lifespan in Drosophila melanogaster. Aging Cell. 2020;19:e13120. doi: 10.1111/acel.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanfi Y, et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 55.Harrison DE, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Honjoh S, Ihara A, Kajiwara Y, Yamamoto T, Nishida E. The sexual dimorphism of dietary restriction responsiveness in Caenorhabditis elegans. Cell Rep. 2017;21:3646–3652. doi: 10.1016/j.celrep.2017.11.108. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Xia K, Yang X, Tang C. Growth strategy of microbes on mixed carbon sources. Nat. Commun. 2019;10:1279. doi: 10.1038/s41467-019-09261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalemba KM, et al. Glycerol induces G6pc in primary mouse hepatocytes and is the preferred substrate for gluconeogenesis both in vitro and in vivo. J. Biol. Chem. 2019;294:18017–18028. doi: 10.1074/jbc.RA119.011033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madeo F, Carmona-Gutierrez D, Hofer SJ, Kroemer G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 2019;29:592–610. doi: 10.1016/j.cmet.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Yazawa H, et al. Beta amyloid peptide (Abeta42) is internalized via the G-protein-coupled receptor FPRL1 and forms fibrillar aggregates in macrophages. FASEB J. 2001;15:2454–2462. doi: 10.1096/fj.01-0251com. [DOI] [PubMed] [Google Scholar]
- 61.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H, et al. The Caenorhabditis elegans AMP-activated protein kinase AAK-2 is phosphorylated by LKB1 and is required for resistance to oxidative stress and for normal motility and foraging behavior. J. Biol. Chem. 2008;283:14988–14993. doi: 10.1074/jbc.M709115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardie DG. AMP-activated protein kinase: maintaining energy homeostasis at the cellular and whole-body levels. Annu. Rev. Nutr. 2014;34:31–55. doi: 10.1146/annurev-nutr-071812-161148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tavernarakis N, Wang SL, Dorovkov M, Ryazanov A, Driscoll M. Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nat. Genet. 2000;24:180–183. doi: 10.1038/72850. [DOI] [PubMed] [Google Scholar]
- 65.Kamath RS, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 66.Simmer F, et al. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr. Biol. 2002;12:1317–1319. doi: 10.1016/S0960-9822(02)01041-2. [DOI] [PubMed] [Google Scholar]
- 67.Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat. Methods. 2010;7:554–559. doi: 10.1038/nmeth.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J. Gen. Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- 70.Kadekar P, Roy R. AMPK regulates germline stem cell quiescence and integrity through an endogenous small RNA pathway. PLoS Biol. 2019;17:e3000309. doi: 10.1371/journal.pbio.3000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spencer WC, et al. A spatial and temporal map of C. elegans gene expression. Genome Res. 2011;21:325–341. doi: 10.1101/gr.114595.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopez M, Nogueiras R, Tena-Sempere M, Dieguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016;12:421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 73.Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153:1435–1447. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacob TC, Kaplan JM. The EGL-21 carboxypeptidase E facilitates acetylcholine release at Caenorhabditis elegans neuromuscular junctions. J. Neurosci. 2003;23:2122–2130. doi: 10.1523/JNEUROSCI.23-06-02122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weir HJ, et al. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab. 2017;26:884–896.e885. doi: 10.1016/j.cmet.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yoon MJ, et al. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 77.Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005;3:e53. doi: 10.1371/journal.pbio.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreno-Arriola E, El Hafidi M, Ortega-Cuellar D, Carvajal K. AMP-activated protein kinase regulates oxidative metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 transcriptional regulators. PLoS ONE. 2016;11:e0148089. doi: 10.1371/journal.pone.0148089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ratnappan R, et al. Germline signals deploy NHR-49 to modulate fatty-acid beta-oxidation and desaturation in somatic tissues of C. elegans. PLoS Genet. 2014;10:e1004829. doi: 10.1371/journal.pgen.1004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naim N, et al. Cell nonautonomous roles of NHR-49 in promoting longevity and innate immunity. Aging Cell. 2021;20:e13413. doi: 10.1111/acel.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bodhicharla R, Devkota R, Ruiz M, Pilon M. Membrane fluidity is regulated cell nonautonomously by Caenorhabditis elegans PAQR-2 and its mammalian homolog AdipoR2. Genetics. 2018;210:189–201. doi: 10.1534/genetics.118.301272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008;32:S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 83.Cunningham KA, et al. Loss of a neural AMP-activated kinase mimics the effects of elevated serotonin on fat, movement, and hormonal secretions. PLoS Genet. 2014;10:e1004394. doi: 10.1371/journal.pgen.1004394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JH, et al. Lipid droplet protein LID-1 mediates ATGL-1-dependent lipolysis during fasting in Caenorhabditis elegans. Mol. Cell Biol. 2014;34:4165–4176. doi: 10.1128/MCB.00722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kennedy JA, Unger SA, Horowitz JD. Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem Pharm. 1996;52:273–280. doi: 10.1016/0006-2952(96)00204-3. [DOI] [PubMed] [Google Scholar]
- 86.Watts JL, Browse J. Genetic dissection of polyunsaturated fatty acid synthesis in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2002;99:5854–5859. doi: 10.1073/pnas.092064799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brock TJ, Browse J, Watts JL. Fatty acid desaturation and the regulation of adiposity in Caenorhabditis elegans. Genetics. 2007;176:865–875. doi: 10.1534/genetics.107.071860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brock TJ, Browse J, Watts JL. Genetic regulation of unsaturated fatty acid composition in C. elegans. PLoS Genet. 2006;2:e108. doi: 10.1371/journal.pgen.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Imanikia S, Sheng M, Castro C, Griffin JL, Taylor RC. XBP-1 remodels lipid metabolism to extend longevity. Cell Rep. 2019;28:581–589 e584. doi: 10.1016/j.celrep.2019.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goudeau J, et al. Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biol. 2011;9:e1000599. doi: 10.1371/journal.pbio.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Owen DM, Rentero C, Magenau A, Abu-Siniyeh A, Gaus K. Quantitative imaging of membrane lipid order in cells and organisms. Nat. Protoc. 2011;7:24–35. doi: 10.1038/nprot.2011.419. [DOI] [PubMed] [Google Scholar]
- 92.Ruiz M, Stahlman M, Boren J, Pilon M. AdipoR1 and AdipoR2 maintain membrane fluidity in most human cell types and independently of adiponectin. J. Lipid Res. 2019;60:995–1004. doi: 10.1194/jlr.M092494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim HM, et al. A two-photon fluorescent probe for lipid raft imaging: C-laurdan. Chembiochem. 2007;8:553–559. doi: 10.1002/cbic.200700003. [DOI] [PubMed] [Google Scholar]
- 94.Amaro M, Reina F, Hof M, Eggeling C, Sezgin E. Laurdan and Di-4-ANEPPDHQ probe different properties of the membrane. J. Phys. D. Appl. Phys. 2017;50:134004. doi: 10.1088/1361-6463/aa5dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamauchi T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 96.Svensk E, et al. PAQR-2 regulates fatty acid desaturation during cold adaptation in C. elegans. PLoS Genet. 2013;9:e1003801. doi: 10.1371/journal.pgen.1003801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamauchi T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 98.Kurtoglu M, et al. Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol. Cancer Ther. 2007;6:3049–3058. doi: 10.1158/1535-7163.MCT-07-0310. [DOI] [PubMed] [Google Scholar]
- 99.Kurtoglu M, Maher JC, Lampidis TJ. Differential toxic mechanisms of 2-deoxy-D-glucose versus 2-fluorodeoxy-D-glucose in hypoxic and normoxic tumor cells. Antioxid. Redox Signal. 2007;9:1383–1390. doi: 10.1089/ars.2007.1714. [DOI] [PubMed] [Google Scholar]
- 100.Zhong D, et al. 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol. Cancer Ther. 2008;7:809–817. doi: 10.1158/1535-7163.MCT-07-0559. [DOI] [PubMed] [Google Scholar]
- 101.Muley P, Olinger A, Tummala H. 2-Deoxyglucose induces cell cycle arrest and apoptosisin colorectal cancer cells independent of its glycolysis inhibition. Nutr. Cancer. 2015;67:514–522. doi: 10.1080/01635581.2015.1002626. [DOI] [PubMed] [Google Scholar]
- 102.Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–597. doi: 10.1016/j.tins.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. A Mediator subunit, MDT-15, integrates regulation of fatty acid metabolism by NHR-49-dependent and -independent pathways in C. elegans. Genes Dev. 2006;20:1137–1149. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang F, et al. An ARC/Mediator subunit required for SREBP control of cholesterol and lipid homeostasis. Nature. 2006;442:700–704. doi: 10.1038/nature04942. [DOI] [PubMed] [Google Scholar]
- 105.Kroger J, et al. Erythrocyte membrane fatty acid fluidity and risk of type 2 diabetes in the EPIC-Potsdam study. Diabetologia. 2015;58:282–289. doi: 10.1007/s00125-014-3421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Devkota R, Henricsson M, Boren J, Pilon M. The C. elegans PAQR-2 and IGLR-2 membrane homeostasis proteins are uniquely essential for tolerating dietary saturated fats. Biochim Biophys. Acta Mol. Cell Biol. Lipids. 2021;1866:158883. doi: 10.1016/j.bbalip.2021.158883. [DOI] [PubMed] [Google Scholar]
- 107.Stiernagle T. Maintenance of C. elegans. WormBook 1–11 10.1895/wormbook.1.101.1 (2006). [DOI] [PMC free article] [PubMed]
- 108.Rieckher M, Tavernarakis N. Caenorhabditis elegans microinjection. Bio Protoc. 2017;7:e2565. doi: 10.21769/BioProtoc.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2:2006 0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 111.Kwon ES, Narasimhan SD, Yen K, Tissenbaum HA. A new DAF-16 isoform regulates longevity. Nature. 2010;466:498–502. doi: 10.1038/nature09184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Skov T, van den Berg F, Tomasi G, Bro R. Automated alignment of chromatographic data. J. Chemometrics. 2006;20:484–497. doi: 10.1002/cem.1031. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The main data supporting the results are available within this article as well as its Supplementary information. Source data including the individual P values, whole western blot image and lifespan raw data are provided with this paper. Source data are provided with this paper.