Abstract
Although the glymphatic system hypothesis is highly popular, it also lacks certain details. In this paper, an attempt was made to present a more clearly defined hypothesis, which is consistent with the past experiment results. The new hypothesis consists of (1) water flux in the brain parenchyma, (2) water and solutes pathway of the perivascular space, and (3) maintenance of this pathway by the network of astrocytes.
Keywords: clearance, glymphatic system, neurofluids
Introduction
Although the exact cause of Alzheimer’s disease is still not known, impaired amyloid beta (Aβ) clearance has been considered to be a possible cause in recent years. Aβ clearance itself has been observed in animal experiments, and it is known to increase in sleeping condition.1 The exact mechanism or pathway of Aβ clearance is also not known, but Iliff et al proposed a hypothesis called the “glymphatic system,” which acts like the lymphatic system in other parts of the body.2
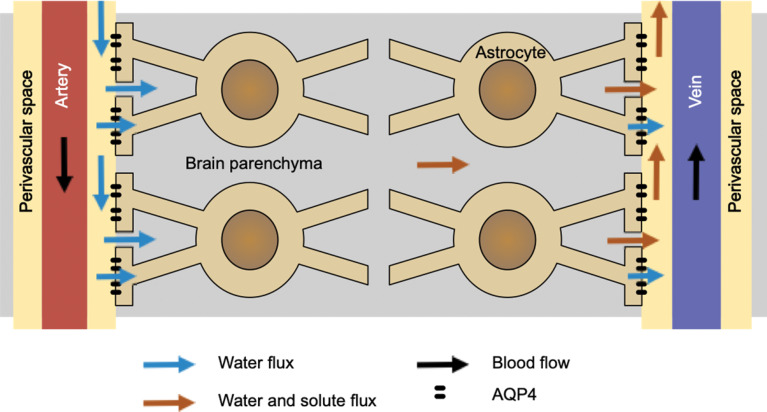
The essence of the glymphatic system may be summarized in Fig. 1.2 The waste clearance system is driven by the water coming through the perivascular space of the artery. It perfuses through the brain parenchyma moving the waste molecules and, finally, move out of the brain through the perivascular space of the vein. This process is somehow helped by the astrocytes, with aquaporin channels on their foot processes. Note that the direction of the venous flow is inverted from the original paper, in order to be consistent with other figures of this paper. Also, in this paper, the word “perivascular space” is used to indicate Virchow-Robin space. Although “paravascular space” was used throughout Iliff’s paper2 for the same meaning, it seems that “perivascular space” is more commonly used for this purpose.
Fig. 1.
This illustrates the concept of the original glymphatic system hypothesis by Iliff et al.2 Note that the direction of venous flow is inverted from the original paper, in order to be consistent with other figures in this paper.
There are several issues regarding this interpretation of the experimental results. For example, the driving force of the water that comes in from the subarachnoid space, and then comes out into the subarachnoid space again, is not clear. Also, if the water moves along this pathway, the overall removal of waste products from the brain tissue is not clear, especially if the subarachnoid space is included in the system of consideration. Whether the water movement is diffusion or bulk flow is another issue. Finally, how exactly astrocytes help the clearance system is not clearly described.
In this work, an attempt was made to present a hypothesis, which can explain these issues in a reasonable way. The proposed hypothesis is not based on new experimental results. Rather, it was designed to be consistent with existing results. The hypothesis itself is presented first, and then interpretation of existing results based on the new hypothesis is presented.
Flux of the Water in the Interstitial Space
In the proposed hypothesis, the source of the water is the capillary, or more specifically, arterial side of it. The driving force is the hydrostatic pressure of the arterial blood. At the venous side of the capillary, the water is re-absorbed in the blood vessel by the osmotic pressure primarily generated by the plasma protein. This overall process is common with the tissues in the other parts of the body. The main difference is that only water comes out of the blood vessel in the brain.
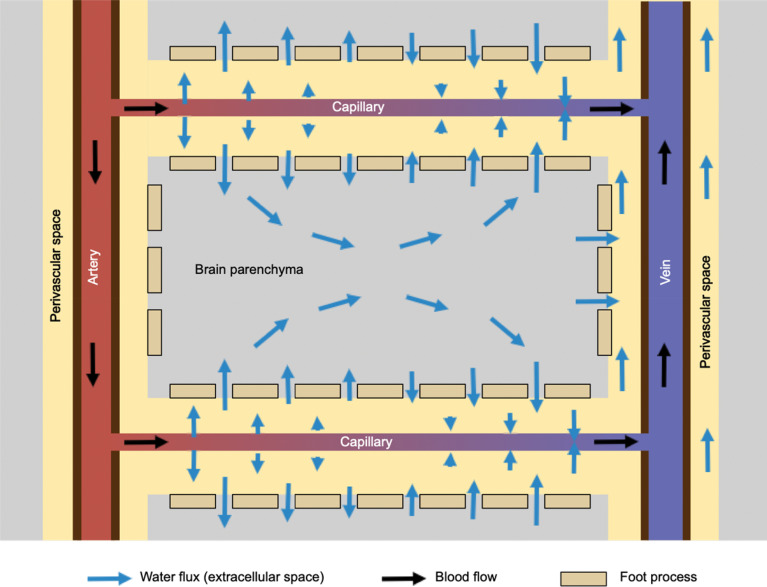
In the brain parenchyma, water molecules move according to osmotic pressure gradient in the tissue. This process is essentially the same as the diffusion process, and each water molecule moves randomly, but the overall flux of the water is directed toward the venous side of the capillary (Fig. 2).
Fig. 2.
This shows the flux of water in the interstitial space in the brain parenchyma. Water comes out of the arterial side of the capillary into perivascular space driven by the hydrostatic pressure of the blood, and then moves into the interstitial space through the gaps of astrocyte foot processes. Water is absorbed at the venous side of the capillary, where hydrostatic pressure is lower. Driving force within the brain parenchyma is primarily the osmotic pressure gradient.
Flux of the Solutes
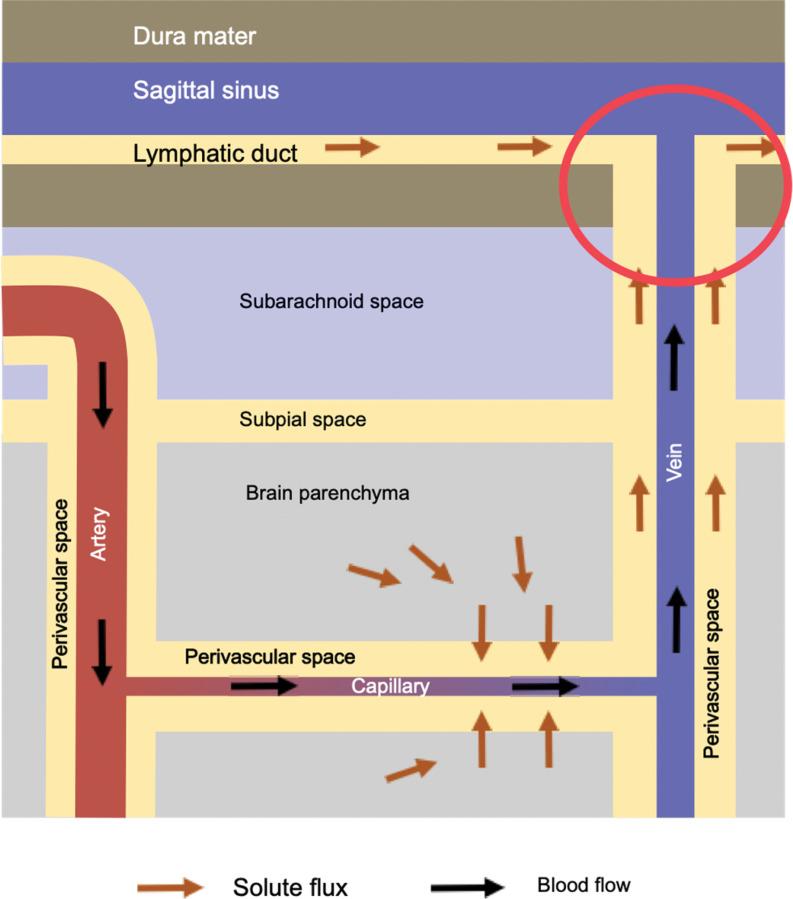
Waste products in the brain tissue are moved out of the brain by the flux of water as mentioned above. They are produced in the brain parenchyma, move toward the venous side of the capillary, and go through the gap of the astrocyte foot processes into the perivascular space. Some of the molecules, like amyloid β, are actively transported into the blood stream.3 The rest of the solutes move along the venous perivascular space. This space is likely to be connected to the dural lymphatic system by the perivascular space of the bridging veins.4 Although the connection of the perivasclular space and the dural lymphatic system (red circle in the Fig. 3) is not directly proven, there are MR images suggesting this pathway.4–7
Fig. 3.
This shows the flux of solutes. They are driven by the water flux shown in Fig. 2. Although not directly proven, the perivenous space is likely to be continuous with the dural lymphatic system (red circle).
The overall pathway of the solutes is brain parenchyma to the venous side of the capillary, through the foot process gap to the perivascular space, through the perivascular space of the vein to the dural lymphatic system (Fig. 3).
Panglial Syncytium
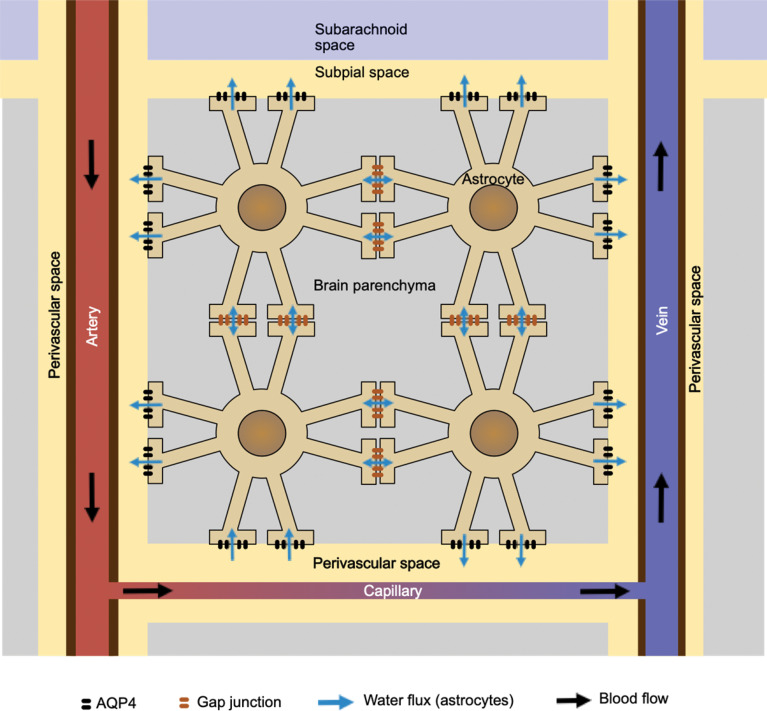
Although it is not so widely known, astrocytes are connected to each other by gap junctions at the foot processes.8 Water molecules and ions move freely across these gap junctions, making the entire astrocyte network a syncytium, as long as water and small ions are concerned. Combined with the aquaporin-4 (AQP4) at the foot processes covering the blood vessels, the astrocyte network provides water pathways connecting the perivascular space at different locations in the brain (Fig. 4).
Fig. 4.
This shows the network of astrocytes, connected to each other with gap junctions at their foot processes. Combined with the AQP4 channels at the foot processes surrounding the perivascular space, the entire network may work as a conduit to distribute water from the capillary to various parts of the perivascular space. AQP4, aquaporin-4.
Since both gap junctions and AQP4 channels are passive gateways for the water molecules, the driving force is osmotic pressure. Although, at most places, osmotic pressure is inward, overall volume of the astrocytes cannot increase, and actual water flux is likely to be driven by the osmotic differences among the locations of the AQP4 channel. The most hypotonic place is at the arterial side of the capillary, where only water comes out of the capillary. As a result, the network of astrocytes may work as a conduit to distribute water from the capillary to various locations of the perivascular space.
Relation between Proposed Hypothesis and Experimental Results in the Literature
Iliff et al. showed that fluorescent and radioactive tracers injected into the subarachnoid space rapidly enter the brain parenchyma.2 They also showed that the rate of this process depends on the molecular weight of the tracer. Based on this fact, they concluded that cerebrospinal fluid (CSF) enters the brain parenchyma. However, there is no clear driving force for this movement. As long as interstitial space is concerned, diffusion is more likely mechanism for both water and the solutes movements. The clearance of solutes from the skull into the cervical lymph nodes may depend more on convective flow as discussed in the later section.
Entry of tracers into the brain parenchyma is dependent on the AQP4 channels. This was also shown by tracer studies, but again, the exact mechanism for this is not clear. One possible explanation is that the astrocyte network maintains the perivascular space by providing water from the capillary to perivasclular space. This process depends on AQP4 channels, as well as the gap junctions among the astrocytes. Without AQP4, the perivasclular space will probably collapse. This view also supports the fact that waste clearance capability changes dynamically as seen in sleeping animals because the amount of water transport to the perivascular space can be changed by controlling the AQP4 channels.
There is a view that continuity of venous perivascular space is not well established histologically.9 However, tracers are shown to move along the veins also, as along the arteries, in vivo experiments.2 If the perivascular spaces are indeed controlled dynamically, there might be discrepancies between in vivo and histological findings.
Finally, there is an issue of diffusion vs. bulk flow. One factor that has to be considered here is the scale of the time and distance. The average displacement distance in the diffusion process is proportional to square root of time, while it is proportional to time in case of bulk flow. In other words, diffusion is relatively fast in short time and distance scale, but as time or distance increases, the relative speed of bulk flow increases. Based on the experiment by Watts et al.,5 it takes days for the contrast agent injected in the subarachnoid space to move out of the brain via the lymphatic system. Assuming that the tracer travels 5 cm in 24 hours, the moving speed is about 0.5 μm/sec, which is much slower than diffusion for the time scale of a second. For example, mannitol molecules travel about 22 um on average in a second, assuming that there is no bulk flow and the diffusion coefficient of mannitol is 5.2 × 10-6 cm2/s. On the other hand, it takes 55 days to move 5 cm by diffusion only.
There are a few points left as unknown in this hypothesis. Since the source of the water is considered to be capillaries, rather than the arterial perivascular space, the function of the arterial perivascular space is now not clear. It might work as a reserve pathway in pathological conditions. Also, in this paper, Weller’s hypothesis9 is not discussed. This hypothesis is based on pathological findings where Aβ deposition is found in the Alzheimer’s disease patients. Since this is related more to pathological conditions than to physiological conditions, it seems to be more appropriate to be discussed in more clinically oriented situations.
Conclusion
So what is the “glymphatic system”? By comparing the proposed hypothesis with the lymphatic system of other parts of the body, certain similarities can be pointed out. The perfusion by the blood is similar between the brain and other parts of the body, and the perivascular space can be considered analogous to the lymphatic system. Since the perivascular space is maintained and/or regulated by the network of the astrocytes, it seems to be natural to consider this combined system consisting of the perivascular space and the astrocyte network, the “glymphatic system”. Of course, this is a hypothesis and needs to be tested through experiments in the future.
Footnotes
Conflicts of Interest
The author has no conflict of interest regarding the topic of this paper.
References
- 1.Tarasoff-Conway JM, Carare RO, Osorio RSet al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015; 11:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iliff JJ, Wang M, Liao Yet al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012; 4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibata M, Yamada S, Kumar SRet al. Clearance of Alzheimer’s amyloid-β1-40 peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 2000; 106:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oshio K, Yui M, Shimizu Set al. The spatial distribution of water components with similar T2 may provide insight into pathways for large molecule transportation in the brain. Magn Reson Med Sci 2021; 20:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watts R, Steinklein JM, Waldman Let al. Measuring glymphatic flow in man using quantitative contrast-enhanced MRI. AJNR Am J Neuroradiol 2019; 40:648–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naganawa S, Ito R, Taoka Tet al. The space between the pial sheath and the cortical venous wall may connect to the meningeal lymphatics. Magn Reson Med Sci 2020; 19:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eide PK, Vatnehol SAS, Emblem KEet al. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep 2018; 8:7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rash JE. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience 2010; 168:982–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weller RO, Massey A, Newman TAet al. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol 1998; 153:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]