Visual Abstract
Key Words: C-C chemokine receptor type 2, extracellular matrix, inflammation, myxomatous valve disease
Abbreviations and Acronyms: Aebp1, adipocyte enhancer binding protein 1; CCR2, C-C chemokine receptor type 2; Cpa3, carboxypeptidase A3; ECM, extracellular matrix; Fbn1, fibrillin 1; Mcpt4, mast cell protease 4; MFS, Marfan syndrome; MVD, myxomatous valve disease
Highlights
-
•
C-C chemokine receptor type 2 genetic knockout reduces mitral valve inflammation and inflammatory proteases in a mouse model of Marfan syndrome.
-
•
C-C chemokine receptor type 2 antagonist RS504393 prevents macrophage infiltration and pathological extracellular matrix remodeling in mitral valves of Fbn1C1039G/+ mice.
-
•
Treatment with RS504393 restores inflammatory chymases and carboxypeptidases in Fbn1C1039G/+ mice.
-
•
Treatment with RS504393 at initiation or at a late phase of myxomatous valve disease ameliorates myxomatous valve degeneration and valve leaflet thickness in Fbn1C1039G/+ mice.
Summary
Myxomatous valve disease (MVD) can lead to cardiac dysfunction and heart failure, yet medical therapies are lacking. C-C chemokine receptor type 2 (CCR2)+ immune cell infiltration promotes mitral valve inflammation in a Marfan syndrome (MFS) mouse model. The CCR2 genetic knockout reduces inflammation with downregulated proteases and improved extracellular matrix integrity. Pharmacological inhibition of CCR2+ cell infiltration by RS504393 prevents the initiation and progression of MVD, indicated by restored protease expression, improved extracellular matrix organization, and reduced valve leaflet thickness in MFS mice. Thus, the CCR2 antagonist RS504393 is a promising therapy for the treatment of MVD in MFS.
Myxomatous valve disease (MVD) is the most common valvular heart disorder, with a prevalence of 2% to 3% of the general population and >10% of individuals >75 years of age.1,2 MVD is a common cause of mitral regurgitation, ventricular dysfunction, arrhythmia, and heart failure, contributing to cardiovascular morbidity and mortality.3 Currently, there is no medical therapy to prevent or reverse MVD other than surgical intervention for valve repair or replacement.1 The key feature of MVD is myxomatous mitral valve degeneration, characterized by valve leaflet thickening and abnormal extracellular matrix (ECM) remodeling, including elastin and collagen fiber disruption and proteoglycan accumulation.4,5 Hence, uncovering the pathological mechanisms behind these events is critical for understanding the pathogenic basis of MVD progression and developing novel therapies for MVD.
Recent work has shown that multiple immune cell populations are present in normal valves and increase in mouse, sheep, and human diseased valves with myxomatous degeneration.6, 7, 8 Notably, increased recruitment of proinflammatory macrophages occurs in myxomatous valves of genetically engineered pigs and human patients with Marfan syndrome (MFS).9 These studies support sterile inflammatory mechanisms in the myxomatous matrix remodeling of MVD. However, how these infiltrating immune cells contribute to pathological ECM remodeling in MVD and whether specific immune cells could be targeted for the treatment of MVD remain unclear.
Circulating C-C chemokine receptor type 2 (CCR2)+ monocytes are recruited to the injured heart and play proinflammatory roles that exacerbate fibrosis and dysfunction in various cardiovascular diseases.10, 11, 12, 13 In mouse models, inhibition of CCR2+ cell recruitment using different approaches, such as CCR2 gene knockout, antagonists, blocking antibodies, and small interfering RNA, has emerged to be therapeutically beneficial for cardiovascular diseases.10,14, 15, 16, 17 Our recent studies showed that the infiltrating immune cells in MVD are predominantly CCR2+ monocytes derived from bone marrow that express major histocompatibility complex class II (MHCII).9 Notably, inhibition of CCR2+ cell infiltration by CCR2 gene ablation reduces valve leaflet thickness and myxomatous valve degeneration in a mouse model of MFS with Fbn1C1039G/+ pathogenic sequence variant.9,18 Hence, pharmacological CCR2 inhibition is an emerging clinical strategy for the treatment of valve disease.
RS504393 is a highly selective CCR2 antagonist, first identified by a large chemical library screen using a high throughput radioligand binding and chemotaxis inhibition assay.19 It has subsequently been shown to have beneficial effects in multiple disease processes in mouse models, including insulin resistance, cancer, and fibrosis in scleroderma, as well as in preclinical trials for chronic obstructive pulmonary disease.20, 21, 22, 23 However, its efficacy in preventing or inhibiting the progression of MVD has not been previously shown.
Methods
Additional information on materials and methods is included in the Supplemental Appendix.
Gene expression profiling
The RNA extracted from microdissected mitral valves of 2-month-old CCR2RFP/+, Fbn1C1039G/+; CCR2RFP/+, and Fbn1C1039G/+; CCR2RFP/RFP mice was subjected to RNA sequencing (RNAseq) individually (n= 4 per genotype).
CCR2 antagonist treatment of Fbn1C1039G/+ MFS mice
The CCR2 antagonist RS504393 (2 mg/kg) was administered to either Fbn1C1039G/+ or Fbn1C1039G/+; CCR2RFP/+ mice by daily intraperitoneal injections during the initiation (1 month) or the progression (2 and 6 months) of MVD. Mice were randomized after genotyping to the experimental cohorts with males and females included at each time point. The sample size used in each experiment was estimated based on SEM measurements and our previous experiments. All mice were maintained in an unbiased fashion and no mouse was excluded from the analysis.
Statistical analysis
Representative histological and fluorescent images were selected reflecting the average level of 3 to 7 individuals. Each data point represents an independent biological sample. All parameters are presented as mean ± SEM. Normal distribution was examined using the Shapiro-Wilk test. Data were analyzed using one- or two-way analysis of variance (ANOVA) with Tukey’s post hoc test for multiple pairwise comparisons using GraphPad Prism 9 software. Mann-Whitney tests were used for comparisons of 2 groups. A value of P < 0.05 was considered statistically significant.
Results
Deficiency of CCR2 reduces immune cell infiltration and pathological ECM remodeling in Fbn1C1039G/+ mouse mitral valves
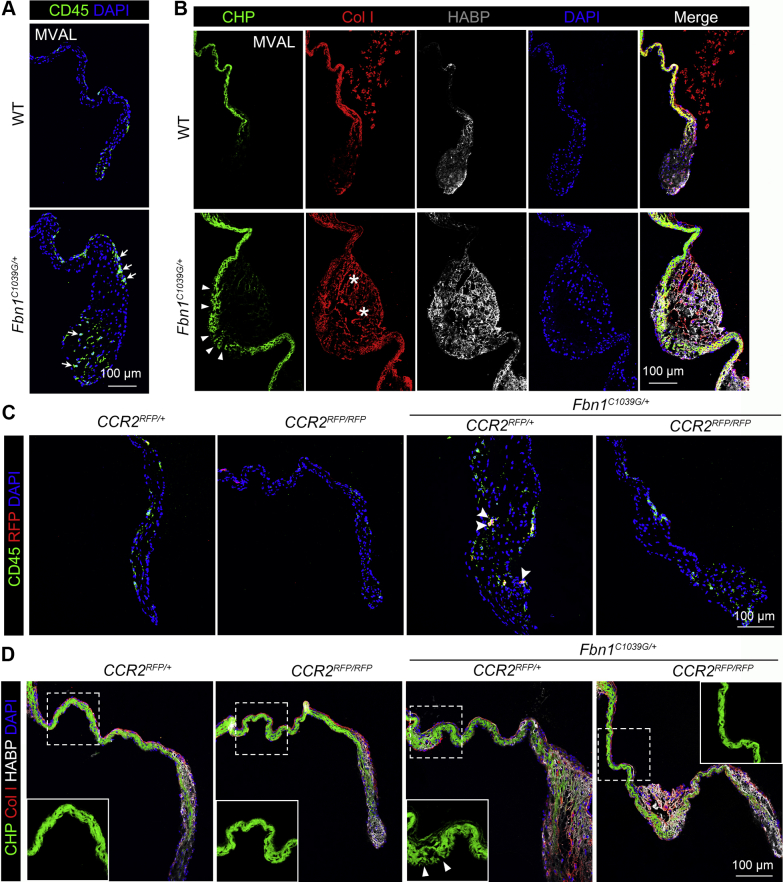
We have previously shown a significant increase of recruited macrophages derived from myeloid lineages in myxomatous mitral valves of MFS mice, pigs, and human patients, whereas genetic loss of CCR2 ameliorated myxomatous valve degeneration in a mouse model of MFS.9,18 Here, examination of pathological ECM changes in the progression of MVD in the MFS Fbn1C1039G/+ mouse model showed increased numbers of CD45+ hematopoietic cells in the mitral valve of Fbn1C1039G/+ mice at 2 months of age (Figure 1A). At the same time, pathologic ECM abnormalities were detected by immunofluorescence in the progressively diseased mitral valves in Fbn1C1039G/+ mice in comparison with wild-type (WT) controls. Increased collagen fiber breaks evident by collagen hybridizing peptide (CHP) staining (Figure 1B, green, arrowheads) indicated collagen fragmentation in MFS mouse valves. Abnormal collagen I deposition (Figure 1B, red, asterisks) was also observed in the mitral valves of Fbn1C1039G/+ mice. Furthermore, hyaluronic acid binding protein (HABP) (Figure 1B, white) deposition is increased in Fbn1C1039G/+ mitral valves, indicating proteoglycan accumulation in MFS mitral valves.
Figure 1.
CCR2 Deficiency Restores Immune Cells and ECM Organization in MFS Valves
(A) CD45+ leukocytes (arrows, green) are significantly increased in mitral valves of 2-month-old Fbn1C1039G/+ mice by immunofluorescence. (B) Immunofluorescence with collagen hybridizing peptide (CHP) (arrowheads, green), collagen I (Col I) (asterisks, red), hyaluronic acid binding protein (HABP) (white) show extracellular matrix (ECM) abnormalities in 2-month-old Fbn1C1039G/+ mice with Marfan syndrome (MFS). (C) CD45+ leukocytes (green) and red fluorescent protein (RFP+) cells (red) are prevalent in mitral valves of Fbn1C1039G/+; CCR2RFP/+ mice vs controls. Fbn1C1039G/+; CCR2RFP/RFP mice have a reduction of both CD45+ leukocytes and RFP+ cells (arrowheads, double positive cells) relative to Fbn1C1039G/+; CCR2RFP/+ controls. (D) Pathological ECM remodeling indicated by immunofluorescence with CHP (green, arrowheads indicating collagen breaks), Col I (red), and HABP (white) was improved by C-C chemokine receptor type 2 (CCR2) deficiency in Fbn1C1039G/+ mice. Nuclei were counterstained with 4’, 6-diamidino-2-phenylindole (DAPI). Scale bar = 100 μm. MVAL = mitral valve anterior leaflet; WT = wild-type.
The presence of CCR2+ cells in regions of pathologic ECM remodeling was confirmed in Fbn1C1039G/+; CCR2RFP/+ mice, and development of ECM abnormalities and mitral valve pathology was prevented in Fbn1C1039G/+; CCR2RFP/RFP (CCR2-deficient) mice (Figures 1C and 1D). The CCR2-deficient MFS mice have reduced overall hematopoietic (CD45+) cells and CCR2 RFP+ cells (Figure 1C), indicating that immune cells overall, and CCR2+ cells specifically, were reduced in mitral valves in MFS mice by CCR2 deficiency. Moreover, loss of CCR2+ immune cells preserved the ECM integrity by improving collagen fiber structure, reducing proteoglycan accumulation, and preventing leaflet thickening in MFS valves (Figure 1D). Taken together, these results show that CCR2 deficiency prevents ECM abnormalities and mitral valve pathogenesis in Fbn1C1039G/+ mice.
Transcriptomic profiling shows that CCR2 deficiency leads to reduced inflammation in MFS mitral valves
To define the molecular and cellular mechanisms underlying how loss of CCR2+ cells prevents pathologic ECM remodeling and valve disease, we performed RNAseq gene expression profiling of mitral valves from 2-month-old CCR2RFP/+ (Chet), Fbn1C1039G/+; CCR2RFP/+ (FhetChet), and Fbn1C1039G/+; CCR2RFP/RFP (FhetCko) mice. At 2 months of age, Fbn1C1039G/+ mitral valves are thickened with collagen fiber breakage, increased proteoglycans, and myeloid cell infiltration.9 Differential gene expression, defined as more than 0.3 or <-0.3 log2 fold-change (1.2 or 0.8 fold-change), was observed for a total of 1,280 genes (571 upregulated and 709 downregulated) in FhetChet diseased valves vs Chet controls (Supplemental Figure 1A). Gene ontology (GO) analysis of biological process using DAVID (Database for Annotation, Visualization, and Integrated Discovery) showed upregulated differentially expressed genes (DEGs) in FhetChet vs Chet were enriched in immune system process, inflammatory response, adaptive immune response, and cellular defense response (Supplemental Figure 1B), which indicated excessive inflammation in 2-month Fbn1C1039G/+ valves compared to controls. Specifically, the upregulated immune genes in FhetChet vs Chet were related to monocyte chemotaxis (Ccl6, Ccl8, Ccl9, Ccl17, Ccr2, Lgals3), leukocyte cell-cell adhesion (Cd24a, Itgam, Itgb2, Sele), positive regulation of cytokine secretion (Clec4n, Cd14, Cd300c2, Fgr, Il1a, Scamp5), and tumor necrosis factor production (Cd14, Ccr5, Cyba, Ptafr). The heatmap showed the upregulation of immune genes in FhetChet diseased valves compared to Chet controls (Supplemental Figure 1C). In addition, the expression of ECM genes (Col4a2, Col18a1, Postn, Aebp1, Fbln2, Hapln4) was increased in Fbn1C1039G/+ valves (Supplemental Figure 1D), indicating a significant change in ECM gene expression associated with increased inflammation in diseased Fbn1C1039G/+ mitral valves. The lists of genes related to specific GO terms are shown in Supplemental Table 1.
To determine the molecular changes of CCR2-deficient;Fbn1C1039G/+ rescued mitral valves relative to Fbn1C1039G/+ diseased valves, Fbn1C1039G/+; CCR2RFP/RFP and Fbn1C1039G/+; CCR2RFP/+ were compared. With the improved valve phenotype, 1,085 genes were differentially expressed (595 upregulated and 490 downregulated) in CCR2-deficient;Fbn1C1039G/+ (FhetCko) rescued valves vs Fbn1C1039G/+; CCR2RFP/+ (FhetChet) diseased valves (Supplemental Figure 2A). Downregulated DEGs in FhetCko vs FhetChet showed enrichment in inflammatory response (Ccl25, Ptgfr, Ciita, Pik3cd, Sele, C3, Crhbp, Il1rl2, Il1b, Kit, Ccr2, Ccr5, Cx3cl1), proteolysis (Rhbdf1, Cpxm2, Mcpt4, Aebp1, Adamts5, C1s1, Capn6, Cpne1, Cfb), and immune system process (Gbp5, Cd300a, Ifih1, Tlr12) (Supplemental Figure 2B), supporting a significant reduction of proinflammatory factors, immune cell–expressed chymases, and carboxypeptidases that regulate ECM integrity in CCR2-deficient MFS mitral valves. We have previously shown that CCR2 deficiency decreased monocyte-derived macrophage infiltration in MFS valves.9 Here, we found that not only macrophages, but also genes characteristic of other immune cell types, such as mast cells, were reduced with CCR2 deficiency in Fbn1C1039G/+ valves. Notably, expression of kit and mast cell protease 4 (mcpt4), which might exert amplifying roles of inflammation dependent on CCR2, were reduced. The lists of genes related to GO terms in this comparison are shown in Supplemental Table 2.
To further examine the gene expression changes that occur with CCR2 deficiency in rescued myxomatous valves, we compared the upregulated genes in FhetChet diseased valves vs Chet control valves with the downregulated genes in FhetCko rescued valves vs FhetChet diseased valves. The overlapping genes are shown in a Venn diagram showing 56 common genes between upregulated DEGs in FhetChet vs Chet valves and downregulated DEGs in FhetCko vs FhetChet valves (Figure 2A). The expression levels of these common genes are shown in a heatmap to visualize their expression across the groups (Figure 2B). Further GO analysis of these common DEGs exhibited enrichment in inflammatory response, immune response, chemotaxis, and regulated exocytosis, as well as chemokine receptor activity (Figure 2C, Supplemental Table 3), indicating a noticeable block of inflammation with loss of CCR2 in Fbn1C1039G/+ diseased valves. Our findings suggest that CCR2 deficiency reversed the many of the gene expression changes of Fbn1C1039G/+ valves, mainly related to inflammation rather than other pathways. Taken together, MFS valves have increased expression of proinflammatory effects and ECM remodeling genes, whereas CCR2 deficiency in these mice specifically reduces inflammatory gene expression along with normalized valve morphology. Thus, our results indicate that immune processes are the main pathogenic drivers of MVD progression.
Figure 2.
CCR2 Knockout Inhibits Inflammation in Fbn1C1039G/+ Mitral Valves
RNA sequencing (RNAseq) was performed from the mitral valves of 2-month-old Fbn1C1039G/+ mice with CCR2RFP/RFP (FhetCko) compared to CCR2RFP/+ (Chet) and Fbn1C1039G/+; CCR2RFP/+ (FhetChet) mice. (A) Venn diagram shows the common 56 differentially expressed genes between FhetChet vs Chet upregulated and FhetCko vs FhetChet downregulated genes. (B) The heatmap of the common differentially expressed genes in Chet, FhetChet, and FhetCko groups. (C) Gene ontology (GO) term analysis of the common genes by DAVID (Database for Annotation, Visualization, and Integrated Discovery). N = 4. BP = biological process; Chet = CCR2RFP/+, FhetChet = Fbn1C1039G/+; CCR2RFP/+, FhetCko = Fbn1C1039G/+; CCR2RFP/RFP; MF = molecular function; other abbreviation as in Figure 1.
CCR2 deficiency reduces inflammatory protease expression in MFS mitral valves
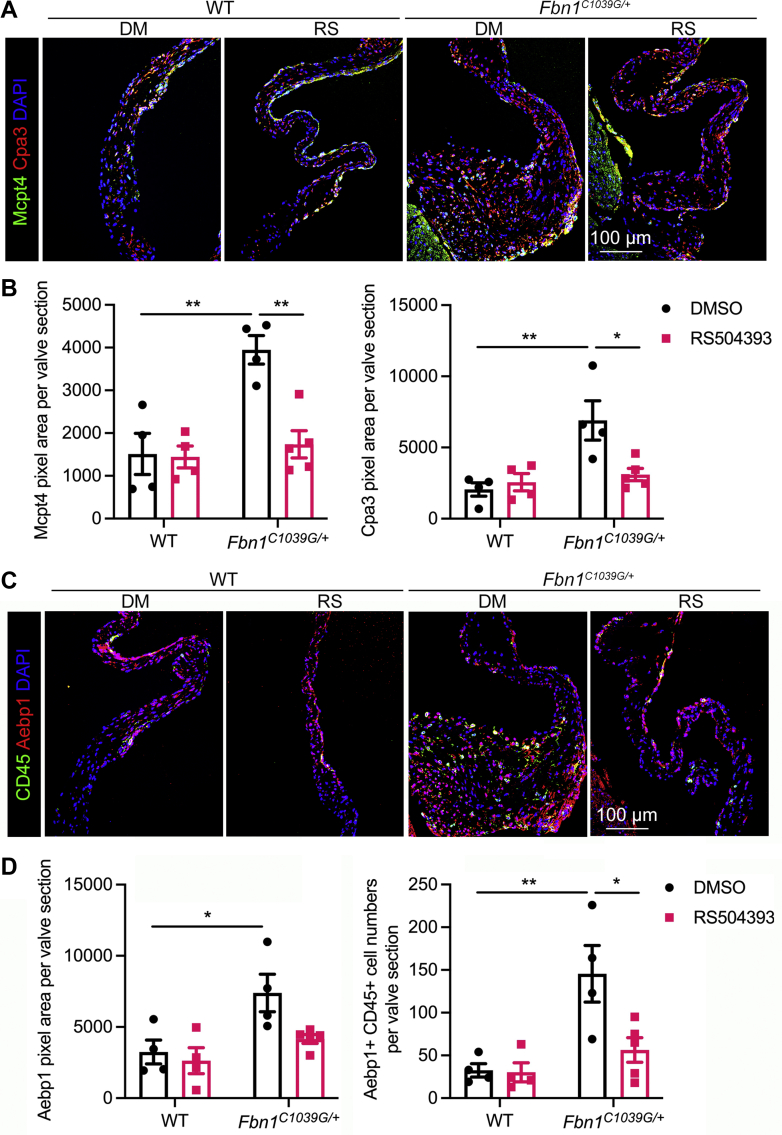
To further examine specific inflammatory changes dependent on CCR2 in Fbn1C1039G/+ valves, we selected genes related to monocyte adhesion and immune cell proteases for validation of mRNA expression by quantitative polymerase chain reaction and protein by immunofluorescence. As expected, the level of CCR2 mRNA expression was increased in Fbn1C1039G/+ valves and was among the significantly decreased genes in the CCR2-deficient mitral valves (Supplemental Figure 3). In addition, the level of C-C motif chemokine ligand 2 (ccl2) was upregulated in Fbn1C1039G/+ valves (Supplemental Figure 3). Interestingly, genes related to mast cells were also among the limited number of genes that were dependent on CCR2 in the Fbn1C1039G/+ valves (Supplemental Table 3). These include selectin E (sele), specific mast cell protease (mcpt4), and adipocyte enhancer binding protein 1 (aebp1), genes, which are upregulated in Fbn1C1039G/+ valves and downregulated with CCR2 deficiency (Supplemental Figure 3). By immunofluorescence, we found increased expression and wide distribution of Mcpt4 at the thickening tips of MFS mitral valves where proteoglycan accumulation and abnormal collagen deposition were observed, compared to controls (Figure 3A, green). Importantly, CCR2 deficiency normalized Mcpt4 expression in Fbn1C1039G/+ valves. Furthermore, Mcpt4 colocalized with expression of carboxypeptidase A3 (Cpa3) (Figure 3A, red), another mast cell–specific protease that was increased in Fbn1C1039G/+; CCR2RFP/+ mice compared to controls, suggesting that an enhancement of these proteases might exert excessive inflammation and proteolytic actions on ECM in the diseased valves. The MFS mice deficient in CCR2 had restored low levels of Mcpt4 and Cpa3 expression (Figure 3B), along with an improvement of immune microenvironment and ECM integrity. Aebp1, also called aortic carboxypeptidase-like protein, was expressed in CD45+ immune cells and other cell types in normal valves with increased expression in diseased mitral valves (Figures 3C and 3D). Moreover, the increased Aebp1+ cells at the area of proteoglycan expansion and collagen dysregulation in MFS valves were decreased by CCR2 deficiency (Figure 3C). Therefore, CCR2-dependent valve inflammation in MFS includes increased expression of mast cell–related chymases and carboxypeptidases in association with pathologic ECM remodeling in myxomatous mitral valves.
Figure 3.
Loss of CCR2 Leads to Reduced Inflammatory Protease Expression
(A) Immunofluorescence indicated increased protein expression of Mcpt4 (green) and Cpa3 (red) specifically from mast cells at the thickening area of MFS valves which was restored by CCR2 knockout. Scale bar = 100 μm. (B) Quantification of immunofluorescence showed Mcpt4 and Cpa3 pixel area was significantly decreased by CCR2 deficiency. (C) Immunofluorescence indicated that Aebp1 (red) was not only expressed in CD45+ immune cells, but was also expressed in other cell types. The increased expression of Aebp1 (red) in MFS valves was inhibited by CCR2 deficiency. The nuclei were counterstained with DAPI. Scale bar = 100 μm. (D) Quantification of immunofluorescence showed Aebp1 pixel area and cell number of Aebp1+ CD45+ immune cells were reduced by CCR2 deficiency. One-way analysis of variance (ANOVA) with Tukey’s post hoc test is used and data are represented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Aebp1 = adipocyte enhancer binding protein 1; Cko = CCR2RFP/RFP; Cpa3 = carboxypeptidase A3; Mcpt4 = mast cell protease 4; other abbreviations as in Figures 1 and 2.
Therapeutic CCR2 blockade with RS504393 reduces CCR2+ immune cell infiltration in Fbn1C1039G/+ myxomatous valves
Our findings suggest that excessive inflammation dependent on CCR2+ cell infiltration is a critical driver of MVD. Hence, to determine if pharmacologic CCR2 inhibition could be an effective therapeutic strategy for the treatment of MVD in MFS, we tested a CCR2-specific antagonist RS504393 in the Fbn1C1039G/+ mouse model (Figure 4A). First, we investigated the effects of RS504393 at the dose of 2 mg/kg/day on disease initiation and prevention by treating 1-month-old mice, when CCR2+ immune cells were not yet increased (Supplemental Figure 4), daily for 30 days and examining mitral valve ECM remodeling and initial thickening in Fbn1C1039G/+ mice at 2 months of age (Figure 4B).10 No obvious changes in behavior, body weight, and gross anatomy were observed in RS504393-treated mice; thus, the drug was well-tolerated consistent with previous reports.10,20
Figure 4.
RS504393 Treatment Decreases CD45+ Immune Cells and MHCII+ Macrophages in Fbn1C1039G/+ Valves
(A) Chemical structure of CCR2 specific inhibitor RS504393. (B) Schematic of RS504393 intraperitoneal daily injection treatment strategy in 1-month-old WT and Fbn1C1039G/+ mice for 1 month. (C) Representative images of immunofluorescence with CD45 (arrows, green) in mitral valves of WT and Fbn1C1039G/+ mice treated with dimethyl sulfoxide (DMSO) (vehicle) or RS504393. Scale bar = 100 μm. (D) Quantification of number of CD45+ cells. (E) Representative images of immunofluorescence detection of CD206+ (green) and MHCII+ (arrows, red) macrophage subpopulations in mitral valves of WT and Fbn1C1039G/+ mice treated with vehicle or RS504393. Scale bar = 100 μm. (F) Quantification of cell number of macrophage subpopulations. Two-way ANOVA with Tukey’s post hoc test is used and data are represented as mean ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001. MVD = myxomatous valve degeneration; MHCII = major histocompatibility complex class II; other abbreviations as in Figures 1, 2, and 3.
To confirm the efficiency of RS504393 treatment in preventing CCR2+ cell infiltration in MVD in mice, CCR2RFP/+ heterozygous reporter mice crossed with Fbn1C1039G/+ mice were treated starting from 1 month of age for 30 days (Supplemental Figure 5A). As expected, vehicle-treated Fbn1C1039G/+; CCR2RFP/+ mice exhibited increased CD45+ hematopoietic cells (Supplemental Figure 5B, green) and CCR2+ RFP+ cell infiltration (Supplemental Figure 5B, red) compared to CCR2RFP/+ controls (Supplemental Figure 5B). In contrast, RS504393 treatment significantly decreased the number of CD45+ leukocytes and CCR2+ RFP+ cells in MFS mice (Supplemental Figures 5C and 5D), confirming that the CCR2 antagonist RS504393 is effective in inhibiting CCR2+ immune cell infiltration in MFS mitral valves.
We then examined the effects on the number of immune cells and macrophage subpopulations in Fbn1C1039G/+ mice intraperitoneally injected with 2 mg/kg RS504393 daily for 1 month. In vehicle-treated controls, Fbn1C1039G/+ valves exhibited an increased number of hematopoietic cells, indicated by CD45 staining, whereas RS504393 treatment dramatically reduced CD45+ cells (Figures 4C and 4D). We have previously shown that the majority of CD45+ cells in the mitral valves are comprised of primarily 2 macrophage subpopulations that differentially expressed CD206 and MHCII, indicative of tissue resident and infiltrating macrophages populations. Vehicle-treated WT controls exhibited distinct localization of the 2 subpopulations, with CD206 (Figure 4E, green) on the flow side and MHCII (Figure 4E, red) at the tips of the mitral valves. Vehicle-treated Fbn1C1039G/+ valves lost the differential localization and showed increased numbers of macrophages colocalized with ECM abnormalities. Strikingly, RS504393 treatment normalized macrophage localization and reduced the numbers of MHCII+ infiltrating macrophages in MFS mice (Figures 4E and 4F). Taken together, inhibition of CCR2 by RS504393 treatment decreases immune cell numbers, including MHCII+ infiltrating macrophages, in MFS mitral valves.
RS504393 treatment prevents pathological ECM remodeling and MVD in Fbn1C1039G/+ mice
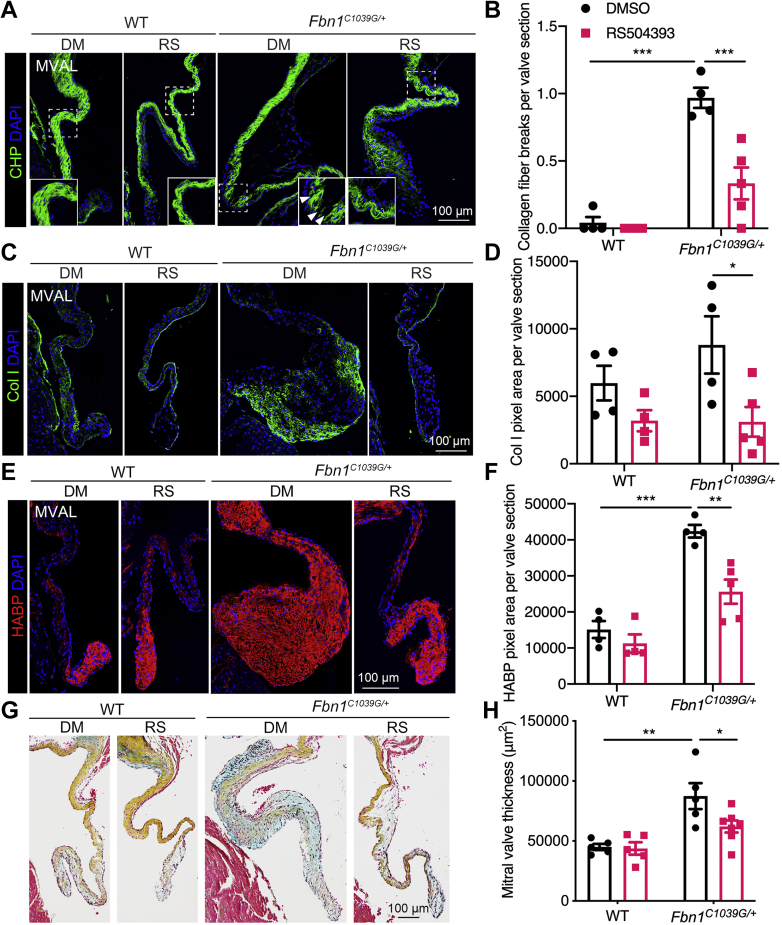
We further examined valve degeneration and characteristic myxomatous ECM abnormalities in the Fbn1C1039G/+ mitral valves by immunofluorescence and Movat pentachrome staining after RS504393 treatment. Vehicle-treated MFS mitral valves exhibited increased collagen breaks, as determined by CHP staining, indicative of increased collagen fiber disruption. In contrast, RS504393 treatment significantly inhibited collagen fragmentation in MFS mitral valves (Figures 5A and 5B). Moreover, abnormal collagen deposition in MFS valves, as determined by collagen I immunostaining, was significantly improved by RS504393 treatment (Figures 5C and 5D). In addition, the proteoglycan accumulation apparent in increased HABP deposition in MFS mitral valves was also dramatically decreased by RS504393 (Figures 5E and 5F). Likewise, ECM structural abnormalities were observed in vehicle-treated, but not RS504393-treated, Fbn1C1039G/+ valves, as determined by Movat pentachrome staining (Figure 5G). Importantly, RS504393 treatment markedly inhibited the mitral valve leaflet thickening and valve degeneration in Fbn1C1039G/+ mice (Figures 5G and 5H). Similar beneficial effects of RS504393 treatment on pathologic ECM remodeling and valve morphology were seen in Fbn1C1039G/+; CCR2RFP/+ mice (Supplemental Figures 6 and 7). Together, these findings show that CCR2 antagonist RS504393 treatment is protective against the initiation and progression of myxomatous valve disease in MFS.
Figure 5.
CCR2 Inhibition Prevents Pathological ECM Remodeling in Fbn1C1039G/+ Valves
(A) Immunofluorescence with CHP (green, arrowheads indicating collagen breaks) showed collagen remodeling in mitral valves of Fbn1C1039G/+ mice treated with DMSO (vehicle) or RS504393. Scale bar = 100 μm. (B) Quantification of collagen fiber breakdown. (C) Col I (green) immunostaining showed collagen I deposition. (D) Quantification of collagen I pixel area in mitral valves. (E) Proteoglycan accumulation is shown in vehicle-treated Fbn1C1039G/+ mice compared with control by immunofluorescence with HABP (red). RS504393 prevented increased proteoglycan accumulation in myxomatous valves. Scale bar = 100 μm. (F) Quantification of HABP pixel area. (G) Representative Movat pentachrome staining of mitral valves of Fbn1C1039G/+ mice treated with vehicle or RS504393 (collagen, yellow; proteoglycan, blue; elastin, black; muscle, red). Scale bar = 100 μm. (H) Quantification of mitral valve thickness. Two-way ANOVA with Tukey’s post hoc test is used and data are represented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figures 1, 2, 3, and 4.
CCR2 inhibition restores the expression of inflammatory proteases in MFS mitral valves
To determine if treatment with the CCR2 antagonist reduces additional indicators of valve inflammation, we examined inflammatory protease expression in RS504393-treated MFS valves. Specific proteases released from mast cells, Mcpt4 and Cpa3, were both increased in vehicle-treated Fbn1C1039G/+ valves, especially at the distal tips in regions with accumulated proteoglycan content and collagen dysregulation. In contrast, RS504393 treatment normalized Mcpt4 and Cpa3 expression and localization coincident with improved ECM integrity (Figures 6A and 6B), suggesting that the pharmacological CCR2 inhibitor recapitulates beneficial effects of CCR2 genetic ablation in myxomatous disease progression. Likewise, histological analysis revealed that mast cells were accumulated in vehicle-treated Fbn1C1039G/+ valves and that RS504393 treatment reduced mast cell numbers (Supplemental Figure 8). Aebp1 expression, increased with ECM abnormalities in vehicle-treated Fbn1C1039G/+ valves, also was normalized with RS504393 treatment (Figures 6C and 6D). Thus, CCR2 inhibition by RS504393 treatment restores the expression and localization of multiple inflammatory proteases in association with pathologic ECM remodeling during myxomatous valve degeneration.
Figure 6.
RS504393 Treatment Prevents Pathologic Induction of Inflammatory Proteases in Fbn1C1039G/+ Valves
(A) Immunofluorescence indicated protein expression of the chymase Mcpt4 (green) and carboxypeptidase Cpa3 (red) in mitral valves of Fbn1C1039G/+ mice treated with CCR2 inhibitor RS504393 compared to DMSO vehicle controls. Mcpt4 and Cpa3 specifically released from mast cells were increased at the thickened area of mitral valves of vehicle-treated Fbn1C1039G/+ mice where pathologic collagen dysregulation and proteoglycan accumulation occur. RS504393 treatment restores their levels and distribution. (B) Quantification of pixel area of Mcpt4 and Cpa3. (C) Immunofluorescence indicated increased protein levels of Aebp1 (red) in regions of pathologic ECM remodeling of Fbn1C1039G/+ valves was inhibited by RS504393 treatment. The nuclei were counterstained with DAPI. Scale bar = 100 μm. (D) Quantification of Aebp1 pixel area and number of Aebp1+ CD45+ cells. Two-way ANOVA with Tukey’s post hoc test is used and data are represented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01. Abbreviations as in Figures 1, 2, 3, and 4.
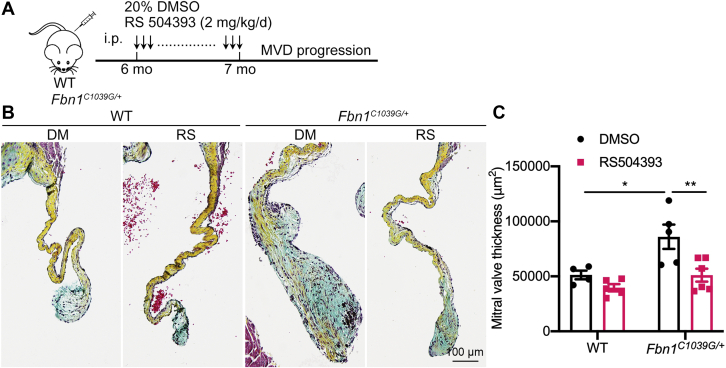
RS504393 treatment ameliorates MVD progression in aged MFS mice
Myxomatous valve features are apparent by 1 month after birth in Fbn1C1039G/+ mice, and valve leaflets become progressively dysmorphic and insufficient with age. By 1 month of age, mitral valve leaflets are thickened with macrophage infiltration and pathological ECM remodeling that is increased by 2 months.9 To determine if pharmacologic CCR2 inhibition can prevent the progression or reverse established mitral valve disease, Fbn1C1039G/+ mice were treated beginning at 6 months of age. Fbn1C1039G/+ and control mice were injected daily for 1 month starting from 6 months of age, when the Fbn1C1039G/+ mice exhibit obvious thickening, inflammation, and pathologic ECM abnormalities (Figure 7A, Supplemental Figure 9). Interestingly, RS504393 treatment daily for 1 month inhibited collagen and proteoglycan abnormalities and valve leaflet thickness in 6-month-old Fbn1C1039G/+ mice, as determined by Movat pentachrome staining (Figures 7B and 7C), indicating beneficial therapeutic effects of the CCR2 inhibitor on aged MFS mice with existing MVD. Notably, the mitral valve dimensions of RS504393-treated Fbn1C1039G/+ mice were not significantly different from age-matched WT controls suggesting that valve pathology is reversed by CCR2 blockade. Together, these studies provide initial evidence that RS504393-mediated CCR2 deficiency can reverse established mitral valve leaflet thickening and restore ECM homeostasis in MFS MVD.
Figure 7.
CCR2 Inhibition Prevents Myxomatous Valve Degeneration In 6-Month-Old MFS Mice
(A) Schematic of RS504393 intraperitoneal daily injection into 6-month-old WT and Fbn1C1039G/+ mice for 1 month. (B) Representative Movat pentachrome staining of mitral valves of WT and Fbn1C1039G/+ mice treated with vehicle or RS504393 (collagen, yellow; proteoglycan, blue; elastin, black; muscle, red). Scale bar = 100 μm. (C) Quantification of mitral valve thickness. Two-way ANOVA with Tukey’s post hoc test is used and data are represented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01. Abbreviations as in Figures 1, 2, 3, and 4.
Discussion
In this study, we have established a central pathogenic role of CCR2+ cell–dependent inflammation in ECM dysregulation in MVD of MFS and show that the pharmacological inhibition of CCR2 prevents the initiation and progression of MVD. Gene expression profiling of normal and diseased mitral valves compared to rescued valves with CCR2 deficiency showed that the main pathologic changes of MVD are related to inflammation. Notably, the increased expression of inflammatory chymases and carboxypeptidases is dependent on CCR2+ cells in the Fbn1C1039G/+ MFS mouse model and is significantly reduced by CCR2 deficiency, along with preserved ECM integrity. Moreover, treatment of Fbn1C1039G/+ mice with the CCR2-specific antagonist, RS504393, prevents the initiation and progression of MVD, resulting in decreased valve leaflet thickening and improved ECM integrity associated with downregulated inflammatory proteases in the Fbn1C1039G/+ MFS mouse model. Taken together, our results support pharmacologic antagonism of CCR2 as an effective treatment for MVD.
Increased immune cells have been detected in heart valve diseases, including calcific aortic valve disease, as well as myxomatous valve disease associated with MFS, Filamin-A knockout, Axin2 knockout, or myocardial infarction–induced insufficiency.6,7,9,24,25 These occur in a variety of species, including mice, sheep, dogs, pigs, and humans, implying common inflammatory mechanisms across species in MVD progression. Further studies indicate that the increased immune cells are predominantly derived from circulating myeloid lineages, which are CCR2+ and MHCII+, although CD206+ macrophages are also increased in myxomatous valves.9 Here, we show that CCR2+ immune cells are required for inflammatory changes and pathological ECM remodeling in the Fbn1C1039G/+ MFS mouse model. CCR2 deficiency reduces gene expression related to the immune response and also rescues ECM abnormalities and valve morphology. However, reduction of ECM and remodeling enzyme gene expression levels was not observed with CCR2 deficiency, supporting a post-transcriptional mechanism for restoration of ECM organization and valve structure. Thus, these data provide evidence for a CCR2-dependent inflammatory mechanism in the pathogenesis of MVD in MFS.
CCR2 deficiency restores the numbers and localization of macrophages and other myeloid lineages in the Fbn1C1039G/+ MFS mice. Macrophages were previously reported as the main infiltrating monocytes in the MFS valves. In this study, pharmacological inhibition of CCR2 decreased MHCII+ infiltrating macrophages and normalized the localization of CD206+ and MHCII+ cells in the mitral valves of the MFS mouse model. Additionally, mast cells with proteolytic functions also are reduced by CCR2 deficiency and have not previously been linked to MVD. The alteration of mast cell–expressed chymase Mcpt4 and carboxypeptidase Cpa3 with CCR2 deficiency is consistent with a previous report showing that loss of CCR2 prevents mast cell migration in abdominal aortic aneurism formation, suggesting that CCR2+ monocytes and mast cells contribute to ECM degradation in MVD.26 Thus, CCR2 is critical for the recruitment of diverse types of immune cells, including not only macrophages but also mast cells in MVD, providing evidence for a novel cellular mechanism in MVD progression. In addition, the observed increases in mast cell–expressed proteases and Aebp1 could directly contribute to pathological ECM remodeling in MVD.
Currently, there is no medical therapy for MVD, and efforts to translate pathophysiological mechanisms identified in animal models to effective treatments have not been entirely successful. Here, we demonstrate a potential medical therapy by CCR2 antagonist RS504393 to prevent both the early and late phases of MVD in MFS. This approach could be relevant for the treatment of human MVD arising from various causes, also characterized by induction of common inflammatory pathways and increased immune cell infiltration.
Study limitations
Although it is difficult to assess the longitudinal relationships between valve function and histological changes in leaflet structure and inflammation in humans, available evidence from postmortem valve tissue supports the presence of significant histological abnormalities before diagnosis and end-stage disease.27 Moreover, comparison of human mitral valve regurgitation detected by echocardiography with histopathology of explanted tissue after repair supports a correlation between valve leaflet thickening and the extent of dysfunction.28 In MFS, MVD often precedes the development of aortic aneurysm, and the dilated aortae in MFS mice and human patients do not exhibit increased immune cells.9 Thus, alternative therapies may be needed to treat aortic dilation characteristic of MFS. Since many CCR2 antagonists, including RS504393, have been tested pre-clinically and are currently in clinical trials, further evidence for safety and efficiency in humans is likely to be forthcoming.
Conclusions
Together, the preclinical demonstration of efficacy and ongoing testing make RS504393 a promising drug for future applications in treatment of MVD with MFS. Further laboratory and clinical studies will be needed to determine whether CCR2 antagonists have broader application to other mitral valve diseases with similar inflammatory-based pathogenesis.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Our preclinical research is focused on mitral valve disease with MVD. Myxomatous mitral valves have increased indicators of inflammation, including increased CCR2+ cell infiltration. Here, we show therapeutic benefits of CCR2 antagonism at both onset of and for well-established MVD in a mouse model of MFS. This work directly relates to the medical knowledge component of the 6 domains promulgated by the Accreditation Council on Graduate Medical Education.
TRANSLATIONAL OUTLOOK: CCR2 antagonism by RS504393 provides an attractive therapeutic approach for MFS patients with MVD. The limitations of this study include using a single CCR2 inhibitor in one mouse model of MVD with Fbn1 mutation, potentially narrowing the application of this approach to treatment of MVD, which can arise from multiple causes. Additionally, the valves in the mouse model are significantly affected histologically and morphologically before the time when valve functional changes can be detected, recapitulating early stage disease. Similarly, human heart valve disease usually develops over the course of many years, during which histological abnormalities occur much earlier than functional deficits necessitating valve replacement or repair. Challenges for clinical translation in humans include the extended time of development of mitral valve disease, often over the course of decades, and genetic heterogeneity of MFS pathogenic sequence variants in human patients. Further studies are needed to determine whether CCR2 antagonists are effective in treating human MVD with potential broader application to other mitral valve diseases.
Funding Support and Author Disclosures
This work has been supported by U.S. National Institutes of Health grant R01HL143881 (to Dr Yutzey) and American Heart Association Career Development Award 933278 (to Dr Xu).
Acknowledgments
The authors thank Hung Chi Liang at Gene Expression Core in Cincinnati Children’s Hospital Medical Center for preparing DNA libraries and DNA Sequencing and Genotyping Core for RNA sequencing; Aditi Paranjpe at Bioinformatics Collaborative Services in Cincinnati Children’s Hospital Medical Center for bioinformatics analysis; and Ronald Vagnozzi at the University of Colorado Anschutz Medical Campus for assistance with immune cell analysis.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental Methods section as well as supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Nishimura R.A., Vahanian A., Eleid M.F., Mack M.J. Mitral valve disease — current management and future challenges. Lancet. 2016;387:1324–1334. doi: 10.1016/S0140-6736(16)00558-4. [DOI] [PubMed] [Google Scholar]
- 2.Rostagno C. Heart valve disease in elderly. World J Cardiol. 2019;11:71–83. doi: 10.4330/wjc.v11.i2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morningstar J.E., Nieman A., Wang C., Beck T., Harvey A., Norris R.A. Mitral valve prolapse and its motley crew-syndromic prevalence, pathophysiology, and progression of a common heart condition. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.020919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine R.A., Hagege A.A., Judge D.P., et al. Mitral valve disease — morphology and mechanisms. Nat Rev Cardiol. 2015;12:689–710. doi: 10.1038/nrcardio.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fornes P., Heudes D., Fuzellier J.F., Tixier D., Bruneval P., Carpentier A. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: a histomorphometric study of 130 excised segments. Cardiovasc Pathol. 1999;8:81–92. doi: 10.1016/s1054-8807(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 6.Sauls K., Toomer K., Williams K., et al. Increased infiltration of extra-cardiac cells in myxomatous valve disease. J Cardiovasc Dev Dis. 2015;2:200–213. doi: 10.3390/jcdd2030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartko P.E., Dal-Bianco J.P., Guerrero J.L., et al. Effect of losartan on mitral valve changes after myocardial infarction. J Am Coll Cardiol. 2017;70:1232–1244. doi: 10.1016/j.jacc.2017.07.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geirsson A., Singh M., Ali R., et al. Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation. 2012;126:S189–S197. doi: 10.1161/CIRCULATIONAHA.111.082610. [DOI] [PubMed] [Google Scholar]
- 9.Kim A.J., Xu N., Umeyama K., et al. Deficiency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in Marfan syndrome. Circulation. 2020;141:132–146. doi: 10.1161/CIRCULATIONAHA.119.042391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel B., Bansal S.S., Ismahil M.A., et al. CCR2(+) monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. J Am Coll Cardiol Basic Trans Science. 2018;3:230–244. doi: 10.1016/j.jacbts.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y., Mouton A.J., Lindsey M.L. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2018;191:15–28. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heo G.S., Kopecky B., Sultan D., et al. Molecular imaging visualizes recruitment of inflammatory monocytes and macrophages to the injured heart. Circ Res. 2019;124:881–890. doi: 10.1161/CIRCRESAHA.118.314030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao X., Shen Y., Zhang R., et al. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci U S A. 2018;115:E4661–E4669. doi: 10.1073/pnas.1720065115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuschner F., Courties G., Dutta P., et al. Silencing of CCR2 in myocarditis. Eur Heart J. 2015;36:1478–1488. doi: 10.1093/eurheartj/ehu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajpai G., Bredemeyer A., Li W., et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019;124:263–278. doi: 10.1161/CIRCRESAHA.118.314028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majmudar M.D., Keliher E.J., Heidt T., et al. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation. 2013;127:2038–2046. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leuschner F., Dutta P., Gorbatov R., et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judge D.P., Biery N.J., Keene D.R., et al. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114:172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirzadegan T., Diehl F., Ebi B., et al. Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle. J Biol Chem. 2000;275:25562–25571. doi: 10.1074/jbc.M000692200. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y.S., Lee M.H., Song H.K., et al. CCR2 antagonism improves insulin resistance, lipid metabolism, and diabetic nephropathy in type 2 diabetic mice. Kidney Int. 2010;78:883–894. doi: 10.1038/ki.2010.263. [DOI] [PubMed] [Google Scholar]
- 21.Mu X.Y., Wang R.J., Yao Z.X., et al. 504393 inhibits M-MDSCs recruiting in immune microenvironment of bladder cancer after gemcitabine treatment. Mol Immunol. 2019;109:140–148. doi: 10.1016/j.molimm.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa M., Yamamoto T. Antifibrogenic effects of C-C chemokine receptor type 2 antagonist in a bleomycin-induced scleroderma model. Exp Dermatol. 2021;30:179–184. doi: 10.1111/exd.14088. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y., Zhang Y., Lian X., et al. Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022;50:D1398–D1407. doi: 10.1093/nar/gkab953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartoli-Leonard F., Zimmer J., Aikawa E. Innate and adaptive immunity: the understudied driving force of heart valve disease. Cardiovasc Res. 2021;117:2506–2524. doi: 10.1093/cvr/cvab273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hulin A., Moore V., James J.M., Yutzey K.E. Loss of Axin2 results in impaired heart valve maturation and subsequent myxomatous valve disease. Cardiovasc Res. 2017;113:40–51. doi: 10.1093/cvr/cvw229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J., Chen H., Liu L., et al. Chemokine (C-C motif) receptor 2 mediates mast cell migration to abdominal aortic aneurysm lesions in mice. Cardiovasc Res. 2012;96:543–551. doi: 10.1093/cvr/cvs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Stallons M.V., Tretter J.T., Hassel K., et al. Calcification and extracellular matrix dysregulation in human postmortem and surgical aortic valves. Heart. 2019;105:1616–1621. doi: 10.1136/heartjnl-2019-314879. [DOI] [PubMed] [Google Scholar]
- 28.Avila-Vanzzini N., Lancellotti P., Fernandez Calix L.A., et al. Histopathological maladaptive changes in the explanted human mitral leaflets correlate with changes in echocardiographic leaflet morphology and the severity of ischemic mitral regurgitation. Eur Heart J Cardiovasc Imaging. Published online March 24, 2022 doi: 10.1093/ehjci/jeac050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.