Depression is the most common neuropsychiatric disorder, affecting 350 million people worldwide; it has been a leading cause of suicide, causing serious harm to family members and consuming a large amount of social resources. The onset time of traditional antidepressants is usually 3–4 weeks, and there is no ameliorating effect in nearly one-third of patients with depression. Therefore, the discovery of fast and effective antidepressants has become an international research hotspot [1]. Currently, there are two pathways for developing novel and more desirable antidepressants. First, the most common strategy is to identify new therapeutic targets by uncovering the underlying mechanisms of depression, resilience to stress, and drugs with ideal anti-depressive but serious side-effects. Secondly, retrofitting existing substances with antidepressant potential is also an important tactic. The natural hallucinogen psilocybin extracted from mushrooms has been reported to have a fast and lasting therapeutic effect on treatment-resistant depression and major depressive disorder in clinical studies [2, 3]. Moreover, it has been suggested that increased spine density at the cellular level and improved brain network integration at the organismal level may underlie the therapeutic effect [4, 5]. Based on this, psychedelic drugs seem to have promise for healing depression. However, their hallucinogenic effects mean that they act as a double-edged sword, which limits their wide application. So, it would be a significant achievement if their hallucinogenic effects could be eliminated while their anti-depressive effects are maintained.
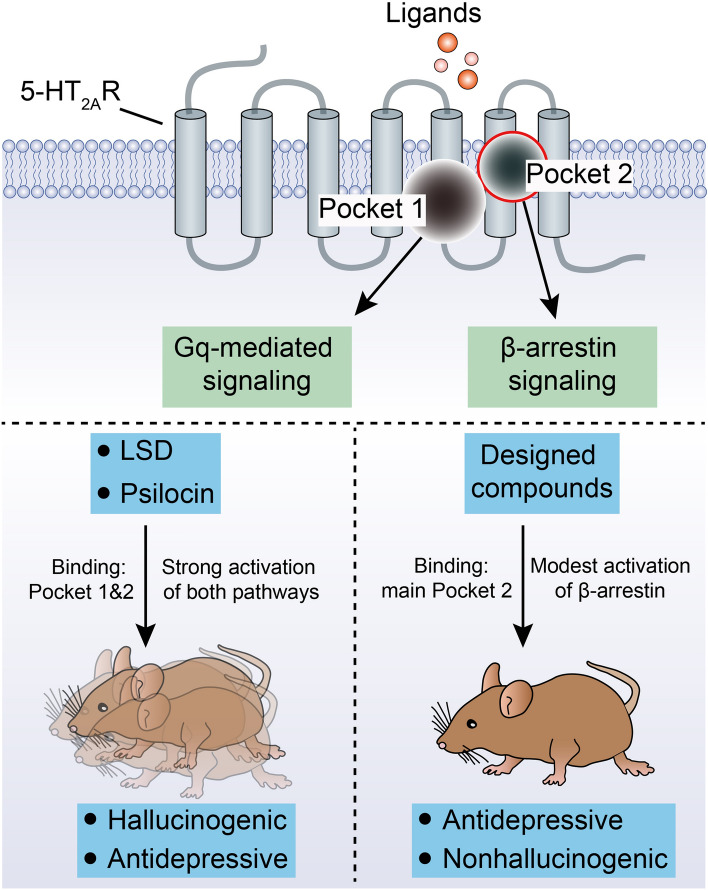
Classic hallucinogens, such as psilocybin and D-lysergic acid diethylamide (LSD) mainly bind to the 5-hydroxytryptamine 2A receptor (5-HT2AR) [6], a G protein coupled receptor. Even with the 5-HT2AR structure in hand [7], and two recent studies reporting non-hallucinogenic psychedelic analogs with antidepressant effects in rodents [6, 8], it remains unclear how to reasonably design such compounds. Besides, whether the hallucinogenic effect is necessary for the anti-depressive effect needs further exploration. Gratifyingly, scientists from China recently explained the possible mechanisms through which hallucinogens generate anti-depressive effects by structural biology, and designed some small molecule drugs that retain antidepressant effects, but without hallucinogenic effects (Fig. 1) [9]. First, the researchers purified 5-HT2ARs, then added the hallucinogenic drugs psilocin (the active metabolite of psilocybin) and LSD, as well as the non-hallucinogenic psychedelic analogs lisuride and serotonin separately. Subsequently, relatively high-resolution density maps of different ligand-receptor complexes were obtained through X-ray crystallography. By comparing the conformations of four different substance-bound 5-HT2AR complexes, the researchers found two binding pockets inside the 5-HT2AR, named the orthosteric binding pocket (OBP) and the extended binding pocket (EBP), which can accommodate and bind to ligands. The hallucinogen LSD can occupy both pockets at the same time, while the hallucinogen psilocin is smaller and can bind to either OBP or EBP. For the two drugs that do not produce hallucinations, although the binding modes of lisuride and serotonin are similar to those of LSD and psilocybin, respectively, both molecules bind EBP less strongly. Functional study showed that the binding of OBP activates the G protein signaling pathway whereas the binding of EBP activates the β-arrestin pathway. The hallucinogenic effect of the drugs may be achieved through the simultaneous strong activation of both signaling pathways. The second binding mode that serotonin and psilocin can be accommodated at the EBP of 5-HT2AR is newly discovered and non-classical. Therefore, designing ligands that bypass the OBP and target the EBP may identify 5-HT2AR ligands that specifically activate the β-arrestin signaling pathway, and these EBP-targeting molecules may not have hallucinogenic effects.
Fig. 1.
Mechanisms of action of hallucinogens and designed compounds. 5-HT2AR is a G protein coupled receptor, which possesses seven transmembrane helices. Inside the receptor, an orthosteric binding pocket (OBP) and an extended binding pocket (EBP) have been identified (Pocket 1 refers to OBP and Pocket 2 refers to EBP), which binds with hallucinogenic drugs psilocin and LSD, as well as the non-hallucinogenic psychedelic analogs lisuride and serotonin. The binding to OBP triggers Gq-mediated signaling while binding to EBP induces activation of the β-arrestin pathway. The hallucinogens LSD and psilocin bind to both the OBP and the EBP, and this leads to strong activation of both the G-protein and the β-arrestin pathway and produces hallucinatory and anti-depressive behaviors in mice. The compounds designed based on the structure of the EBP mainly bind to EBPs and prefer to activate the β-arrestin pathway. Meanwhile, these compounds show significant anti-depressive effects, but do not cause hallucinations [9].
Based on the structural characteristics of the EBP in the 5-HT2AR, the researchers designed a molecule named IHCH-7086 that mainly binds to the EBP and avoids binding to the OBP. By analyzing the crystal structure of the IHCH-7086-bound 5-HT2AR complex, it was found that the binding site of IHCH-7086 in the 5-HT2AR is consistent with the assumption, and there is a significant preference for β-arrestin signaling. Subsequently, with the help of a magnetometer-based detection system, the researchers examined the effects of IHCH-7086 and IHCH-7079 (analogues of IHCH-7086) on the head twitch response (HTR) in mice, which aims to evaluate the hallucinogenic effects of the designed small molecules, and found that even high doses of IHCH-7086 and IHCH-7079 did not induce HTR in mice. Finally, the researchers found that both IHCH-7086 and IHCH-7079 significantly improve depression-like behavior in mice subjected to acute restraint stress or corticosterone injection, and an antagonist of 5-HT2ARs (MDL100907) blocks this improvement. Overall, the specific and modest 5-HT2AR arrestin-biased activation may be sufficient for antidepressant effects, but not for hallucinogenic action.
This work not only illuminates the molecular mechanisms underlying the anti-depressive effects of hallucinogens in depth, but also provides important theoretical guidance into the development of fast- and long-acting antidepressants. However, there are some interesting issues worth discussing. First of all, chronic social defeat stress and chronic mild stress are more classical and widely-used rodent models of depression [10, 11]. Beyond this, the forced swim test alone, which is similar to the tail suspension test, is gradually being recognized as a paradigm for evaluating the stress-coping response, not depression-like behavior [12]. Therefore, drawing support from widely-used rodent models of depression and evaluating multiple indicators of depression, such as anhedonia and social avoidance will make this significant work more persuasive for the treatment of depression. Second, hallucinations in humans are often defined as high-confidence false visual or auditory percepts, and visual and auditory hallucinations have been reported in mice [13, 14], so if subsequent studies of designed psychedelic analogs include the detection of sensory-related hallucinations, they will increase the awareness of novel designed drugs. Third, this study demonstrated that strong activation of both the G protein signaling pathway and the β-arrestin pathway have hallucinogenic and antidepressant effects, while modest β-arrestin pathway activation only has an anti-depressive effect. Therefore, is there a regulatory mechanism specific to hallucinogenic effects? Also, the effect of activation of the G protein pathway alone on hallucinogenic responses and depressive-like behaviors in mice is worth exploring.
Recently, it has been suggested that this study provides a roadmap for eliminating hallucinations in healing through a mind-shift approach [15, 16]. Furthermore, this study provides a good example for the development of new medications for mental illness through a strategy to circumvent side-effects. Ketamine, an NMDAR antagonist, has gained much attention due to its rapid and long-acting antidepressant effects. However, because of the abuse potential and side-effects such as hallucinations [17], its widespread use is limited, especially for the treatment of depression. Besides, in a phase III trial, assisted therapy with 3,4-methylenedioxymethamphetamine (MDMA) significantly improved the symptoms of patients with severe post-traumatic stress disorder. Just like ketamine, MDMA is also an illegal drug due to its strong hallucinogenic effects and other side-effects. Therefore, analog molecules may be designed based on the detailed structural features of the target sites, which confer therapeutic properties without side-effects. Consequently, structure-based non-hallucinogenic psychedelic analogs may be promising medications for the treatment of neuropsychiatric diseases.
Acknowledgements
This Research Highlight was supported by grants from the National Natural Science Foundation of China (82090032 and 31830033), the Key Area Research and Development Program of Guangdong Province (2018B030334001 and 2018B030340001), Guangdong Basic and Applied Basic Research Foundation (2020A1515110565), and the Science and Technology Program of Guangzhou (202007030013).
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Li Z, Ruan M, Chen J, Fang Y. Major depressive disorder: Advances in neuroscience research and translational applications. Neurosci Bull. 2021;37:863–880. doi: 10.1007/s12264-021-00638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carhart-Harris RL, Bolstridge M, Rucker J, Day CMJ, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry. 2016;3:619–627. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- 3.Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry. 2021;78:481–489. doi: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28:844–851. doi: 10.1038/s41591-022-01744-z. [DOI] [PubMed] [Google Scholar]
- 5.Shao LX, Liao C, Gregg I, Davoudian PA, Savalia NK, Delagarza K, et al. Psilocybin induces rapid and persistent growth of dendritic spines in frontal cortex in vivo. Neuron. 2021;109:2535–2544.e4. doi: 10.1016/j.neuron.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, et al. Psychedelic-inspired drug discovery using an engineered biosensor. Cell. 2021;184:2779–2792.e18. doi: 10.1016/j.cell.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Che T, Panova O, DiBerto JF, Lyu J, Krumm BE, et al. Structure of a hallucinogen-activated gq-coupled 5-HT 2A serotonin receptor. Cell. 2020;182:1574–1588.e19. doi: 10.1016/j.cell.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. 2021;589:474–479. doi: 10.1038/s41586-020-3008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao D, Yu J, Wang H, Luo Z, Liu X, He L, et al. Structure-based discovery of nonhallucinogenic psychedelic analogs. Science. 2022;375:403–411. doi: 10.1126/science.abl8615. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chem Neurosci. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmack K, Bosc M, Ott T, Sturgill JF, Kepecs A. Striatal dopamine mediates hallucination-like perception in mice. Science. 2021;372:eabf4740. doi: 10.1126/science.abf4740. [DOI] [PubMed] [Google Scholar]
- 14.Marshel JH, Kim YS, Machado TA, Quirin S, Benson B, Kadmon J, et al. Cortical layer-specific critical dynamics triggering perception. Science. 2019;365:eaaw5202. doi: 10.1126/science.aaw5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Service RF. Psychedelics without hallucinations? Science. 2022;375:370. doi: 10.1126/science.ada0540. [DOI] [PubMed] [Google Scholar]
- 16.Osman S. A mind-changing approach to the therapeutic use of psychedelics. Nat Struct Mol Biol. 2022;29:189. doi: 10.1038/s41594-022-00753-3. [DOI] [PubMed] [Google Scholar]
- 17.Morgan CJA, Curran HV, Drugs ISCO. Ketamine use: A review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]