Abstract
Major advances have been made over the past few decades in identifying and managing disorders of consciousness (DOC) in patients with acquired brain injury (ABI), bringing the transformation from a conceptualized definition to a complex clinical scenario worthy of scientific exploration. Given the continuously-evolving framework of precision medicine that integrates valuable behavioral assessment tools, sophisticated neuroimaging, and electrophysiological techniques, a considerably higher diagnostic accuracy rate of DOC may now be reached. During the treatment of patients with DOC, a variety of intervention methods are available, including amantadine and transcranial direct current stimulation, which have both provided class II evidence, zolpidem, which is also of high quality, and non-invasive stimulation, which appears to be more encouraging than pharmacological therapy. However, heterogeneity is profoundly ingrained in study designs, and only rare schemes have been recommended by authoritative institutions. There is still a lack of an effective clinical protocol for managing patients with DOC following ABI. To advance future clinical studies on DOC, we present a comprehensive review of the progress in clinical identification and management as well as some challenges in the pathophysiology of DOC. We propose a preliminary clinical decision protocol, which could serve as an ideal reference tool for many medical institutions.
Keywords: Clinical decision, Disorders of consciousness, Acquired brain injury, Identification, Management
Introduction
Disorders of consciousness (DOC) can develop after acquired brain injury (ABI), consisting of coma, unresponsive wakefulness syndrome (previously known as the vegetative state) (VS/UWS), and minimally conscious state (MCS) [1]. Such a clinical setting deserves adequate attention because misdiagnosis/missed diagnosis might lead to incorrect medical decisions, which can further result in expensive healthcare and societal expenses, or even ethical concerns [2]. Many studies have been conducted to decode the neurophysiological mechanism of consciousness, such as understanding the neural circuits and transmitters, and establishing the evaluation indicators. To decode the neuroradiological signals in patients with DOC, a multitude of evaluation tools such as positron emission tomography (PET), structural or functional magnetic resonance imaging (MRI), and electroencephalography (EEG) are available [3]. These achievements have fueled a shift from direct observation to objective evaluation and have even enabled dynamic evaluation in a temporal continuum. Current advances in understanding the etiology of DOC have restored our hope for meaningful recovery in some “abandoned” patients. A sizable fraction of patients with DOC has benefited from the premise that diagnostic accuracy is improving.
With the advancement of this issue, some therapeutic methods for increasing consciousness recovery in patients with DOC became available. Nowadays, clinical management using invasive or noninvasive approaches is available, albeit there are no guidelines or regulations on how to do so, either in the acute or in the subacute-to-chronic periods of DOC (for classification criteria, see below) [4]. Unfortunately, certain potential methods, such as spinal cord stimulation (SCS), brain-computer interfaces (BCIs), and other physical therapy paradigms, are still in the clinical trial phase and hence are not supported by the official consensus [5–7]. However, evidence-based clinical studies are now shifting from retrospective data analyses to prospective randomized controlled trials (RCTs), and interdisciplinary or multi-modality, even multi-scale data are providing hope for the depth profiling of consciousness phenotypes [8]. As always, clinicians are now confronted with issues such as dynamic changes in temporal and spatial scale indicators, varied patterns of precision management, and time-variant brain activity in ABI patients, as well as regional heterogeneities. Nonetheless, no unified clinical decision-making methodology is widely used around the world.
The purpose of this review is to provide an overview of the advances in pathogenesis, diagnostic and therapeutic options, and prospects for recent research in patients with DOC. We highlight the important concerns that have perplexed neurologists and neurorehabilitation specialists, as well as the remaining hurdles in neuroimaging and electrophysiology. We provide a preliminary experience-based protocol that could be adapted in most tertiary medical institutions to identify and manage DOC in patients with ABI.
Pathophysiology
Brain Regions
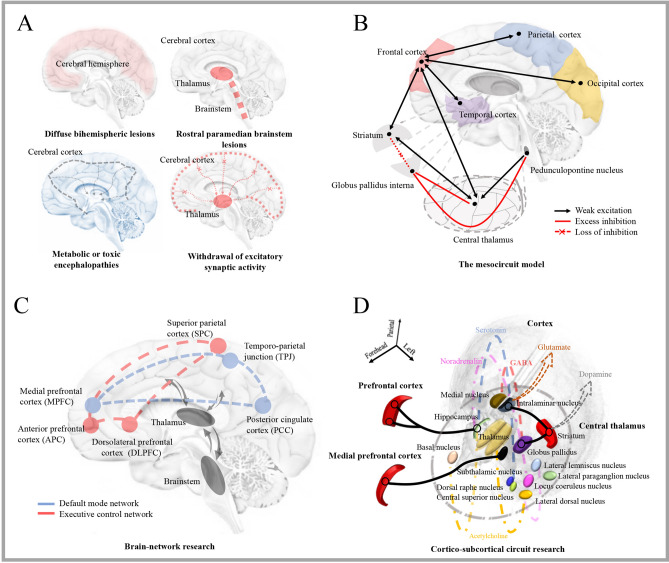
Global injuries, such as diffuse hemispheric lesions, bilateral brainstem lesions or bilateral diencephalopathy with brainstem involvement, metabolic or toxic encephalopathies that cause widespread connection dysfunction, and focal brain injuries that lead to widespread brain dysfunction between the corticothalamic system and basal ganglia and limbic system are the widely used descriptions of DOC pathophysiology in ABI patients (Fig. 1A). These result in the direct loss of neural structure, as well as a decrease in input to neocortical and thalamic neurons and a reduction in neuronal firing rates. The frequency with which this occurs indicates that the withdrawal of excitatory neurotransmission results in a passive hyperpolarizing phenomenon—the dominance of K+ leakage currents in the neuronal membrane potential. This phenomenon is also known as “disfacilitation”. Significantly, the “ABCD” model (“A” represents the EEG power spectrum which is restricted to <1 Hz, “B” represents the spontaneously generated bursting for seconds at a rate of ~5–9 Hz, “C” represents the partial restoration of neocortical membrane potentials and coincident bursting of deafferented thalamic neurons, and “D” represents the restoration of the normal EEG power spectrum with a peak in the alpha frequency range of ~8–13 Hz) was presented to characterize the degree of thalamocortical disfacilitation (see reference [9] for details) at the consciousness level.
Fig. 1.
Perspective of the pathophysiological interpretation of DOC. Although the mechanism of DOC after ABI is not fully understood, the interpretation has transformed from a single brain region to a brain network, from a macro or micro perspective to a comprehensive perspective. A The traditional explanation is largely based on the location of lesions in brain regions. From the cerebral hemisphere injury to the brainstem arousal system, and the reduction of excitation between the cortical and subcortical structures. B The mesocircuit model presents a common mechanism which is the down-regulation of the anterior forebrain mesocircuit. A reduction of thalamocortical and thalamostriatal outflow is caused by the withdrawal of afferent drive to the medium spiny neurons of the striatum, and the discharge threshold cannot be reached. Then the loss of active inhibition from the striatum to the globus pallidus internus, which provides active inhibition to their synaptic targets. C Brain network theory holds that the functional activation of a set of areas within the default mode network (core network) and the executive control network (high-level network) is necessary for maintaining consciousness. D The cortico-subcortical circuits (from the thalamus to the cortex or the striatum) and several neurotransmitters released by the thalamic nucleus are associated with DOC, which provides a new viewpoint of the pathophysiology of DOC.
The Mesocircuit Model
To account for state transitions across the DOC continuum, the microcircuit model (Fig. 1B) has been proposed [1]. Anterior forebrain function is significantly down-regulated in this model as a result of widespread disconnection or neurotmesis, and the central thalamus plays an important role as progressive deafferentation is directly proportional to the level of anatomical damage [10]. This model provides a conceptual framework for similar shift patterns of activation from the frontal cortex to the striatum in diverse stages of consciousness, such as general anesthesia, wake-sleep, and DOC in ABI patients [11]. In this model [12], the level of consciousness varies with some regular patterns, and it can be used to unify the pathological evaluation of treatment effects: First, down-regulation of anterior forebrain activity is inconsistent with the etiological spectrum of zolpidem-responsive patients [1]. Second, the reaction to drugs and stimulations is associated with the activation of frontocentral or central thalamic areas, which is frequently seen in the return of consciousness in ABI patients [9]. Third, this model has a shared final pathway that supports the production and regulation of awareness. Unfortunately, determining ways to link brain-wide central thalamic status to network-level mechanisms remains difficult.
Brain Network
The frontoparietal control system (Fig. 1C) includes two sub-networks, the default mode network (DMN) and the executive control network (ECN), which can be utilized to decode the mechanism of consciousness [13]. The original investigation found that the integrity of functional connectivity within the DMN is inversely linked with various levels of consciousness impairment [14]. A subsequent study found that the structural architecture of the DMN is proportional to the severity of DOC [15]. Another multimodal study has revealed that the metabolic integrity of the DMN performs better than other brain networks in decoding the pathogenesis of DOC [16]. The essential regions of the DMN, particularly the posterior cingulate cortex and precuneus, have been found to be substantially linked with the state of consciousness [17, 18]. As a result, the destruction of DMN structure or function can be regarded as a DOC biomarker. In DOC patients, recent research has revealed that DMN alterations, mostly driven by the precuneus and inferior parietal cortex, are due to diminished inhibition of the striatum and are accompanied by decreased coupling from the striatum to the thalamus [19]. Nonetheless, relying primarily on the DMN in the origin of consciousness is analogous to seeing only one area at the total brain voxel level. Recently, independent component analysis permitted the detection of ECN information, in which the breakdown of functional connectivity, dynamic changes of connections, and structural destruction have been found to be associated with consciousness. Further network interaction studies have revealed that different consciousness settings are associated with decreased connectivity between the ECN and the DMN, with a dual-task paradigm playing an essential role [20]. Therefore, the evolution of consciousness can be regarded as the re-emergence of the connectivity of functional networks or rising from a comatose, VS/UWS, or MCS condition to a normal condition.
Meanwhile, white matter fiber analyses have revealed that cortico-cortical and sub-cortico-cortical structures are physically destroyed in DOC patients, while subcortical functional connections are intact. Subsequent studies suggest that the structural damage of the basal ganglia, thalamus, and frontal cortex [21] is significantly associated with consciousness problems [21]. A meta-analysis study found that the corpus callosum (splenium and body) is a more reliable region of interest (ROI) for quantitative evaluation in the reflection of consciousness [22]. Following that, it was discovered that the precuneus (DMN core node) has the strongest structural connectivity with the thalamus and that differences between these regions represent dynamic variations in consciousness [23]. A recent DMN dissection investigation suggests that a fiber tract located near the inferior arm of the cingulum and connecting to the precuneus and medial temporal lobe plays a significant role in the phenotypes of consciousness [24]. These findings show that when it comes to understanding the pathophysiology of DOC, the functional and structural networks are not only indispensable but also complementary.
Cortico-Subcortical Circuits
Consciousness can be understood in three processes (Fig. 1D): (1) cortico-subcortical coupling, (2) pathway of cortical stimulation governed by the central thalamus, and (3) stimulation of the thalamic nucleus by cortical activity. Although the role of deep nuclei in the origin, changes, and even quantification of brain activity in consciousness processing has not been fully investigated [25], several neural circuits that are thought to have a pathophysiological impact on DOC have been identified: (1) activation of the thalamus, activation of the ascending reticular activating system, and re-establishment of the cortico-striatal-thalamic-cortical loop; (2) promotion of inhibitory connection between the DMN and external networks by activation of the salience network (SN); (3) increase in activity and connectivity within the brain network via the norepinephrine pathway; and (4) increase in activity within the DMN via the serotonin pathway. Meanwhile, some notable markers [e.g., lactate dehydrogenase levels in the cerebrospinal fluid reveal structural and non-structural defects [26], the opposite effects of dopamine and serotonin on functional networks [27], and the serum tau [28] and neurofilament light chain (NFL) levels reflect structural damage [29]] are in progress to reveal stronger clues in the pathophysiological decoding of altered consciousness. As an old Chinese proverb goes: “One tree does not make a forest”, and DOC pathogenesis can now be deciphered on a scale of separately but tightly-integrated levels of a hierarchy ranging from the micro-scale of a gene, protein, synapse, and neuron to the macro-scale of a neural circuit, brain area, and transmission pathway.
Identification
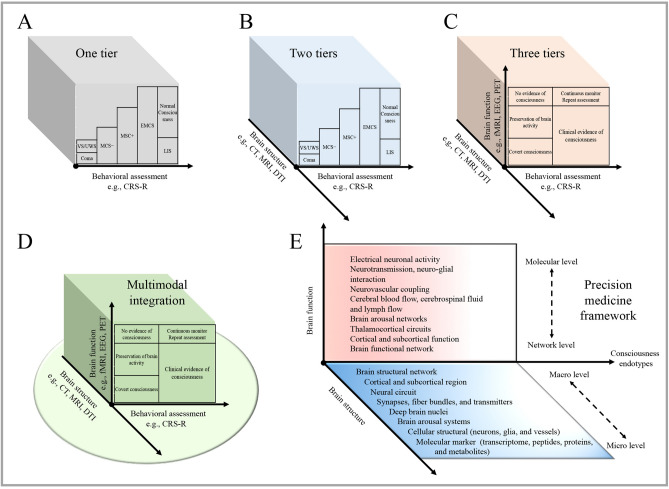
A novel framework for classifying consciousness was proposed by the Neurocritical Care Society’s Curing Coma Campaign in 2010 [30]. Such a tiered multidimensional framework reflects the precision of the current identification process by integrating modern approaches of phenomenology. Each subdirectory of this framework is discussed below (Fig. 2).
Fig. 2.
Advances in identification methods. A The traditional behavioral assessment tools (e.g., CRS-R scales) enable the clinical classification of DOC patients into Coma, VS/UWS, MCS−, MCS+, and EMCS, in which the EMCS, ILS, and normal consciousness can be differentiated by using the traditional assessment protocol. B Taking brain structural neuroimages (e.g., CT, MRI, and DTI) and clinical examination into consideration, assessments were designed to meet the possibilities in low-resource settings, which can increase the accuracy and effectiveness. C Adding the assessment of brain function (e.g., fMRI and EEG), and allowing detection of covert consciousness in unresponsive patients. In patients with no behavioral evidence, fMRI or EEG evidence of command-following indicate covert consciousness, and fMRI or EEG responses within an association cortex during passive stimuli indicate covert cortical processing. D A multimodal approach was proposed to optimize the identification accuracy of DOC, which can help to further screen out the preservation of brain activity. E Future challenges are applying the reasonable integration and specification of all relevant biological and physiological data and allowing clinicians to follow the change of consciousness endotypes over time. CRS-R, Coma Recovery Scale-Revised; CT, computed tomography; DTI, diffusion tensor imaging; EEG, electroencephalography; EMCS, emerged from the MCS; LIS, locked-in syndrome; MCS, minimally conscious state; MRI, magnetic resonance imaging; PET, positron emission tomography; UWS, unresponsive wakefulness syndrome; VS, vegetative state.
Behavioral Assessment
The American Congress of Rehabilitation Medicine conducted a systematic review of behavioral assessment scales for DOC in early 2010 [31] and provided evidence-based recommendations for its clinical use based on reliability and validity. If there is only one option, the Coma Recovery Scale-Revised (CRS-R) is preferable because it meets all the Aspen Workgroup criteria. In the past, the CRS-R was extensively recommended for acute or subacute-to-chronic DOC assessment [1, 9], and was recommended by the American Academy of Neurology guidelines in 2018 [32] and the European Academy of Neurology (EAN) guidelines in 2020 [33]. The guidelines propose the concept of delayed recovery of consciousness (recovery >1 year after damage) with the help of CRS-R and advocate abandoning the concept of permanent VS/UWS. Measurable CRS-R evidence of volitional responses has been found in ~40% of individuals who had previously been diagnosed with VS/UWS.
CRS-R adaptation and validation studies have been conducted, and a total of 14 language revision versions have been reported. The next consideration is the best time to evaluate. Early identification of DOC is linked to prognosis prediction and establishing the aims of future care decisions. A prospective study has shown that CRS-R scores at admission effectively distinguish patients’ outcomes at hospital discharge after severe brain injury [34]. Later, the optimization of evaluation is considered. Many strategies, such as using the patient’s name to assess auditory function, using a mirror to assess visual fixation, using supplementary BCIs, and measuring the auditory situation in complex listening environments, have resulted in varying degrees of optimization in the assessment of DOC. A further RCT study indicated that individualized objects are more effective at eliciting responses from MCS patients [35]. There may be some advantages to using a repeated CRS-R examination technique, with a minimum of six repetitions in traumatic brain injury (TBI) patients and five in non-TBI patients, which can reduce misdiagnosis within one week. The CRS-R should be used 5 times a week, preferably daily, to reduce the rate of misdiagnosis [36]. Although the neurological examination is still the most often used tool for DOC detection, the use of CRS-R in the acute phase of severe ABI is limited since those patients require prolonged sedation and continuous monitoring.
Neuroimaging Techniques
Currently, three major neuroimaging strategies for assessing consciousness are available [3]: first, resting-state measurements of brain activity, which do not require active participation from patients and evaluate their sensory feedback; second, a passive sensory paradigm to measure brain response to external stimuli, which takes into account the information-processing capacity of the brain’s primary active region; and third, a command-following or a communication paradigm to measure dynamic brain activity, that records willful modulation of the brain under task-state conditions (rarely used in the clinic, but often in scientific research). The important question is how to implement it appropriately after a preliminary understanding of the classification of neuroimaging applicability. In general, the most important rule is to appropriately distinguish between acute and chronic DOC in patients with ABI. The acute period of DOC, according to a recent EAN guideline, is the first 28 days after damage, followed by the subacute-to-chronic periods, and the persistent lower levels of consciousness at one year is a transition time point from persistent to permanent VS/UWS.
We have a conservative view regarding “the more neuroimaging application, the better” during the acute phase of DOC. The usefulness of traditional head computed tomography (CT) cannot be overstated because it is widely available and allows for quick data capture. To the best of our knowledge, CT can assist in predicting the early death of individuals with acute DOC in a variety of ABIs, including TBI, hemorrhage, and hypoxic-ischemic encephalopathy (HIE). However, due to its low sensitivity, applying CT alone to detect most DOC-causing entities has rarely been seen in clinical applications. In a nutshell, MRI is superior because it has significantly higher accuracy and provides evidence for treatment or withdrawal of life-support in a patient with severe ABI within a specific time frame. Nowadays, T2-weighted, susceptibility-weighted, and fluid-attenuated inversion recovery (FLAIR) imaging lesions can be used to predict coma duration, residual function, and long-term prognosis after a TBI. Despite this, individuals with significant micro-bleeding in the brainstem [37] can unexpectedly recover consciousness. At this point, relying solely on the bleeding phenomena of neuroimaging to describe the pathophysiology of DOC is insufficiently rigorous.
To the best of our knowledge, preventing misinterpretation, recognizing reversible harm, and offering an accurate prognosis of awakening are key goals in the acute phase of DOC, which drives us to use sophisticated neuroimaging. The most impressive achievement has been the use of diffusion tensor imaging (DTI) measurements in the acute phase of ABI. Using DTI, researchers discovered that broad disconnection of white matter (particularly the fornix) following severe brain injury is strongly correlated with DOC [38], and abnormal structural connectivity between the basal ganglia, frontal cortex, and thalamus has been detected in DOC [21]. The degree of inter-hemispherical disconnection such as the separation of integrality is an independent biomarker of consciousness [39]. According to one encouraging DTI study, using machine learning algorithms might achieve a maximum of 100% accuracy in discriminating the thalamic tracts reaching the frontal, parietal, and sensorimotor regions [40]. DTI technology has advanced further by using high-resolution techniques to map structural connections in ABI patients by reconstructing fiber tracts in the white matter. An initial investigation indicated that the integrity of the ascending arousal network is diminished in individuals with traumatic coma [41], and the axonal pathway from the brainstem to the hypothalamus and thalamus is destroyed [42]. According to one recent study, reduced connectivity in subcortical arousal pathways is a sensitive indicator of DOC [43]. Therefore, DTI technology can be used to determine the preserved connectome in individual ABI patients and provide evidence for future decision-making. Even though there is no clear opinion on acute DOC assessment, efforts are ongoing to develop a combination of multimodal techniques (CT, MRI, and DTI), build a large-scale database, and share data globally.
Because the patient’s condition is not critical in the subacute-to-chronic phase of DOC, the use of advanced but not complicated neuroimaging is feasible. A feast of progress during the last few decades can be summed up as follows: (1) paradigm shifts from simple to complex [44], (2) method shifts from region-of-interest to whole-brain, (3) technique shifts from fledgling to sophisticated, (4) data collection shifts from depictive to quantitative, and (5) model development shifts from unitary to multi-dimensional. Assessing consciousness in a brain with significant structural or functional damage generally entails capturing independent signals of brain processes.
The use of PET in diagnosing metabolic abnormalities in the brain is a cornerstone of neuroimaging advancement. The earlier functional imaging modalities for assessing DOC, 18F-fluorodeoxyglucose (FDG), and H215O, are commonly-used molecular labels in PET practice. Previous FDG-PET investigations found a 40%–50% reduction in whole-brain metabolism in DOC patients [45], as well as reduced cortical effective connectivity in VS/UWS [46]. According to H215O-PET investigations, consciousness recovery appears to coincide with the restoration of functional connections between the thalamus and cortex [47, 48]. During voxel-based PET analysis, it was discovered that DOC patients have a widespread frontal-parietal network (FPN) deficiency that includes midline and lateral association cortices (the anterior cingulate, mediofrontal, and posterior cingulate or precuneus linked with the prefrontal or posterior parietal cortex). Whether processing intrinsic or extrinsic stimuli, the disruption pattern exhibits a certain degree of homogeneity and continuity at the network level [48]. Then, comparative studies of PET activation in different DOC states revealed that MCS patients have greater metabolic preservation of FPN than controls [49]. A recent FDG-PET comparison of MCS subcategorization discovered that the MCS+ group had higher metabolism than the MCS− group in the left middle temporal lobe, which is essential in semantic processing [50]. A pilot investigation using (11C) flumazenil PET revealed that the γ-amino butyric acid (GABA) receptor binding potential value can be applied as an early evaluation marker of consciousness recovery in VS/UWS and MCS patients [51]. According to recent PET investigations, the degree of metabolic modification within the association cortices (particularly broad FPN and connections to thalamic nuclei) rather than the primary sensory cortices is tightly proportional to the stage of consciousness. PET has revealed first-hand evidence of several intriguing conditions such as cortical processing in VS, auditory processing in VS, pain perception in MCS, preserved brain activity in MCS, and cognitive motor dissociation (CMD) or hidden awareness among many others. A comparative investigation has even shown that 18F-FDG PET can be used to predict the long-term recovery of patients with UWS, and the predictive value appears to be more accurate than the later-described functional MRI (fMRI) [52]. At this point, it is no surprise that the EAN recommendation emphasizes the great sensitivity and specificity of paraclinical PET in distinguishing between VS/UWS and MCS patients.
Structural MRI was the most direct method of visualizing brain abnormalities to make a diagnosis of DOC. Concerning lesion mapping investigations, unconsciousness-related lesions are closely located in the brain’s deep structure, and the value of mapping tegmental arousal nuclei cannot be overstated, given that the rostral brainstem area is substantially correlated with the presence of coma [53]. In addition, because patients with DOC frequently have diffuse cortical lesions, the proposed lesion-network mapping technique will assist in the investigation of distributed cortical networks associated with arousal. This enables automated tissue segmentation for volumetric or morphometric analyses. A recent regional volumetric information analysis applying a boosting tree technique achieved a classification accuracy of 90%–98% for UWS and MCS [54]. Disruption of white matter tracts within the DMN and the fibers connecting the DMN to the thalamus has been used as a barometer to measure the consciousness level in structural brain network studies [15]. Accumulating data suggests that the thalamocortical connection patterns can accurately differentiate between DOC and consciousness [40]. However, it is unknown whether patients with white matter injury in the DMN distribution area are more likely to develop DOC than patients with diffuse white matter injury. In addition, the diffuse anomalies in structural connection matrices of personalized DOC classification have not been described [21].
Resting-state fMRI has the benefit of a single procedure by calculating the temporal correlation (Pearson’s R-value) of blood oxygen level-dependent (BOLD) signal time courses. The seed ROI and defined voxels of a particular region or functional network are often used to produce statistical maps. Therefore, the data-driven approach provides a means of network re-invention rather than an a priori template. A multicenter study discovered that functional connectivity at the group level within the DMN, FPN, SN, auditory, sensorimotor, and visual networks is associated with CRS-R scores and top-to-bottom processing abilities in MCS, and it can properly categorize virtually all VS patients [55, 56]. Although these disparities occur when the DOC and control groups are compared rather than the DOC subgroups, the validity of DMN-based assessment is most reliable as it has been validated across multiple cohorts. In addition, the DMN has been advocated as the preferable functional diagnostic network due to its negative correlations with awareness, reduction in MCS patients, and inversion (complementarily realizing the link to other networks) in VS patients. Recent studies have confirmed that non-DMN correlations are a sign of a prolonged coma or UWS, and that inter-network DMN correlations/anti-correlations are required for patients recovering consciousness following TBI [57]. Furthermore, investigations have indicated that the dynamic equilibrium between excitatory connections toward the posterior cingulate cortex (an intrinsic component of the DMN) and its feedback projections play a critical role in consciousness alteration [58].
In addition, it is beneficial for DOC patients to use MRI to evaluate time-varying BOLD signals. An initial study found that the connection configuration occurs less frequently in VS patients than in controls [59], and a multicenter study established that consciousness is based on the whole-brain capacity to sustain rich dynamics [60]. The subsequent research suggested that integrating dynamic functional connectivity in a connectome-based predictive modeling strategy can predict CRS-R scores and reduce individual heterogeneity in statistical analysis [61]. Then, in a longitudinal fMRI study, it was discovered that patients who regain consciousness have a large increase in the dynamic variance of network measures, as opposed to those whose responsiveness just improved [62]. Thus, cross-species primate mechanisms can be represented by a computational model of the whole brain that evaluates the global synchronization and metrics (structure-function relationships, functional connections, or integration and segregation states) between consciousness and unconsciousness [63]. A dynamic reconfiguration, fragmentation, and integrated model of the whole-brain network indicates that unconsciousness is caused by the disorganization of hierarchical fragmentation patterns between cortical modules [64]. More recently, another finding has revealed that the spatial and temporal hierarchical distinctions in neuronal activity are coupled to the dynamic flexibility of the brain and that its dysfunction is a novel marker of consciousness loss [65]. Taken together, these data have established numerous theories and highlighted the indispensable components of neuronal activity that are integrated into the temporospatial dynamic of whole-brain activity.
Collectively, the field of advanced structural and functional MRI is maturing to the point where it can accomplish two objectives with a single action. Beyond being a diagnostic tool, MRI can shed light on brain correlates, reflect the neurophysiological stage, and find biomarkers of recovery. Nowadays, hypothesis-based and data-driven approaches are used to develop recovery time scales for various types of ABI using structural and fMRI biomarkers. For example, applying data-driven techniques to identify the resting-state fMRI characteristics in the 3-month [66] or 1-year (accuracy reaching 90%) [67] recovery of DOC permits the classification of recovery ability in patients following ABI. The emerging evidence from MRI reveals that the quantified white matter value is a strong predictor of recovery of consciousness, allowing for the identification of a threshold value below which no patient with ABI may regain consciousness [68]. Recent graph theory and dynamic functional connectivity studies have revealed that the breakdown of the sub-state of brain connectivity integration can serve as a biomarker for the loss and recovery of consciousness [69]. All in all, recovery from DOC was previously thought to be correlated with brain network connections but is now conceptualized as relying on dynamic interactions between subcortical and cortical networks.
The multidimensional framework is gradually displacing lesion-based detection, and the implementation of the multimodal technique is approaching or exceeding the conventional gold standard. To begin, defining a single implementation criterion remains a hurdle when developing a multidimensional model. Debate on consciousness at the molecular level is lacking, as the current circulating neurotransmitters or releasers found in ABI patients do not meet clinical biomarker standards in DOC studies (i.e., mostly single-arm, open-label, phase I studies). Despite voxel-wise functional connectivity analyses that have been proposed to decode co-occurring patterns of brain activity, it appears that ROI-based analyses are preferred because they avoid the heterogeneity of ABI during the normal registration or segmentation processes [54]. Second, the optimization of modern technologies continues to require extensive data enrichment. A recent 7.0 T MRI study reported increased density in the area of the temporoparietal junction in MCS patients compared to those with VS/UWS [70], and connections in the structural subnetwork, such as the frontal cortex, limbic system, and occipital and parietal lobes, are significantly decreased in DOC patients [71]. However, the evidence is preliminary and fledgling, consisting simply of a few case reports. Third, how can the best DOC identification be achieved through a comparison of various combination forms? Now, a range of multimodal techniques is available for identifying DOC, such as using fMRI and EEG in acute severe TBI, combining FDG-PET and fMRI in severe TBI, and using DTI and FDG-PET to subcategorize MCS as MCS+ or MCS−). However, a recent study has reported that combining static and dynamic indicators does not improve classification accuracy, and the superiority of various combination forms has rarely been compared. Finally, the multidisciplinary model has not received adequate popularization. A preliminary strategy for effective multimodal integration that incorporates neuroimaging and molecular biology data is believed to exist. Only a few studies have examined the role of molecular markers in determining how the variable number tandem repeat (VNTR) polymorphism of the Period3 (Per3) gene can alter impairment and residual cognitive abilities by enhancing the preservation of major brain connections [72].
All told, Table 1 provides a summary of existing neuroimaging assessment approaches, with the benefits summarized as follows: (1) achieving objective clinical recording of ABI damage, (2) expanding scientific understanding of the neurological correlates of consciousness, and (3) providing new information for therapeutic efforts, and can serve as indicators for curative efficacy.
Table 1.
Advantages and limitations of currently available assessment techniques in DOC after ABI.
| Techniques | Uses in DOC | Analysis | Advantages | Limitations | |
|---|---|---|---|---|---|
| Non-intervention imaging assessment | |||||
| CT/MRI (T1, T2, FLAIR, and SWI) [3, 9] | Lesion mapping | To assess the integrity of brain structure | Voxel-wise association between the lesion and DOC | Fast availability, relatively economical, and integration and sharing of routine diagnosis and treatment process of clinical ABI | Poor prognostic ability, poor visualization of underlying changes |
| Volumetry or morphometry | Automated segmentation. ROI/voxel-wise GLM association between volume and DOC | ||||
| PET (18F-FDG, H215O and 11C-flumazenil) [9, 51] | To detect metabolic processes or blood flow changes, even evaluate the GABAergic system |
18F-FDG: metabolic activity by MIBH/MCP, ROI-based analysis of the voxel maps of values for glucose consumption in DOC. H215O: regional CBF, or CBF distribution in DOC 11C-flumazenil: GABAA receptor binding potential values and LTG ratio in DOC |
Early detection of patients with relative preservation of brain metabolism, reduced misdiagnosis rates, and lower risk of withdrawal of life-sustaining therapy; used as a pharmacodynamic biomarker of targeted therapeutic interventions | Limited accessibility and irradiation in nature, and intake of radioactive elements are required | |
| DTI (DWI) [22, 40] | To assess the integrity of white matter fiber tracts | Voxel/tract/ROI-wise association between diffusion parameter and DOC | Early, non-invasive, quantitative evaluation of white matter injury (loss of fibers and anatomical disruption of the tracts), is useful as a critical tool in revealing the anatomical basis of DOC | Partial specificity is lost if applied alone | |
| fMRI [3, 60, 61] | rs-FC | To assess brain activity and explore functional connections between brain areas | Association between ROI-to-ROI, ROI-to-voxel, network-to-network, voxel-to-Fisher Z-transformed Pearson’s R and level of consciousness | Early detection of the disruption of brain networks in the DOC, and finding the functional connections within the DMN and between the DMN and ECN is crucial for diagnosis and prognosis | Deficiency of standardized acquisition process and analysis methods, and heterogeneity and the sample size of some studies are limited |
| Dynamic FC | Association between the portion of rs-MRI acquisition time spent in particular brain connectivity configuration and level of consciousness | Lends itself to a variety of signal processing techniques for data analysis, which reduces fluctuations during the acquisition period | Need rigorous acquisition technology and analytical method, and difficult to prioritize one over another | ||
| fNIRS [171] | To assess brain activity across the cortex and explore functional connections in the brain | Comparing the measured patterns to patterns induced by self-performance of the same task in DOC | This technology has the unique value of detecting residual cognitive capacity in patients with prolonged DOC by active command-driven motor imagery | Small sample size, preliminary, and relatively insufficient to detect residual consciousness | |
| EEG [86] | To record electrical activity in the brain, and explore neural oscillations/interactions to a stimulus onset | Analysis of spectral power, coherence, entropy, phase synchronization, sleeping pattern, and amplitude and latency of stimulus-evoked responses in DOC, and several functional brain network indexes show correlation with the level of consciousness | Economical, portable, and easy for bedside clinical testing, helps to track individual patients longitudinally. The simplest method both in acute and chronic stages of DOC | The heterogeneity of case series and from different times, and lack of a common classification method and reaching an agreement | |
| Integrated intervention technology and imaging assessment | |||||
| TMS-EEG [131] | To explore the changes in brain dynamics, and further probe the degree of brain activity related to consciousness | Use of PCI to differentiate MCS from VS, and TEP, PCI, and GMFP are used to evaluate the level of consciousness | A more objective evaluation of consciousness. Can be used to evaluate TMS therapeutic efficiency | Heterogeneity, no significant changes are found in VS patients | |
| TMS-fMRI [172] | To assess the hemodynamic response and establish the causal link between different regions in the brain | Measures ratio of oxygenated to deoxygenated hemoglobin and TMS-evoked regional activity and network connectivity in DOC | High spatial resolution, reveals the causal relationship of specific regions in a disturbed brain | Expensive, small sample size, requires specialized equipment and sophisticated analytical methods, and difficult to design placebo-controlled trials | |
| TMS-PET [173] | To measure the causal relationships of metabolic changes at the cellular level across networks | Measures the “standardized uptake value” units in DOC | Can identify the location of specific areas active during rest and task performance | Irradiation required, insensitive to changes in task performance, and difficult to design placebo-controlled trials | |
| tDCS/tACS-EEG [174] | To establish the causal link between different brain areas and explore the changes in brain networks | Analyzes EEG-derived functional and effective connectivity, and relevant brain network dynamics in DOC | A useful approach to improve diagnosis and concurrent with therapeutic effect | Evidence from small sample size and no control group | |
| DBS/SCS-fNIRS [175] | To evaluate the dynamics of brain activity and therapeutic effects | Assesses the real-time changes in brain activity during DBS or SCS in DOC | Can induce significant cerebral blood volume changes in the cortex | Requires specific equipment, requires high-level experimental skills, and small sample size | |
CT, computed tomography; MRI, magnetic resonance imaging; fMRI, functional MRI; ROI, region of interest; PET, positron emission tomography; MIBH, metabolic index of the best-preserved hemisphere; MCP, metabolic covariance pattern; CBF, cerebral blood flow; LTG, local-to-global; DTI, diffusion tensor imaging; DTT, diffusion tensor tractography; DWI, diffusion-weighted imaging; rs, resting stage; FC, functional connectivity; DMN, default mode network; ECN, executive control network; fNIRS, functional near-infrared spectroscopy; EEG, electroencephalography; TMS, transcranial magnetic stimulation; PCI, perturbational complexity index; TEP, TMS-evoked potential; GMFP, global mean field power; tDCS, transcranial direct current stimulation; tACS, transcranial alternating current stimulation; DBS, deep brain stimulation; SCS, spinal cord stimulation.
Electroencephalography Technique
The complementary role of EEG in the acute phase of DOC should be emphasized because it not only avoids the subjectivity of behavioral testing but also has the advantages of simplicity and convenience when compared to neuroimaging. According to a recent study, EEG-reactivity markers can improve the sensitivity in predicting 1-year survival in patients with DOC at admission [73]. Multiple EEG paradigms, including resting stage and sensory stimulation, have confirmed consistency between EEG and behavioral evaluation tools, and the information on intact thalamocortical integrity indicated by EEG signals has been uniformly confirmed by FDG-PET imaging. Signals of consciousness detected by EEG always take precedence over behavioral reactions, making it a valuable technique for identifying and predicting consciousness recovery (Fig. 3). Initially, the degree of thalamocortical disconnection, which is the cause of consciousness impairment, can be seen on the EEG spectrogram. The preliminary quantitative EEG investigation then provides evidence that evaluating functional connectivity can be useful for diagnostic and prognostic purposes in the acute phase of DOC. The quantitative information from EEG signals has been found to correspond with the functional connectivity signals derived from fMRI. In TBI patients, increased EEG entropy (or EEG complexity) has been reported in MCS rather than coma or VS/UWS [74], and the spectral power is proportional to the level of consciousness in subarachnoid hemorrhage (SAH) or hypoxic-ischemic encephalopathy (HIE) patients. Finally, a recent multicenter study reported that EEG criteria applied in HIE could be applied equally to patients with various etiologies of DOC (stroke, TBI, or toxic/metabolic/infectious), and that sample capacity, rather than etiological heterogeneity, plays a crucial role in predictive accuracy [75]. Therefore, it is feasible to implement qualitative EEG assessments at each stage of DOC. The main technical challenge is that electrophysiological data are easily influenced by metabolic abnormalities, medicines, and surgical wounds, particularly during the acute period of ABI.
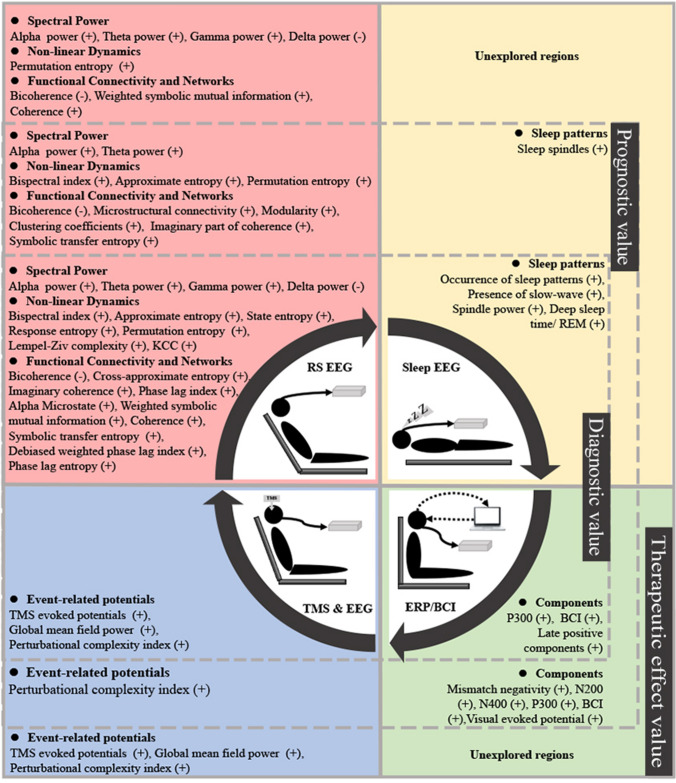
Fig. 3.
EEG parameters. Applying EEG to patients with DOC can be realized in four forms: resting stage EEG (RS EEG; pink), sleep EEG (yellow), event-related potentials/brain-computer interface (ERP/BCI; green), and transcranial magnetic stimulation EEG (TMS & EEG). For example, frequency, power, and correlation are the quantitative indicators, and functional connectivity and coherence are the spatial indicators reflecting the impairment of consciousness, the degree of recovery, and the therapeutic effect.
The milestone role of EEG in subacute-to-chronic DOC is that it brings the information to the bedside, and its advances can be summarized as (1) from wave-pattern recognition to quantitative analysis, (2) from single characteristic attribution to the overall discussion of connectivity and networks, (3) from the correlation of individual paradigms to the stratification of hierarchical paradigms, and (4) from the conventional single-layer, graph theoretical analysis approach to the multiplex, multilayer network analysis approaches. It is critical for detecting signals of reactivity in DOC patients, and the current stimulus-related paradigms are: (1) comparison of the application vs non-use of stimuli, (2) different types of stimulation paradigms [e.g., auditory (global effect, chirp-modulated tones, own name, music or emotion, and single-word, phrase, and sentence levels), visual (eyes open or closed/sleep, fixation, pursuit, and gaze-independent audiovisual), tactile, olfactory, peripheral nerve (median nerve electrical stimulation), mental imagery, or willed and odd-ball (encompassing visual, auditory, and tactile) paradigms], (3) the application of electrophysiological signals combined with other means, such as EEG, PET, and fMRI). Auditory stimulation has received the most attention since it is easier to provide to DOC patients [76], while tactile and olfactory stimulation has received less attention. Specifically, these paradigms can be used to identify DOC by recording positive or negative EEG waveforms that have a strong correlation with the stimulus (single or averaged repeated stimulation), and different types of waves (P300 and N400 reflect higher-order processing) in response to a stimulus [77, 78]. Previous meta-analysis studies have found that late-evoked potentials (the P300 wave) are a powerful predictor of consciousness recovery in patients with prolonged DOC [79]. A subsequent study found that N400 was far more sensitive than P300 in the same field [80] and that the presence of P300 alone cannot consistently discriminate between VS/UWS and MCS [81]. Furthermore, recent evidence suggests that the transcranial magnetic stimulation (TMS)-EEG paradigm reveals sleep-like cortical OFF-periods in the sleep stages of UWS/VS patients, and not in healthy participants [82]. The use of a multimodal paradigm in the evaluation of DOC (different performances in the auditory and vibrotactile stimulation tasks) has been emphasized [83]. Recently, simplification gave insight into the construction of a unique paradigm. The most convincing example was a multicenter study reporting that EEG reactivity to eye-opening stimuli is superior to tactile, noxious, and even evoked potentials (including ERPs) [84]. Furthermore, a recent study reported that assessing the hand-blink reflex, an index of the functional processing of higher-order cortical areas to the sensory-motor integration network, can be used to distinguish MCS from UWS [85].
To date, analytical techniques for EEG data include directed transfer function, imaginary coherence, coherence, and phase lag index from EEG signals, and collaboration between physicians, engineers, physicists, technicians, and mathematicians [86]. Entropy, for example, is a quantitative measure of regularity, with greater values indicating a close-to-awake state and lower values indicating an unconscious state; the mathematical function is applied to study the relationship between EEG fluctuation frequencies and consciousness level. Various computer techniques for calculating entropy, such as approximation entropy, Lempel-Ziv complexity, or cross-entropy, have been developed and show promise in either identifying UWS/VS and MCS or assessing their relationship with clinical ratings. Regardless of data variances and inhomogeneities, EEG network analysis can be used to distinguish UWS/VS from MCS. Compared to generalized partial directed coherence and the directed transfer function, investigations have indicated that the partial coherence is enough to distinguish UWS/VS from MCS [87]. The percentage of alpha microstates spent inside the combination indices (power in alpha and delta frequency bands, entropy, and microstates) has been shown to be the most effective in distinguishing UWS/VS from MCS [88]. At this point, a non-obvious interpretation of the outcomes of the investigation is better since it does not disregard the underlying mechanism of integrative functions or exceptional rearrangement.
There is still space for EEG to advance because it naturally has the advantages of low cost and portability, as well as the capacity to study patients longitudinally. According to the evidence, EEG-based signals are consistent with information from behavioral assessments or MRI data. In individuals with ABI, the architecture derived from EEG characteristics can predict long-term recovery from DOC [89]. Changes in synchronization in EEG signals show the return of consciousness after severe brain injury; this is consistent with findings in metabolism reflected in FDG-PET and structural connections found by DTI [90]. Examples are two excellent recent EEG studies: in one, 15% of clinically unresponsive patients had EEG evidence in response to spoken motor commands in the first few days after ABI [91]; in the other, using a hierarchical linguistic processing paradigm in high-density EEG improved the accuracy rate of residual consciousness identification (from 47% to 87%) [92]. However, unified technical languages that can be applied to a broader spectrum of EEG research, let alone a consensus on an EEG decision protocol in patients with ABI, are still lacking. Although the integrated information theory of consciousness can be applied in consciousness research among other decoding approaches, it still has a long way to go in clinical transformation and application. Therefore, the challenges of this field stem not from the development of fresh methods, but from the insufficiently homogeneous application of existing signal data, the availability of advanced EEG techniques, and the validation of mass analytical approaches.
Management
An authoritative assessment of treatment strategies published in 2019 established the theoretical foundation for managing DOC [4]. However, there are still obstacles to providing first-choice drugs or the first-line treatment of DOC in ABI patients. Several RCTs, open-label studies, and even case reports have presented the data available for DOC treatments without taking stratification into account. In addition to the limitations (requiring large-sample multicenter RCT, greater individualization and precision, and side-effects to be recorded and reported), we highlight the following current challenges: (1) The pronounced effect of a few individuals may distort the overall findings, (2) more valid subdivided CRS-R measurements that have been proved to stratify the phases of awareness, and (3) a well-designed technique that explains inclusion criteria and withdrawal scheme.
Pharmacological Management
Amantadine
Amantadine, a dopamine agonist and an N-methyl-D-aspartic acid receptor (NMDA) antagonist, is the only prescription drug that has completed a class II RCT. It hastens the process of consciousness recovery, although this slows down during the two weeks following a four-week course of therapy [93]. Its beneficial effect in encouraging DOC recovery has been studied in patients with traumatic VS/UWS and MCS, as well as in patients with non-traumatic DOC, such as post-anoxic MCS [94]. However, amantadine is not beneficial for all levels of traumatic DOC. An RCT investigation found that amantadine monotherapy did not affect consciousness in patients with moderate to severe TBI during the first week or at 6-month follow-up [95]. Nonetheless, both observational and retrospective controlled trials have indicated that amantadine can improve consciousness in patients with UWS following a significant cerebral hemorrhage, and the action is unaffected by the site of bleeding [96, 97]. Furthermore, recent case-control research found that combining amantadine with cerebrolysin had a synergistic impact in patients with DOC following ABI [98]. At this point, it is not surprising that the American Academy of Neurology guidelines recommended using amantadine for DOC in 2018 [32].
Methylphenidate
Methylphenidate, one of the most widely recommended awaking medicines in patients with ABI [99], has previously been shown to impact consciousness recovery in the early stages of trauma. According to a PET study, methylphenidate improves awareness via enhancing the cerebral glucose metabolism of the posteromedial parietal cortex [100]. However, a meta-analysis study found no significant effect of standardized methylphenidate administration in individuals with DOC following brain damage [101]. Despite this, this neurostimulator has been shown to have a structural basis for medicating the neuronal network of consciousness, and no data on its harmful effects have been recorded. Therefore, methylphenidate is recommended as a DOC pharmacological option in a recent review [9].
Apomorphine
Apomorphine, a powerful dopaminergic agonist that is being used as a last resort in Parkinson’s Disease (PD), has been shown in a case report to improve consciousness recovery from MCS [102]. In detail, a dopaminergic deficit (similar to the pathophysiological mechanism of PD) [103] can be reversed in patients with MCS by apomorphine infusion, and it is not a spontaneous event, as discontinuation of this agent on day 18 (contrast day) or day 84 (planned termination day) may result in DOC. The subsequent multicenter trial demonstrated that continuous subcutaneous apomorphine administration is possible, safe, and efficacious for improving consciousness in individuals with VS or MCS following severe TBI [104]. However, verifying whether these preliminary findings are accurate and beneficial requires further clinical trials.
Zolpidem
Zolpidem, a hypnotic drug that can restore aberrant cell metabolism after brain injury, has been shown to have the counterintuitive transient effect of arousing patients with low consciousness [105]. Several clinical reports have been published over the last 20 years, each one delving more into the therapeutic efficacy of zolpidem in DOC. It began with an unexpected awakening from VS, which disappeared after withdrawal and reappeared on repeated administration [106, 107]; then, an association between sub-sedative doses of zolpidem and recovery from DOC [108] was demonstrated, and it was accidentally discovered that a higher dose (30 mg instead of 10 mg) improves the curative effect [109]. To date, the combined application of multi-modal approaches is relatively rare. Intrinsically, the clinical evidence for using zolpidem in patients with DOC stems from two reliable clinical trials: in one [110], the clear signs of EEG cortical arousal were detected after zolpidem intake and this was unquestionably related to functional improvement; in the other [111], ~5% of patients with MCS or VS/UWS responded to zolpidem, but the specific zolpidem responder type of ABI could not be identified. Impressively, these issues have been addressed in recent studies or case reports, such as using FDG-PET, EEG, fMRI, or brain perfusion single‐photon emission computed tomography (SPECT) [112] in the evaluation of DOC patients’ response to zolpidem therapy and using magnetoencephalography (MEG) technology to investigate the subtle effects of zolpidem [113] in patients with DOC after TBI. Metabolic activation of prefrontal areas could explain zolpidem’s paradoxical impact on DOC, and the perfusion pattern of focal or multifocal cortical abnormalities revealed by SPECT strongly indicates zolpidem’s therapeutic response. MEG studies revealed a decrease in power in the theta–alpha (4–12 Hz) and lower beta (15–20 Hz) frequency bands of unmedicated patients, while individuals ingesting zolpidem had a higher beta or lower gamma (20–43 Hz) frequency band. These findings corroborate the mesocircuit hypothesis by indicating that zolpidem acts on the globus pallidus internus and affects the thalamocortical connection via thalamic disinhibition. As detailed in a recent review [114], the use of zolpidem may not only increase arousal in VS and MCS patients, but it may also be promising in select patients with intact brain structures, such as an intact white matter connection and deep and superficial gray matter.
Non-pharmacological Interventions
Given the low efficiency of pharmacological intervention in promoting awareness, non-pharmacological methods appear to be dominant in the therapy for patients with DOC after ABI [4]. Based on the literature and current usage, a recent proposal was made that all patients with DOC can benefit from non-invasive brain stimulation [115] and transcranial direct current stimulation (tDCS) should be prioritized over repetitive transcranial magnetic stimulation (rTMS) [116].
Transcranial Direct Current Stimulation (tDCS)
A double-blind RCT in 2014 [117] revealed Class II evidence that short-duration tDCS of the dorsolateral prefrontal cortex (DLPFC) improves consciousness in patients with MCS, establishing the superiority of tDCS over alternative neuromodulation approaches in the management of DOC. Unfortunately, no therapeutic effects have been reported in individuals with UWS when tDCS is used in a group setting. Only a case report study showed a patient who came with the diagnosis of UWS was diagnosed as being in MCS− following the tDCS management [118]. Another study replaced tDCS with transcranial random noise stimulation in the DLPFC, finding that only one patient evolved from VS to MCS, and it is not an efficient treatment for VS/UWS [119].
Nonetheless, some findings from past tDCS trials should be highlighted here. First, one study revealed that the extent of therapeutic effects may be related to the severity and duration of pathology rather than the location of stimulation [120]. Second, an RCT reported that using tDCS once a day for 5 consecutive days increases consciousness recovery for up to 1 week after the stimulation [121]. Another RCT demonstrated that home-based 4-week tDCS improves consciousness recovery in chronic MCS patients [122]. Then, in a subsequent RCT, it was reported that repeated tDCS of the left DLPFC for 10 days produces considerable behavioral improvements, which can be seen on the P300 ERP [123]. Finally, in terms of therapeutic response, behavioral improvements after tDCS involve the anatomical and functional preservation of the DLPFC, precuneus, and thalamus. Furthermore, EEG studies emphasize that neuronal activity in the prefrontal area of DOC patients plays a crucial role in the response to tDCS. In addition, a recent EEG study found that multifocal frontoparietal tDCS has varying behavioral effects in individuals with DOC [124]. However, using tDCS to excite the orbitofrontal cortex, the primary motor cortex (M1), or precuneus is not considered promising up until this point. Thus, the prefrontal cortex (particularly the left DLPFC) appears to be a preferable target for tDCS stimulation since there is a connection between the prefrontal cortex and the thalamus, and disinhibition of thalamocortical communication may contribute to recovery from DOC. It is worth noting that, despite the benefits of low cost and easy management of tDCS devices, the International Federation of Clinical Neurophysiology has stated unequivocally that current evidence does not allow making any Level A (definite efficacy) recommendations for therapeutic use in patients with DOC [125]. It is still unclear how to execute tDCS optimally among DOC subgroups while avoiding incorrect application or potential abuse or misuse of this method in future clinical practice.
Repeated Transcranial Magnetic Stimulation (rTMS)
The evidence on the behavioral effects of rTMS is marginally inferior to that of tDCS. In a case report, meaningful behaviors increased following rTMS, and markers of consciousness—the alpha, beta, and delta frequency bands of EEG—were enhanced [126]. Another piece of evidence has suggested that manipulating slow-wave activity with rTMS reveals residual patterns of connections within cortical-thalamocortical loops in patients with DOC, although there is still variation in methodology and outcomes in those studies [127]. Previous RCTs failed to provide evidence of a therapeutic effect of 20-Hz rTMS of M1 for 5 consecutive days in chronic VS [128], whereas a subsequent sham-controlled study suggested that rTMS applied to M1 improves cerebral blood flow (CBF) velocity in the bilateral middle cerebral artery [129]. Then, an RCT suggested that 20-Hz rTMS delivered to M1 for 10 min every day for 5 days improves arousal in DOC [130]. Furthermore, EEG-based TMS-evoked connectivity investigations have shown that rTMS can successfully regulate effective connection in MCS patients but not in VS patients [131].
On the one hand, the DLPFC has been used as a therapeutic target in several uncontrolled rTMS trials. A previous study found that applying 10-Hz multi-session rTMS to the left DLPFC is particularly effective for patients with MCS [132]. Another study found that a single session of 10-Hz rTMS over the right DLPFC not only enhances awareness but also restores cortical connections in UWS [133]. Moreover, a recent study revealed the feasibility and efficacy of 10-Hz rTMS of the right DLPFC for 20 days in early DOC to speed up recovery from VS [134]. On the other hand, a case report found that applying rTMS over the ascending reticular activating system was associated with an increase in neural tract volume of the right prefrontal lobe, resulting in an improvement in consciousness during the application period [135]. To date, the most encouraging aspects are the safety considerations for the use of rTMS after ABI. For one thing, there was no severe adverse effect of prolonged use in two long-term follow-up cases [136], and for another, recent clinical trials indicated that the rTMS protocol is a relatively safe choice if the risk of seizure induction can be monitored [137].
Deep Brain Stimulation (DBS)
A preliminary double-blind alternating crossover study has provided evidence that the mechanisms by which DBS promotes functional recovery can be viewed as compensation for damaged arousal regulation via bilateral DBS of the central thalamus [138]. Recent evidence suggests that the overall changes in DOC patients are related to the activation of the DMN cortex via the thalamocortical-basal and tegmental loops, which are successfully affected by DBS [139]. Previously, the findings of an open-label prospective multi-institutional trial indicated that bilateral thalamic DBS can improve the clinical status of patients with DOC, although neither returned to a completely aware state [140]. Another prospective open-label study proposed DBS selection criteria, which included the presence of somatosensory evoked potentials, motor, and brainstem auditory evoked potentials, and glucose metabolism in the brain is not affected by the low metabolic level, and that DBS could be an effective treatment option for VS or MCS patients if the criteria were met [141]. With the assistance of DBS in the management of DOC, the chance of recovery in TBI is almost twice that of non-TBI, and the recovery from MCS is more evident than VS and can be considered even in the long term (>5 years) [142]. Notably, DBS cannot be used during the spontaneous recovery period following DOC (which should not be <6 months for VS and one year for MCS) [143]. To prevent ambiguity in distinguishing between the effects of spontaneous recovery and DBS, the time interval between the damage and the DBS implant should be fully considered in future research.
Indeed, DBS experiences have been confined to a tiny number of UWS of MCS patients due to the rarity of spontaneous recovery. Nonetheless, curable individuals consistently demonstrate persistent responses to DBS, such as command-following or conversation, and there are carryover effects indicating that the brain remains above baseline in DOC patients [4]. The central thalamus is a reasonable target for DBS due to its extensive linkages to the brainstem and frontal cortex. However, because thalamic connections are susceptible to cell death in patients with VS, there is scant direct evidence that DBS reverses this widespread cell death [144]. At this point, the regulation of long-range network interactions [145] rather than the arousal system [146] is a more appropriate explanation for the effect of DBS. Because the distinct features and connections between the subnuclei of the central thalamus are not fully understood, it is difficult to find a first-choice target for DBS, and no sham-controlled RCT on DBS in patients with DOC has been published so far.
Vagal Nerve Stimulation (VNS)
The feasibility of VNS in patients with DOC has been demonstrated in two case studies: one is the first successful case of VNS with inspiring results from the clinical condition and the functional network [147], and the other is a UWS patient who improved to MCS and demonstrated enhanced brain connection patterns [148]. In addition, a landmark study proposed six precise processes of VNS in the care of DOC patients: (1) thalamic activation, (2) ascending reticular activating system activation, (3) regulation of the cortico-striatal-thalamic-cortical loop, (4) increased inhibition between the DMN and external networks by activating the SN, (5) increased activity within the DMN via the serotonin pathway, and (6) increased connectivity or activity in networks via the norepinephrine pathway [25]. Recently, an open-label pilot transcutaneous auricular VNS (taVNS) study that used arterial spin labeling in fMRI to evaluate the CBF of DOC patients reported that intact auditory function is a requirement for taVNS responders, and taVNS facilitates the reaction to auditory stimuli in patients with DOC [149].
Sensory Stimulation (SS)
SS has been recommended not only for its ability to sustain maintained connections but also for its ability to improve dendritic development and synaptic plasticity following ABI [150]. Although the feasibility, safety, and economic value of SS have improved its relevance in clinical and research contexts, earlier effectiveness studies were primarily based on theoretical benefit rather than empirical efficiency. A reasonable explanation for the current discussions over using SS in normal care is that it is adequate to increase brain responsiveness but not sufficient to restore consciousness in patients with DOC (particularly MCS) [151]. This raises the question of whether the SS program will go beyond improving consciousness and so realize more disability rescue. When the behavioral evaluation result of SS is negative, several paraclinical assessment methods, such as fMRI and EEG, have recently become accessible for identifying and assessing the SS-associated increase in consciousness and brain activity. The central autonomic network system model, for example, is a useful tool for quantifying the influence of SS [152], and electrodermal reactivity is a differentiating measure of emotional engagement elicited by music stimuli [153]. Another niche worthy of concern is that DOC patients can enjoy the opportunity of economical rehabilitation when the SS is applied and will continue to remain so for many years. Therefore, SS seems to be a reasonable continuous management tool, from initial clinical discharge to rehospitalization rehabilitation, and even long-term follow-up. One example of this is a blinded crossover study that explored familiar auditory sensory training supplied by families, clinicians, and assistants of all levels for seven years post-injury, and the implication was that appropriately adding SS to the assessment is crucial to detect a subtle change during DOC management [154]. Of course, every response should be rigorously monitored; soliciting feedback and adjusting stimulation at the appropriate time are critical in the development of verified management schemes.
Given all of that, the following are recommended characteristics of SS intervention: (1) emotional and autobiographical stimuli connected to patients, (2) the ability to not only excite but also record probable changes in consciousness (differentiating spontaneous and stimulus-response activity), and (3) the ability to cause changes [155]. However, the wide range of existing heterogeneities, such as different clinical settings, different stages of the disease, and distinct levels of consciousness impairment, as well as the modality, contents, frequency, and intensity of stimulation, eventually lead to incompatibility with previous evidence.
Brain-computer Interfaces (BCIs)
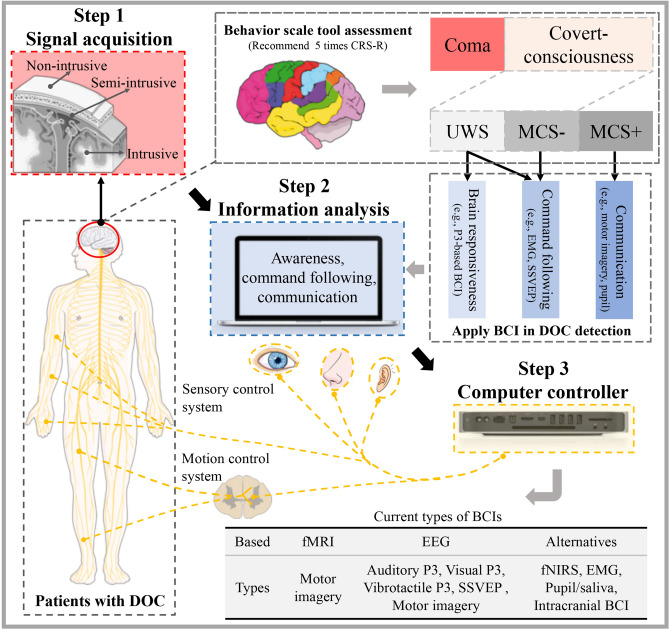
BCIs are appropriate for patients with UWS and MCS in terms of potential awareness detection (covert consciousness) and command following, as well as providing an assistive means of communication and environment control in those patients with slight impairment of consciousness [156]. After the release of a pivotal work in 2006 [157], the view that negative findings can never be interpreted as the absence of consciousness has become established in subsequent therapy of DOC patients using fMRI-based BCIs. Significantly, the EEG-based BCIs are affordable and portable, and are more suitable in the evaluation period of ABI patients. A hierarchical scheme of BCIs could be fully considered (Fig. 4), as there is currently no consensus regarding the use of BCIs in patients with DOC.
Fig. 4.
A three-step hierarchical scheme for clinical brain-computer interfaces (BCIs). The first step is signal acquisition. Three kinds of BCI signal acquisition methods can be implemented: non-intrusive, semi-intrusive, and intrusive. BCI technology can be used to capture covert-consciousness information in patients with UWS or MCS−. The second step is information analysis. In this step, the brain responsiveness or command pursuit information can be decoded from patients with UWS, the command pursuit information can be decoded from patients with MCS−, and communication information can be decoded from patients with MCS+. The third step is computer control. The control of sensory and/or motor systems in a patient with DOC can be realized by the BCI system. The gray table in the lower right corner presents the current types of BCI. BCI, brain-computer interface; CRS-R, Coma Recovery Scale-Revised; DOC, disorder of consciousness; EEG, electroencephalography; EMG, electromyography; fMRI, functional magnetic resonance imaging; fNIRS, functional near-infrared spectroscopy; MCS, minimally conscious state; SSVEP, steady-state visual evoked potential; UWS, unresponsive wakefulness syndrome.
A sequence of paradigms is available in the EEG-based BCIs during the identification of patients with DOC. The initial evidence for BCIs indicated that the use of active sniffing tools to control the speller is equivalent to the use of P3 BCIs to control the speller in patients with locked-in syndrome (LIS), and a subsequent study indicated that this assistive device can assist in stopping the ongoing music by vigorous breathing (although no command is followed by motor output) [158]. Electromyography (EMG) is another method for identifying voluntary action. In previous clinical investigations, supra-threshold EMG activity associated with command-following has been reported in MCS patients [159], and the high false-negative rate associated with EMG technology is a clinical problem. In addition, recording pupil reactions and salivary pH of BCIs in the management of DOC is feasible, as they are simple and adaptable to a variety of settings, as MCS patients are able to follow commands via pupil size measured by a bedside camera [160] and LIS patients can communicate via salivary pH [161]. Unfortunately, only a few case reports or small-scale clinical trials support BCIs, and the study of BCI for DOC application is rare. Several challenges including persistent ethical concerns, the cost of extended promotion, and the need for sophisticated data processing and analytic methods need to be addressed in the future.
New Fledgling Managements
Although some exploratory research has been conducted on the efficacy of various neuro-stimulants in patients with DOC (180 days following injury), there is currently no statistically significant evidence linking dosage to meaningful consciousness improvement. A few uncontrolled studies have reported that intrathecal baclofen may speed up consciousness recovery [162], midazolam has been reported to enhance recovery from MCS [163], and ziconotide may be a candidate for reversing VS/UWS [164].
As a unique alternative to DBS, low intensity focused ultrasonic pulses can induce direct neuromodulation of deep brain nuclei without disrupting the surroundings [165]. SCS is now considered to be an alternative invasive approach, yet the pathophysiological mechanisms underpinning SCS remain unknown. The initial results indicated that the SCS technique could enhance CBF in patients with MCS or VS [166]. Then, one EEG study suggested that SCS can modulate brain function in MCS patients by activating the thalamocortical neural network [5], while another EEG study indicated that low-frequency oscillations in the frontal and occipital regions were improved and became more complex following SCS. In addition, two pioneering case reports suggest that vestibular stimulation may aid in the improvement of awareness in MCS patients [167]. A recent comprehensive review established that head-up tilt can improve awareness in patients with DOC, but additional study is needed to establish a definitive effect [168].
In terms of multidisciplinary interventions, a recent pilot study examined the efficacy of combining amantadine and rTMS in patients with DOC, finding that the combination achieved double the significant gains achieved with rTMS alone [169]. In addition, combining an interdisciplinary team protocol with zolpidem (10 mg) medication has been proposed as a synergistic approach for optimizing the care of patients with extended DOC [170].
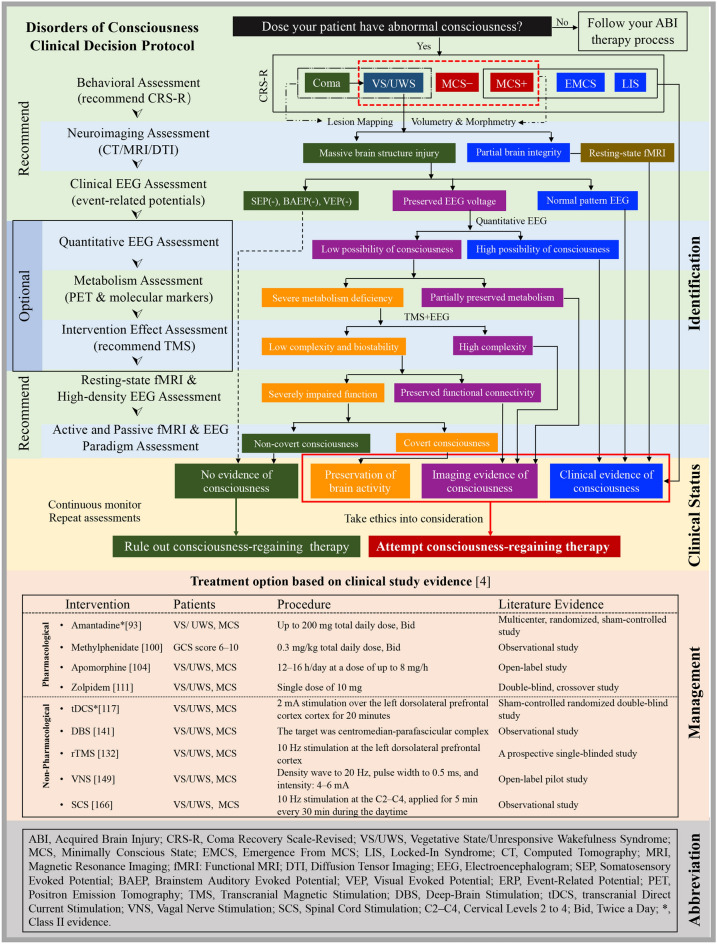
An Experience-based Protocol for Clinical Decision
In the contemporary medical paradigm, exaggerating or underestimating the recovery potential of DOC patients may result in inappropriate treatment withdrawal, catastrophic economic and social loss, and unrealistic expectations by their families. Gradually, obtaining a more independent life and improving quality of life have been the primary focus of attention in patients with DOC. To the best of our knowledge, the neuroimaging and electrophysiological studies of DOC have not been restricted to identification purposes, as they can provide a screening biomarker of consciousness recovery and feedback on management efficacy. Meanwhile, an increasing body of evidence in the effective management of DOC has been reported by RCT studies. Therefore, we extracted and combined the characteristics of these studies with our clinical experience and then designed a preliminary protocol for identifying and managing patients with DOC after TBI.
The primary motive of our “Disorder of Consciousness Clinical Decision Protocol” was to reduce misdiagnosis rates, and to identify more patients who have the opportunity of awakening. As shown in Fig. 5, a sequential approach is proposed to improve the detection rate of covert consciousness and guide the selection of therapies. A behavioral assessment is the first step toward personalized diagnosis, and initial diagnostic categories can be established according to the characteristics of patients. Then the patient’s brain structure is assessed, to provide a high-resolution characterization of the grey and white matter alterations following ABI. An EEG assessment then follows, as it is convenient for bedside assessment and is a reliable approach to characterize and predict states of consciousness [92]. To our knowledge, it has already been found that VS/UWS patients who can localize auditory stimuli have higher levels of brain metabolism and increased fMRI connectivity between the visual and frontoparietal areas, rather than increased EEG signals in auditory localization [3]. Subsequently, neuroimaging work-up is strongly recommended to overcome the deficits of bedside EEG techniques. Multimodal imaging protocols provide complementary data to reduce misdiagnosis rates and provide surrogate biomarkers of early responsiveness. Passive paradigms measure brain responses to external stimuli without the participation of the patient, while active paradigms record willful modulation of brain activity by the patient in response to a command. At this point, our protocol brings in an improved classification of clinical DOC (that is, patients with severely impaired brain activity and poor prognosis can be identified early; VS/UWS patients with a capacity for covert consciousness can be detected with neuroimaging and may benefit from adapted care plans to promote recovery).
Fig. 5.
Illustration of a clinical decision-making protocol for patients with DOC after ABI. An experience-based protocol is proposed to optimize the multimodal approaches, improve the accuracy of consciousness diagnosis, and guide therapy selection based on high-quality evidence. Green boxes indicate patients with irreparable brain activity and poor prognosis; orange boxes indicate patients with an uncertain capacity for regaining consciousness (e.g., VS/UWS patients with preserved brain metabolism or mismatching neuroimaging results); purple boxes indicate patients with multimodal neuroimaging evidence of consciousness or preserved brain activity who requires consciousness-regaining therapy; blue boxes indicate patients with clinical signs of consciousness. In addition, the results of clinical treatment trials with high-quality evidence are listed in the table. This multimodal protocol can be applied (e.g., the sequence of the assessments combined in reasonable order) by different institutions.
In addition, the treatment of patients with DOC is challenging as many case reports or open-label studies cannot be translated directly into clinical practice. However, the high-quality evidence that has been published since 2013 provides a reference for clinical management. Present evidence suggests that patients with DOC might benefit from neuromodulation interventions from days to years after the brain injury. Although only two interventions (amantadine and tDCS) show class II evidence, the promising effects in several RCT studies can provide a good reference when we are in a dilemma. Therefore, we can adopt this protocol or modified versions in the future to establish a personalized decision-making method for DOC in patients with ABI.
Conclusions
The core objective in identifying and managing DOC in patients with ABI is to consistently improve accuracy and effectiveness. On the one hand, the identification of DOC is multidimensional, and a multimodal or multidisciplinary framework that integrates advanced neuroimaging and electrophysiological technologies; on the other hand, multiple interventions can be applied to the management of patients with DOC, of which amantadine and tDCS have provided class II evidence, zolpidem is also of high quality, and non-pharmacological management appears to be effective. Despite the increasing availability of interventions, their effectiveness remains to be determined in large double-blind RCT studies. Clarifying the mechanism of consciousness, building unique and clinically precise identification techniques, and improving the awakening rate are still difficult tasks to solve. We should never neglect any type of fledgling approach and should evaluate more verifiable RCT trials, especially new findings in awakening experiments.
Search Strategy and Review Criteria
We searched the PubMed database for English language literature published up to September 1st, 2021 that used the terms “disorders of consciousness”, “coma”, “vegetative state”, “minimally conscious state”, “unresponsive wakefulness syndrome”, “brain injury”, “neuroimaging”, “PET”, “MRI”, “EEG”, “diagnosis”, “identification”, “management”, “treatment”, and “outcome”. We reviewed the full text of all original articles, reviews, case reports, comments, early‐release publications, associated citations, and relevant papers mentioned in the references. Articles were selected for inclusion based on their topical representativeness and methodological rigor, as judged by the latest journal impact factor or journal authority.
Acknowledgements
This work was supported by the Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), ZJ Lab, Shanghai Center for Brain Science and Brain-Inspired Technology, and National Major Pre-Research Project (pilot project) (IDF151042).
Conflict of interest
The authors claim that there are no conflicts of interest.
Footnotes
Rui-Zhe Zheng and Zeng-Xin Qi have contributed equally to this work.
Contributor Information
Xue-Hai Wu, Email: wuxuehai2013@163.com.
Ying Mao, Email: maoying@fudan.edu.cn.
References
- 1.Giacino JT, Fins JJ, Laureys S, Schiff ND. Disorders of consciousness after acquired brain injury: The state of the science. Nat Rev Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- 2.Septien S, Rubin MA. Disorders of consciousness: Ethical issues of diagnosis, treatment, and prognostication. Semin Neurol. 2018;38:548–554. doi: 10.1055/s-0038-1667384. [DOI] [PubMed] [Google Scholar]
- 3.Sanz LRD, Thibaut A, Edlow BL, Laureys S, Gosseries O. Update on neuroimaging in disorders of consciousness. Curr Opin Neurol. 2021;34:488–496. doi: 10.1097/WCO.0000000000000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thibaut A, Schiff N, Giacino J, Laureys S, Gosseries O. Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 2019;18:600–614. doi: 10.1016/S1474-4422(19)30031-6. [DOI] [PubMed] [Google Scholar]
- 5.Bai Y, Xia X, Li X, Wang Y, Yang Y, Liu YF, et al. Spinal cord stimulation modulates frontal delta and gamma in patients of minimally consciousness state. Neuroscience. 2017;346:247–254. doi: 10.1016/j.neuroscience.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Pan J, Xie Q, Qin P, Chen Y, He Y, Huang H, et al. Prognosis for patients with cognitive motor dissociation identified by brain-computer interface. Brain. 2020;143:1177–1189. doi: 10.1093/brain/awaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pool JW, Siegert RJ, Taylor S, Dunford C, Magee W. Evaluating the validity, reliability and clinical utility of the Music therapy Sensory Instrument for Cognition, Consciousness and Awareness (MuSICCA): Protocol of a validation study. BMJ Open. 2020;10:e039713. doi: 10.1136/bmjopen-2020-039713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang T. Recent progress in basic and clinical research on disorders of consciousness. Neurosci Bull. 2018;34:589–591. doi: 10.1007/s12264-018-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edlow BL, Claassen J, Schiff ND, Greer DM. Recovery from disorders of consciousness: Mechanisms, prognosis and emerging therapies. Nat Rev Neurol. 2021;17:135–156. doi: 10.1038/s41582-020-00428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maxwell WL, Pennington K, MacKinnon MA, Smith DH, McIntosh TK, Wilson JTL, et al. Differential responses in three thalamic nuclei in moderately disabled, severely disabled and vegetative patients after blunt head injury. Brain. 2004;127:2470–2478. doi: 10.1093/brain/awh294. [DOI] [PubMed] [Google Scholar]
- 11.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiff ND. Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modolo J, Hassan M, Wendling F, Benquet P. Decoding the circuitry of consciousness: From local microcircuits to brain-scale networks. Netw Neurosci. 2020;4:315–337. doi: 10.1162/netn_a_00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJF, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. 2010;133:161–171. doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Espejo D, Soddu A, Cruse D, Palacios EM, Junque C, Vanhaudenhuyse A, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol. 2012;72:335–343. doi: 10.1002/ana.23635. [DOI] [PubMed] [Google Scholar]
- 16.Rosazza C, Andronache A, Sattin D, Bruzzone MG, Marotta G, Nigri A, et al. Multimodal study of default-mode network integrity in disorders of consciousness. Ann Neurol. 2016;79:841–853. doi: 10.1002/ana.24634. [DOI] [PubMed] [Google Scholar]
- 17.Qin P, Wu X, Huang Z, Duncan NW, Tang W, Wolff A, et al. How are different neural networks related to consciousness? Ann Neurol. 2015;78:594–605. doi: 10.1002/ana.24479. [DOI] [PubMed] [Google Scholar]
- 18.di Perri C, Bahri MA, Amico E, Thibaut A, Heine L, Antonopoulos G, et al. Neural correlates of consciousness in patients who have emerged from a minimally conscious state: A cross-sectional multimodal imaging study. Lancet Neurol. 2016;15:830–842. doi: 10.1016/S1474-4422(16)00111-3. [DOI] [PubMed] [Google Scholar]
- 19.Coulborn S, Taylor C, Naci L, Owen AM, Fernández-Espejo D. Disruptions in effective connectivity within and between default mode network and anterior forebrain mesocircuit in prolonged disorders of consciousness. Brain Sci. 2021;11:749. doi: 10.3390/brainsci11060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lemaire JJ, Pontier B, Chaix R, El Ouadih Y, Khalil T, Sinardet D, et al. Neural correlates of consciousness and related disorders: From phenotypic descriptors of behavioral and relative consciousness to cortico-subcortical circuitry. Neuro-Chirurgie. 2022;68:212–222. doi: 10.1016/j.neuchi.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Weng L, Xie QY, Zhao L, Zhang RB, Ma Q, Wang JJ, et al. Abnormal structural connectivity between the basal ganglia, thalamus, and frontal cortex in patients with disorders of consciousness. Cortex. 2017;90:71–87. doi: 10.1016/j.cortex.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Wei RL, Peng GP, Zhou JJ, Wu M, He FP, et al. Correlations between diffusion tensor imaging and levels of consciousness in patients with traumatic brain injury: A systematic review and meta-analysis. Sci Rep. 2017;7:2793. doi: 10.1038/s41598-017-02950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham SI, Tomasi D, Volkow ND. Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum Brain Mapp. 2017;38:938–956. doi: 10.1002/hbm.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skandalakis GP, Komaitis S, Kalyvas A, Lani E, Kontrafouri C, Drosos E, et al. Dissecting the default mode network: Direct structural evidence on the morphology and axonal connectivity of the fifth component of the cingulum bundle. J Neurosurg. 2020;134:1334–1345. doi: 10.3171/2020.2.JNS193177. [DOI] [PubMed] [Google Scholar]
- 25.Briand MM, Gosseries O, Staumont B, Laureys S, Thibaut A. Transcutaneous auricular vagal nerve stimulation and disorders of consciousness: A hypothesis for mechanisms of action. Front Neurol. 2020;11:933. doi: 10.3389/fneur.2020.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vázquez JA, Adducci M, Godoy Monzón D, Iserson KV. Lactic dehydrogenase in cerebrospinal fluid may differentiate between structural and non-structural central nervous system lesions in patients with diminished levels of consciousness. J Emerg Med. 2009;37:93–97. doi: 10.1016/j.jemermed.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Conio B, Martino M, Magioncalda P, Escelsior A, Inglese M, Amore M, et al. Opposite effects of dopamine and serotonin on resting-state networks: Review and implications for psychiatric disorders. Mol Psychiatry. 2020;25:82–93. doi: 10.1038/s41380-019-0406-4. [DOI] [PubMed] [Google Scholar]
- 28.Bagnato S, Andriolo M, Boccagni C, Sant’Angelo A, D’Ippolito ME, Galardi G. Dissociation of cerebrospinal fluid amyloid-β and tau levels in patients with prolonged posttraumatic disorders of consciousness. Brain Inj 2018, 32: 1056–1060. [DOI] [PubMed]
- 29.Bagnato S, Grimaldi LME, di Raimondo G, Sant’Angelo A, Boccagni C, Virgilio V, et al. Prolonged cerebrospinal fluid neurofilament light chain increase in patients with post-traumatic disorders of consciousness. J Neurotrauma 2017, 34: 2475–2479. [DOI] [PubMed]
- 30.Kondziella D, Menon DK, Helbok R, Naccache L, Othman MH, Rass V, et al. A precision medicine framework for classifying patients with disorders of consciousness: Advanced classification of consciousness endotypes (ACCESS) Neurocrit Care. 2021;35:27–36. doi: 10.1007/s12028-021-01246-9. [DOI] [PubMed] [Google Scholar]
- 31.Brain Injury Interdisciplinary Special Interest Group American Congress of Rehabilitation Medicine, Seel RT, Sherer M, Whyte J, Katz DI, Giacino JT, et al. Assessment scales for disorders of consciousness: Evidence-based recommendations for clinical practice and research. Arch Phys Med Rehabil 2010, 91: 1795–1813. [DOI] [PubMed]
- 32.Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S, et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91:450–460. doi: 10.1212/WNL.0000000000005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondziella D, Bender A, Diserens K, van Erp W, Estraneo A, Formisano R, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27:741–756. doi: 10.1111/ene.14151. [DOI] [PubMed] [Google Scholar]
- 34.Portaccio E, Morrocchesi A, Romoli AM, Hakiki B, Taglioli MP, Lippi E, et al. Score on coma Recovery Scale-Revised at admission predicts outcome at discharge in intensive rehabilitation after severe brain injury. Brain Inj. 2018;32:730–734. doi: 10.1080/02699052.2018.1440420. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Wang J, Heine L, Huang W, Wang J, Hu N, et al. Personalized objects can optimize the diagnosis of EMCS in the assessment of functional object use in the CRS-R: A double blind, randomized clinical trial. BMC Neurol. 2018;18:38. doi: 10.1186/s12883-018-1040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Hu X, Hu Z, Sun Z, Laureys S, Di H. The misdiagnosis of prolonged disorders of consciousness by a clinical consensus compared with repeated coma-recovery scale-revised assessment. BMC Neurol. 2020;20:343. doi: 10.1186/s12883-020-01924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izzy S, Mazwi NL, Martinez S, Spencer CA, Klein JP, Parikh G, et al. Revisiting grade 3 diffuse axonal injury: Not all brainstem microbleeds are prognostically equal. Neurocrit Care. 2017;27:199–207. doi: 10.1007/s12028-017-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Yang Y, Chen S, Ge M, He J, Yang Z, et al. White matter integrity correlates with residual consciousness in patients with severe brain injury. Brain Imaging Behav. 2018;12:1669–1677. doi: 10.1007/s11682-018-9832-1. [DOI] [PubMed] [Google Scholar]
- 39.Ferraro S, Nigri A, Nava S, Rosazza C, Sattin D, Sebastiano DR, et al. Interhemispherical anatomical disconnection in disorders of consciousness patients. J Neurotrauma. 2019;36:1535–1543. doi: 10.1089/neu.2018.5820. [DOI] [PubMed] [Google Scholar]
- 40.Zheng ZS, Reggente N, Lutkenhoff E, Owen AM, Monti MM. Disentangling disorders of consciousness: Insights from diffusion tensor imaging and machine learning. Hum Brain Mapp. 2017;38:431–443. doi: 10.1002/hbm.23370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snider SB, Bodien YG, Frau-Pascual A, Bianciardi M, Foulkes AS, Edlow BL. Ascending arousal network connectivity during recovery from traumatic coma. Neuroimage Clin. 2020;28:102503. doi: 10.1016/j.nicl.2020.102503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edlow BL, Haynes RL, Takahashi E, Klein JP, Cummings P, Benner T, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72:505–523. doi: 10.1097/NEN.0b013e3182945bf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snider SB, Bodien YG, Bianciardi M, Brown EN, Wu O, Edlow BL. Disruption of the ascending arousal network in acute traumatic disorders of consciousness. Neurology. 2019;93:e1281–e1287. doi: 10.1212/WNL.0000000000008163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song M, He J, Yang Y, Jiang T. Combination of biomedical techniques and paradigms to improve prognostications for disorders of consciousness. Neurosci Bull. 2021;37:1082–1084. doi: 10.1007/s12264-021-00724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, et al. Willful modulation of brain activity in disorders of consciousness. N Engl J Med. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- 46.Laureys S, Goldman S, Phillips C, Bogaert PV, Aerts J, Luxen A, et al. Impaired effective cortical connectivity in vegetative state: Preliminary investigation using PET. Neuroimage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- 47.Laureys S, Faymonville ME, Luxen A, Lamy M, Franck G, Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1791. doi: 10.1016/S0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- 48.Bodien YG, Chatelle C, Edlow BL. Functional networks in disorders of consciousness. Semin Neurol. 2017;37:485–502. doi: 10.1055/s-0037-1607310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boly M, Faymonville ME, Schnakers C, Peigneux P, Lambermont B, Phillips C, et al. Perception of pain in the minimally conscious state with PET activation: An observational study. Lancet Neurol. 2008;7:1013–1020. doi: 10.1016/S1474-4422(08)70219-9. [DOI] [PubMed] [Google Scholar]
- 50.Aubinet C, Cassol H, Gosseries O, Bahri MA, Larroque SK, Majerus S, et al. Brain metabolism but not gray matter volume underlies the presence of language function in the minimally conscious state (MCS): MCS+ versus MCS− neuroimaging differences. Neurorehabil Neural Repair. 2020;34:172–184. doi: 10.1177/1545968319899914. [DOI] [PubMed] [Google Scholar]
- 51.Qin P, Wu X, Duncan NW, Bao W, Tang W, Zhang Z, et al. GABAA receptor deficits predict recovery in patients with disorders of consciousness: A preliminary multimodal [11C]Flumazenil PET and fMRI study. Hum Brain Mapp. 2015;36:3867–3877. doi: 10.1002/hbm.22883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stender J, Gosseries O, Bruno MA, Charland-Verville V, Vanhaudenhuyse A, Demertzi A, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet. 2014;384:514–522. doi: 10.1016/S0140-6736(14)60042-8. [DOI] [PubMed] [Google Scholar]
- 53.Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, et al. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016;87:2427–2434. doi: 10.1212/WNL.0000000000003404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Annen J, Frasso G, Crone JS, Heine L, Perri C, Martial C, et al. Regional brain volumetry and brain function in severely brain-injured patients. Ann Neurol. 2018;83:842–853. doi: 10.1002/ana.25214. [DOI] [PubMed] [Google Scholar]
- 55.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demertzi A, Antonopoulos G, Heine L, Voss HU, Crone JS, de Los Angeles C, et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain. 2015;138:2619–2631. doi: 10.1093/brain/awv169. [DOI] [PubMed] [Google Scholar]
- 57.Threlkeld ZD, Bodien YG, Rosenthal ES, Giacino JT, Nieto-Castanon A, Wu O, et al. Functional networks reemerge during recovery of consciousness after acute severe traumatic brain injury. Cortex. 2018;106:299–308. doi: 10.1016/j.cortex.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crone JS, Schurz M, Höller Y, Bergmann J, Monti M, Schmid E, et al. Impaired consciousness is linked to changes in effective connectivity of the posterior cingulate cortex within the default mode network. Neuroimage. 2015;110:101–109. doi: 10.1016/j.neuroimage.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.di Perri C, Amico E, Heine L, Annen J, Martial C, Larroque SK, et al. Multifaceted brain networks reconfiguration in disorders of consciousness uncovered by co-activation patterns. Hum Brain Mapp. 2018;39:89–103. doi: 10.1002/hbm.23826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demertzi A, Tagliazucchi E, Dehaene S, Deco G, Barttfeld P, Raimondo F, et al. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci Adv 2019, 5: eaat7603. [DOI] [PMC free article] [PubMed]
- 61.Cao B, Chen Y, Yu R, Chen L, Chen P, Weng Y, et al. Abnormal dynamic properties of functional connectivity in disorders of consciousness. Neuroimage Clin. 2019;24:102071. doi: 10.1016/j.nicl.2019.102071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crone JS, Lutkenhoff ES, Vespa PM, Monti MM. A systematic investigation of the association between network dynamics in the human brain and the state of consciousness. Neurosci Conscious 2020, 2020: niaa008. [DOI] [PMC free article] [PubMed]
- 63.Hahn G, Zamora-López G, Uhrig L, Tagliazucchi E, Laufs H, Mantini D, et al. Signature of consciousness in brain-wide synchronization patterns of monkey and human fMRI signals. Neuroimage. 2021;226:117470. doi: 10.1016/j.neuroimage.2020.117470. [DOI] [PubMed] [Google Scholar]
- 64.Standage D, Areshenkoff CN, Nashed JY, Hutchison RM, Hutchison M, Heinke D, et al. Dynamic reconfiguration, fragmentation, and integration of whole-brain modular structure across depths of unconsciousness. Cereb Cortex. 2020;30:5229–5241. doi: 10.1093/cercor/bhaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Signorelli CM, Uhrig L, Kringelbach M, Jarraya B, Deco G. Hierarchical disruption in the cortex of anesthetized monkeys as a new signature of consciousness loss. Neuroimage. 2021;227:117618. doi: 10.1016/j.neuroimage.2020.117618. [DOI] [PubMed] [Google Scholar]
- 66.Wu X, Zou Q, Hu J, Tang W, Mao Y, Gao L, et al. Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci. 2015;35:12932–12946. doi: 10.1523/JNEUROSCI.0415-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song M, Yang Y, He J, Yang Z, Yu S, Xie Q, et al. Prognostication of chronic disorders of consciousness using brain functional networks and clinical characteristics. eLife 2018, 7: e36173. [DOI] [PMC free article] [PubMed]
- 68.Galanaud D, Perlbarg V, Gupta R, Stevens RD, Sanchez P, Tollard E, et al. Assessment of white matter injury and outcome in severe brain trauma: A prospective multicenter cohort. Anesthesiology. 2012;117:1300–1310. doi: 10.1097/ALN.0b013e3182755558. [DOI] [PubMed] [Google Scholar]
- 69.Luppi AI, Golkowski D, Ranft A, Ilg R, Jordan D, Menon DK, et al. Brain network integration dynamics are associated with loss and recovery of consciousness induced by sevoflurane. Hum Brain Mapp. 2021;42:2802–2822. doi: 10.1002/hbm.25405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tan X, Gao J, Zhou Z, Wei R, Gong T, Wu Y, et al. Spontaneous recovery from unresponsive wakefulness syndrome to a minimally conscious state: Early structural changes revealed by 7-T magnetic resonance imaging. Front Neurol. 2018;8:741. doi: 10.3389/fneur.2017.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan X, Zhou Z, Gao J, Meng F, Yu Y, Zhang J, et al. Structural connectome alterations in patients with disorders of consciousness revealed by 7-tesla magnetic resonance imaging. Neuroimage Clin. 2019;22:101702. doi: 10.1016/j.nicl.2019.101702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bedini G, Bersano A, D’Incerti L, Marotta G, Rosazza C, Rossi Sebastiano D, et al. Period3 gene in disorder of consciousness: The role of neuroimaging in understanding the relationship between genotype and sleep. A brief communication. J Neurol Sci. 2017;381:220–225. doi: 10.1016/j.jns.2017.08.3253. [DOI] [PubMed] [Google Scholar]
- 73.Meiron O, Barron J, David J, Jaul E. Neural reactivity parameters of awareness predetermine one-year survival in patients with disorders of consciousness. Brain Inj. 2021;35:453–459. doi: 10.1080/02699052.2021.1879398. [DOI] [PubMed] [Google Scholar]
- 74.Gosseries O, Schnakers C, Ledoux D, Vanhaudenhuyse A, Bruno MA, Demertzi A, et al. Automated EEG entropy measurements in coma, vegetative state/unresponsive wakefulness syndrome and minimally conscious state. Funct Neurol. 2011;26:25–30. [PMC free article] [PubMed] [Google Scholar]
- 75.Müller M, Rossetti AO, Zimmermann R, Alvarez V, Rüegg S, Haenggi M, et al. Standardized visual EEG features predict outcome in patients with acute consciousness impairment of various etiologies. Crit Care. 2020;24:680. doi: 10.1186/s13054-020-03407-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren S, Shao H, He S. Interaction between conscious and unconscious information-processing of faces and words. Neurosci Bull. 2021;37:1583–1594. doi: 10.1007/s12264-021-00738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, et al. Preserved feedforward but impaired top-down processes in the vegetative state. Science. 2011;332:858–862. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- 78.Kotchoubey B, Lang S, Mezger G, Schmalohr D, Schneck M, Semmler A, et al. Information processing in severe disorders of consciousness: Vegetative state and minimally conscious state. Clin Neurophysiol. 2005;116:2441–2453. doi: 10.1016/j.clinph.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 79.Daltrozzo J, Wioland N, Mutschler V, Kotchoubey B. Predicting coma and other low responsive patients outcome using event-related brain potentials: A meta-analysis. Clin Neurophysiol. 2007;118:606–614. doi: 10.1016/j.clinph.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 80.Steppacher I, Eickhoff S, Jordanov T, Kaps M, Witzke W, Kissler J. N400 predicts recovery from disorders of consciousness. Ann Neurol. 2013;73:594–602. doi: 10.1002/ana.23835. [DOI] [PubMed] [Google Scholar]
- 81.Sitt JD, King JR, El Karoui I, Rohaut B, Faugeras F, Gramfort A, et al. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain. 2014;137:2258–2270. doi: 10.1093/brain/awu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosanova M, Fecchio M, Casarotto S, Sarasso S, Casali AG, Pigorini A, et al. Sleep-like cortical OFF-periods disrupt causality and complexity in the brain of unresponsive wakefulness syndrome patients. Nat Commun. 2018;9:4427. doi: 10.1038/s41467-018-06871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Annen J, Mertel I, Xu R, Chatelle C, Lesenfants D, Ortner R, et al. Auditory and somatosensory P3 are complementary for the assessment of patients with disorders of consciousness. Brain Sci. 2020;10:748. doi: 10.3390/brainsci10100748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Estraneo A, Fiorenza S, Magliacano A, Formisano R, Mattia D, Grippo A, et al. Multicenter prospective study on predictors of short-term outcome in disorders of consciousness. Neurology. 2020;95:e1488–e1499. doi: 10.1212/WNL.0000000000010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calabrò RS, Chillura A, Billeri L, Cannavò A, Buda A, Molonia F, et al. Peri-personal space tracing by hand-blink reflex modulation in patients with chronic disorders of consciousness. Sci Rep. 2020;10:1712. doi: 10.1038/s41598-020-58625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bai Y, Lin Y, Ziemann U. Managing disorders of consciousness: The role of electroencephalography. J Neurol. 2021;268:4033–4065. doi: 10.1007/s00415-020-10095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Höller Y, Thomschewski A, Bergmann J, Kronbichler M, Crone JS, Schmid EV, et al. Connectivity biomarkers can differentiate patients with different levels of consciousness. Clin Neurophysiol. 2014;125:1545–1555. doi: 10.1016/j.clinph.2013.12.095. [DOI] [PubMed] [Google Scholar]
- 88.Stefan S, Schorr B, Lopez-Rolon A, Kolassa IT, Shock JP, Rosenfelder M, et al. Consciousness indexing and outcome prediction with resting-state EEG in severe disorders of consciousness. Brain Topogr. 2018;31:848–862. doi: 10.1007/s10548-018-0643-x. [DOI] [PubMed] [Google Scholar]
- 89.Arnaldi D, Terzaghi M, Cremascoli R, de Carli F, Maggioni G, Pistarini C, et al. The prognostic value of sleep patterns in disorders of consciousness in the sub-acute phase. Clin Neurophysiol. 2016;127:1445–1451. doi: 10.1016/j.clinph.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 90.Thengone DJ, Voss HU, Fridman EA, Schiff ND. Local changes in network structure contribute to late communication recovery after severe brain injury. Sci Transl Med 2016, 8: 368re5. [DOI] [PubMed]
- 91.Claassen J, Doyle K, Matory A, Couch C, Burger KM, Velazquez A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380:2497–2505. doi: 10.1056/NEJMoa1812757. [DOI] [PubMed] [Google Scholar]
- 92.Gui P, Jiang Y, Zang D, Qi Z, Tan J, Tanigawa H, et al. Assessing the depth of language processing in patients with disorders of consciousness. Nat Neurosci. 2020;23:761–770. doi: 10.1038/s41593-020-0639-1. [DOI] [PubMed] [Google Scholar]
- 93.Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366:819–826. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- 94.Schnakers C, Hustinx R, Vandewalle G, Majerus S, Moonen G, Boly M, et al. Measuring the effect of amantadine in chronic anoxic minimally conscious state. J Neurol Neurosurg Psychiatry. 2008;79:225–227. doi: 10.1136/jnnp.2007.124099. [DOI] [PubMed] [Google Scholar]
- 95.Ghalaenovi H, Fattahi A, Koohpayehzadeh J, Khodadost M, Fatahi N, Taheri M, et al. The effects of amantadine on traumatic brain injury outcome: A double-blind, randomized, controlled, clinical trial. Brain Inj. 2018;32:1050–1055. doi: 10.1080/02699052.2018.1476733. [DOI] [PubMed] [Google Scholar]
- 96.Gao Y, Ma L, Liang F, Zhang Y, Yang L, Liu X, et al. The use of amantadine in patients with unresponsive wakefulness syndrome after severe cerebral hemorrhage. Brain Inj. 2020;34:1084–1088. doi: 10.1080/02699052.2020.1780315. [DOI] [PubMed] [Google Scholar]
- 97.Gao Y, Zhang Y, Li Z, Ma L, Yang J. Persistent vegetative state after severe cerebral hemorrhage treated with amantadine: A retrospective controlled study. Medicine. 2020;99:e21822. doi: 10.1097/MD.0000000000021822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee S, Lee HH, Lee Y, Lee J. Additive effect of cerebrolysin and amantadine on disorders of consciousness secondary to acquired brain injury: A retrospective case-control study. J Rehabil Med 2020, 52: jrm00025. [DOI] [PubMed]
- 99.DeMarchi R, Bansal V, Hung A, Wroblewski K, Dua H, Sockalingam S, et al. Review of awakening agents. Can J Neurol Sci. 2005;32:4–17. doi: 10.1017/S0317167100016826. [DOI] [PubMed] [Google Scholar]
- 100.Kim YW, Shin JC, An YS. Effects of methylphenidate on cerebral glucose metabolism in patients with impaired consciousness after acquired brain injury. Clin Neuropharmacol. 2009;32:335–339. doi: 10.1097/WNF.0b013e3181b40678. [DOI] [PubMed] [Google Scholar]
- 101.Martin RT, Whyte J. The effects of methylphenidate on command following and yes/no communication in persons with severe disorders of consciousness: A meta-analysis of n-of-1 studies. Am J Phys Med Rehabil. 2007;86:613–620. doi: 10.1097/PHM.0b013e3181154a84. [DOI] [PubMed] [Google Scholar]
- 102.Fridman EA, Calvar J, Bonetto M, Gamzu E, Krimchansky BZ, Meli F, et al. Fast awakening from minimally conscious state with apomorphine. Brain Inj. 2009;23:172–177. doi: 10.1080/02699050802649662. [DOI] [PubMed] [Google Scholar]
- 103.Hughes AJ, Lees AJ, Stern GM. Apomorphine test to predict dopaminergic responsiveness in parkinsonian syndromes. Lancet. 1990;336:32–34. doi: 10.1016/0140-6736(90)91531-E. [DOI] [PubMed] [Google Scholar]
- 104.Fridman EA, Krimchansky BZ, Bonetto M, Galperin T, Gamzu ER, Leiguarda RC, et al. Continuous subcutaneous apomorphine for severe disorders of consciousness after traumatic brain injury. Brain Inj. 2010;24:636–641. doi: 10.3109/02699051003610433. [DOI] [PubMed] [Google Scholar]
- 105.Machado C, Estévez M, Rodriguez-Rojas R. Zolpidem efficacy and safety in disorders of consciousness. Brain Inj. 2018;32:530–531. doi: 10.1080/02699052.2018.1429664. [DOI] [PubMed] [Google Scholar]
- 106.Whyte J, Myers R. Incidence of clinically significant responses to zolpidem among patients with disorders of consciousness: A preliminary placebo controlled trial. Am J Phys Med Rehabil. 2009;88:410–418. doi: 10.1097/PHM.0b013e3181a0e3a0. [DOI] [PubMed] [Google Scholar]
- 107.Ciurleo R, Bramanti P, Calabrò RS. Pharmacotherapy for disorders of consciousness: Are ‘awakening’ drugs really a possibility? Drugs. 2013;73:1849–1862. doi: 10.1007/s40265-013-0138-8. [DOI] [PubMed] [Google Scholar]
- 108.Sutton JA, Clauss RP. A review of the evidence of zolpidem efficacy in neurological disability after brain damage due to stroke, trauma and hypoxia: A justification of further clinical trials. Brain Inj. 2017;31:1019–1027. doi: 10.1080/02699052.2017.1300836. [DOI] [PubMed] [Google Scholar]
- 109.Calabrò RS, Aricò I, de Salvo S, Conti-Nibali V, Bramanti P. Transient awakening from vegetative state: Is high-dose zolpidem more effective? Psychiatry Clin Neurosci. 2015;69:122–123. doi: 10.1111/pcn.12215. [DOI] [PubMed] [Google Scholar]
- 110.Machado C, Estévez M, Rodríguez R, Pérez-Nellar J, Chinchilla M, DeFina P, et al. Zolpidem arousing effect in persistent vegetative state patients: Autonomic, EEG and behavioral assessment. Curr Pharm Des. 2014;20:4185–4202. [PubMed] [Google Scholar]
- 111.Whyte J, Rajan R, Rosenbaum A, Katz D, Kalmar K, Seel R, et al. Zolpidem and restoration of consciousness. Am J Phys Med Rehabilitation. 2014;93:101–113. doi: 10.1097/PHM.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 112.Khalili H, Rakhsha A, Ghaedian T, Niakan A, Masoudi N. Application of brain perfusion SPECT in the evaluation of response to zolpidem therapy in consciousness disorder due to traumatic brain injury. Indian J Nucl Med. 2020;35:315–320. doi: 10.4103/ijnm.IJNM_97_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sripad P, Rosenberg J, Boers F, Filss CP, Galldiks N, Langen KJ, et al. Effect of zolpidem in the aftermath of traumatic brain injury: An MEG study. Case Rep Neurol Med. 2020;2020:8597062. doi: 10.1155/2020/8597062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tucker C, Sandhu K. The effectiveness of zolpidem for the treatment of disorders of consciousness. Neurocrit Care. 2016;24:488–493. doi: 10.1007/s12028-015-0227-5. [DOI] [PubMed] [Google Scholar]
- 115.Shou Z, Li Z, Wang X, Chen M, Bai Y, Di H. Non-invasive brain intervention techniques used in patients with disorders of consciousness. Int J Neurosci. 2021;131:390–404. doi: 10.1080/00207454.2020.1744598. [DOI] [PubMed] [Google Scholar]
- 116.Bourdillon P, Hermann B, Sitt JD, Naccache L. Electromagnetic brain stimulation in patients with disorders of consciousness. Front Neurosci. 2019;13:223. doi: 10.3389/fnins.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Thibaut A, Bruno MA, Ledoux D, Demertzi A, Laureys S. tDCS in patients with disorders of consciousness: Sham-controlled randomized double-blind study. Neurology. 2014;82:1112–1118. doi: 10.1212/WNL.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 118.Thibaut A, Chatelle C, Vanhaudenhuyse A, Martens G, Cassol H, Martial C, et al. Transcranial direct current stimulation unveils covert consciousness. Brain Stimul. 2018;11:642–644. doi: 10.1016/j.brs.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 119.Mancuso M, Abbruzzese L, Canova S, Landi G, Rossi S, Santarnecchi E. Transcranial random noise stimulation does not improve behavioral and neurophysiological measures in patients with subacute vegetative-unresponsive wakefulness state (VS-UWS) Front Hum Neurosci. 2017;11:524. doi: 10.3389/fnhum.2017.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Angelakis E, Liouta E, Andreadis N, Korfias S, Ktonas P, Stranjalis G, et al. Transcranial direct current stimulation effects in disorders of consciousness. Arch Phys Med Rehabil. 2014;95:283–289. doi: 10.1016/j.apmr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 121.Thibaut A, Wannez S, Donneau AF, Chatelle C, Gosseries O, Bruno MA, et al. Controlled clinical trial of repeated prefrontal tDCS in patients with chronic minimally conscious state. Brain Inj. 2017;31:466–474. doi: 10.1080/02699052.2016.1274776. [DOI] [PubMed] [Google Scholar]
- 122.Martens G, Lejeune N, O’Brien AT, Fregni F, Martial C, Wannez S, et al. Randomized controlled trial of home-based 4-week tDCS in chronic minimally conscious state. Brain Stimul. 2018;11:982–990. doi: 10.1016/j.brs.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Song W, Du J, Huo S, Shan G, Li R. Transcranial direct current stimulation in patients with prolonged disorders of consciousness: Combined behavioral and event-related potential evidence. Front Neurol. 2017;8:620. doi: 10.3389/fneur.2017.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martens G, Kroupi E, Bodien Y, Frasso G, Annen J, Cassol H, et al. Behavioral and electrophysiological effects of network-based frontoparietal tDCS in patients with severe brain injury: A randomized controlled trial. Neuroimage Clin. 2020;28:102426. doi: 10.1016/j.nicl.2020.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol. 2017;128:56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 126.Piccione F, Cavinato M, Manganotti P, Formaggio E, Storti SF, Battistin L, et al. Behavioral and neurophysiological effects of repetitive transcranial magnetic stimulation on the minimally conscious state: A case study. Neurorehabil Neural Repair. 2011;25:98–102. doi: 10.1177/1545968310369802. [DOI] [PubMed] [Google Scholar]
- 127.Pisani LR, Naro A, Leo A, Aricò I, Pisani F, Silvestri R, et al. Repetitive transcranial magnetic stimulation induced slow wave activity modification: A possible role in disorder of consciousness differential diagnosis? Conscious Cogn. 2015;38:1–8. doi: 10.1016/j.concog.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 128.Cincotta M, Giovannelli F, Chiaramonti R, Bianco G, Godone M, Battista D, et al. No effects of 20 Hz-rTMS of the primary motor cortex in vegetative state: A randomised, sham-controlled study. Cortex. 2015;71:368–376. doi: 10.1016/j.cortex.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 129.Xia X, Liu Y, Bai Y, Liu Z, Yang Y, Guo Y, et al. Long-lasting repetitive transcranial magnetic stimulation modulates electroencephalography oscillation in patients with disorders of consciousness. Neuroreport. 2017;28:1022–1029. doi: 10.1097/WNR.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 130.He F, Wu M, Meng F, Hu Y, Gao J, Chen Z, et al. Effects of 20 Hz repetitive transcranial magnetic stimulation on disorders of consciousness: A resting-state electroencephalography study. Neural Plast. 2018;2018:5036184. doi: 10.1155/2018/5036184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xia X, Wang Y, Li C, Li X, He J, Bai Y. Transcranial magnetic stimulation-evoked connectivity reveals modulation effects of repetitive transcranial magnetic stimulation on patients with disorders of consciousness. Neuroreport. 2019;30:1307–1315. doi: 10.1097/WNR.0000000000001362. [DOI] [PubMed] [Google Scholar]
- 132.Xia X, Bai Y, Zhou Y, Yang Y, Xu R, Gao X, et al. Effects of 10 Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in disorders of consciousness. Front Neurol. 2017;8:182. doi: 10.3389/fneur.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Naro A, Russo M, Leo A, Bramanti P, Quartarone A, Calabrò RS. A single session of repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex in patients with unresponsive wakefulness syndrome: Preliminary results. Neurorehabil Neural Repair. 2015;29:603–613. doi: 10.1177/1545968314562114. [DOI] [PubMed] [Google Scholar]
- 134.Ge X, Zhang Y, Xin T, Luan X. Effects of 10 Hz repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in the vegetative state. Exp Ther Med. 2021;21:206. doi: 10.3892/etm.2021.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jang SH, Kwon YH. Effect of repetitive transcranial magnetic stimulation on the ascending reticular activating system in a patient with disorder of consciousness: A case report. BMC Neurol. 2020;20:37. doi: 10.1186/s12883-020-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pape TLB, Rosenow JM, Patil V, Steiner M, Harton B, Guernon A, et al. RTMS safety for two subjects with disordered consciousness after traumatic brain injury. Brain Stimul. 2014;7:620–622. doi: 10.1016/j.brs.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 137.Kletzel SL, Aaronson AL, Guernon A, Carbone C, Chaudhry N, Walsh E, et al. Safety considerations for the use of transcranial magnetic stimulation as treatment for coma recovery in people with severe traumatic brain injury. J Head Trauma Rehabil. 2020;35:430–438. doi: 10.1097/HTR.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 139.Lemaire JJ, Sontheimer A, Pereira B, Coste J, Rosenberg S, Sarret C, et al. Deep brain stimulation in five patients with severe disorders of consciousness. Ann Clin Transl Neurol. 2018;5:1372–1384. doi: 10.1002/acn3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Magrassi L, Maggioni G, Pistarini C, di Perri C, Bastianello S, Zippo AG, et al. Results of a prospective study (CATS) on the effects of thalamic stimulation in minimally conscious and vegetative state patients. J Neurosurg. 2016;125:972–981. doi: 10.3171/2015.7.JNS15700. [DOI] [PubMed] [Google Scholar]
- 141.Chudy D, Deletis V, Almahariq F, Marčinković P, Škrlin J, Paradžik V. Deep brain stimulation for the early treatment of the minimally conscious state and vegetative state: Experience in 14 patients. J Neurosurg. 2018;128:1189–1198. doi: 10.3171/2016.10.JNS161071. [DOI] [PubMed] [Google Scholar]
- 142.Giacino JT, Katz DI, Whyte J. Neurorehabilitation in disorders of consciousness. Semin Neurol. 2013;33:142–156. doi: 10.1055/s-0033-1348960. [DOI] [PubMed] [Google Scholar]
- 143.Schiff ND, Fins JJ. Deep brain stimulation and cognition: Moving from animal to patient. Curr Opin Neurol. 2007;20:638–642. doi: 10.1097/WCO.0b013e3282f1c6e4. [DOI] [PubMed] [Google Scholar]
- 144.Schiff ND. Central thalamic deep-brain stimulation in the severely injured brain: Rationale and proposed mechanisms of action. Ann N Y Acad Sci. 2009;1157:101–116. doi: 10.1111/j.1749-6632.2008.04123.x. [DOI] [PubMed] [Google Scholar]
- 145.Gonzalez-Escamilla G, Muthuraman M, Ciolac D, Coenen VA, Schnitzler A, Groppa S. Neuroimaging and electrophysiology meet invasive neurostimulation for causal interrogations and modulations of brain states. Neuroimage. 2020;220:117144. doi: 10.1016/j.neuroimage.2020.117144. [DOI] [PubMed] [Google Scholar]
- 146.Garcia-Rill E, Luster B, D’Onofrio S, Mahaffey S, Bisagno V, Urbano FJ. Pedunculopontine arousal system physiology - Deep brain stimulation (DBS) Sleep Sci. 2015;8:153–161. doi: 10.1016/j.slsci.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu YT, Yang Y, Wang LB, Fang JL, Chen YY, He JH, et al. Transcutaneous auricular vagus nerve stimulation in disorders of consciousness monitored by fMRI: The first case report. Brain Stimul. 2017;10:328–330. doi: 10.1016/j.brs.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 148.Corazzol M, Lio G, Lefevre A, Deiana G, Tell L, André-Obadia N, et al. Restoring consciousness with vagus nerve stimulation. Curr Biol. 2017;27:R994–R996. doi: 10.1016/j.cub.2017.07.060. [DOI] [PubMed] [Google Scholar]
- 149.Yu Y, Yang Y, Gan S, Guo S, Fang J, Wang S, et al. Cerebral hemodynamic correlates of transcutaneous auricular vagal nerve stimulation in consciousness restoration: An open-label pilot study. Front Neurol. 2021;12:684791. doi: 10.3389/fneur.2021.684791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Megha M, Harpreet S, Nayeem Z. Effect of frequency of multimodal coma stimulation on the consciousness levels of traumatic brain injury comatose patients. Brain Inj. 2013;27:570–577. doi: 10.3109/02699052.2013.767937. [DOI] [PubMed] [Google Scholar]
- 151.Cheng L, Cortese D, Monti MM, Wang F, Riganello F, Arcuri F, et al. Do sensory stimulation programs have an impact on consciousness recovery? Front Neurol. 2018;9:826. doi: 10.3389/fneur.2018.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Riganello F, Cortese MD, Arcuri F, Quintieri M, Dolce G. How can music influence the autonomic nervous system response in patients with severe disorder of consciousness? Front Neurosci. 2015;9:461. doi: 10.3389/fnins.2015.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Magliacano A, de Bellis F, Galvao-Carmona A, Estraneo A, Trojano L. Can salient stimuli enhance responses in disorders of consciousness? A systematic review. Curr Neurol Neurosci Rep. 2019;19:98. doi: 10.1007/s11910-019-1018-8. [DOI] [PubMed] [Google Scholar]
- 154.Sullivan EG, Guernon A, Blabas B, Herrold AA, Pape TLB. Familiar auditory sensory training in chronic traumatic brain injury: A case study. Disabil Rehabil. 2018;40:945–951. doi: 10.1080/09638288.2016.1277403. [DOI] [PubMed] [Google Scholar]
- 155.Padua L, Cuccagna C, Pazzaglia C. Novel sensory paradigms for neuromodulation in disorders of consciousness in traumatic brain injury. Curr Opin Neurol. 2019;32:844–849. doi: 10.1097/WCO.0000000000000747. [DOI] [PubMed] [Google Scholar]
- 156.Gibson RM, Owen AM, Cruse D. Brain-computer interfaces for patients with disorders of consciousness. Prog Brain Res. 2016;228:241–291. doi: 10.1016/bs.pbr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 157.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 158.Charland-Verville V, Lesenfants D, Lee S, Noirhomme Q, Ziegler E, Chatelle C, et al. Detection of response to command using voluntary control of breathing in disorders of consciousness. Front Hum Neurosci. 2014;8:1020. doi: 10.3389/fnhum.2014.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lesenfants D, Habbal D, Chatelle C, Schnakers C, Laureys S, Noirhomme Q. Electromyographic decoding of response to command in disorders of consciousness. Neurology. 2016;87:2099–2107. doi: 10.1212/WNL.0000000000003333. [DOI] [PubMed] [Google Scholar]
- 160.Stoll J, Chatelle C, Carter O, Koch C, Laureys S, Einhäuser W. Pupil responses allow communication in locked-in syndrome patients. Curr Biol. 2013;23:R647–R648. doi: 10.1016/j.cub.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 161.Wilhelm B, Jordan M, Birbaumer N. Communication in locked-in syndrome: Effects of imagery on salivary pH. Neurology. 2006;67:534–535. doi: 10.1212/01.wnl.0000228226.86382.5f. [DOI] [PubMed] [Google Scholar]
- 162.Pistoia F, Sacco S, Sarà M, Franceschini M, Carolei A. Intrathecal baclofen: Effects on spasticity, pain, and consciousness in disorders of consciousness and locked-in syndrome. Curr Pain Headache Rep. 2015;19:466. doi: 10.1007/s11916-014-0466-8. [DOI] [PubMed] [Google Scholar]
- 163.Carboncini MC, Piarulli A, Virgillito A, Arrighi P, Andre P, Tomaiuolo F, et al. A case of post-traumatic minimally conscious state reversed by midazolam: Clinical aspects and neurophysiological correlates. Restor Neurol Neurosci. 2014;32:767–787. doi: 10.3233/RNN-140426. [DOI] [PubMed] [Google Scholar]
- 164.Lanzillo B, Loreto V, Calabrese C, Estraneo A, Moretta P, Trojano L. Does pain relief influence recovery of consciousness? A case report of a patient treated with ziconotide. Eur J Phys Rehabil Med. 2016;52:263–266. [PubMed] [Google Scholar]
- 165.Monti MM, Schnakers C, Korb AS, Bystritsky A, Vespa PM. Non-invasive ultrasonic thalamic stimulation in disorders of consciousness after severe brain injury: A first-in-man report. Brain Stimul. 2016;9:940–941. doi: 10.1016/j.brs.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 166.Yamamoto T, Watanabe M, Obuchi T, Kobayashi K, Oshima H, Fukaya C, et al. Spinal cord stimulation for vegetative state and minimally conscious state: Changes in consciousness level and motor function. Acta Neurochir Suppl. 2017;124:37–42. doi: 10.1007/978-3-319-39546-3_6. [DOI] [PubMed] [Google Scholar]
- 167.Vanzan S, Wilkinson D, Ferguson H, Pullicino P, Sakel M. Behavioural improvement in a minimally conscious state after caloric vestibular stimulation: Evidence from two single case studies. Clin Rehabil. 2017;31:500–507. doi: 10.1177/0269215516646167. [DOI] [PubMed] [Google Scholar]
- 168.Ng H, King A. A systematic review of head-up tilt to improve consciousness in people with a prolonged disorder of consciousness. Clin Rehabil. 2021;35:13–25. doi: 10.1177/0269215520946696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bender Pape TL, Herrold AA, Livengood SL, Guernon A, Weaver JA, Higgins JP, et al. A pilot trial examining the merits of combining amantadine and repetitive transcranial magnetic stimulation as an intervention for persons with disordered consciousness after TBI. J Head Trauma Rehabilitation. 2020;35:371–387. doi: 10.1097/HTR.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 170.Delargy M, O’Connor R, McCann A, Galligan I, Cronin H, Gray D, et al. An analysis of the effects of using Zolpidem and an innovative multimodal interdisciplinary team approach in prolonged disorders of consciousness (PDOC) Brain Inj. 2019;33:242–248. doi: 10.1080/02699052.2018.1537008. [DOI] [PubMed] [Google Scholar]
- 171.Kurz EM, Wood G, Kober SE, Schippinger W, Pichler G, Müller-Putz G, et al. Towards using fNIRS recordings of mental arithmetic for the detection of residual cognitive activity in patients with disorders of consciousness (DOC) Brain Cogn. 2018;125:78–87. doi: 10.1016/j.bandc.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 172.Guller Y, Giacino J. Potential applications of concurrent transcranial magnetic stimulation and functional magnetic resonance imaging in acquired brain injury and disorders of consciousness. Brain Inj. 2014;28:1190–1196. doi: 10.3109/02699052.2014.920527. [DOI] [PubMed] [Google Scholar]
- 173.Bodart O, Gosseries O, Wannez S, Thibaut A, Annen J, Boly M, et al. Measures of metabolism and complexity in the brain of patients with disorders of consciousness. Neuroimage Clin. 2017;14:354–362. doi: 10.1016/j.nicl.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Straudi S, Bonsangue V, Mele S, Craighero L, Montis A, Fregni F, et al. Bilateral M1 anodal transcranial direct current stimulation in post traumatic chronic minimally conscious state: A pilot EEG-tDCS study. Brain Inj. 2019;33:490–495. doi: 10.1080/02699052.2019.1565894. [DOI] [PubMed] [Google Scholar]
- 175.Zhang Y, Yang Y, Si J, Xia X, He J, Jiang T. Influence of inter-stimulus interval of spinal cord stimulation in patients with disorders of consciousness: A preliminary functional near-infrared spectroscopy study. Neuroimage Clin. 2017;17:1–9. doi: 10.1016/j.nicl.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]