Abstract
Objective
To compare the clinical characteristics of Stanford type B aortic intramural hematoma (IMH) and Stanford type B aortic dissection (AD), and to identify the differences between thoracic endovascular aortic repair (TEVAR) and medical management (MM) in the Stanford type B IMH patients.
Methods
A retrospective observational study was conducted in patients treated between January 2015 and December 2016. The clinical characteristics and CT images of patients with type B IMH and type B AD were compared, and the clinical characteristics and CT images of patients in the type B IMH group who were treated with TEVAR and MM were compared.
Results
A total of 176 patients were included in this study, including 62 patients of type B IMH and 114 patients of type B AD. Five patients died in the IMH group and three in the AD group. The proximal hematoma or entry tear in both groups was mainly located in the descending aorta, and the proportion of the iliac artery involved in the AD group was significantly higher than that in the IMH group (31.6% vs. 8.1% P < 0.05). There were 50 MM patients and 12 TEVAR patients in the IMH group. No death occurred in the TEVAR group, while five patients in the MM group died. Seven patients in the MM group had disease progression vs. 12 in the TEVAR group (P < 0.05). The patients in the TEVAR group had more intima lesions than those in the MM group (83.3% vs. 30.0%, P < 0.05). TEVAR group involved more iliac artery hematoma than MM group (33.3% vs. 2.0%, P < 0.05). The maximum thickness of hematoma in TEVAR group was 14.9 ± 3.4 mm, which was significantly larger than that of MM group (10.2 ± 2.8 mm) (P < 0.05).
Conclusion
In the diagnosis of IMH, patients' symptoms and high-risk signs of CTA should be paid attention to. TEVAR therapy should be actively considered on the basis of effective medical management when there are intima lesions (ULP/PAU), increased aortic diameter and hematoma thickness, extensive hematoma involvement, and pleural effusion.
Keywords: intramural hematoma, TEVAR, medical management, aortic dissection, maximum aortic diameter
Introduction
Aortic intramural hematoma (IMH) has similar clinical manifestations to aortic dissection (AD) and penetrating aortic ulcer (PAU), so it is called acute aortic syndrome (AAS), of which IMH accounts for about 10%–30% of the total (1). In 1920, Krukenberg et al. first described IMH as “aortic dissection without intimal rupture” (2). Similar to AD, IMH can be divided into Stanford type A (hematoma involving the ascending and/or descending aorta) and Stanford type B (hematoma involving only the descending aorta), with the latter accounting for 50%–85% (3).
The initiating factors leading to the formation of IMH have not yet been fully clarified, but hypertension, atherosclerosis and smoking undoubtedly play an important role (4). The current mainstream view is that the hemorrhage in the aortic wall originates from the spontaneous rupture of the aortic vasa vasorum, resulting in the formation of a hematoma. Some scholars also believe that the tiny breach on the aortic intima or PAU is an important reason for the formation of IMH. Other possible mechanisms include pathological proliferation and spontaneous rupture of microvessels in atherosclerotic plaques (5). After an intermural hemorrhage, the aortic wall becomes weak, but the intima of the artery remains intact without rupture or internal diaphragm formation.
The clinical manifestations of IMH patients are similar to AD, mainly with the sudden onset of chest and back laceration pain, and most patients have a history of hypertension. CTA is the preferred method for imaging examination, which can not only confirm the diagnosis, but also know the hematoma range, diameter and whether there are microscopic lesions in the intima, providing a basis for subsequent treatment (6). It is generally believed that for Stanford type A IMH, surgery should be performed as soon as possible to prevent severe complications. For Stanford type B IMH, most patients can choose medical management (MM). When the IMH disease progresses, surgical intervention should be considered, and thoracic endovascular aortic repair (TEVAR) is the preferred treatment method (7).
Relevant studies indicate that 88% of type A IMH patients will progress to AD, while the proportion of type B IMH patients will progress to AD is 3%–14%. The natural course of IMH is mostly stable or absorbed after conservative treatment, but 15%–20% of patients will progress to AD or even aortic rupture, and other complications include pleural effusion and cardiac tamponade (1). This study will compare the clinical characteristics of type B IMH and type B AD, as well as the disease progression and outcomes of patients with type B IMH in the TEVAR group and MM group, in order to analyze the characteristics of the disease, CT image characteristics, treatment methods and prognosis.
Materials and methods
Patients and study design
In this study, patients admitted to the Department of Vascular Surgery of the First Affiliated Hospital of Anhui Medical University from January 2015 to December 2016 were collected, including 114 patients with type B AD and 62 patients with type B IMH. Complicated or high-risk type B AD were treated with TEVAR. All type B IMH patients were initially treated with medication on admission, primarily to control blood pressure, heart rate and pain. TEVAR treatment was actively considered for complicated cases of type B IMH in our center. PAU, ulcer like projection (ULP), intramural blood pooling were subjects for elective TEVAR. 68 patients in the AD group were treated with TEVAR, 12 patients in the IMH group were treated with TEVAR and 50 patients were treated with MM. This study was approved by the ethics committee of our hospital, and all patients gave informed consent. The exclusion criteria of this study included: transfer, withdrawal from treatment, severe complications and short survival period. A total of five patients in the type B AD group were excluded, and three patients in the type B IMH group were excluded.
Data collection and analysis
This study firstly compared the clinical characteristics and CT image differences between type B IMH and type B AD patients, and then compared the clinical and CT image characteristics of MM group and TEVAR group in type B IMH patients.The collected indicators included demographic data, past history, chronicity classification, onset symptoms, positive findings in physical examination, CT imaging characteristics, treatment method, therapeutic outcome, in-hospital complications, in-hospital mortality, etc.
Definitions and follow-up
All patients were evaluated with CTA on admission. The diagnostic criteria for AD is a typical double-channel aorta with a visible intimal tear or flap. Typical IMH on CTA imaging showed smooth, crescent-shaped aortic artery wall (CT value: 60–80 HU) or thickened annular aortic wall (>5 mm) with longitudinal extension, without intimal laceration or double-lumen flow concave (4). CTA was followed up once before discharge, three months after discharge, 12 months after discharge and 24 months after discharge. High-risk type B AD refers to the presence of any of the following risk factors: refractory pain, refractory hypertension, bloody pleural effusion, aortic diameter >40 mm, false lumen diameter >22 mm, radiographic only malperfusion, entry tear: lesser curve location, and readmission. Complicated type B AD include rupture and malperfusion. Complicated type B IMH was defined as recurrent pain, enlarged or thickened hematoma, mal-perfusion, maximum aortic diameter ≥55 mm, evolution to classic dissection or aneurysm, aortic rupture or impending rupture.
Statistical analysis
Statistical analysis was performed with SPSS, release 19.0 for Windows. Categorical variables were compared using χ2 tests or Fisher's exact test. Continuous variables were compared using Student's t test or Mann-Whitney U-test. All reported P values are two-sided, and P values <0.05 were considered to be statistically significant.
Results
Comparison of clinical characteristics of type B IMH and type B AD
A total of 176 patients were included in this study, including 62 cases of type B IMH and 114 cases of type B AD. In the type B IMH group, there were 40 males and 22 females. In the type B AD group, there were 96 males and 18 females. There was a significant difference in gender composition between the two groups, and the proportion of males in the type B AD group was significantly higher (84.2% vs. 64.5%; P = 0.003). The average age of the type B IMH group was 68.4 ± 12.3 years, which was significantly higher than that of the type B AD group (58.3 ± 11.6 years) (P = 0.031). The risk factors included hypertension, diabetes mellitus, dyslipidemia, smoking history, coronary heart disease, pulmonary disease and chronic renal insufficiency. The proportion of chronic renal insufficiency in type B AD group was significantly higher than that in type B IMH group (15.8% vs. 3.2%; P = 0.012). Chronicity classification was formulated by Society for Vascular Surgery/Society of Thoracic Surgeons (SVS/STS), including hyperacute (<24 h); acute (1 to 14 days); subacute (15 to 90 days); and chronic (>90 days). The difference in chronicity classification between the two groups was not statistically significant. The initial symptoms of both groups were mainly chest and back pain, and other symptoms included abdominal pain, loss of consciousness, dyspnea, etc. The comparison of malperfusion in type B AD and type B IMH patients included lower limb, mesenteric, celiac and renal malperfusion. The renal malperfusion in type B AD group was significantly higher than that in type B IMH group (17.5% vs. 4.8%; P = 0.017). Serum creatinine in the type B AD group was also higher than that in the IMH group, but the difference between the two groups was not statistically significant (89.1 ± 31.1 vs. 82.8 ± 30.9; P = 0.200). In terms of admission hemodynamics, there was no significant difference in systolic blood pressure, diastolic blood pressure and heart rate between the two groups. 12 patients in the type B IMH group were finally treated with TEVAR, and 68 patients in the type B AD group were treated with TEVAR. There was no significant difference in in-hospital complications between the two groups, mainly including pulmonary infection, myocardial infarction, stroke, cardiac tamponade, and aortic rupture. Five patients died in the type B IMH group (three died of aortic rupture, one died of cerebral infarction, and one died of esophageal cancer), and three patients died in the type B AD group (two postoperatively from aortic rupture and one intraoperatively). In-hospital mortality in the type B IMH group was higher than in the type B AD group, but there was no significant difference between the two groups (8.1% vs. 2.6%; P = 0.098). All these outcomes are depicted in Table 1.
Table 1.
Clinical characteristics of type B IMH and type B AD.
| Category | Type B IMH | Type B AD | P value |
|---|---|---|---|
| Number of patients | 62 | 114 | |
| Male gender | 40 (64.5) | 96 (84.2) | 0.003 |
| Age, years | 68.4 ± 12.3 | 58.3 ± 11.6 | 0.031 |
| Risk factors | |||
| Hypertension | 58 (93.5) | 106 (93.0) | 1.000 |
| Diabetes mellitus | 4 (6.5) | 12 (10.5) | 0.369 |
| Dyslipidemia | 14 (22.6) | 32 (28.1) | 0.429 |
| History of smoking | 23 (37.1) | 59 (51.8) | 0.063 |
| Coronary heart disease | 5 (8.1) | 15 (13.2) | 0.309 |
| Pulmonary disease | 3 (4.8) | 9 (7.9) | 0.649 |
| Chronic renal insufficiency, eGFR < 60 ml/min/1.73 m2 | 2 (3.2) | 18 (15.8) | 0.012 |
| Chronicity classificationa | |||
| Hyperacute | 25 (40.3) | 33 (28.9) | 0.125 |
| Acute | 19 (30.6) | 30 (26.3) | 0.540 |
| Subacute | 18 (29.0) | 50 (43.9) | 0.054 |
| Chronic | 0 (0) | 1 (0.9) | 1.000 |
| Initial symptom | |||
| Chest and back pain | 45 (72.6) | 90 (78.9) | 0.340 |
| Abdominal pain | 14 (22.6) | 18 (15.8) | 0.265 |
| Loss of consciousness | 1 (1.6) | 3 (2.6) | 1.000 |
| Dyspnea | 1 (1.6) | 2 (1.8) | 1.000 |
| Other | 1 (1.6) | 1 (0.9) | 1.000 |
| Malperfusion | |||
| Lower limb | 1 (1.6) | 6 (5.3) | 0.435 |
| Mesenteric | 1 (1.6) | 4 (3.5) | 0.804 |
| Celiac | 0 (0) | 2 (1.8) | 0.541 |
| Renal | 3 (4.8) | 20 (17.5) | 0.017 |
| Serum creatinine, umol/l | 82.8 ± 30.9 | 89.1 ± 31.1 | 0.200 |
| Hemodynamics on admission | |||
| SBP, mm Hg | 142.0 ± 35.1 | 150.1 ± 37.5 | 0.064 |
| DBP, mm Hg | 92.4 ± 19.5 | 95.5 ± 18.4 | 0.231 |
| Heart rate | 90.7 ± 16.8 | 91.8 ± 21.5 | 0.346 |
| TEVAR | 12 (19.3) | 68 (59.6) | <0.001 |
| In-hospital complications | |||
| Pulmonary infection | 3 (4.8) | 5 (4.3) | 1.000 |
| MI | 1 (1.6) | 3 (2.6) | 1.000 |
| Stroke | 1 (1.6) | 2 (1.8) | 1.000 |
| Cardiac tamponade | 1 (1.6) | 1 (0.9) | 1.000 |
| Aortic rupture | 3 (4.8) | 2 (1.8) | 0.483 |
| In-hospital mortality | 5 (8.1) | 3 (2.6) | 0.098 |
AD, aortic dissection; DBP, diastolic blood pressure; IMH, intramural hematoma; MI, myocardial infarction; SBP, systolic blood pressure; TEVAR, thoracic endovascular aortic repair.
Hyperacute, <24 h; acute, 1 to 14 days; subacute, 15 to 90 days; and chronic, >90 days. Society for Vascular Surgery/Society of Thoracic Surgeons (SVS/STS) chronicity classification of aortic dissection.
Categorical variables are presented as number (%). Continuous variables are presented as mean ± standard deviation.
Comparison of CT imaging of type B IMH and type B AD
The CT imaging differences between type B IMH and type B AD groups were further compared. From the initial CTA at the time of admission, the proximal hematoma or rupture in both groups was mainly located in the descending aorta. In terms of the extent of disease involvement, the proportion of type B AD involving the iliac artery was significantly higher than that in the type B IMH group (31.6% vs. 8.1%; P < 0.001). There was no significant difference in the maximum diameter of the aorta between the two groups, and the maximum thickness of the intramural hematoma in the type B IMH group was 12.7 ± 5.3 mm on average. There were 15 and 25 patients with pleural effusion in the type B IMH group and type B AD group, respectively (24.2% vs. 21.9; P = 0.732). All these outcomes are depicted in Table 2. Imaging data of malperfusion and pleural effusion in type B AD and type B IMH patients can be seen in Figure 1.
Table 2.
Ct imaging of type B IMH and type B AD.
| Category | IMH | AD | P value |
|---|---|---|---|
| Number of patients | 62 | 114 | |
| Location of proximal hematoma or entry tears | |||
| Descending aorta | 58 (93.5) | 110 (96.5) | 0.605 |
| Abdominal aorta | 4 (6.5) | 4 (3.5) | 0.605 |
| Distal location of the lesion | |||
| Descending aorta | 16 (25.8) | 14 (12.3) | 0.023 |
| Abdominal aorta | 41 (66.1) | 64 (56.1) | 0.197 |
| Iliac artery | 5 (8.1) | 36 (31.6) | <0.001 |
| MAD, mm | 41.2 ± 7.5 | 39.4 ± 6.9 | 0.146 |
| Maximum haematoma thickness, mm | 12.7 ± 5.3 | / | / |
| Pleural effusion | 15 (24.2) | 25 (21.9) | 0.732 |
AD, aortic dissection; IMH, intramural hematoma; MAD, maximum aortic diameter.
Categorical variables are presented as number (%). Continuous variables are presented as mean ± standard deviation.
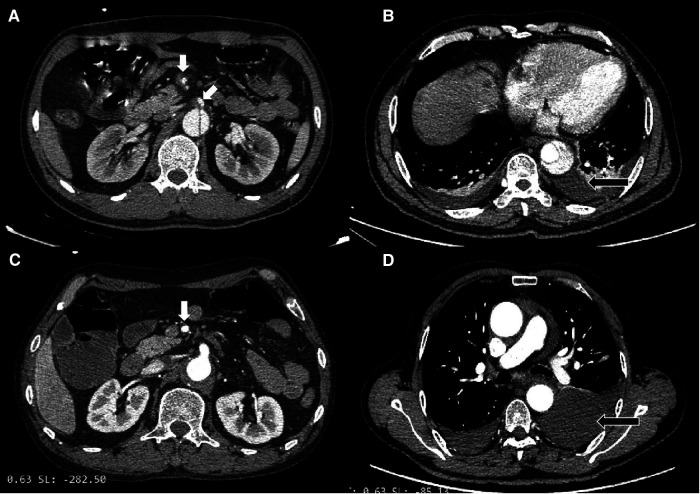
Figure 1.
(A) Type B AD with superior mesenteric artery malperfusion. (B) Type B AD with pleural effusion. (C) Type B IMH with superior mesenteric artery malperfusion. (D) Type B IMH with pleural effusion.
Comparison of clinical characteristics between TEVAR group and MM group in type B IMH
Patients with type B IMH were divided into TEVAR group and MM group, and the clinical characteristics of the two groups were compared. There was no significant difference in gender composition and age between the two groups. Among the risk factors, the proportion of patients with dyslipidemia in the TEVAR group was significantly higher (66.7% vs. 12.0%; P < 0.001). The difference in chronicity classification between the two groups was not statistically significant between hyperacute phase and acute phase. A higher proportion of patients in the MM group were in the subacute phase compared with the TEVAR group (36.0% vs. 0%; P = 0.035). The initial symptoms were mainly chest and back pain, and one patient in the TEVAR group had loss of consciousness. There were no significant differences in systolic blood pressure, diastolic blood pressure, and heart rate between the two groups at admission. In terms of blood pressure fluctuation, the maximum daily increase of blood pressure in the TEVAR group was significantly higher than that in the MM group (32.1 ± 7.8 vs. 20.4 ± 9.4; P = 0.045). There was no significant difference in in-hospital complications between the two groups, mainly including pulmonary infection, myocardial infarction, stroke, cardiac tamponade, and aortic rupture. There were no deaths during hospitalization in the TEVAR group, compared with five deaths in the MM group (The reason is mentioned above). One-year mortality was higher in the MM group than in the TEVAR group, but the difference was not significant (12.0% vs. 8.3%; P = 1.000). All these outcomes are depicted in Table 3.
Table 3.
Clinical characteristics of TEVAR group and MM group.
| Category | MM | TEVAR | P value |
|---|---|---|---|
| Number of patients | 50 | 12 | |
| Male gender | 33 (66.0) | 7 (58.3) | 0.513 |
| Age, years | 63.7 ± 12.7 | 61.0 ± 15.1 | 0.527 |
| Risk factors | |||
| Hypertension | 48 (96.0) | 10 (83.3) | 0.166 |
| Diabetes mellitus | 2 (4.0) | 2 (16.7) | 0.166 |
| Dyslipidemia | 6 (12.0) | 8 (66.7) | <0.001 |
| History of smoking | 17 (34.0) | 6 (50.0) | 0.485 |
| Coronary heart disease | 3 (6.0) | 2 (16.7) | 0.246 |
| Pulmonary disease | 2 (4.0) | 1 (8.3) | 0.482 |
| Renal insufficiency, eGFR < 60 ml/min/1.73 m2 | 1 (2.0) | 1 (8.3) | 0.352 |
| Chronicity classificationa | |||
| Hyperacute | 19 (38.0) | 6 (50.0) | 0.665 |
| Acute | 13 (26.0) | 6 (50.0) | 0.204 |
| Subacute | 18 (36.0) | 0 (0) | 0.035 |
| Chronic | 0 (0) | 0 (0) | / |
| Initial symptom | |||
| Chest and back pain | 35 (70.0) | 10 (83.3) | 0.569 |
| Abdominal pain | 13 (26.0) | 1 (8.3) | 0.352 |
| Loss of consciousness | 0 (0) | 1 (8.3) | 0.194 |
| Dyspnea | 1 (2.0) | 0 (0) | 1.000 |
| Other | 1 (2.0) | 0 (0) | 1.000 |
| Hemodynamics on admission | |||
| SBP, mm Hg | 139.2 ± 38.4 | 145.4 ± 36.9 | 0.076 |
| DBP, mm Hg | 89.4 ± 21.5 | 95.4 ± 19.7 | 0.102 |
| Heart rate | 88.1 ± 17.3 | 93 ± 18.0 | 0.213 |
| In-hospital complications | |||
| Pulmonary infection | 2 (4.0) | 1 (8.3) | 0.482 |
| MI | 1 (2.0) | 0 (0) | 1.000 |
| Stroke | 1 (2.0) | 0 (0) | 1.000 |
| Cardiac tamponade | 1 (2.0) | 0 (0) | 1.000 |
| Aortic rupture | 3 (6.0) | 0 (0) | 1.000 |
| In-hospital mortality | 5 (10.0) | 0 (0) | 0.573 |
| One-year mortality | 6 (12.0) | 1 (8.3) | 1.000 |
DBP, diastolic blood pressure; MM, medical management; SBP, systolic blood pressure; TEVAR, thoracic endovascular aortic repair.
Categorical variables are presented as number (%). Continuous variables are presented as mean ± standard deviation.
Comparison of CT imaging between TEVAR group and MM group intype B IMH
To further investigate the differences in CT imaging characteristics between the two groups of patients with type B IMH, the following data were compared in this study: the number and location of intima lesions, the initial location and range of hematoma, the size of aorta and hematoma, CT value of hematoma, pleural effusion and other indicators and their reexamination results. The results showed that most of the patients in the TEVAR group had intima lesions (including ULP and PAU), which were significantly higher than those in the MM group (83.3% vs. 30.0%; P = 0.002). The intima lesions in the TEVAR group were mostly multiple, whereas the intima lesions in the MM group were mostly solitary. There was no significant difference between the two groups in the location of intimal lesions, which were mainly in the descending aorta. The proximal hematoma location was similar between the two groups, and most of them were located in the descending aorta (96.0% vs. 83.3%; P = 0.166). However, the TEVAR group had a wider range of hematoma involvement, with a higher proportion involving the iliac artery, and there was a significant difference between the two groups (33.3% vs. 2.0%, P = 0.004). The mean maximum diameter of the aorta in the TEVAR group was significantly larger than that in the MM group (43.6 mm ± 8.1 vs. 38.5 mm ± 7.2; P = 0.035). The maximum thickness of the hematoma was 14.9 ± 3.4 mm in the TEVAR group and 10.2 ± 2.8 mm in the MM group, the former was significantly larger than the latter (P = 0.043). There was no significant difference in the CT value of hematoma between the two groups, while the proportion of pleural effusion in the TEVAR group was significantly higher than that in the MM group (75.0% vs. 12.0%; P < 0.001). CT reexamination during hospitalization showed that a total of seven patients in the MM group had disease progression, including two patients with enlarged or thickened hematoma, and four patients with AD or aortic aneurysm. These seven patients were ultimately treated conservatively. The TEVAR group decided to receive TEVAR due to the progression of the lesion. The specific reasons were as follows: expanded hematoma scope and increased thickness in four patients, progression to AD in six patients, and increased pleural effusion in two patients. All these outcomes are depicted in Table 4.
Table 4.
Ct imaging of TEVAR group and MM group.
| Category | MM | TEVAR | P value |
|---|---|---|---|
| Number of patients | 50 | 12 | |
| Intima lesion (ULP/PAU) | 15 (30.0) | 10 (83.3) | 0.002 |
| Single lesion | 12 (24.0) | 2 (16.7) | 0.872 |
| Multiple lesions | 3 (6.0) | 8 (66.7) | <0.001 |
| Location of major intima lesions | |||
| Descending aorta | 12 (24.0) | 7 (58.3) | 0.049 |
| Abdominal aorta | 3 (6.0) | 3 (25.0) | 0.146 |
| Location of proximal hematoma | |||
| Descending aorta | 48 (96.0) | 10 (83.3) | 0.166 |
| Abdominal aorta | 2 (4.0) | 2 (16.7) | 0.166 |
| Location of distal hematoma | |||
| Descending aorta | 13 (26.0) | 3 (25.0) | 1.000 |
| Abdominal aorta | 36 (72.0) | 5 (41.7) | 0.098 |
| Iliac artery | 1 (2.0) | 4 (33.3) | 0.004 |
| MAD, mm | 38.5 ± 7.2 | 43.6 ± 8.1 | 0.035 |
| Maximum haematoma thickness, mm | 10.2 ± 2.8 | 14.9 ± 3.4 | 0.043 |
| CT value of hematoma, HU | 65.3 ± 2.6 | 67.2 ± 4.6 | 0.156 |
| Pleural effusion | 6 (12.0) | 9 (75.0) | <0.001 |
| Reexamination of CT | |||
| Disease progression | 7 (14.0) | 12 (100.0) | <0.001 |
| Enlarged or thickened hematoma | 2 (4.0) | 4 (33.3) | 0.011 |
| Progression to AD or aneurysm | 4 (8.0) | 6 (50.0) | 0.002 |
| Increased pleural effusion | 0 (0) | 2 (16.7) | 0.035 |
| Other | 1 (2.0) | 0 (0) | 1.000 |
| No change or shrinkage of the hematoma | 43 (86.0) | 0 (0) | <0.001 |
AD, aortic dissection; MM, medical management; PAU, penetrating aortic ulcer; TEVAR, thoracic endovascular aortic repair; ULP, ulcer-like projection.
Categorical variables are presented as number (%). Continuous variables are presented as mean ± standard deviation.
Discussion
IMH is one of the common emergencies in vascular surgery. According to the International Registration of Aortic Dissection (IRAD) statistics, about 58% of IMH patients are classified as Stanford type B (8). This study first compared the clinical characteristics of type B IMH and type B AD. There were significant differences in gender composition between the two groups, and the proportion of males in AD group was significantly higher. The average age of the IMH group was significantly higher than that of the AD group, suggesting that the age of onset of IMH was later or the progression of the disease was slower. Studies have shown that female patients with acute aortic syndrome are older, with atypical symptoms and high mortality (9). The comparison of risk factors and common comorbidities between the two groups showed that there was no significant difference between the two groups except for chronic renal insufficiency. This may be due to the fact that the lesions of some AD patients involved the renal artery, while IMH was less likely to have poor organ perfusion and had a better long-term prognosis (10). The initial symptoms were mainly chest and back pain, and other symptoms included abdominal pain, loss of consciousness, dyspnea and so on. The admission CT images of the patients showed that AD lesions involved a wider range, and the proportion of involved iliac arteries was significantly higher than that in the IMH group. Considering the pathogenesis of AD, high-pressure blood flow travels under the intima of the rupture, so it may involve a wider range. While the hematoma of IMH is limited by the adventitia and branch vessels, the range of involvement is smaller.
The current mainstream view for type B IMH is that conservative treatment is the mainstay of the disease (11). In fact, some patients will eventually develop into dissection, aneurysm or even rupture during the follow-up period (12). The clinical and CT imaging characteristics that suggest the need for TEVAR are the focus of this study. Among the 62 IMH patients, 12 patients were finally treated with TEVAR. There was no significant difference in gender composition and age between TEVAR group and MM group. The proportion of patients with dyslipidemia in the TEVAR group was significantly increased, and increased blood lipids is one of the risk factors for endometrial lesions (such as atherosclerosis). Studies have found that if IMH has the following high-risk signs on CT imaging: ULP, increased diameter of aorta, increased thickness of hematoma, intermural blood pool and pericardium/pleural effusion, it is easy to progress to AD, aneurysm or rupture (4). IMH has a unique pathophysiological mechanism compared with AD. In addition to the traditional viewpoint that IMH originates from the rupture of the vasa vasorum, a series of studies have confirmed that a considerable part of IMH is AD with thrombosis of the false lumen. Microscopic intimal rupture can be seen in CT images, which can be further confirmed by reexamination of CTA. The CT value of a typical IMH hematoma is usually 60–70HU, and the thickness of the hematoma is generally greater than 7 mm (13). In this study, most patients in the TEVAR group had intima lesions (including ULP and PAU), and most of them were multiple lesions, which increased significantly compared with the MM group and were mainly located in the descending aorta. The TEVAR group involved a wider range of hematoma and a higher proportion involved the iliac artery. The maximum diameter of aorta, the thickness of hematoma and the ratio of pleural effusion in the TEVAR group were significantly higher than those in the MM group. The above indicators are all risk factors suggesting that the progression of IMH requires surgical intervention, and should be paid close attention in future clinical work. A number of studies have identified high-risk CTA signs of IMH progression (14). Schlatter et al. believed that ULP was associated with complications of IMH, including aneurysm, dissection, rupture, vessel wall thickening, increased surgery rate and mortality. The diameter of ULP is in the range of 10–20 mm and the depth is in the range of 5–10 mm, which is related to the progression of dissection, aneurysm and rupture. Hematoma thickness greater than 11–16 mm is associated with complications (15). Park et al.'s study suggested that type B IMH with a diameter greater than 41 mm is associated with a higher risk (16). Wu et al.'s study suggested that pleural/pericardial effusion was related to IMH complications, such as dissection, aneurysm, surgery and death (17).
All IMH patients were initially treated with medication on admission, primarily to control blood pressure, heart rate and pain. As previously mentioned, a significant proportion of IMH is pseudoluminal thrombotic AD, and many cases are associated with intimal lesions such as ULP and PAU. In the case of unstable blood pressure control, the shear force of the intima impacted by blood flow becomes larger, and the original intima lesions may form a rupture or the previously closed rupture may reopen, forming AD. This further suggests the importance of blood pressure control in IMH medical management (10).
IRAD data show that 5% of Stanford type B IMH require surgical treatment. The main indications are pain, uncontrollable blood pressure, increased thickness of hematoma, combined with aortic PAU, false lumen oppressing the true lumen or various sign of rupture. Active endovascular stent treatment for such Stanford type B IMH was recommended (18). According to the latest 2022 ACC/AHA guidelines, in patients with uncomplicated type B IMH, medical therapy as the initial management strategy is recommended (19). Erbel et al.'s study showed that 61%–91% of patients with uncomplicated IMH had stable or reduced hematoma after medical management, and when complicated conditions such as pericardial effusion, shock, and aneurysm occurred, surgical intervention should be actively considered (20). In this study, all patients in the TEVAR group were treated with TEVAR due to disease progression, the specific reasons included the enlarged or thickened hematoma in four patients, the progression to AD in six patients, and the increase of pleural effusion in two patients. The surgical indications were similar to those in the above literature, and no death occurred in patients treated with TEVAR. A total of seven patients in the MM group had disease progression, including two with hematoma expansion and thickness increase, four with AD or aortic aneurysm. After clinical and imaging evaluation, they were found to be unsuitable for TEVAR. Five patients died in the MM group (three from aortic rupture, one from cerebral infarction, and one from esophageal cancer). The above data suggest that for patients with Stanford type B IMH, it is not only necessary to detect high-risk factors in CT imaging for surgical intervention in time, for patients who do not have these risk factors temporarily, follow-up should also be strengthened.
A systematic analysis showed that the in-hospital mortality of type B AD with different treatment methods was 0%–27% (median 7%) with medical management, 13%–17% (median 16%) with open surgery, and 0%–18% (median 6%) with TEVAR (8). Mesar et al. treated 67 type B IMH patients with medical management after admission, 34 patients failed within 14 days of admission, and 14 patients failed after 14 days of admission. Finally, only 19 patients were successfully treated, and the failure rate of medical management was 71.6%. While the thickness of intramural hematoma was an important factor for medical management failure (21). In the studies of Bischoff et al. and Schoenhoff et al., the failure rate of medical management in patients with type B IMH was 68.3% and 60.0%, respectively. These studies showed that the failure rate of medical management alone was higher (22, 23).
In a multicenter retrospective study of 41 patients with type B IMH, 31 patients were treated with TEVAR and 10 patients were treated with medical management alone. During the 12-month follow-up period, survival rate was lower in the medical management group than in the TEVAR group (77% vs. 85%), with no statistical difference between the two groups. However, patients in the TEVAR group had significantly lower rates of aortic disease progression and adverse aortic events than in the medical management group (24). Li et al. included 56 type B IMH patients in their study, and considered that TEVAR was suitable for patients with the maximum diameter of aorta above 45 mm, the thickness of hematoma above 10 mm, and patients with persistent chest and back pain after drug treatment. All 33 patients in the TEVAR group were successfully operated, and there was no death in hospital. Of the 23 patients in the medical management group, six patients progressed to AD and two died (25). A meta-analysis comparing TEVAR and medical management, including nine studies with a total of 327 patients with type B IMH, showed that TEVAR treatment reduced the probability of long-term progression to dissection and aortic rupture (26). It is worth noting that there are also some unfavorable factors in the treatment of IMH with TEVAR, mainly due to the weak blood vessel wall caused by the hematoma, which may not be able to withstand the pressure of the balloon expansion and stent release during the TEVAR surgery. TEVAR surgery also has a series of other risks, so it is not advisable to blindly expand the indications (27).
Conclusion
In conclusion, Stanford type B IMH is one of the common emergencies in vascular surgery, and it has certain pathophysiological characteristics compared with Stanford type B AD. In the diagnosis of IMH, patients' symptoms and high-risk signs of CTA should be paid attention to. TEVAR therapy should be actively considered on the basis of effective medical management when there are intima lesions (ULP/PAU), increased aortic diameter and hematoma thickness, extensive hematoma involvement, and pleural effusion.
Funding
The authors disclosed the receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Natural Science Research Project of Universities in Anhui Province (KJ2018A0663).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Clinical Medical Research Ethics Committee of the First Affiliated Hospital of Anhui Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors participated in data acquisition. ZZ, FL and ZH contributed to the conception and design of the study. FL, HW and TC did the data analysis and interpretation. ZZ, FL, ZH, YZ and HW contributed to drafting and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Tolboom H, de Beaufort HWL, Smith T, Vos JA, Smeenk HG, Heijmen RH. Endovascular repair of complicated type b aortic intramural haematoma: a single centre long term experience. Eur J Vasc Endovasc Surg. (2022) 63(1):52–8. 10.1016/j.ejvs.2021.09.045 [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Liu Y, Chen B, Jiang J, Shi Y, Ma T, et al. Clinical features and outcomes after endovascular therapy for penetrating aortic ulcer and intramural hematoma. Vascular. (2022) 30(2):191–98. 10.1177/17085381211012573 [DOI] [PubMed] [Google Scholar]
- 3.Alomari IB, Hamirani YS, Madera G, Tabe C, Akhtar N, Raizada V. Aortic intramural hematoma and its complications. Circulation. (2014) 129(6):711–16. 10.1161/CIRCULATIONAHA.113.001809 [DOI] [PubMed] [Google Scholar]
- 4.Kruse MJ, Johnson PT, Fishman EK, Zimmerman SL. Aortic intramural hematoma: review of high-risk imaging features. J Cardiovasc Comput Tomogr. (2013) 7(4):267–72. 10.1016/j.jcct.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 5.Sorber R, Hicks CW. Diagnosis and management of acute aortic syndromes: dissection, penetrating aortic ulcer, and intramural hematoma. Curr Cardiol Rep. (2022) 24(3):209–16. 10.1007/s11886-022-01642-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maslow A, Atalay MK, Sodha N. Intramural hematoma. J Cardiothorac Vasc Anesth. (2018) 32(3):1341–62. 10.1053/j.jvca.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 7.Tian C, Shu C, Luo M, Fang K, Zhang Y. Survival and aortic remodeling outcomes in patients with type b aortic intramural hematoma in the endovascular era: an observational cohort study. J Vasc Surg. (2022) 76(1):70–8. 10.1016/j.jvs.2022.01.143 [DOI] [PubMed] [Google Scholar]
- 8.Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute aortic dissection and intramural hematoma. JAMA. (2016) 316(7):754. 10.1001/jama.2016.10026 [DOI] [PubMed] [Google Scholar]
- 9.Sandhu HK, Tanaka A, Charlton-Ouw KM, Afifi RO, Miller CC, III, Safi HJ, et al. Outcomes and management of type a intramural hematoma. Ann Cardiothorac Surg. (2016) 5(4):317–27. 10.21037/acs.2016.07.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JA, Arnaoutakis GJ, Kilic A, Gleason TG, Aranda Michel E, Sultan I. Medical and surgical management of acute type b aortic intramural hematoma. J Card Surg. (2020) 35(9):2324–30. 10.1111/jocs.14823 [DOI] [PubMed] [Google Scholar]
- 11.Abdu RW, Long GW, Baker D, Boudiab E, Callahan RE, Studzinski DM, et al. Intramural hematoma of the thoracic aorta: a single-institution, 12-year experience. J Vasc Surg. (2022) 75(6):1872–81. 10.1016/j.jvs.2021.12.077 [DOI] [PubMed] [Google Scholar]
- 12.Piazza M, Squizzato F, Porcellato L, Casali E, Grego F, Antonello M. Predictors of intervention in acute type b aortic penetrating ulcer and intramural hematoma. Semin Thorac Cardiovasc Surg. (2022) 22:S1043–0679. 00190-3. 10.1053/j.semtcvs.2022.07.009 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg JB, Kim JB, Sundt TM. Current understandings and approach to the management of aortic intramural hematomas. Semin Thorac Cardiovasc Surg. (2014) 26(2):123–31. 10.1053/j.semtcvs.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Song J. Update in acute aortic syndrome: intramural hematoma and incomplete dissection as new disease entities. J Cardiol. (2014) 64(3):153–61. 10.1016/j.jjcc.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 15.Schlatter T, Auriol J, Marcheix B, Lebbadi M, Marachet MA, Dang-Tran KD, et al. Type b intramural hematoma of the aorta: evolution and prognostic value of intimal erosion. J Vasc Interv Radiol. (2011) 22(4):533–41. 10.1016/j.jvir.2010.10.028 [DOI] [PubMed] [Google Scholar]
- 16.Park GM, Ahn JM, Kim DH, Kang JW, Song JM, Kang DH, et al. Distal aortic intramural hematoma: clinical importance of focal contrast enhancement on ct images. Radiology. (2011) 259(1):100–08. 10.1148/radiol.11101557 [DOI] [PubMed] [Google Scholar]
- 17.Wu MT, Wang YC, Huang YL, Chang RS, Li SC, Yang P, et al. Intramural blood pools accompanying aortic intramural hematoma: ct appearance and natural course. Radiology. (2011) 258(3):705–13. 10.1148/radiol.10101270 [DOI] [PubMed] [Google Scholar]
- 18.Harris KM, Braverman AC, Eagle KA, Woznicki EM, Pyeritz RE, Myrmel T, et al. Acute aortic intramural hematoma. Circulation. (2012) 126(11_suppl_1):S91–6. 10.1161/CIRCULATIONAHA.111.084541 [DOI] [PubMed] [Google Scholar]
- 19.Isselbacher EM, Preventza O, Black JR, Augoustides JG, Beck AW, Bolen MA, et al. 2022 Acc/aha guideline for the diagnosis and management of aortic disease: a report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. Circulation. (2022) 24:e334–e482. 10.1161/CIR.0000000000001106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European society of cardiology (ESC). Eur Heart J. (2014) 35(41):2873–926. 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 21.Mesar T, Lin MJ, Kabir I, Dexter DJ, Rathore A, Panneton JM. Medical therapy in type b aortic intramural hematoma is associated with a high failure rate. J Vasc Surg. (2020) 71(4):1088–96. 10.1016/j.jvs.2019.07.084 [DOI] [PubMed] [Google Scholar]
- 22.Bischoff MS, Meisenbacher K, Wehrmeister M, Böckler D, Kotelis D. Treatment indications for and outcome of endovascular repair of type b intramural aortic hematoma. J Vasc Surg. (2016) 64(6):1569–79. 10.1016/j.jvs.2016.05.078 [DOI] [PubMed] [Google Scholar]
- 23.Schoenhoff FS, Zanchin C, Czerny M, Makaloski V, Gahl B, Carrel T, et al. Aorta related and all-cause mortality in patients with aortic intramural haematoma. Eur J Vasc Endovasc Surg. (2017) 54(4):447–53. 10.1016/j.ejvs.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 24.Piffaretti G, Lomazzi C, Benedetto F, Pipito N, Castelli P, Trimarchi S, et al. Best medical treatment and selective stent-graftrepair for acute type b aortic intramural hematoma. Semin Thorac Cardiovasc Surg. (2018) 30(3):279–87. 10.1053/j.semtcvs.2018.02.006 [DOI] [PubMed] [Google Scholar]
- 25.Li DL, Zhang HK, Cai YY, Jin W, Chen XD, Tian L, et al. Acute type b aortic intramural hematoma: treatment strategy and the role of endovascular repair. J Endovasc Ther. (2010) 17(5):617–21. 10.1583/10-3125.1a [DOI] [PubMed] [Google Scholar]
- 26.Chakos A, Twindyawardhani T, Evangelista A, Maldonado G, Piffaretti G, Yan TD, et al. Endovascular versus medical management of type b intramural hematoma: a meta-analysis. Ann Cardiothorac Surg. (2019) 8(4):447–55. 10.21037/acs.2019.06.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel HJ, Williams DM, Upchurch GR, Dasika NL, Deeb GM. The challenge of associated intramural hematoma with endovascular repair for penetrating ulcers of the descending thoracic aorta. J Vasc Surg. (2010) 51(4):829–35. 10.1016/j.jvs.2009.11.050 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.