Abstract
Environmental pollution caused by polycyclic aromatic hydrocarbons (PAHs) involves a high-risk and have received considerable attention due to their carcinogenic, teratogenic, and mutagenic properties. Phenanthrene (PHE) is a low molecular weight PAH, which has three benzene rings. It is one of the most common PAH found in contaminated environments mainly due to its low volatilization ability and hydrophobic character. A PHE degrading bacterium was isolated from an industrial contaminated soil using enrichment culture techniques. Based on macroscopic, microscopic examination and phylogenetic analysis, this bacterium was classified as Stenotrophomonas indicatrix and named strain CPHE1. Several authors have reported about bacteria stains, which can degrade PHE, but this is the first time where the ability of S. indicatrix to biodegrade and mineralize PHE has been demonstrated.
Keywords: Polycyclic aromatic hydrocarbons, Phenanthrene, Degrading-bacterium, Whole genome sequence, Stenotrophomonas indicatrix
Introduction
PAHs come mainly from incomplete combustion of organic matter (OM), due to anthropogenic causes (waste incineration, use of fossil fuels, vehicle emissions, home heating, industrial processes, etc.) or from natural causes (volcanic eruptions, forest fires, etc.). However, PAHs come also from natural origin because of geochemical processes that can occur in sedimentary OM, such as aromatization, decarboxylation and defunctionalization (Abdel-Shafy and Mansour 2016; Perra et al. 2009). PAHs are considered as persistent organic pollutants (POPs) due to their lipophilic and hydrophobic character, their low volatility, and their capacity for bioaccumulation in ecosystems. They present long half-lives in soils, sediments, and biota (Badejo et al. 2013; Khan et al. 2019).
The PAHs ubiquity poses a risk to human health. They can interfere with the function of cellular membranes, causing genotoxicity, as well as carcinogenic and immunosuppressant effects (Abdel-Shafy and Mansour 2016; Bateni et al. 2022).
PHE has been selected as representative compound of PAHs for several reasons: It is extensively detected in the environment; it is considered as landmark substance in order to detect PAH pollution, since the PHE structure is present in the majority of PAHs, and it is the smallest PAHs that contains two types of regions known as bay and k, responsible for their toxicity (Sánchez-Trujillo et al. 2014; Ghosal et al. 2016; Luo et al. 2022). Hence, it is essential the removal of PHE from the environment. Microbial degradation is an efficient technology to convert PHE into non-toxic substances (Lara-Moreno et al. 2021).
Stenotrophomonas indicatrix CPHE1, isolated from an industrial soil, efficiently removes PHE from an aqueous solution. CPHE1 strain could use PHE as carbon and energy source and degraded around 100% and mineralizing 38.2% PHE after 30 d in solution (initial concentration 10 mg L−1) (Fig. 1). In case of biodegradation assay, PHE concentration was monitored using gas chromatographer (Agilent GC 6890 N) combined with a mass spectrometer (MS, Agilent MD 5975B). In the study of PHE mineralization, PHE was quantified through evolution of the 14CO2 produced, using a liquid scintillation counter (Beckman Instruments Inc., Fullerton, California, model L55000TD). Both methods were described by Lara-Moreno et al. (2021).
Fig. 1.
Solid line shows PHE biodegradation (left axis) and bar chart mineralization (right axis) process in solution after inoculation with S. indicatrix CPHE1. Standard deviation (vertical bars)
The identity of the studied strain was determined by a comparative BLAST analysis of 16S rRNA gene. We found that 16S rRNA gene shares 99,7% similarity with the 16S rRNA genes of S. indicatrix sp. (Weber et al. 2018). Previously, Lara-Moreno et al. (2021) classified the degrading bacteria as S. maltophilia CPHE1 (NCBI number: MT138842), but later, a more in-depth genetic study revealed that this specie is closer phylogenetically to Stenotrophomas indicatrix. This result is in line with the study carried out by Weber et al. (2018) classified for the first time this species as S. indicatrix, but they had previously classified it as S. maltophilia due to their high genomic similarity.
As far as we know, only two studies have published detailing genomic information regarding PHE degradation route in Stenotrophomonas genus (Kumari et al. 2017; Elufisan et al. 2020). However, no genome of a Stenotrophomonas genus with the capability to mineralize PHE has been sequenced. The genome of S. indicatrix CPHE1 has been sequenced to obtain useful genetic information about the genes involved in PHE biodegradation pathway. The CPHE1 genome was sequenced by MicrobesNG company (Birmingham, UK) using Illumina next-generation sequencing data at a minimum coverage of 30x (https://microbesng.com/). The generated sequencing reads were assembled using SPAdes genome assembler version 3.14 (https://cab.spbu.ru/software/spades/). The bacterial genome has been annotated using RAST tool (Rapid Annotation Subsystem Technology) and NCBI Prokaryotic Genome Annotation Pipeline. It is composed by 163 contigs for a total of 4,553,664 bp and a G + C content of 66.1%. A total of 4137 coding sequences are present in the genome. The genome also encodes 2 16S rRNAs, 4 5S rRNAs, 2 23S rRNAs and 64 tRNAs (Table 1).
Table 1.
Genomic features of S. indicatrix CPHE1
| Features | |
|---|---|
| Size (bp) | 4.553.664 |
| Contigs | 163 |
| GC content (%) | 66.1 |
| rRNAs (5S, 16S, 23S) | 4, 2, 2 |
| tRNA | 64 |
The genome contains 15 genes responsible for the metabolism of aromatic compounds: quinate, benzoate and gentisate degradation, catechol branch of beta-ketoadipate and homogentisate pathway and salicylate and gentisate catabolism. Some enzymes responsible for these kinds of metabolisms have been previously described in the PAHs biodegradation route (Rodríguez-Salazar et al. 2020).
In addition, 105 genes are associated with the motility and chemotaxis. It is known that these genes could help the bacterium to locate the pollutant and increase the mass transfer, enhancing and facilitating the biodegradation process (Ibrar et al. 2022). Moreover, it was demonstrated that bacteria have the chemotaxis toward aromatic compounds which can be unleashed via sensing their intermediate metabolites of tricarboxylic acid cycle. These genes could help bacteria to find aromatic compounds, and the degradation of aromatic compounds can produce more chemo-effectors to attract bacteria (Lu et al. 2022). Regarding the genes involved in resistance to antibiotics and toxic compounds, we found 80 candidate genes implicated in this function. The presence of these kinds of genes could provide the ability to grow in the presence of certain aromatic compounds as sole carbon and energy sources (Safari et al. 2019).
Oxygenases are a group of enzymes able to oxidize a substrate, transferring an atom of oxygen from molecular oxygen (O2) to substrate. Monooxygenase and dioxygenase enzymes have a crucial role in the PAHs degradation. They have been described as responsible for catalyzing the first step of the aromatic hydrocarbons degradation pathway, and hence, as specific markers of PAHs pollution (Fuentes et al. 2014). Without oxygenase activity, hydrocarbons could not be metabolized into central metabolic routes (Bagi et al. 2022). Twenty-two oxygenases have been identified in the genome sequence of S. indicatrix CPHE1. Oxygenases are classified in nine monooxygenases and 13 dioxygenases.
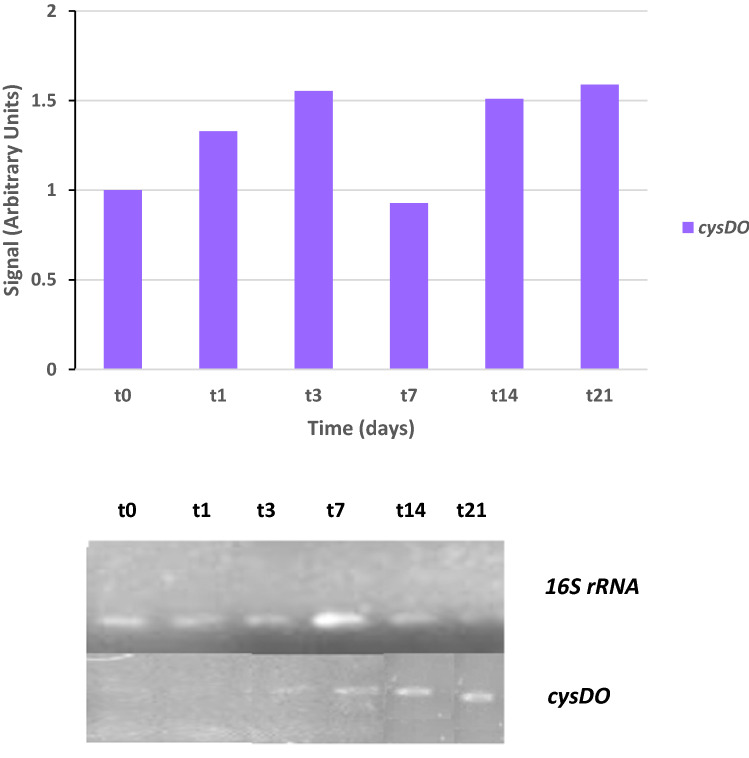
Semi-quantitative RT-PCR was used to predict the dioxygenase responsible for PHE biodegradation. Several authors have employed these techniques to confirm the involvement of genes in the biodegradation pathway of different pollutants (Churchill et al. 2008; di Canito et al. 2018; Thanh et al. 2019). The results showed that only one dioxygenase, cysteine dioxygenase (cysDO), was expressed in the presence of PHE from the third day of treatment, with a length of 588 bp. Quantification of cysDO expression in relation to the 16S rRNA and negative control (t0) signal is shown in the histograms (Fig. 2). This dioxygenase could be the responsible for initiation of the PHE mineralization pathway that proceeds through phenanthrene-3,4-dihydrodiol (Stingley et al. 2004).
Fig. 2.
RT-PCR analysis of cysteine dioxygenases gene expression in S. indicatrix CPHE1. cDNAs were synthesized from RNA at different times (to, t1, t3, t7, t14 and t21). PCR with specific primers for cysDO (30 cycles) and 16S rRNA (13 cycles) gene were performed. 16S rRNA expression was used as RNA-loading control for these different samples. Dioxygenase quantification expression in relation to the 16S rRNA and negative control (t0) signal is shown in the above histograms (in arbitrary units)
The follow step usually is catalyzed by dehydrogenases. Dehydrogenases are member of the oxidoreductive group of enzymes. They can oxidize a substrate by reducing an electron acceptor, generally NADH/NADPH/FAD/FMN. Dehydrogenases are known for their participation in the second step of PHE biodegradation, transforming phenanthrene-3,4-dihydrodiol into 3,4-dihydroxyphenanthrene (Stingley et al. 2004). We identified 119 proteins with dehydrogenase function in the genome of CPHE1 strain.
In conclusion, the genome of S. indicatrix CPHE1 contributes to elucidate and facilitate the studies about the mechanism of biodegradation of PHE in the Stenotrophomonas genus which is little known.
Acknowledgements
This work was supported by the Spanish Ministry of Economy and Competitiveness under the research project CMT2017-82472-C2-1-R (AEI/FEDER, UE) and by grants from the Spanish Ministry of Education, Culture and Sports for her FPU fellowship (FPU15/03740) and University of Seville for her Margarita Salas grant funded by the European Union’s Next Generation EU.
Author contributions
ALM contributed to writing—original draft preparation, conceptualization, methodology, investigation, EM contributed to resources, funding acquisition, FM contributed to resources, supervision, JLGP contributed to date curation, JV contributed to investigation, validation, supervision, writing.
Data availability
The sequencing data generated in this study were deposited in the National Center for Biotechnology Information (NCBI), under the BioProject ID PRJNA868539. The whole genome shotgun of S. indicatrix CPHE1 has been deposited in DDBJ/ENA/GenBank under the accession number JANQDV000000000. The version described in this paper is version JANQDV010000000. The raw data were deposited in the Sequence Read Archive (SRA) under the accession number SRR21098193.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Informed consent
We affirm that all the authors have seen, prepared, and agreed to the submission of the paper and their inclusion of name(s) as co-author(s). We also declare that there are no conflicts of interest for the same.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Alba Lara-Moreno, Email: lara@irnas.csic.es.
Jaime Villaverde, Email: jvillaverde@irnase.csic.es.
References
- Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet. 2016;25(1):107–123. doi: 10.1016/j.ejpe.2015.03.011. [DOI] [Google Scholar]
- Badejo AC, Choi CW, Badejo AO, Shin KH, Hyun JH, Lee YG, Kim S, Park KS, Kim SH, Jung KH, Chung YH, Chai YG. A global proteome study of Mycobacterium gilvum PYR-GCK grown on pyrene and glucose reveals the activation of glyoxylate, shikimate and gluconeogenetic pathways through the central carbon metabolism highway. Biodegradation. 2013;24(6):741–752. doi: 10.1007/s10532-013-9622-9. [DOI] [PubMed] [Google Scholar]
- Bagi A, Knapik K, Baussant T. Abundance and diversity of n-alkane and PAH-degrading bacteria and their functional genes—potential for use in detection of marine oil pollution. Sci Total Environ. 2022;810:152238. doi: 10.1016/j.scitotenv.2021.152238. [DOI] [PubMed] [Google Scholar]
- Bateni F, Mehdinia A, Lundin L, Hashtroudi MS. Distribution, source and ecological risk assessment of polycyclic aromatic hydrocarbons in the sediments of northern part of the Persian Gulf. Chemosphere. 2022;295:133859. doi: 10.1016/j.chemosphere.2022.133859. [DOI] [PubMed] [Google Scholar]
- Churchill PF, Morgan AC, Kitchens E. Characterization of a pyrene-degrading Mycobacterium sp. strain CH-2. J Environ Sci Health Part B Pesticides Food Contaminants Agric Wastes. 2008;43(8):698–706. doi: 10.1080/03601230802388801. [DOI] [PubMed] [Google Scholar]
- di Canito A, Zampolli J, Orro A, D’Ursi P, Milanesi L, Sello G, Steinbüchel A, di Gennaro P. Genome-based analysis for the identification of genes involved in o-xylene degradation in Rhodococcus opacus R7. BMC Genomics. 2018;19(1):1–17. doi: 10.1186/s12864-018-4965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elufisan TO, Rodríguez-Luna IC, Oyedara OO, Sánchez-Varela A, Hernández-Mendoza A, Gonzalez ED, Paz-González AD, Muhammad K, Rivera G, Villalobos-Lopez MA, Guo X. The Polycyclic Aromatic Hydrocarbon (PAH) degradation activities and genome analysis of a novel strain Stenotrophomonas sp. Pemsol isolated from Mexico. Microbiology. 2020;8:e8102. doi: 10.7717/peerj.8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes S, Méndez V, Aguila P, Seeger M. Bioremediation of petroleum hydrocarbons: catabolic genes, microbial communities, and applications. Appl Microbiol Biotechnol. 2014;98(11):4781–4794. doi: 10.1007/s00253-014-5684-9. [DOI] [PubMed] [Google Scholar]
- Ghosal D, Ghosh S, Dutta TK, Ahn Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): a review. Front Microbiol. 2016;7:1369. doi: 10.3389/fmicb.2016.01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrar M, Khan S, Hasan F, Fang X. Biosurfactants and chemotaxis interplay in microbial consortium-based hydrocarbons degradation. Environ Sci Pollut Res. 2022;29(17):24391–24410. doi: 10.1007/s11356-022-18492-9. [DOI] [PubMed] [Google Scholar]
- Khan AHA, Ayaz M, Arshad M, Yousaf S, Khan MA, Anees M, Sultan A, Nawaz I, Iqbal M. Biogeochemical cycle, occurrence and biological treatments of polycyclic aromatic hydrocarbons (PAHs) Iran J Sci Technol Transa A Sci. 2019;43(3):1393–1410. doi: 10.1007/s40995-017-0393-8. [DOI] [Google Scholar]
- Kumari S, Regar RK, Bajaj A, Ch R, Satyanarayana GNV, Mudiam MKR, Manickam N. Simultaneous biodegradation of polyaromatic hydrocarbons by a Stenotrophomonas sp.: characterization of nid genes and effect of surfactants on degradation. Indian J Microbiol. 2017;57(1):60–67. doi: 10.1007/s12088-016-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Moreno A, Morillo E, Merchán F, Villaverde J. A comprehensive feasibility study of effectiveness and environmental impact of PAH bioremediation using an indigenous microbial degrader consortium and a novel strain Stenotrophomonas maltophilia CPHE1 isolated from an industrial polluted soil. J Environ Manag. 2021;289:112512. doi: 10.1016/j.jenvman.2021.112512. [DOI] [PubMed] [Google Scholar]
- Lu Q, Sun X, Jiang Z, Cui Y, Li X, Cui J. Effects of Comamonas testosteroni on dissipation of polycyclic aromatic hydrocarbons and the response of endogenous bacteria for soil bioremediation. Environ Sci Pollut Res. 2022 doi: 10.1007/s11356-022-21497-z. [DOI] [PubMed] [Google Scholar]
- Luo C, Hu X, Bao M, Sun X, Li F, Li Y, Liu W, Yang Y. Efficient biodegradation of phenanthrene using Pseudomonas stutzeri LSH-PAH1 with the addition of sophorolipids: alleviation of biotoxicity and cometabolism studies. Environ Pollut. 2022;301:119011. doi: 10.1016/j.envpol.2022.119011. [DOI] [PubMed] [Google Scholar]
- Perra G, Renzi M, Guerranti C, Focardi SE. Polycyclic aromatic hydrocarbons pollution in sediments: distribution and sources in a lagoon system (Orbetello, Central Italy) Transitional Waters Bull. 2009;3(1):45–58. doi: 10.1285/i1825229Xv3n1p45. [DOI] [Google Scholar]
- Rodríguez-Salazar J, Almeida-Juarez AG, Ornelas-Ocampo K, Millán-López S, Raga-Carbajal E, Rodríguez-Mejía JL, Muriel-Millán LF, Godoy-Lozano EE, Rivera-Gómez N, Rudiño-Piñera E, Pardo-López L. Characterization of a novel functional trimeric catechol 1,2-dioxygenase from a Pseudomonas stutzeri isolated from the Gulf of Mexico. Front Microbiol. 2020;11:1–17. doi: 10.3389/fmicb.2020.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safari M, Yakhchali B, Shariati JV. Comprehensive genomic analysis of an indigenous Pseudomonas pseudoalcaligenes degrading phenolic compounds. Sci Rep. 2019;9(1):12736. doi: 10.1038/s41598-019-49048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Trujillo MA, Lacorte S, Villaverde J, Barata C, Morillo E. Decontamination of polycyclic aromatic hydrocarbons and nonylphenol from sewage sludge using hydroxypropyl-β-cyclodextrin and evaluation of the toxicity of leachates. Environ Sci Pollut Res. 2014;21:507–517. doi: 10.1007/s11356-013-1930-4. [DOI] [PubMed] [Google Scholar]
- Stingley RL, Khan AA, Cerniglia CE. Molecular characterization of a phenanthrene degradation pathway in Mycobacterium vanbaalenii PYR-1. Biochem Biophys Res Commun. 2004;322:133–146. doi: 10.1016/j.bbrc.2004.07.089. [DOI] [PubMed] [Google Scholar]
- Thanh LTH, Thi TVN, Shintani M, Moriuchi R, Dohra H, Loc NH, Kimbara K. Isolation and characterization of a moderate thermophilic Paenibacillus naphthalenovorans strain 4B1 capable of degrading dibenzofuran from dioxin-contaminated soil in Vietnam. J Biosci Bioeng. 2019;128(5):571–577. doi: 10.1016/j.jbiosc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Weber M, Schünemann W, Fuß J, Kämpfer P, Lipski A. Stenotrophomonas lactitubi sp. nov. and Stenotrophomonas indicatrix sp. nov., isolated from surfaces with food contact. Int J Syst Evol Microbiol. 2018;68(6):1830–1838. doi: 10.1099/ijsem.0.002732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequencing data generated in this study were deposited in the National Center for Biotechnology Information (NCBI), under the BioProject ID PRJNA868539. The whole genome shotgun of S. indicatrix CPHE1 has been deposited in DDBJ/ENA/GenBank under the accession number JANQDV000000000. The version described in this paper is version JANQDV010000000. The raw data were deposited in the Sequence Read Archive (SRA) under the accession number SRR21098193.