Neuropathic pain is a major health problem affecting up to 10% of the general population, resulting from a lesion or dysfunction of the somatosensory nervous system, including peripheral nerve injury (PNI) [1]. However, neuropathic pain is difficult to treat because of its obscure mechanisms. Diverse types of neuropathic pain have been modelled in rodents to study its underlying mechanisms and identify novel therapeutic targets. The spinal nerve ligation (SNL) and the spared nerve injury (SNI) models are widely used for PNI-induced pain. These models are considered to mimic peripheral nerve injuries including major surgery and post-traumatic peripheral painful neuropathic states in human patients [2]. Of note, neuropathic pain in the SNL model could be gradually resolved after several (usually 5–10) weeks, while chronic pain would last for >5 months in SNI mice. Thus, it is critical to study the mechanisms underlying the resolution of neuropathic pain, which could lead to effective therapeutic strategies. Recently, Kohno et al. [3] have revealed that a specialized sub-population of microglia in the spinal cord is responsible for resolving neuropathic pain in a modified SNL mouse model in which the L4 spinal nerve was cut and pain symptoms were fully resolved after 5 weeks.
Microglia are resident immune cells in the brain and spinal cord, and are important for maintaining the homeostasis of the central nervous system. It is well established that microglial activation and microgliosis in the spinal cord are critical for both the initiation and maintenance of chronic pain [4, 5]. Recent studies suggest that microglia are heterogeneous and contribute to the resolution of neuroinflammation in the brain and spinal cord [6, 7]. Increasing evidence indicates that microglia also play a role in the resolution of chronic pain [5, 8]. However, the identity of the pain-resolving microglia and the mechanism by which they resolve neuropathic pain are still unclear. Combining flow cytometry analyses, fluorescent activated cell sorting (FACS) and RNA-seq of spinal microglia, diphtheria toxin receptor (DTR)-mediated targeted cell depletion, phagocytosis assays, and conditional KO mouse models, Kohno et al. [3] demonstrated that specialized CD11c+ microglia in the spinal dorsal horn resolve neuropathic pain via insulin-like growth factor-1 (IGF-1) signaling. Furthermore, these specialized CD11c+ microglia express the AXL receptor tyrosine kinase and phagocyte myelin debris to release IGF-1, which mediates the resolution of neuropathic pain (Fig. 1).
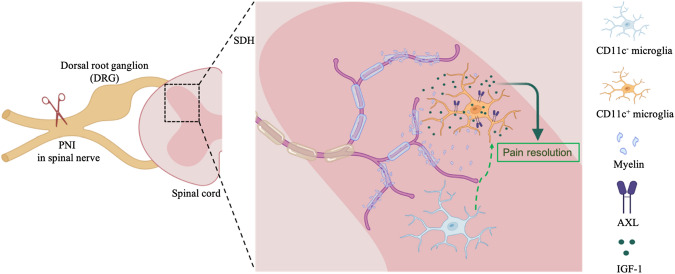
Fig. 1.
Specialized CD11c+ microglia in the spinal dorsal horn resolve neuropathic pain via insulin-like growth factor-1 (IGF-1) signaling. Peripheral nerve injury (PNI) causes the degeneration of primary afferents of DRG neurons and the shedding of myelin sheaths from damaged afferents. A subset of microglia phagocyte the myelin debris and CD11c+ microglia appear in the spinal dorsal horn (SDH). These specialized CD11c+ microglia express the AXL receptor tyrosine kinase (AXL) and release IGF-1 to resolve pain.
To determine whether CD11c+ microglia are necessary for the remission of neuropathic pain, Kohno et al. [3] took advantage of Itgax (integrin aX, also known as CD11c)-Venus mice and found that CD11c-positive (CD11c+, also Venus+) microglia emerged and increased in spinal dorsal horn after PNI. Further behavioral testing combined with flow cytometry analyses revealed that CD11chigh microglia in the spinal dorsal horn peaked in the fifth week after PNI, when neuropathic pain was resolved in this modified SNL model. It seems that the number of CD11chigh microglia was positively correlated with the remission of mechanical allodynia in PNI mice. Moreover, Kohno et al. [3] used a DTR-mediated CD11c+ cell-depletion strategy in Itgax-DTR-EGFP mice, and demonstrated that mice with depletion of CD11c+ spinal microglia from day 14 after PNI failed to recover from neuropathic pain. These results indicate that CD11c+ microglia in the dorsal horn are required for the resolution of neuropathic pain.
Next, Kohno et al. [3] illustrated that IGF-1 is released by CD11c+ microglia and required for the recovery from neuropathic pain. They conducted FACS sorting and RNA sequencing of spinal microglia from Itgax-Venus mice, and found that Igf1 was strongly expressed in CD11chigh spinal microglia and peaked at day 35, while PNI-induced pain was resolved. To determine whether IGF1 is required for resolving neuropathic pain, Kohno et al. conditionally knocked out (CKO) Igf1 in CD11c+ cells using Itgax-Cre;Igf1flox/flox mice or in CX3CR1+ microglia/macrophages using Cx3cr1CreERT2;Igf1flox/flox mice, and found that these Igf1 CKO mice did not recover from neuropathic pain induced by PNI. Moreover, intrathecal administration of IGF1-neutralizing antibodies prevented pain recovery from PNI, while intrathecal injection of recombinant IGF-1 promoted pain recovery in PNI mice. Therefore, these data suggest that IGF-1 derived from spinal CD11c+ microglia are both necessary and sufficient for the recovery from neuropathic pain (Fig. 1).
Kohno et al. took further steps to explore the molecular mechanism controlling the appearance of CD11c+ spinal microglia after PNI [3]. Microglial activation in the spinal cord after nerve injury depends on the activity of both unmyelinated C-fiber and myelinated A-fiber primary sensory neurons in the dorsal root ganglions (DRGs) [9]. Kohno et al. injected conjugated saporin to selectively kill myelinated and unmyelinated DRG fibers in naïve mice; interestingly, CD11c+ microglia were robustly induced in the dorsal horn only with myelinated afferents injured by the injection of CTB-saporin. Their results also showed that CD11c+ spinal microglia were able to engulf myelin debris and contained phagocytosed myelin particles after PNI revealed by electron microscopy and phagocytosis assays. Notably, the engulfment of myelin debris was sufficient to induce spinal microglia expressing CD11c. Furthermore, Kohno et al. confirmed that AXL—a receptor tyrosine kinase associated with phagocytosis [10]—was expressed in CD11chigh spinal microglia of PNI mice, and AXL was important for Igf1 up-regulation and the recovery from neuropathic pain after PNI. These data indicate that phagocytosis of shredded myelin sheaths from damaged primary afferents is critical for the induction of CD11c+ microglia in the spinal dorsal horn after PNI (Fig. 1).
Since CD11c+ spinal microglia remained high after 5 weeks of PNI when pain has fully resolved, Kohno et al. [3] further investigated their function using a targeted depletion strategy. Unexpectedly, depletion of CD11c+ spinal microglia or knocking down Igf1 expression in spinal microglia at 5 weeks after PNI lead to the recurrence of pain. Similarly, intrathecal injection of IGF-1 neutralizing antibody or an IGF1 receptor inhibitor also induced pain hypersensitivity. These results suggest that ongoing release of IGF1 from CD11c+ spinal microglia is required to maintain pain resolution in the neuropathic state after PNI.
Taken together, Kohno et al. [3] used multiple approaches to comprehensively evaluate the function of CD11c+ spinal microglia in the resolution of neuropathic pain. Previous studies have demonstrated that microglial activation in the spinal cord plays important roles in the pathogenesis of neuropathic pain in male but not in female animals [11]. Here, Kohno et al. present the first evidence that specialized CD11c+ microglia in the spinal dorsal horn are critical for the resolution of neuropathic pain via releasing IGF-1, in a sex-independent manner. This study also provides a new strategy for neuropathic pain treatment by targeting specialized microglia and IGF-1 signaling. Since IGF-1 receptors are widely expressed in many cell types including neurons and glial cells [12], the cells targeted by IGF-1 need to be further investigated.
The study by Kohno et al. calls for future research to understand the emergence and function of specialized microglia in chronic pain. It is vital to determine how pain-resolving microglia could be induced under long-lasting chronic pain conditions, such as in the SNI model in which neuropathic pain could last for at least 5 months. Interestingly, a subset of microglia that express Axl and Igf1 have been detected in the spinal cord of SNI mice after 5 months, revealed by single-cell RNA sequencing [13]. Further studies are warranted to determine whether pain-resolving CD11c+ microglia could be induced and the AXL-IGF1 axis could contribute to the resolution of neuropathic pain in SNI mice. In addition, a recent study showed that spinal cord macrophages also contributed to resolving spinal neuroinflammation and pain in mice after injury [14]. To rule out the contribution of CD11c+ cells from peripheral monocytes, Kohno et al. used bone marrow–chimera mice to establish the local origin of CD11c+ spinal microglia for the resolution of neuropathic pain [3]. Overall, future studies identifying the molecular signaling that regulates CD11c+ microglia will be helpful for a better understanding of the roles of microglia in pain resolution and provide novel targets for neuropathic pain treatment.
Acknowledgements
This research highlight was supported by the National Key R&D Program of China (2021ZD0202703), Zhejiang Provincial Natural Science Foundation (LZ18C090002), and the National Natural Science Foundation of China (81971050 and 82171206).
Contributor Information
Shengmei Zhu, Email: smzhu20088@zju.edu.cn.
Zhen-Zhong Xu, Email: xuzz@zju.edu.cn.
References
- 1.Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: From mechanisms to treatment. Physiol Rev. 2021;101:259–301. doi: 10.1152/physrev.00045.2019. [DOI] [PubMed] [Google Scholar]
- 2.Price TJ, Basbaum AI, Bresnahan J, Chambers JF, de Koninck Y, Edwards RR, et al. Transition to chronic pain: Opportunities for novel therapeutics. Nat Rev Neurosci. 2018;19:383–384. doi: 10.1038/s41583-018-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohno K, Shirasaka R, Yoshihara K, Mikuriya S, Tanaka K, Takanami K, et al. A spinal microglia population involved in remitting and relapsing neuropathic pain. Science. 2022;376:86–90. doi: 10.1126/science.abf6805. [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Tsuda M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19:138–152. doi: 10.1038/nrn.2018.2. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: Detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100:1292–1311. doi: 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shemer A, Erny D, Jung S, Prinz M. Microglia plasticity during health and disease: An immunological perspective. Trends Immunol. 2015;36:614–624. doi: 10.1016/j.it.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, et al. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557:724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sideris-Lampretsas G, Malcangio M. Microglial heterogeneity in chronic pain. Brain Behav Immun. 2021;96:279–289. doi: 10.1016/j.bbi.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, et al. Injured sensory neuron–derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:94–101. doi: 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Happonen KE, Burrola PG, O’Connor C, Hah N, Huang L, et al. Microglia use TAM receptors to detect and engulf amyloid β plaques. Nat Immunol. 2021;22:586–594. doi: 10.1038/s41590-021-00913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mogil JS. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat Rev Neurosci. 2020;21:353–365. doi: 10.1038/s41583-020-0310-6. [DOI] [PubMed] [Google Scholar]
- 12.Krieger CC, Neumann S, Gershengorn MC. TSH/IGF1 receptor crosstalk: Mechanism and clinical implications. Pharmacol Ther. 2020;209:107502. doi: 10.1016/j.pharmthera.2020.107502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tansley S, Uttam S, Ureña Guzmán A, Yaqubi M, Pacis A, Parisien M, et al. Single-cell RNA sequencing reveals time- and sex-specific responses of mouse spinal cord microglia to peripheral nerve injury and links ApoE to chronic pain. Nat Commun. 2022;13:843. doi: 10.1038/s41467-022-28473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niehaus JK, Taylor-Blake B, Loo L, Simon JM, Zylka MJ. Spinal macrophages resolve nociceptive hypersensitivity after peripheral injury. Neuron. 2021;109:1274–1282.e6. doi: 10.1016/j.neuron.2021.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]